Abstract
Introduction
Exercise improves gait in Parkinson disease (PD), but whether exercise differentially affects people with PD with (freezers) and without freezing of gait (non-freezers) remains unclear. This study examines exercise’s effects on gait performance, neural correlates related to these effects, and potential neural activation differences between freezers and non-freezers during motor imagery (MI) of gait.
Methods
Thirty-seven participants from a larger exercise intervention completed behavioral assessments and functional magnetic resonance imaging (fMRI) scans before and after a 12-week exercise intervention. Gait performance was characterized using gait velocity and stride length, and a region of interest (ROI) fMRI analysis examined task-based blood oxygen-level dependent (BOLD) signal changes of the somatomotor network (SMN) during MI of forward (IMG-FWD) and backward (IMG-BWD) gait.
Results
Velocity (F(1,34)=55.04, p<0.001) and stride length (F(1,34)=77.58, p<0.001) were significantly lower for backward versus forward walking in all participants. The ROI analysis showed freezers had lower BOLD signal compared to non-freezers in the cerebellum (F(1,32)=7.01, p=0.01), primary motor (left: F(1,32)=7.09, p=0.01; right: F(1,32)=7.45, p=0.01), and primary sensory (left: F(1,32)=9.59, p=0.004; right: F(1,32)=8.18, p=0.007) cortices during IMG-BWD only. The evidence suggests the exercise intervention did not affect gait or BOLD signal during MI.
Conclusion
While all participants had significantly slower and shorter backward velocity and stride length, respectively, the exercise intervention had no effect. Similarly, BOLD signal during MI did not change with exercise; however, freezers had significantly lower BOLD signal during IMG-BWD compared to non-freezers. This suggests potential decreased recruitment of the SMN during MI of gait in freezers.
Keywords: Parkinson disease, Freezing of Gait, Cerebellum, Neuroimaging
Introduction
Gait impairment is a common feature of Parkinson disease (PD) and is characterized by short, asymmetric steps and slow velocity. Gait decrements are evident in both forward and backward walking and are associated with an increase in debilitating falls [1]. In addition to the aforementioned gait impairments, approximately 50% of individuals with PD experience freezing of gait (FOG) at some point during the disease, with prevalence increasing with increased disease severity [2, 3]. Individuals with PD and FOG (freezers) describe freezing episodes as feeling like their feet are stuck to the floor or as an inability to take another step forward [4]. Freezers have an even greater risk of falls and further gait decrements compared to people with PD without FOG (non-freezers) [1]. By addressing gait impairment in people with PD, we may be able to reduce fall risk and improve quality of life.
One approach to improving gait in PD is exercise. Many forms of exercise are associated with gait improvements in PD [5]; however, few studies have separated freezers and non-freezers to determine if exercise differentially affects gait performance in these populations. Further, we understand very little about how changes in gait relate to underlying brain activity and changes therein. To address these gaps, this study investigated how exercise affects gait, the neural correlates related to these effects, and potential differences in blood oxygen-level dependent (BOLD) signal changes between freezers and non-freezers.
We used a motor imagery (MI) paradigm during functional magnetic resonance imaging (fMRI) to investigate changes in brain activation after 12 weeks of exercise in people with PD, both freezers and non-freezers. Because multiple brain regions overlap between MI and motor execution [6], MI is a useful proxy to investigate how gait-related brain activity changes with exercise. Previous work from our lab noted differences in cerebellar activity [7] but similar cerebellar volumes when comparing freezers and non-freezers [8]. In this paper, we further our examination of the role of the cerebellum in FOG and focus our analyses on the somatomotor network (SMN) [9, 10]. The cerebellar component of the SMN is of particular interest because it includes areas known to be active during gait MI [6]. Further, these cerebellar regions are functionally related to primary motor (M1) and primary sensory (S1) cortices [11]. Prior research shows consistent involvement of S1 during MI, but debate over M1 involvement continues [6]. M1 is, however, part of the SMN [9]. While changes in gait are likely reflected in changes in M1 output during walking, whether similar changes occur during MI remains controversial. Therefore, we opted to explore regions of the SMN with known involvement in MI and functional connection to M1, while also examining M1 directly. We conducted a region of interest (ROI) analysis on cerebellar SMN areas, as well as S1 and M1 cortical regions. We hypothesized that, 1) the 12-week exercise intervention would improve gait performance in freezers more than non-freezers, 2) any improvement in gait performance would correspond with an increase in cerebellar BOLD signal, and 3) freezers would have significantly lower BOLD signal in cerebellar ROIs during MI, regardless of exercise.
Methods
Participants
All participants were part of a larger prospective, controlled exercise intervention study (ClinicalTrials.gov Identifier: NCT01768832) [12] where participants were recruited and assigned to the exercise intervention currently enrolling. Participants met the following inclusion criteria: 1) clinical diagnosis of idiopathic PD, 2) clear benefit from levodopa, 3) Hoehn & Yahr stage I–IV, 4) ability to walk independently for at least three meters, and 5) score of ≥24 on the Mini Mental Status Exam (MMSE). Further, the present analyses required participants to have completed baseline and post-test behavioral assessments and MRI scans, and the MRI data needed to be of sufficient quality for successful cortical and cerebellar segmentation with FreeSurfer software (v5.3.0, http://surfer.nmr.mgh.harvard.edu/) and the Spatially Unbiased Infratentorial (SUIT) toolbox [13]. Of the 98 participants who completed the study, 37 participants met all inclusion criteria (Fig. 1).
Figure 1.
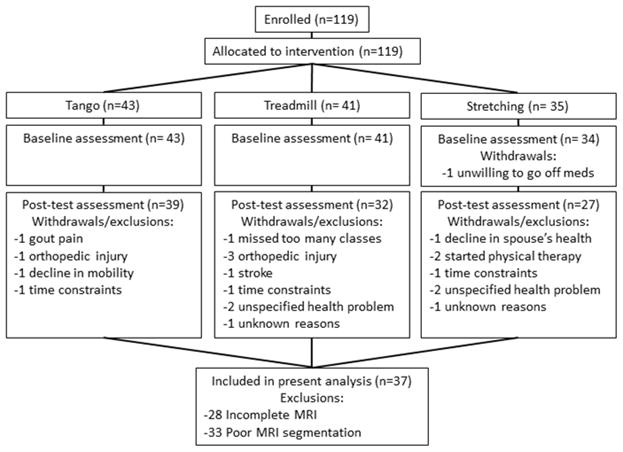
Consort diagram.
Participants completed behavioral assessments and MRI scans while OFF medications, defined as being ≥12 hours since their last dose of anti-Parkinson medication. Freezers (n=13) and non-freezers (n=24) were differentiated using question one of the New Freezing of Gait Questionnaire (NFOG-Q), which asks whether an individual has experienced a freezing episode within the past month [14]. The Human Research Protection Office of Washington University in St Louis approved this study, and all participants provided written informed consent.
Procedures
All participants completed a baseline behavioral assessment and MRI scan; participated in 12 weeks of treadmill walking (n=11), tango dance (n=13), or guided stretching (n=13); and completed a post-test evaluation comprised of the same behavioral assessment and MRI scan as at baseline. All assessments were performed within 5 weeks prior to starting and following completion of the exercise intervention (mean(SD): baseline:16.2(9.3) days; post-test:14.8(8.0) days). Participants were told to not change their regular exercise routine during the time between baseline evaluation and post-test evaluation, except for adding the exercise intervention provided as part of the study.
Behavioral assessments included gait analysis with an instrumented GAITRite walkway (CIR Systems, Franklin, NJ). Participants walked forward and backward across the GAITRite at a comfortable pace. Three trials of each gait direction were combined to calculate average forward and backward velocities and stride lengths. Participants were also assessed with the Movement Disorder Society Unified Parkinson’s Disease Rating Scale Section III (MDS-UPDRS-III) [15]. The MDS-UPDRS-III was video recorded and scored by a rater blinded to treatment group and time point. Importantly, the un-blinded rater’s score was used for the rigidity item (question 3.4) which could not be determined from the video.
Before the fMRI scan, participants practiced walking 15 and 30-foot long paths both forward and backward on level ground outside the scanner. Walking conditions were performed twice (8 total attempts). After two trials of a condition, participants sat in a chair with eyes closed and imagined performing the walking task just completed. Imagined tasks were performed twice and provided practice for the fMRI task. Additionally, prior to the MRI scan session, at baseline only, participants completed the full version of the Kinesthetic and Visual Imagery Questionnaire (KVIQ-20) [16], which is a validated measure of how well an individual is able to imagine performing movements with their body. This measure was only administered at baseline assessment as motor imagery training was not part of the interventions and was therefore expected to remain constant over the course of the study.
All fMRI scans were performed on a Siemens TRIO 3T scanner at Washington University in St. Louis School of Medicine. Participants completed T1-weighted (T1-W) structural scans (TR=2400ms, TI=1000ms, TE=3.16ms, FA=8°, 0.9mm3 voxels, 8:09min), T2-weighted (T2-W) structural scans (TR=3200ms, TE=455ms, 1.0mm3 voxels, 4:43min), and BOLD sensitized eco-planar MRI scans (TR=2200ms, TE=27ms, 4mm voxels, FA=90°, 7:20min).
For the baseline and post-test MRI sessions, participants completed one T1-W, one T2-W, and two fMRI scans. During the fMRI scans, participants viewed task instructions from a screen projected onto a head coil-mounted mirror. Task instructions were presented using E-Prime (V2.0, Psychology Software Tools, Inc, Sharpsburg, PA), and an MRI-compatible eye tracker showed when eyes were open or closed. Trial direction (FWD or BWD) and length (15 or 30 feet) was displayed before each trial. Participants were told to close their eyes and imagine performing the instructed walking task, as they had previously outside the scanner. Participants indicated the start and end of task performance by pressing a button on an MRI compatible button box (Mag Design and Engineering, Redwood City, CA, USA). Rest periods lasted 15.4 seconds with eyes open, fixated on a crosshairs on the screen. Rests alternated with imagined walking trials of variable lengths dependent upon the participant’s response. For the two fMRI scans, imagined forward (IMG-FWD) and imagined backward (IMG-BWD) walking conditions were intermixed, and each condition was presented 6 times (3 times per scan).
MRI Pre-Processing
BrainVoyager QX (v2.8.2.2523, Brain Innovation, Maastricht, The Netherlands) was used to process fMRI data. For all imagine scans, the first two volumes were discarded. Sinc interpolation was used to correct for 3D head movement and slice-time correction. Scans with more than 2 degrees or 2 mm of movement were removed from further analyses. Temporal high-pass filtering was also used to account for signal drift. T1-W scans were transformed into Talaraich space [17], and all functional scans (baseline and post-test) were co-registered to the participant-specific baseline T1-W scan. All tasks were modeled using an event-related design and the canonical hemodynamic response function.
Instructions in E-Prime were spaced at specific time intervals, but the length of each imagined walking trial was dictated by the time a participant took to imagine the instructed task (i.e. time between button presses). E-Prime allowed 19.8 seconds for any imagined walking trial; however, some participants‘ eyes remained closed for longer than the allotted time. In these instances, the imagined trial was included in analyses, but any subsequent tasks for which the instructions were missed due to the closed eyes were excluded from analyses. After removing the first two volumes from each scan, up to the next seven volumes of imagined task were included. This prevented inclusion of volumes with signal attenuation. As in previous research, trials of the same direction (forward, backward) but different distances (15ft, 30ft) were combined into IMG-FWD or IMG-BWD [7]. In order to have sufficient data for inclusion in this study, of the possible 12, 12, and 26 IMG-FWD, IMG-BWD, and Rest, respectively, participants needed at least half of each trial type to be included.
Region of Interest Analyses
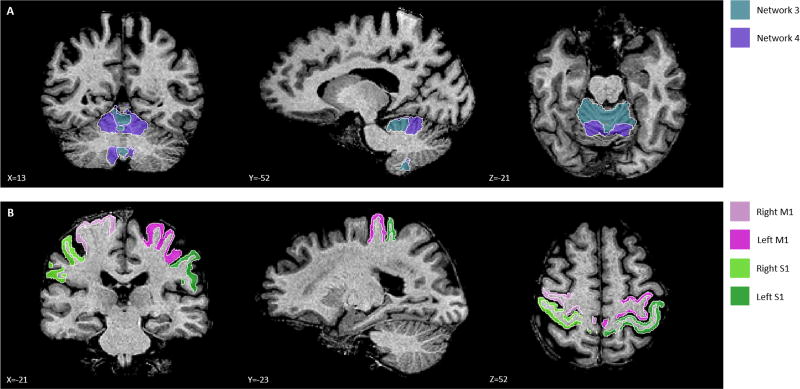
We conducted an ROI analysis with a-priori defined regions for the SMN [9]. We focused on cerebellar regions associated with the SMN, as well as M1 and S1 cortical regions (Fig. 2) [10]. Baseline structural scans were automatically segmented using FreeSurfer and the SUIT toolbox as previously described [8]. Each participant’s baseline scan was segmented with the SUIT toolbox using Buckner’s 17 networks [10]. Only cerebellar regions associated with networks 3 and 4 were analyzed. The cerebellar ROI associated with network 3 corresponds to the dorsal, cortical SMN, and the cerebellar ROI associated with network 4 corresponds to the ventral, cortical SMN. A custom MATLAB (R2016a, The MathWorks Inc., Natick, Massachusetts, USA) program converted the ROI coordinates from participant space to Talaraich space, using the Talaraich transform matrix generated by BrainVoyager.
Figure 2.
Regions of Interest. (a) Network 3 and network 4 in the cerebellum and (b) M1 and S1 in the cortex.
Cortical regions were analyzed unilaterally (right-M1, left-M1, right-S1, left-S1) while cerebellar regions were not separated into left and right hemispheres. All cortical ROIs were traced by hand onto each participant’s baseline T1-W scan. M1 consisted of the pre-central gyrus, extending from its most superior portion to the lateral sulcus [18, 19]. Similarly, S1 consisted of the post-central gyrus, extending from the most superior portion of the gyrus to the lateral sulcus [18, 19]. Both regions were traced bilaterally by individuals blinded to the demographics of the participants.
Statistics
Using BrainVoyager, average beta-weights per participant were extracted from each ROI for rest, IMG-FWD, and IMG-BWD. Baseline and post-test scan sessions were extracted separately. IMG-FWD and IMG-BWD beta-weights were normalized to rest by subtracting the average rest beta-weight from the respective scan session for each individual. Normalizing allowed for analysis of the change in beta-weight from rest associated with the imagery task. Specifically, a negative normalized beta-weight suggests a decrease in activity during the imagery task compared to rest, where as a positive beta-weight would indicate an increase in activity compared to rest. Repeated measures analyses of covariance (RM-ANCOVA) were conducted with within-subjects factors of time (baseline, post-test) and condition (forward, backward) and between-subjects factor of freezing status (freezer, non-freezer). Exercise group was used as a covariate to control for potential effects of the different exercise interventions. Residuals for gait characteristics and beta-weights were graphed with boxplots. Individuals with residuals ≥3 interquartile ranges away from the end of a box were investigated as potential outliers. Gait characteristics and beta-weights were analyzed separately. All statistics were performed using IBM-SPSS statistics (version 24, IBM Analytics, Armonk, New York, USA). Cortical and cerebellar analyses were treated as separate analyses. We used Bonferroni correction for multiple comparisons. Accordingly, for the cortical analyses we set significance at α≤0.01, and for the gait characteristics and cerebellar analyses, we set significance at α≤0.02.
Results
Freezers and non-freezers were matched well at baseline (Table 1). The two groups significantly differed on the NFOG-Q score as expected since non-freezers by definition score a 0 and freezers score ≥1. Freezers and non-freezers were similar in baseline LEDD, and there were no differences in change in LEDD between freezers and non-freezers from baseline to post-test (t=0.10, p=.92).
Table 1.
Baseline Demographics.
| Demographics | Freezers (n=13) | Non-Freezers (n=24) | p-value |
|---|---|---|---|
| Age, mean (SD) | 65.13 (10.44) | 65.81 (8.03) | 0.83 |
| Sex, % female | 23 | 46 | 0.17 |
| MDS-UPDRSIII√, median (range) | 36 (17–52) | 34 (7–43) | 0.40 |
| MMSE√, median (range) | 29 (25–30) | 29 (27–30) | 0.52 |
| Duration of diagnosis, mean (SD) | 6.1 (5.0) | 4.3 (4.3) | 0.25 |
| N-FOGQ, median (range) | 10 (3–20) | 0 (0) | <0.001 * |
| MRI Head MotionΩ, mean (SD) | 1.05 (0.27) | 1.08 (0.39) | 0.82 |
| Baseline LEDD√ϔ, median (range) | 600 (0, 1600) | 600 (0, 2000) | 0.62 |
| KVIQ Visual, mean (SD) | 34.8 (9.0) | 38.1 (8.0) | 0.27 |
| KVIQ Kinesthetic, mean (SD) | 34.8 (9.0) | 38.1 (8.0) | 0.27 |
significant difference between freezers and non-freezers
maximum head translation (X-, Y-, Z-plane) in mm or rotation (X-, Y-, Z-axis) in degrees
Mann-Whitney U test for non-parametric data
LEDD measured in mg
We ran separate RM-ANCOVAs for velocity and stride length. For velocity, there was a main effect of condition (F(1, 34)=55.04, p<0.001) indicating that all participants had slower backward gait velocities compared to forward gait (Table 2). For stride length, there was a main effect of condition (F(1, 34)=77.58, p<0.001), indicating that both freezers and non-freezers took shorter steps during backward gait (Table 2). There was no significant effect of time, indicating that exercise did not significantly improve gait in either freezers or non-freezers. There were no extreme outliers.
Table 2.
Gait Characteristics for non-freezers and freezers both before (baseline) and after (post-test) 12 weeks of exercise. All values represent mean (SD) without removing outliers.
| Baseline | Post-test | ||||
|---|---|---|---|---|---|
| Gait Characteristic | Condition | Non-Freezer | Freezer | Non-Freezer | Freezer |
| Velocity (m/s)† | Forward | 1.17 (0.18) | 1.05 (0.19) | 1.19 (0.19) | 1.10 (0.16) |
| Backward | 0.70 (0.19) | 0.63 (0.21) | 0.80 (0.33) | 0.75 (0.20) | |
| Stride Length (m)† | Forward | 1.30 (0.19) | 1.20 (0.20) | 1.33 (0.20) | 1.23 (0.20) |
| Backward | 0.78 (0.19) | 0.68 (0.24) | 0.86 (0.26) | 0.79 (0.23) | |
significant condition effect, p<0.001
All participants had at least 13 usable Rest trials (range:13–26), 6 usable IMG-FWD trials (range: 8–12), and 6 usable IMG-BWD trials (range: 6–12) from the baseline and post-test MRI scans. Separate RM-ANCOVAs were run for network 3 and 4 cerebellar regions, and M1 and S1 (bilaterally). The RM-ANCOVA for network 3 showed a significant condition*freezing status interaction (F(1,34)=5.60, p=0.02), such that freezers had lower beta-weights during IMG-BWD compared to non-freezers. There were no significant effects for network 4. For M1 and S1, we noted a significant condition*freezing status interaction for left S1 only (F(1,34)=8.90, p=0.005), suggesting that freezers had significantly lower beta-weights during IMG-BWD compared to non-freezers. All other cortical ROIs showed trends towards significant condition*freezing status interactions (left M1: F(1,34)=6.29, p=0.02; right M1: F(1,34)=5.56, p=0.02; right S1: F(1,34)=6.57, p=0.02). No significant effect of time was seen for any ROI.
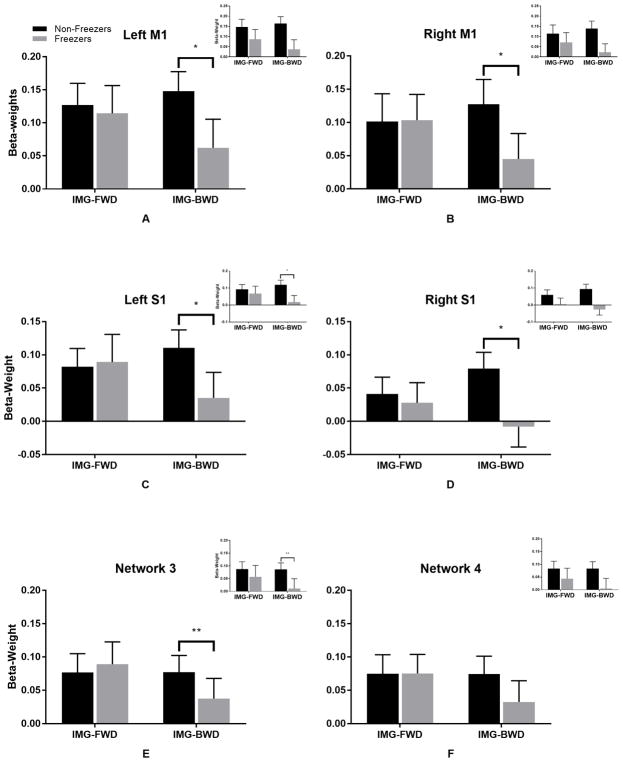
After removing two participants, one freezer and one non-freezer, as potential outliers, we repeated the ROI analyses. Both participants were outliers for ≥2 ROIs. Removing outliers did not affect the cerebellar ROI results (network 3: condition*freezing status- F(1,32)=7.01, p=0.01; network 4: no significant results); but the cortical ROI results were affected, such that all four ROIs had a significant condition*freezing status interaction (left M1: F(1,32)=7.09, p=0.01; right M1: F(1,32)=7.45, p=0.01; left S1: F(1,32)=9.59, p=0.004; right S1: F(1,32)=8.18, p=0.007), again suggesting that freezers compared to non-freezers had lower beta-weights during IMG-BWD (Fig. 3).
Figure 3.
Beta-weights. Bars represent mean beta-weight for IMG-FWD and IMG-BWD, normalized to rest and collapsed across time for non-freezers and freezers, with two outliers removed. Error bars represent standard error of the mean. Plots represent results after removing one freezer and one non-freezers as potential outliers for a) left M1, b) right M1, c) left S1, d) right S1, e) network 3, and f) network 4. All insets show results with all participants included.
*p<0.012
**p<0.025
Discussion
This study examined differences in exercise response between freezers and non-freezers, both in BOLD signal and motor performance. Our data show condition*freezing status interactions across all cortical ROIs and cerebellar network 3 regions, as well as significant differences in forward and backward gait. In line with our hypotheses, freezers compared to non-freezers showed lower beta-weights during IMG-BWD in all SMN ROIs except network 4. Our findings corroborate known sensorimotor processing deficits in freezers [20] that may be due to altered functional connectivity in cerebellar [21] and sensorimotor networks [22]. Surprisingly only IMG-BWD, not IMG-FWD, showed lower cerebellar BOLD signal in freezers. However, we believe our results may indicate the beginning of neural network changes specific to freezers because these differences were only apparent for the more difficult of the two walking tasks. Freezers with a longer duration of PD diagnosis (12.3 years) than our sample had significantly lower cerebellar activation during MI of all gait types [23]. The lower beta-weights during IMG-BWD in freezers suggests that there may be a progression of cerebellar activity reduction starting with more complicated gait tasks. In addition, cognitive deficits may impair freezers’ ability to imagine walking backward. Though participants practiced the task outside the scanner, backward walking is challenging due to visuospatial constraints and lack of daily practice. Freezers’ spatial processing deficits may compound the task’s difficulty, forcing them to rely on other networks to perform the task. As PD progresses, freezers may use the cerebellum less as MI becomes progressively more challenging. In line with this idea, our results for M1 and S1 were similar to those for the cerebellum. Similar reductions in M1 activity for all imagined gait types [23] and decreased interhemispheric connectivity of S1 in freezers [24] have been previously reported. The progressive reduction in cerebellar activity may reflect changes to the SMN as a whole.
We hypothesized there would be a time effect for both the ROI and gait performance analyses because research indicates that regular exercise affects resting-state networks [25]. Conversely, there were no significant changes in either gait performance or beta-weights. However, while not statistically significant, all participants improved backward gait velocity and stride length after exercise. On average, backward gait velocity increased by 0.11m/s, and stride length increased by 0.09m, regardless of freezing status. This corroborates findings on exercise benefits in people with PD [26] and suggests that these benefits occur regardless of freezing status. We note that forward gait did not show similar trends, likely because our participants had little room for improvement. Average forward gait velocity for healthy older adults falls between 0.9m/s and 1.2m/s [27–29], and our freezers and non-freezers were at 1.06m/s and 1.18m/s, respectively.
We chose to examine the SMN for neural correlates related to exercise-induced gait changes because the SMN likely contributes to motor output. Unfortunately, the trends of improvement during backward gait did not correspond with trends towards changes in beta-weights. This underscores the possibility of progressive decreased use of the SMN during MI for freezers as well as previous research showing exercise-induced gait changes correlating with changes in non-motor networks [25, 30]. One possibility is that regular exercise improves movement self-confidence [31], allowing for decreased inhibition that results in performance improvements. Indeed, studies in older adults with mild cognitive impairment suggest that the fronto-executive network, known for involvement in learning and action-outcome associations, may be most affected by exercise [25]. Exercise may weaken cognitive associations between backward walking and injury, allowing individuals to move with less fear and more confidence.
Our study is not without limitations. Our sample size is a potential limiting factor; however, our effects remain after Bonferroni correction for multiple comparisons. We also note that our sample was mild to moderate in disease severity, so the results of this study may not generalize to individuals with more severe PD. This was a necessary inclusion criterion as study assessments were performed OFF medications, and participants needed to fully participate in the exercise program. However, we propose that our results may represent early stages of neural network changes in freezers. Finally, we acknowledge that 12 weeks is a short amount of time for an exercise intervention and may not be long enough to induce measurable changes in gait or in the SMN.
Nevertheless, our results further understanding of freezing of gait in Parkinson disease. We provide evidence that may suggest possible progressive reduction in activity of regions associated with the SMN in freezers. Additionally, we note that changes in motor symptoms may not be directly related to changes in the somatomotor network. This highlights the fact that PD is not just a movement disorder. Rather, it affects multiple neural networks such that changes in one aspect of the disease may be related to a change in another disease aspect. Current treatment options for PD focus on mitigating symptoms; however, a more holistic approach could have greater effects on patient outcomes. Future research should focus on longitudinal analyses of Buckner’s 17 networks in both freezers and non-freezers, over the span of years, to better characterize network changes associated with disease progression. This could help with early detection of potential freezers and help further understanding of how different aspects of the disease are related to one another, providing a framework for development of more holistic treatment.
Highlights.
Freezers and non-freezers had similar forward and backward gait velocity.
Somatomotor network activity was lower in freezers during imagined backward gait.
Gait velocity and somatomotor network activity did not change after regular exercise.
Acknowledgments
We thank all members of the Movement Science Research Center and the Neuroimaging Laboratory for their contributions to data collection and analyses, including Martha J. Hessler and Richard G. Nagel. We are especially grateful to our participants and their care partners. This work was supported by the National Institutes of Health [NICHD T32HD007434, NINDS R01NS077959]; the Greater St. Louis Chapter of the American Parkinson Disease Association (APDA); Parkinson Study Group and the Parkinson’s Disease Foundation’s Advancing Parkinson’s Treatments Innovations Grant; and the APDA Center for Advanced Parkinson Disease Research at Washington University in St. Louis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with Parkinson’s disease. Exp Neurol. 2005;193(2):504–21. doi: 10.1016/j.expneurol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Lloret S, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destee A, Meissner WG, Schelosky L, Tison F, Rascol O. Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol. 2014;71(7):884–90. doi: 10.1001/jamaneurol.2014.753. [DOI] [PubMed] [Google Scholar]
- 3.Giladi N, McDermott MP, Fahn S, Przedborski S, Jankovic J, Stern M, Tanner C G. Parkinson Study. Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology. 2001;56(12):1712–21. doi: 10.1212/wnl.56.12.1712. [DOI] [PubMed] [Google Scholar]
- 4.Giladi N, Nieuwboer A. Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord. 2008;23(Suppl 2):S423–5. doi: 10.1002/mds.21927. [DOI] [PubMed] [Google Scholar]
- 5.Shen X, Wong-Yu IS, Mak MK. Effects of Exercise on Falls, Balance, and Gait Ability in Parkinson’s Disease: A Meta-analysis. Neurorehabil Neural Repair. 2016;30(6):512–27. doi: 10.1177/1545968315613447. [DOI] [PubMed] [Google Scholar]
- 6.Hetu S, Gregoire M, Saimpont A, Coll MP, Eugene F, Michon PE, Jackson PL. The neural network of motor imagery: An ALE meta-analysis. Neuroscience and Biobehavioral Reviews. 2013;37(5):930–949. doi: 10.1016/j.neubiorev.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Peterson DS, Pickett KA, Duncan R, Perlmutter J, Earhart GM. Gait-Related Brain Activity in People with Parkinson Disease with Freezing of Gait. Plos One. 2014;9(3) doi: 10.1371/journal.pone.0090634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers PS, McNeely ME, Koller JM, Earhart GM, Campbell MC. Cerebellar Volume and Executive Function in Parkinson Disease with and without Freezing of Gait. Journal of Parkinson’s Disease. 2017;7(1):9p. doi: 10.3233/JPD-161029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–45. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and Overlapping Functional Zones in the Cerebellum Defined by Resting State Functional Connectivity. Cerebral Cortex. 2010;20(4):953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earhart GM, Duncan RP, Huang JL, Perlmutter JS, Pickett KA. Comparing interventions and exploring neural mechanisms of exercise in Parkinson disease: a study protocol for a randomized controlled trial. Bmc Neurology. 2015;15 doi: 10.1186/s12883-015-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46(1):39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 14.Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, Giladi N. Reliability of the new freezing of gait questionnaire: Agreement between patients with Parkinson’s disease and their carers. Gait & Posture. 2009;30(4):459–463. doi: 10.1016/j.gaitpost.2009.07.108. [DOI] [PubMed] [Google Scholar]
- 15.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N U.R.T.F. Movement Disorder Society. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 16.Schuster C, Lussi A, Wirth B, Ettlin T. Two assessments to evaluate imagery ability: translation, test-retest reliability and concurrent validity of the German KVIQ and Imaprax. BMC Med Res Methodol. 2012;12:127. doi: 10.1186/1471-2288-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talairach TPJ. Co-Planar Stereotaxic Atlas of the Human Brain. 1. Thieme; 1988. [Google Scholar]
- 18.Felten DLJRF. Netter’s Atlas of Human Neuroscience. 1. Icon Learning Systems; Teterboro, New Jersey: 2003. [Google Scholar]
- 19.DeArmond SJ, Fusco MM, Dewey MM. Structure of the human brain: a photographic atlas. 3. Oxford University Press; New York: 1989. [Google Scholar]
- 20.Almeida QJ, Frank JS, Roy EA, Jenkins ME, Spaulding S, Patla AE, Jog MS. An evaluation of sensorimotor integration during locomotion toward a target in Parkinson’s disease. Neuroscience. 2005;134(1):283–93. doi: 10.1016/j.neuroscience.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 21.Wang M, Jiang S, Yuan Y, Zhang L, Ding J, Wang J, Zhang J, Zhang K, Wang J. Alterations of functional and structural connectivity of freezing of gait in Parkinson’s disease. J Neurol. 2016;263(8):1583–92. [Google Scholar]
- 22.Canu E, Agosta F, Sarasso E, Volonte MA, Basaia S, Stojkovic T, Stefanova E, Comi G, Falini A, Kostic VS, Gatti R, Filippi M. Brain structural and functional connectivity in Parkinson’s disease with freezing of gait. Hum Brain Mapp. 2015;36(12):5064–78. doi: 10.1002/hbm.22994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maillet A, Thobois S, Fraix V, Redoute J, Le Bars D, Lavenne F, Derost P, Durif F, Bloem BR, Krack P, Pollak P, Debu B. Neural substrates of levodopa-responsive gait disorders and freezing in advanced Parkinson’s disease: a kinesthetic imagery approach. Hum Brain Mapp. 2015;36(3):959–80. doi: 10.1002/hbm.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenka A, Naduthota RM, Jha M, Panda R, Prajapati A, Jhunjhunwala K, Saini J, Yadav R, Bharath RD, Pal PK. Freezing of gait in Parkinson’s disease is associated with altered functional brain connectivity. Parkinsonism Relat D. 2016;24:100–106. doi: 10.1016/j.parkreldis.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Huang P, Fang R, Li BY, Chen SD. Exercise-Related Changes of Networks in Aging and Mild Cognitive Impairment Brain. Front Aging Neurosci. 2016;8:47. doi: 10.3389/fnagi.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2008;23(5):631–40. doi: 10.1002/mds.21922. [DOI] [PubMed] [Google Scholar]
- 27.Langlois JA, Keyl PM, Guralnik JM, Foley DJ, Marottoli RA, Wallace RB. Characteristics of older pedestrians who have difficulty crossing the street. Am J Public Health. 1997;87(3):393–7. doi: 10.2105/ajph.87.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: reference values and determinants. Age Ageing. 1997;26(1):15–9. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- 29.Paulson S, Gray M. Parameters of gait among community-dwelling older adults. J Geriatr Phys Ther. 2015;38(1):28–32. doi: 10.1519/JPT.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 30.Maidan I, Rosenberg-Katz K, Jacob Y, Giladi N, Hausdorff JM, Mirelman A. Disparate effects of training on brain activation in Parkinson disease. Neurology. 2017;89(17):1804–1810. doi: 10.1212/WNL.0000000000004576. [DOI] [PubMed] [Google Scholar]
- 31.Woodman T, Hardy L. The relative impact of cognitive anxiety and self-confidence upon sport performance: a meta-analysis. J Sports Sci. 2003;21(6):443–57. doi: 10.1080/0264041031000101809. [DOI] [PubMed] [Google Scholar]