Abstract
Background:
The paraventricular nucleus of the thalamus (PVT) is a limbic brain structure that affects ethanol drinking, but the neurochemicals transcribed in this nucleus that may participate in this behavior have yet to be fully characterized. The neuropeptide, pituitary adenylate cyclase-activating polypeptide (PACAP), is known to be transcribed in other limbic areas and to be involved in many of the same behaviors as the PVT itself, possibly including ethanol drinking. It exists in two isoforms, PACAP-38 and PACAP-27, with the former expressed at higher levels in most brain regions. The purpose of this study was to characterize PACAP in the PVT and to assess its response to ethanol drinking.
Methods:
First, ethanol-naïve, Sprague-Dawley rats were examined using quantitative real-time PCR and immunohistochemistry, to characterize PACAP mRNA and peptide throughout the rostro-caudal axis of the PVT. Next, ethanol-naïve, vGLUT2-GFP transgenic mice were examined using immunohistochemistry, to identify the neurochemical phenotype of the PACAPergic cells in the PVT. Finally, Long-Evans rats were trained to drink 20% ethanol under the intermittent-access paradigm and then examined with quantitative real-time PCR and immunohistochemistry, to determine the effects of ethanol on endogenous PACAP in the PVT.
Results:
Gene expression of PACAP was detected across the entire PVT, denser in the posterior than the anterior portion of this nucleus. The protein isoform, PACAP-27, was present in a high percentage of cell bodies in the PVT, again particularly in the posterior portion, while PACAP-38 was instead dense in fibers. All PACAP-27+ cells co-labeled with glutamate, which itself was identified in the majority of PVT cells. Ethanol drinking led to an increase in PACAP gene expression and in levels of PACAP-27 in individual cells of the PVT.
Conclusions:
The present study characterizes the PVT neuropeptide, PACAP, and its understudied protein isoform, PACAP-27, and demonstrates that it is involved in pharmacologically-relevant ethanol drinking. This indicates that PACAP-27 should be further investigated for its possible role in ethanol drinking.
Keywords: anterior paraventricular nucleus of the thalamus, intermittent access, PACAP, PACAP-38, posterior paraventricular nucleus of the thalamus
The paraventricular nucleus of the thalamus (PVT) is a limbic brain structure known to have an integral role in motivated behavior (Millan et al., 2017) and recently shown to participate specifically in ethanol drinking (Barson et al., 2015, Barson et al., 2017, Dayas et al., 2008, Pandey et al., 2017). Believed to contain glutamate as its primary classical neurotransmitter (identified by vesicular-glutamate-transporter 2, vGLUT2) (Frassoni et al., 1997, Moutsimilli et al., 2008), the PVT has also been found to express several neuropeptides, specifically enkephalin, neurotensin, substance P, and corticotropin-releasing hormone (Arluison et al., 1994, Peng et al., 2017), but it likely also contains others. This nucleus is considered to have two major subregions, the anterior PVT (aPVT) and posterior PVT (pPVT), which exhibit overlapping but distinct afferent and efferent connectivity (Li and Kirouac, 2008, Peng and Bentivoglio, 2004, Vertes and Hoover, 2008) and participate differentially in motivated behavior. For example, we have recently shown with microinjections of specific neuropeptides that activation of the aPVT promotes pharmacologically-relevant ethanol drinking (Barson et al., 2015, Barson et al., 2017), while activation of the pPVT instead inhibits this drinking (Pandey et al., 2017). Thus, the PVT subregions are differentially involved in ethanol intake, and this may be due to neurochemicals in the cells of the PVT itself, which have so far been incompletely characterized.
One neurochemical that may be located in the PVT is pituitary adenylate cyclase-activating polypeptide (PACAP), a neuropeptide structurally similar to vasoactive intestinal peptide, that has been identified in various limbic nuclei and, like the PVT itself, is implicated in a range of motivated and stress-related behaviors (Hsu et al., 2014, Miles et al., 2017, Shen et al., 2013). Very limited evidence suggests that PACAP, like the PVT, may also be involved in ethanol drinking (Miyata et al., 1989, Tanaka et al., 2010). While studies using immunoreactivity have yet to identify PACAP in cell bodies of the PVT, in situ hybridization studies examining the PACAP precursor (ADCYAP1) have detected PACAP mRNA in the pPVT, with the aPVT not examined (Skoglosa et al., 1999, Murase et al., 1995). Studies also find PACAP to be particularly dense in the hypothalamus, including its paraventricular nucleus (PVN), as well as other limbic areas such as the amygdala, bed nucleus of the stria terminalis, and hippocampus (Hannibal, 2002, Murase et al., 1995). In cultured C6 glioma cells, PACAP receptor levels are increased by ethanol exposure (He et al., 2002), suggesting that PACAP itself may be responsive to ethanol intake, but this has not yet been investigated. Together, the literature suggests that PACAP could be responsive to ethanol exposure and may be located throughout the PVT, consistent with its expression in other limbic regions.
As there are two known isoforms of PACAP, it is possible that one or both are located in cells of the PVT. Originally isolated from the ovine hypothalamus, PACAP was initially identified as a 38-amino acid peptide (PACAP-38), which strongly stimulated 3’−5’-cyclic adenosine monophosphate (cAMP) accumulation in cultured rat anterior pituitary cells (Miyata et al., 1989). Subsequently, a second PACAP isoform, corresponding to the N-terminal 27 residues of PACAP-38 (PACAP-27) was isolated and shown to induce cAMP accumulation at comparable levels (Miyata et al., 1990). These isoforms are believed to be independently translated from ADCYAP1 (Miyata et al., 1990), with PACAP-38 comprising the major portion of PACAP in the brain (Masuo et al., 1993). Because of this, most studies involving PACAP have focused on PACAP-38.
The major goals of this study were twofold: first, to characterize the expression of PACAP in cells of the PVT; and second, to determine how PACAP in the PVT responds to ethanol. To accomplish this, we examined both gene and protein expression of PACAP throughout the rostro-caudal axis of the PVT, explored the neurotransmitter phenotype of the PACAPergic cells, and then investigated PACAP gene and protein expression following pharmacologically-relevant ethanol drinking. We hypothesized that PACAP would be located throughout the PVT, with PACAP-38 as the principal isoform in this nucleus; that PACAP would be co-expressed with glutamate; and that PACAP levels would be altered following ethanol drinking, indicating that PACAP is involved in this motivated behavior.
MATERIALS AND METHODS
Subjects
Adult, male Sprague-Dawley rats (N = 10; 8 weeks old; Charles River Laboratories International, Inc., Malvern, PA, USA); adult male vGLUT2-GFP (green fluorescent protein) transgenic mice on a C57BL/6J background (N = 3; 8 weeks old; bred in house from original mice generously donated by Dr. Ole Keihn) (Caldeira et al., 2017, Borgius et al., 2014, Borgius et al., 2010); and adult, male Long-Evans rats (N = 28; 8 weeks old; Charles River Laboratories) were housed in an AAALAC-accredited facility, on a 12-hour light/dark cycle (lights off at 0900 h for rats; lights on at 0700 h for mice). They were given at least one week to acclimate to the facility and rats were handled daily prior to the start of experiments. All animals received ad libitum chow (Laboratory Rodent Diet 5001, Lab Diet, St. Louis, MO, USA) and water throughout the study. All rats were individually housed, to maintain consistent conditions across groups, matched with those required for ethanol-drinking groups. Mice were group housed, to minimize stress, as they were maintained in the facility for a longer duration. The strains and lines employed in this study were selected due to their common usage in alcohol research (Long-Evans, Sprague-Dawley, C57BL/6J), with the inclusion of these multiple models enabling broader conclusions to be drawn from consistencies or contradictions in the findings. Experiments were approved by the Institutional Animal Care and Use Committee of Drexel University College of Medicine and followed the NIH Guide for the Care and Use of Laboratory Animals.
Experimental Protocols
Experiment 1:
To determine if PACAP mRNA could be detected across the PVT, Sprague-Dawley rats (N = 5) were sacrificed in the middle of the dark cycle via rapid decapitation to examine mRNA of PACAP in the anterior/middle PVT (a/mPVT) and middle/posterior PVT (m/pPVT) using quantitative real-time PCR (qRT-PCR). Food was removed 60 minutes prior to sacrifice.
Experiment 2:
To identify the PACAP isoform(s) present in cell bodies of the PVT subregions, Sprague-Dawley rats (N = 5) were anesthetized and perfused in the middle of the dark cycle for immunofluorescent histochemical analysis of PACAP-27, PACAP-38, and the nuclear stain 4’,6-diamidino-2-phenylindole (DAPI). To confirm the presence of PACAP protein in PVT cells using a different antibody, thus enhancing scientific rigor, alternate brain slices were used for immunohistochemical analysis with an antibody that labels both PACAP isoforms. Food was removed 60 minutes prior to sacrifice.
Experiment 3:
To establish that PACAP co-localizes with the primary classical neurotransmitter of the PVT, glutamate, vGLUT2-GFP mice (N = 3) were anesthetized and perfused at the end of the light cycle for immunohistochemical analysis of PACAP-27, vGLUT2, and DAPI.
Experiment 4:
To determine if levels of PACAP mRNA are affected by pharmacologically-relevant ethanol drinking, Long-Evans rats were trained to drink 20% ethanol under the intermittent-access two-bottle-choice paradigm or were maintained on water and chow only (n = 8/group). During the fifth week of training, starting 45 min into daily access and with food removed 60 minutes prior, they were sacrificed via rapid decapitation to examine mRNA of PACAP in the a/mPVT and m/pPVT using qRT-PCR. We have previously shown that Long-Evans rats drinking under this model have stable ethanol intake by week 4 of training and that their peak intake occurs during the first 30 minutes of daily ethanol access (Pandey et al., 2017).
Experiment 5:
To confirm that PACAP protein is also affected by pharmacologically-relevant ethanol drinking, Long-Evans rats were trained to drink 20% ethanol or were maintained on water and chow only (n = 6/group). During the fifth week of training, starting 100 minutes into daily access and with food removed 60 minutes prior, they were perfused for immunohistochemical analysis of PACAP-27, PACAP-38, and DAPI.
Ethanol drinking
Rats trained to drink ethanol drank unsweetened 20% v/v ethanol under the intermittent-access two-bottle-choice paradigm adapted from Wise (1973) and Simms (2008), which involved access to ethanol during three 24-hour-sessions per week in addition to ad libitum water and chow. One hour after dark onset, one of their two 16 oz Macrolon bottles of water (Ancare, Bellmore, NY, USA) was replaced with a 9 oz polycarbonate bottle of ethanol (Ancare), and after 24 hours, the ethanol was replaced with the second bottle of water. This was repeated each Monday, Wednesday, and Friday for a total of 13 complete sessions. Relative bottle position was alternated at each session to prevent side preference and all bottles were fitted with non-drip sipper tubes of equal size. Animals were weighed on Tuesdays and Saturdays. To determine blood ethanol concentration (BEC), trunk blood was obtained from the group tested for quantitative real-time PCR (Experiment 4), which was sacrificed 45 minutes after the start of daily ethanol access. The blood was then centrifuged and plasma extracted and tested using an Analox AM1 Alcohol Analyzer (Lunenburg, MA, USA). Rats used as water controls were treated identically except that their two bottles always contained water.
Quantitative Real-Time PCR
Dissections of the a/mPVT, m/pPVT, and PVN were taken for analysis with qRT-PCR. The middle PVT (mPVT) was bisected and grouped with the aPVT and pPVT, as finer division of the subregions was not possible for these experiments. Immediately after sacrifice, the brain was placed with the ventral surface facing up in a matrix slicing guide set on ice. Three coronal cuts were made, starting with the middle optic chiasm (Bregma −1.5 mm) (Paxinos and Watson, 2005). The second cut was 1.0 mm caudal to this, yielding a slice (Bregma −1.5 to −2.5 mm) for microdissection of the a/mPVT and PVN, and the third cut was 1.0 mm caudal to that, yielding a slice (Bregma −2.5 to −3.5 mm) for the m/pPVT. Under a microscope, on a slide placed on a petri dish filled with ice, the a/mPVT and m/pPVT were dissected as inverted isosceles triangles directly ventral to the dorsal third ventricle, approximately 0.8 mm wide at the base, while the PVN was dissected as an inverted isosceles triangle, 1.0 mm bilateral to the third ventricle and between the fornix structures. The sections were stored at −20 °C in RNAlater (Qiagen Inc., Valenia, CA, USA) until extraction of RNA.
As previously described (Pandey et al., 2017), total RNA from each brain section was purified using the RNeasy Mini Kit (Qiagen Inc.) and DNA was removed using RNase-free DNase 1 (Qiagen Inc.). The yield was quantified with a NanoDrop Lite spectrophotometer (Thermo Electron North America LLC, Madison WI), with resulting A260/A280 ratios between 2.00 and 2.26, indicating high purity. cDNA was reverse transcribed using SuperScript® VILO™ Master Mix (Invitrogen, Grand Island, NY, USA) in a SimpliAmp™ Thermal Cycler (Applied Biosystems, Waltham, MA, USA), using 1 μg of RNA from each sample. Minus RT controls were synthesized by denaturing the reverse transcriptase. For qRT-PCR, the SYBR Green PCR core reagents kit (Applied Biosystems, Grand Island, NY, USA) was used in MicroAmp® Fast Optical 96-Well Reaction Plates (Applied Biosystems), with 12.5 ng of cDNA template in a 25 μl reaction volume. The reaction was carried out on a StepOnePlus Real-Time PCR System (Applied Biosystems), under the conditions of 2 min at 50 °C, 10 min at 95 °C, 40 cycles of 15 s at 95 °C, and 1 min at 60 °C. Each sample was run in triplicate, and each run included a no-template and negative RT control. Target gene expression was quantified relative to cyclophilin-A using the relative quantification method (ΔΔCT). Primers were designed with the NCBI Primer design tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Ye et al., 2012), and purchased from Invitrogen at ThermoFisher Scientific (Grand Island, NY, USA). The primers, used at a 200 nM concentration, were cyclophilin-A forward: 5′-GTGTTCTTCGACATCACGGCT-3′, reverse: 5′-CTGTCTTTGGAACTTTGTCTGCA-3′; and PACAP forward: 5′-GCCTCTCTGGTTGTGATTCCA-3′, reverse: 5′-GGTCATTCGCGGCTAGGAA-3′.
Immunohistochemistry
Rats were deeply anesthetized with 75 mg/kg ketamine (Fort Dodge Animal Health, Overland Park, KS, USA) and 10 mg/kg xylazine (LLOYD Incorporated, Shenandoah, IA, USA) (i.p.) and perfused transcardially with 60 ml of ice-cold 0.9% sodium chloride followed by 240 ml of 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Mice were deeply anesthetized with 150 mg/kg ketamine and 10 mg/kg xylazine (i.p.) and perfused transcardially with 30 ml of ice-cold 0.9% sodium chloride followed by 50 ml of 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Brains were then removed, post-fixed in 4% paraformaldehyde for 24 hours at 4 00B0C, transferred to 30% sucrose for 2 − 4 days at 4 °C, and then frozen at −80 °C. They were sliced coronally on a cryostat at 30 μm and the sections were stored at −20 °C in antifreeze solution (37.5% ethylene glycol, 20% sucrose in 0.03 M phosphate buffered saline (PBS)). Free-floating sections were processed for immunohistochemistry to label PACAP. Every sixth section through the PVT was taken for analysis, resulting in approximately 18 sections per brain/analysis for rats and 10 sections per brain/analysis for mice. Serial sections were taken for distinct analyses.
To label PACAP-27 and PACAP-38, sections were rinsed for 10 min in 0.1% hydrogen peroxide to remove endogenous peroxidase activity. After rinsing in 0.1 M PBS, they were blocked for 90 min in 5% normal goat serum containing 0.5% Triton X-100 in PBS and incubated overnight at 4 °C in polyclonal guinea pig anti-PACAP-27 antibody (1:250, Peninsula Laboratories International, Inc, San Carlos, CA, USA; cat# T-5039.0050, lot# 040119–1) and, for rat tissue, also polyclonal rabbit anti-PACAP-38 antibody (1:250, Peninsula Laboratories International, Inc, San Carlos, CA, USA; cat# T-4473.0050, lot# A14722 and A16369). The specificity of these antibodies has previously been validated using both immunohistochemistry and western blot (Ahnaou et al., 2006, Koh et al., 2003, Moore et al., 2012). After this, the slices were rinsed in PBS and then incubated for 2 h in goat anti-guinea pig (Alexa Fluor® 555) (1:200, Abcam, Cambridge, MA, USA; cat# ab150186, lot# GR128511–1) and goat anti-rabbit (Alexa Fluor® 488) (1:200, Abcam, Cambridge, MA, USA; cat# ab150077, lot# GR239034–1) secondary antibodies in block solution. Pilot experiments were run to determine optimal staining conditions. Alternate sections did not show immunofluorescence when processed with the primary or secondary antibody omitted. Sections were mounted on slides and dried overnight in the dark, coverslipped with ProLong® Diamond Antifade Mountant with DAPI (Life Technologies, Carlsbad, CA, USA) and allowed to set for 24 hours prior to imaging.
To label both PACAP isoforms with a single antibody, sections were initially processed as above, instead using 10% normal donkey serum for block solution. They were then incubated for 40 hours at 4 °C in polyclonal rabbit anti-PACAP antibody (1:50, Abcam, Cambridge, MA, USA; cat# ab174982, lot# GR138714–12) in block solution. After this, the slices were rinsed with PBS and incubated for 90 min with biotinylated donkey anti-rabbit secondary antiserum (1:500, Vector Laboratories, Burlingame, CA, USA) in block solution. Sections were then incubated with avidin–biotin–horseradish peroxidase complex (1:300, Vectastain Elite ABC Kit, Vector Laboratories) in PBS for 60 min. After rinses with PBS, the tissue was reacted with 3,3′-diaminobenzidine tetrahydrochloride (DAB) as a chromogen. After additional rinses with dH2O and then PBS, the sections were mounted on slides (Fisherbrand Superfrost Plus, Fisher Scientific, Pittsburgh, PA, USA) and dried overnight. They were then dehydrated with a graded ethanol ladder, cleared with Shandon Xylene Substitute (Fisher Scientific), and coverslipped with Permount mounting medium (Fisher Scientific).
Immunofluorescent brain slices were imaged for analysis with a Leica DM5500 automated microscope (Buffalo Grove, IL, USA) and the images were captured with an Olympus DP71 high resolution digital color camera (Waltham, MA, USA) with Slidebook V6 image acquisition and analysis software (3i, Denver, CO, USA). These analyses were confirmed with a Leica SP8 VIS/405 HyVolution confocal microscope (Buffalo Grove, IL, USA). Brain slices processed with DAB were imaged for analysis with a Zeiss Axio Lab.A1 microscope and the images captured with an Axiocam and ZEN software (Carl Zeiss Microscopy, LLC, Thornwood, NY, USA). Exposure conditions were the same for each slice processed with each primary antibody. Due to the anatomical precision of this technique, sections could be defined as aPVT (−0.84 to −2.04 mm for rat; −0.22 to −0.93 mm for mouse), mPVT (−2.05 mm to −2.99 mm for rat; −0.94 to −2.17 mm for mouse), or pPVT (−3.00 to −4.08 mm for rat; −2.18 to −2.45 mm for mouse) relative to bregma (Paxinos and Franklin, 2004, Paxinos and Watson, 2005). Staining was quantified by an evaluator blind to group membership. Cells stained with PACAP-27, PACAP-38, and vGLUT2 were counted manually. Cells single-labeled with DAPI or DAB were counted using NIH software, ImageJ version 1.48v for Windows (Schneider et al., 2012). To do this, the image in the appropriate channel was converted to grayscale, the PVT was isolated and the threshold adjusted, with the same limits used for each slice/antibody, and software counts were initially matched to manual counts. The average number of labeled cells per slice for each antibody was determined for each subregion. To measure integrated density for PACAP-27 and PACAP-38, the image in the appropriate channel was converted to grayscale in ImageJ, the PVT was isolated, and the measure function employed. Integrated density evaluated all fluorescence, both cell bodies and fibers. To measure PACAP-27 cell fluorescence intensity, the integrated density of individual cells was measured in ImageJ and the mean fluorescence of background readings, multiplied by the area of the selected cell, was subtracted from this cell integrated density measurement. This was repeated for 20 cells at the same level in each subregion of each animal and the average number of labeled cells was determined for each subregion.
Data Analysis
Differences between two PVT subregions were analyzed by paired-samples t-tests with two tails. Comparisons between groups were made using independent-samples t-tests with two tails. To examine specific antibody labeling, a one-way ANOVA was used. To examine labeling of PACAP, a repeated-measures or mixed ANOVA was used, with subregion and isoform as the repeated measures and drinking group as the between-subjects measure, where appropriate. Significant main effects were followed up with a Sidak pairwise comparison test and significant interaction effects were followed up with additional one-way or mixed ANOVAs (with PVT subregion as the repeated measure and drinking group as the between-subjects measure) and a Sidak pairwise comparison test when appropriate. Linear regression was used to examine the relationship between ethanol intake and BEC and cell fluorescence intensity. Significance was determined at p < 0.05. Data are reported as mean ± standard error of the mean (S.E.M.).
RESULTS
Experiment 1: Gene expression of PACAP in the PVT
To examine PACAP mRNA across the PVT, the a/mPVT and m/pPVT from rats (N = 5) were analyzed using qRT-PCR. Comparing gene expression between the subregions, a significant difference was detected [t(4) = −3.20, p < 0.05], with levels of PACAP mRNA being higher in the m/pPVT than in the a/mPVT (Figure 1). Thus, PACAP mRNA is detectable across the PVT but is denser in the posterior portion of the PVT.
Fig 1.
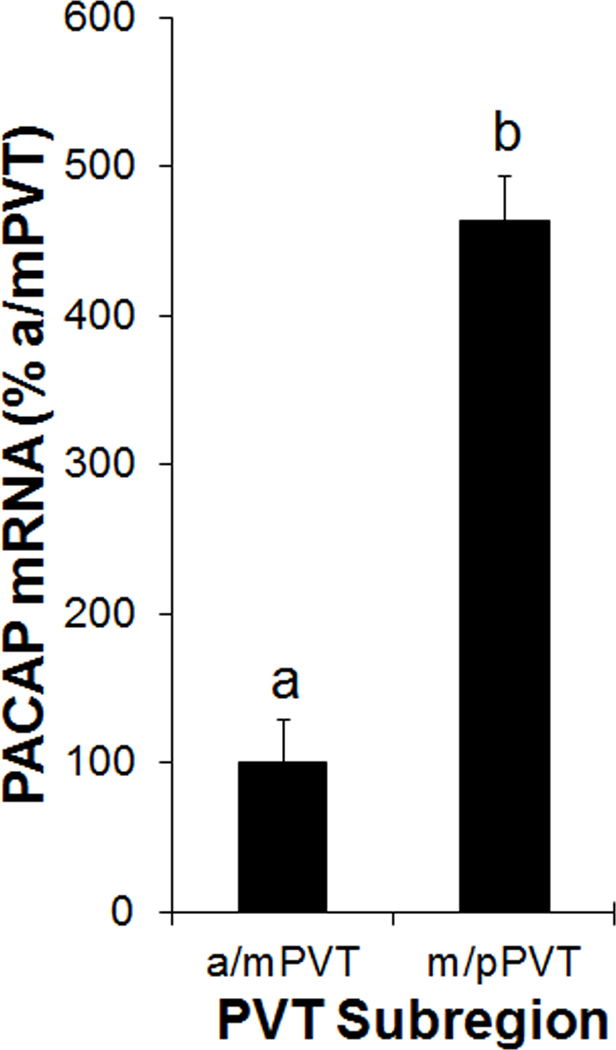
Gene expression of pituitary adenylate cyclase-activating polypeptide (PACAP) in the paraventricular nucleus of the thalamus (PVT) of Sprague-Dawley rats (N = 5), as assessed using quantitative real-time PCR (Experiment 1). Bars labeled with the same letter are not significantly different from each other. Values are mean ± SEM. Abbreviations: a/mPVT, anterior/middle paraventricular nucleus of the thalamus; m/pPVT, middle/posterior paraventricular nucleus of the thalamus.
Experiment 2: Protein expression of PACAP-27 and PACAP-38 in the PVT
To characterize PACAP protein in the PVT, rats (N = 5) were examined using immunofluorescent histochemistry with validated antibodies to label PACAP-27, PACAP-38, and cellular nuclei (DAPI) in the aPVT, mPVT, and pPVT. Comparing the overall density of labeling of the PACAP isoforms across subregions, the analysis revealed no significant main effect of isoform [F(1, 4) = 2.47, not significant (ns)] or subregion [F(2, 8) = 1.17, ns], indicating that overall PACAP labeling in the PVT was similar between PACAP-38 and PACAP-27 and across the aPVT, mPVT, and pPVT.
Notably, while PACAP-38 was primarily located in fibers in the PVT, PACAP-27 was primarily detected in soma of the PVT; thus, the PACAP isoforms were additionally analyzed by counting immunopositive cells. In examining general cellular nuclei across the PVT subregions, there were no significant main effects for the average number of DAPI+ cells per 30 μm slice [F(2, 8) = 3.82, ns], with 428 ± 20 DAPI+ cells counted per slice across the PVT. Therefore, analyses involving PACAP-38+ and PACAP-27+ cells were made as a percentage of DAPI+ cells in each PVT subregion. In contrast to labeling density, the percentage of DAPI+ cells that co-labeled with PACAP was significantly different between the isoforms and across the subregions (Figure 2A, B). Specifically, there was a significant main effect for PACAP isoform [F(1, 4) = 912.18, p < 0.001], with greater co-labeling of DAPI+ with PACAP-27 compared to PACAP-38 (44% vs 10%). Notably, cells containing PACAP-38 always co-labeled with PACAP-27. There was also a trend for a significant main effect for PVT subregion [F(2, 8) = 3.79, p = 0.07], with pairwise comparisons revealing that the mPVT had more overall PACAP co-labeling than the aPVT (p < 0.05). Finally, there was also a significant interaction effect between PACAP isoform and PVT subregion [F(2, 8) = 7.08, p < 0.05]. Follow-up of this effect, first through examination of specific co-labeling of DAPI with PACAP-27, revealed that there was again a significant main effect of subregion [F(2, 8) = 5.00, p < 0.05], with pairwise comparisons showing that this was due to significantly more PACAP-27 co-labeling in the mPVT compared to the aPVT (47% vs 37%, p < 0.05). Although it did not attain statistical significance, there was also more co-labeling in the pPVT compared to the aPVT (48% vs. 37%). Examination of specific co-labeling of DAPI with PACAP-38, in contrast, revealed that there was no significant difference across subregions [F(2, 8) = 1.09, ns]. These results focus attention on PACAP-27 as being present in a high percentage of cell bodies of the PVT, particularly in the mPVT and pPVT, and suggest that PACAP mRNA in the PVT may be primarily translated into PACAP-27.
Fig 2.
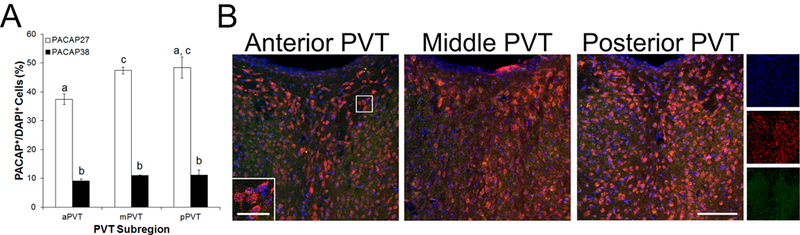
Cells expressing pituitary adenylate cyclase-activating polypeptide (PACAP) protein and 4’,6-diamidino-2-phenylindole (DAPI) in the paraventricular nucleus of the thalamus (PVT) of Sprague-Dawley rats (N = 5), as assessed using immunofluorescent histochemistry (Experiment 2). A. Quantification of cells expressing PACAP-27 and PACAP-38 as a percentage of cells expressing DAPI in the anterior PVT (aPVT), middle PVT (mPVT), and posterior PVT (pPVT). Bars labeled with the same letter are not significantly different from each other. Values are mean ± SEM. B. Confocal photomicrographs showing PACAP-27 (red), PACAP-38 (green), and DAPI (blue) in the aPVT, mPVT, and pPVT. Inset is a higher magnification of the image marked with a white square. Images on the far right are single-channel photomicrographs of the triple-labeled image of the posterior PVT. Scale bars = 100 μm in main image and 30 μm in inset.
To confirm the identification of PACAP in soma of the PVT, alternate slices from the same rats (N = 5) were examined immunohistochemically using an antibody that targeted both PACAP-27 and PACAP-38 (Figure 3A, B). This antibody again labeled numerous cells in the PVT. Analyzing the number of PACAP+ cells, a significant main effect was found for PVT subregion [F(2, 8) = 7.54, p < 0.05], with pairwise comparisons revealing that this was due to significantly greater labeling in the pPVT compared to the aPVT (p < 0.05). These results with an additional antibody strongly support the idea that PACAP is densely expressed in cell bodies of the PVT, particularly in the posterior portion of the PVT.
Fig 3.
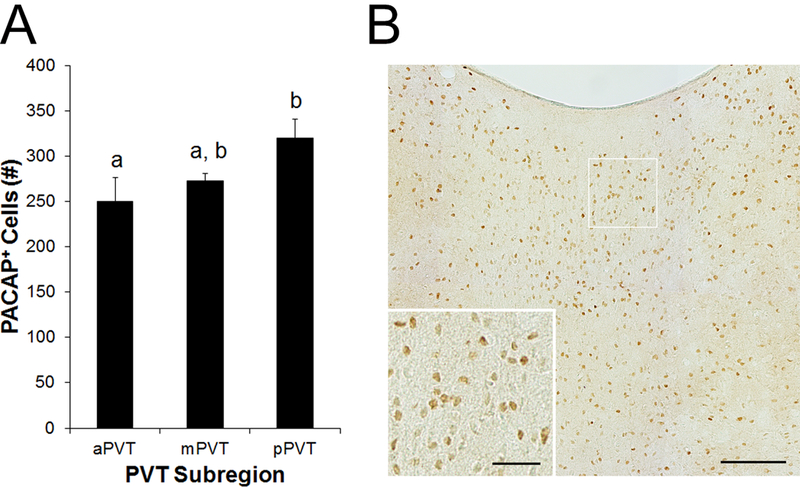
Cells expressing pituitary adenylate cyclase-activating polypeptide (PACAP) protein in the paraventricular nucleus of the thalamus (PVT) of Sprague-Dawley rats (N = 5), as assessed using immunohistochemistry with an antibody that targets both PACAP isoforms (Experiment 2). A. Quantification of cells expressing PACAP in the anterior PVT (aPVT), middle PVT (mPVT), and posterior PVT (pPVT). Bars labeled with the same letter are not significantly different from each other. Values are mean ± SEM. B. Photomicrograph showing PACAP in the mPVT. Inset is a higher magnification of the image marked with a white square. Scale bars = 100 μm in main image and 30 μm in inset.
Experiment 3: Co-localization of PACAP-27 and glutamate in the PVT
To assess the neurotransmitter phenotype of the PACAP-27+ cells in the PVT, determining if they are co-localized with the major classical neurotransmitter of the PVT, immunohistochemistry was used on tissue from vGLUT2-GFP mice (N = 3) to examine PACAP-27, vGLUT2, and DAPI in the aPVT, mPVT, and pPVT. Consistent with the findings in rats, nearly half of all DAPI+ cells co-labeled with PACAP-27, although this percentage was fairly uniform across all three PVT subregions (43 – 47%) (Table 1). Notably, every PACAP-27+ cell co-labeled with vGLUT2. Moreover, the percentage of DAPI+ cells that co-labeled with vGLUT2 was very high, ranging from 75 – 78% across the PVT (Table 1). Despite this dense glutamatergic labeling, more than half of all vGLUT2+ cells in the PVT co-labeled with PACAP-27 (57 – 60%) (Table 1 and Figure 4). These results demonstrate that PACAPergic cells in the PVT are glutamatergic and that, across all subregions of the PVT, the majority of cells contain glutamate.
Table 1. Pituitary adenylate cyclase-activating polypeptide 27 and glutamate in the paraventricular thalamus.
Quantification of cells expressing pituitary adenylate cyclase-activating polypeptide-27 (PACAP-27), vesicular-glutamate-transporter 2 (vGLUT2), and 4’,6-diamidino-2-phenylindole (DAPI) in the overall paraventricular nucleus of the thalamus (PVT), as well as the anterior PVT (aPVT), middle PVT (mPVT), and posterior PVT (pPVT) of vGLUT2-GFP transgenic mice (N = 3), using immunofluorescent histochemistry (Experiment 3). Values are mean ± SEM.
| Region | PACAP-27/DAPI (%) | vGLUT2/DAPI (%) | PACAP-27/vGLUT2 (%) |
|---|---|---|---|
| PVT | 44.4 ± 5.0 | 75.7 ± 2.5 | 58.3 ± 4.9 |
| aPVT | 47.0 ± 8.6 | 78.2 ± 4.6 | 59.6 ± 9.9 |
| mPVT | 43.1 ± 4.3 | 74.9 ± 2.6 | 57.3 ± 3.8 |
| pPVT | 46.4 ± 2.4 | 77.8 ± 3.6 | 59.7 ± 3.7 |
Fig 4.
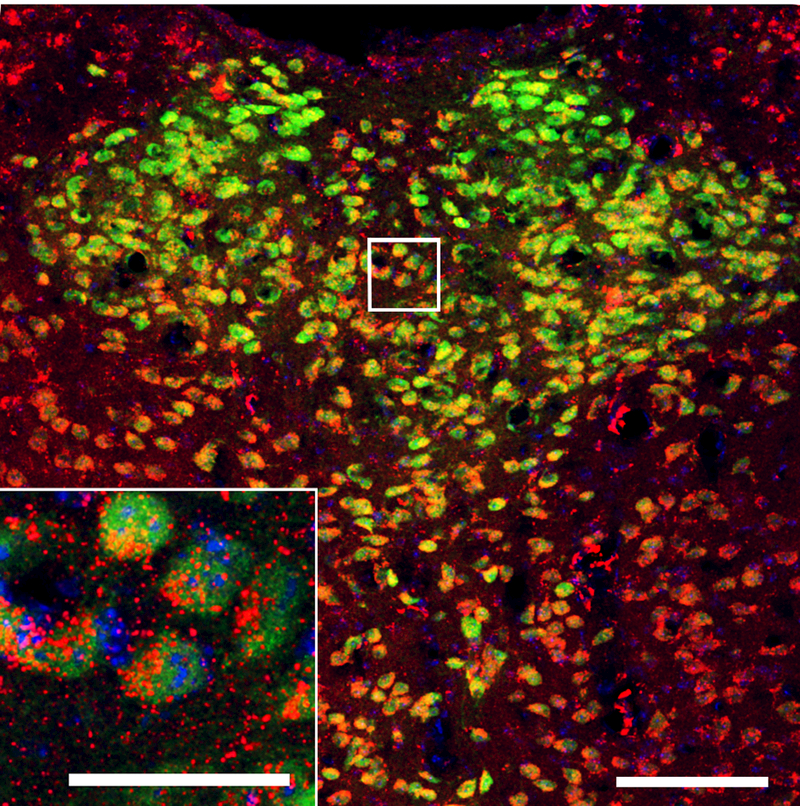
Confocal photomicrograph showing cells expressing pituitary adenylate cyclase-activating polypeptide-27 (PACAP-27, red) protein, vesicular-glutamate-transporter 2 (vGLUT2, green), and 4’,6-diamidino-2-phenylindole (DAPI, blue) in the paraventricular nucleus of the thalamus (PVT) of male vGLUT2-GFP transgenic mice (Experiment 3). Inset is a higher magnification of the image marked with a white square. Scale bars = 100 μm in main image and 30 μm in inset.
Experiment 4: Response of PACAP gene expression in the PVT to ethanol drinking
Having established that PACAP is present throughout the PVT, the next step was to determine if levels of PACAP mRNA in the PVT are affected by ethanol drinking. Rats were trained to drink 20% ethanol using the intermittent access model or were maintained on water and chow only (n = 8/group), and gene expression was then examined using qRT-PCR. Rats with access to ethanol drank an average of 5.4 ± 0.9 g/kg/d. On the day of sacrifice, they consumed 1.4 ± 0.3 g/kg ethanol in 45 minutes and achieved BECs of 59 ± 14 mg/dl, which were significantly predicted by their 45-minute ethanol intake (R2 = +0.81, p < 0.01). Starting by examining levels of PACAP mRNA in the PVT, a main effect was found for subregion [F(1, 14) = 25.25, p < 0.001], with gene expression being higher overall in the m/pPVT than in the a/mPVT, as in Experiment 1. Turning to the effect of ethanol drinking on levels of PACAP mRNA in the PVT, there was a strong trend for a main effect of drinking condition [F(1, 14) = 4.02, p = 0.07], with ethanol stimulating PACAP expression by 42% in the a/mPVT and 88% in the m/pPVT (Figure 5). In comparison, in another brain region where PACAP is heavily expressed, the PVN in the hypothalamus, there was no effect of ethanol drinking [t(14) = 0.13, ns]. Thus, ethanol intake appears to stimulate gene expression of PACAP and this effect is anatomically-specific, occurring in the PVT but not the PVN.
Fig 5.
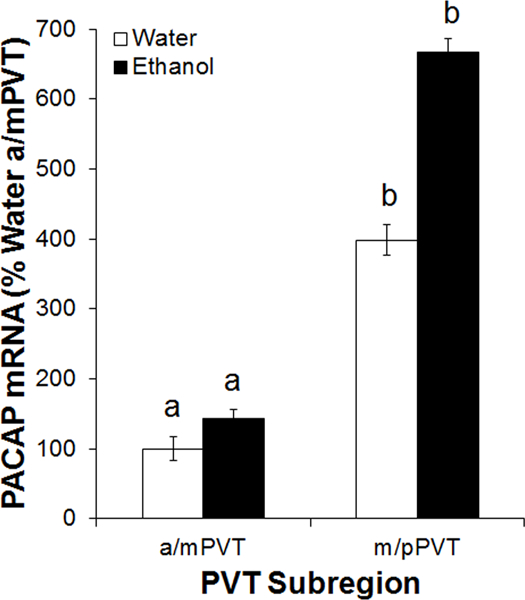
Gene expression of pituitary adenylate cyclase-activating polypeptide (PACAP) in the paraventricular nucleus of the thalamus (PVT) of male Long-Evans rats, trained to drink 20% ethanol under the intermittent-access two-bottle-choice paradigm or maintained on water and chow only (n = 8/group), as assessed using quantitative real-time PCR (Experiment 4). Bars labeled with the same letter are not significantly different from each other. Values are mean ± SEM. Abbreviations: a/mPVT, anterior/middle paraventricular nucleus of the thalamus; m/pPVT, middle/posterior paraventricular nucleus of the thalamus.
Experiment 5: Response of PACAP-27 and PACAP-38 protein expression in the PVT to ethanol drinking
To ascertain if ethanol drinking preferentially affects a specific isoform of PACAP, a second group of rats was trained to drink 20% ethanol using the intermittent access model or was maintained on water and chow only (n = 6/group). Immunohistochemistry was then used to examine PACAP-27, PACAP-38, and DAPI. Rats with access to ethanol drank an average of 4.1 ± 0.6 g/kg/d. On the day of sacrifice, they consumed 1.5 ± 0.2 g/kg ethanol in 100 minutes. Looking first at the percentage of DAPI+ cells that co-labeled with PACAP, there were no main effects of drinking condition on co-labeling with PACAP-27 [F(1, 10) = 0.03, ns] or PACAP-38 [F(1, 10) = 1.57, ns], suggesting that the number of PACAP+ cells was not affected by ethanol. Moreover, there was no significant effect of ethanol drinking on the density of labeling of PACAP-38 [F(1, 10) = 2.53, ns] which, as before, was found primarily in fibers (Figure 6A). Instead, ethanol significantly increased the density of labeling of PACAP-27 [F(1, 10) = 5.95, p < 0.05]. While the lack of interaction between drinking condition and PVT subregion [F(2, 20) = 0.78, ns] indicated that this occurred across the PVT (Figure 6A), it was notable that PACAP-27 labeling was increased by 29% in the pPVT, compared to 15% in the aPVT and 18% in the mPVT. This increased labeling of PACAP-27 in the PVT was additionally observed as a greater level of fluorescence in individual PACAP-27+ cells in the PVT of ethanol compared to water drinkers [F(1, 10) = 5.95, p < 0.05], with a lack of interaction between drinking condition and PVT subregion [F(2, 20) = 2.50, ns] again indicating that the increase occurred across the PVT (Figure 6B, C). Notably, among ethanol drinkers, average cell fluorescence intensity for PACAP-27 was very strongly predicted by average level of daily drinking (g/kg/d) (R2 = +0.93, p < 0.01). Thus, ethanol drinking specifically increases levels of PACAP-27 in individual cells of the PVT, increasing PACAP protein in cells that already produce this neuropeptide.
Fig 6.
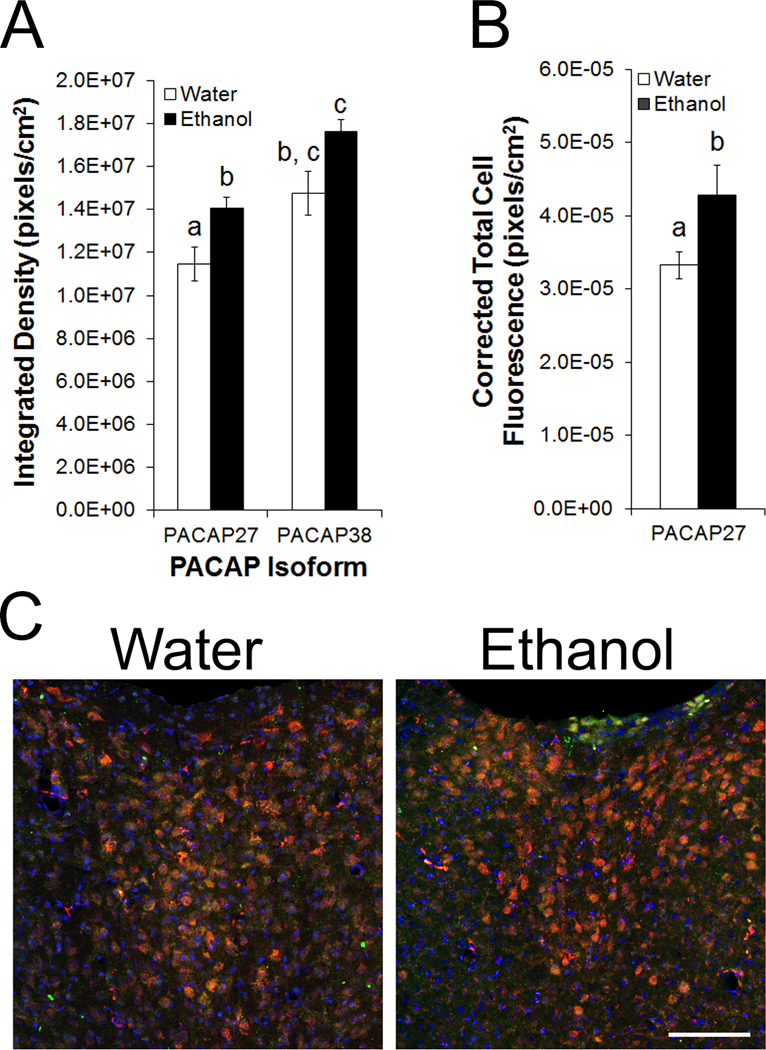
Pituitary adenylate cyclase-activating polypeptide (PACAP) protein in the paraventricular nucleus of the thalamus (PVT) of male Long-Evans rats, trained to drink 20% ethanol or maintained on water and chow only (n = 6/group), as assessed using immunofluorescent histochemistry (Experiment 5). A. Density of immunofluorescence for PACAP-27 and PACAP-38 in the PVT. B. Total immunofluorescence for PACAP-27 in individual cells in the PVT. Bars labeled with the same letter are not significantly different from each other. Values are mean ± SEM. C. Confocal photomicrographs showing cells expressing PACAP-27 (red), PACAP-38 (green), and 4’,6-diamidino-2-phenylindole (DAPI, blue) in the mPVT of ethanol and water drinkers. Scale bar = 100 μm.
DISCUSSION
The results of this study identify a neuropeptide that is densely expressed in the PVT and is responsive to ethanol drinking. Specifically, they show that PACAP is present throughout the rostro-caudal extent of the PVT in both rats and mice. In addition, the less ubiquitous of its isoforms, PACAP-27, is expressed in nearly half of all PVT cells, is particularly dense in the posterior portion of the PVT, and is contained in glutamatergic cells. Importantly, the results show that levels of PACAP-27 in cells of the PVT are increased following pharmacologically-relevant ethanol drinking. These results focus attention on changes in PACAP-27 in the PVT as a novel consequence of ethanol drinking.
Our results show that PACAP is dense in both cell bodies and fibers throughout the PVT subregions. This was demonstrated with both qRT-PCR and immunohistochemistry, using well-validated antibodies directed against the specific PACAP isoforms as well as one directed against both PACAP isoforms. Previous research using in situ hybridization identified PACAP mRNA in the pPVT, but did not examine the aPVT (Skoglosa et al., 1999, Murase et al., 1995), and immunohistochemical research using a different antibody believed to be directed against both isoforms reported that PACAP protein was dense only in fibers of the PVT (Hannibal, 2002, Koves et al., 1991). These latter results are difficult to interpret, as they did not specify the rostro-caudal level or number of sections examined, and thus may have sampled only the aPVT, where PACAP expression is lower. By examining serial sections throughout the entire PVT and using several different antibodies, we demonstrate here that PACAP is not only dense in fibers as reported (Hannibal, 2002, Koves et al., 1991), but is also expressed in nearly half of all PVT cells. This is consistent with the presence of PACAP in soma of other limbic regions (Koves et al., 1991, Masuo et al., 1993), suggesting that the PVT shares this neurochemical feature with its larger brain network.
A central finding of the present experiments is the identification of PACAP-27 as the major PACAP isoform in cell bodies of the PVT. This is in contrast with much of the rest of the brain, where PACAP-38 comprises the major portion of total PACAP protein (Masuo et al., 1993) and is more frequently examined in studies of behavioral function. The limited literature on PACAP-27 suggests that it regulates feeding behavior and the sympathetic response (Mungan et al., 1992, Nandha et al., 1991, Vu et al., 2015), both of which are also affected by the PVT (Huang et al., 1988, Millan et al., 2017). It should be noted that, while the majority of PACAP-27+ cells in the PVT may be neurons, PACAP has also been identified in glia (Hirose et al., 2005, van Landeghem et al., 2007), so future research should determine which specific cell types in the PVT contain PACAP-27. Our discovery of the less ubiquitous PACAP isoform in the PVT is perhaps not unexpected in light of evidence that it is also present in cell bodies of some other regions of the limbic system, including the supraoptic nucleus and the PVN in the hypothalamus, as well as the periaqueductal gray (Kivipelto et al., 1992), and PACAP-27 binding has been found to be dense in the PVT itself (Masuo et al., 1992). In line with this finding that the PVT is one of a restricted number of brain areas containing PACAP-27+ cells, this nucleus also demonstrates other neurochemical traits that differentiate it from other brain regions. For example, while the orexin/hypocretin-induced promotion of excessive substance intake occurs primarily via the orexin 1 receptor in most of the limbic system, it occurs primarily via the orexin 2 receptor in the PVT (Barson and Leibowitz, 2017). Thus, while the PVT shares a number of traits with other limbic regions, it also exhibits its own, distinguishing characteristics.
In characterizing the neurotransmitter phenotype of the PACAPergic cells of the PVT, we found that they are glutamatergic, and that glutamate is in fact contained in more than 75% of all cells in the PVT. While it has been noted in multiple studies that vGLUT2 exists at high levels in neurons of the PVT (Moutsimilli et al., 2008, Zhang and van den Pol, 2017), our results provide the first direct evidence that a much higher percentage of PVT neurons contain glutamate than the 30% estimated by an earlier study using an older anti-glutamate antibody (Frassoni et al., 1997). As with PACAP, it is possible that vGLUT2 is present not only in neurons but also in glia (Danik et al., 2005, Li et al., 2013). While we used in the present study the general nuclear stain, DAPI, which does not allow us to distinguish between these cell types, we expect based on research suggesting that the glia-to-neuron ratio in the PVT is about one-to-one (Bandeira et al., 2009), that most neurons in the PVT express glutamate and a significant portion of glutamatergic neurons co-express PACAP-27.
Importantly, our results show for the first time that levels of PACAP in the PVT are increased by pharmacologically-relevant ethanol drinking. This occurs in an anatomically-specific manner, in the PVT but not the PVN, and in an isoform-specific manner, for PACAP-27 but not PACAP-38. Moreover, this increase occurs within PACAP-27+ soma, confirming that it is affecting the cells of the PVT itself. It is also directly related to the amount of ethanol consumed over several weeks of access. Prior research connecting ethanol with PACAP has shown in cultured C6 glioma cells that acute ethanol exposure increases gene expression of the PACAP receptor (He et al., 2002), suggesting that ethanol affects PACAP release, in addition to expression. Future research should determine if the changes observed in the present study also occur in response to ethanol under other conditions, such as after experimenter-administered ethanol, with dependence, and in females. Gene expression of PACAP in the limbic system has also been found to be upregulated by other, related circumstances. Specifically, PACAP in the bed nucleus of the stria terminalis is increased by self-administration of cocaine, and also by chronic variable stress (Hammack et al., 2009, Miles et al., 2017). In turn, there is evidence that PACAP-27 may provide a negative feedback signal to curb ethanol intake. Compared to wild-type mice, PACAP knockout mice have a significantly greater preference for ethanol and increased ethanol-induced conditioned place preference (Tanaka et al., 2010), indicating that endogenous PACAP suppresses ethanol intake and reward. The present findings also have clinical relevance, as suggested by evidence that polymorphisms of the PACAP receptor in humans are associated with problematic alcohol use (Dragan et al., 2017, Kovanen et al., 2010). Therefore, in response to ethanol drinking, the rise in levels of PACAP-27 in cells of the PVT could represent a homeostatic mechanism for regulating intake.
Given their divergent anatomical connections and roles in behavior, it is notable that the posterior compared to the anterior portion of the PVT in rats showed heavier expression of PACAP and a slightly greater increase in levels of PACAP-27 in response to ethanol drinking. Given our recent finding that neuropeptide-induced activation of the pPVT inhibits ethanol drinking (Pandey et al., 2017), this lends further support to the idea that PACAP-27 could curb ethanol intake. We propose that PACAP release from the PVT subregions may also contribute to their other known effects, particularly in the case of the pPVT. For example, compared with the aPVT, the pPVT sends denser anatomical projections to the bed nucleus of the stria terminalis (Li and Kirouac, 2008) and is more strongly linked with the stress response (Barson and Leibowitz, 2015, Bhatnagar et al., 2003, Li et al., 2010) and drug-seeking behavior (Matzeu et al., 2016). Notably, the bed nucleus of the stria terminalis is known to receive dense PACAPergic afferent fibers (Kozicz et al., 1997) and PACAP here is found to increase anxiety-like behavior and promote drug seeking, much like the pPVT itself (Hammack et al., 2009, Miles et al., 2017). In contrast to the pPVT, the aPVT sends denser anatomical projections to the suprachiasmatic nucleus (Moga et al., 1995) and is more strongly linked with control of the circadian system (Yan and Silver, 2016). In fact, extracellular PACAP in the suprachiasmatic nucleus is known to control circadian rhythms (Michel et al., 2006). Taken together, the published literature suggests that the roles of the PVT subregions in behavior may occur, in part, through their release of PACAP, with this PVT neuropeptide perhaps making a greater contribution to stress-, alcohol-, and drug-related behavior due to its heavier expression in the pPVT than the aPVT.
Together, our results identify the involvement of the peptide, PACAP-27, in pharmacologically-relevant ethanol drinking. They show that it is densely expressed in cells of the PVT, an area of the brain recently found to participate in this behavior (Barson et al., 2015, Barson et al., 2017, Pandey et al., 2017). Adding to clinical evidence implicating the PACAP receptor in ethanol drinking, our findings, showing anatomically-specific effects of this selectively-expressed PACAP isoform, indicate that PACAP-27 should be further investigated for its role in ethanol drinking.
ACKNOWLEDGMENTS
This research was supported by the National Institute on Alcohol Abuse and Alcoholism under Award Number R00AA021782 (J.R.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors have no conflict of interest to declare. We would like to thank Dr. Ole Keihn and Charlotta Borgius at the Karolinska Institutet for providing the original vGLUT2-GFP mice and Dr. Kimberly Dougherty and Lihua Yao at Drexel University College of Medicine for providing the specific vGLUT2-GFP mice used in this work. We thank Priyanka Shah for assistance in the analysis of the vGLUT2-GFP immunohistochemistry.
REFERENCES
- Ahnaou A, Yon L, Arluison M, Vaudry H, Hannibal J, Hamon M, Adrien J & Bourgin P (2006) Immunocytochemical distribution of VIP and PACAP in the rat brain stem: implications for REM sleep physiology. Ann N Y Acad Sci, 1070, 135–142. [DOI] [PubMed] [Google Scholar]
- Arluison M, Brochier G, Vankova M, Leviel V, Villalobos J & Tramu G (1994) Demonstration of peptidergic afferents to the bed nucleus of the stria terminalis using local injections of colchicine. A combined immunohistochemical and retrograde tracing study. Brain Res Bull, 34, 319–337. [DOI] [PubMed] [Google Scholar]
- Bandeira F, Lent R & Herculano-Houzel S (2009) Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci U S A, 106, 14108–14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Ho HT & Leibowitz SF (2015) Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol, 20, 469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR & Leibowitz SF (2015) GABA-induced inactivation of dorsal midline thalamic subregions has distinct effects on emotional behaviors. Neurosci Lett, 609, 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR & Leibowitz SF (2017) Orexin/Hypocretin System: Role in Food and Drug Overconsumption. Int Rev Neurobiol, 136, 199–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Poon K, Ho HT, Alam MI, Sanzalone L & Leibowitz SF (2017) Substance P in the anterior thalamic paraventricular nucleus: promotion of ethanol drinking in response to orexin from the hypothalamus. Addict Biol, 22, 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Lazar E, Pych L & Vining C (2003) Chronic stress alters behavior in the conditioned defensive burying test: role of the posterior paraventricular thalamus. Pharmacol Biochem Behav, 76, 343–349. [DOI] [PubMed] [Google Scholar]
- Borgius L, Nishimaru H, Caldeira V, Kunugise Y, Low P, Reig R, Itohara S, Iwasato T & Kiehn O (2014) Spinal glutamatergic neurons defined by EphA4 signaling are essential components of normal locomotor circuits. J Neurosci, 34, 3841–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgius L, Restrepo CE, Leao RN, Saleh N & Kiehn O (2010) A transgenic mouse line for molecular genetic analysis of excitatory glutamatergic neurons. Mol Cell Neurosci, 45, 245–257. [DOI] [PubMed] [Google Scholar]
- Caldeira V, Dougherty KJ, Borgius L & Kiehn O (2017) Spinal Hb9::Cre-derived excitatory interneurons contribute to rhythm generation in the mouse. Sci Rep, 7, 41369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danik M, Cassoly E, Manseau F, Sotty F, Mouginot D & Williams S (2005) Frequent coexpression of the vesicular glutamate transporter 1 and 2 genes, as well as coexpression with genes for choline acetyltransferase or glutamic acid decarboxylase in neurons of rat brain. J Neurosci Res, 81, 506–521. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Mcgranahan TM, Martin-Fardon R & Weiss F (2008) Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry, 63, 152–157. [DOI] [PubMed] [Google Scholar]
- Dragan WL, Czerski PM & Dragan M (2017) PAC1 receptor (ADCYAP1R1) genotype and problematic alcohol use in a sample of young women. Neuropsychiatr Dis Treat, 13, 1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frassoni C, Spreafico R & Bentivoglio M (1997) Glutamate, aspartate and co-localization with calbindin in the medial thalamus. An immunohistochemical study in the rat. Exp Brain Res, 115, 95–104. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM & May V (2009) Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology, 34, 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J (2002) Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol, 453, 389–417. [DOI] [PubMed] [Google Scholar]
- He DY, Vagts AJ, Yaka R & Ron D (2002) Ethanol induces gene expression via nuclear compartmentalization of receptor for activated C kinase 1. Mol Pharmacol, 62, 272–280. [DOI] [PubMed] [Google Scholar]
- Hirose M, Hashimoto H, Shintani N, Nakanishi M, Arakawa N, Iga J, Niwa H, Miyazaki J & Baba A (2005) Differential expression of mRNAs for PACAP and its receptors during neural differentiation of embryonic stem cells. Regul Pept, 126, 109–113. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Kirouac GJ, Zubieta JK & Bhatnagar S (2014) Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Front Behav Neurosci, 8, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZS, Varner KJ, Barman SM & Gebber GL (1988) Diencephalic regions contributing to sympathetic nerve discharge in anesthetized cats. Am J Physiol, 254, R249–256. [DOI] [PubMed] [Google Scholar]
- Kivipelto L, Absood A, Arimura A, Sundler F, Hakanson R & Panula P (1992) The distribution of pituitary adenylate cyclase-activating polypeptide-like immunoreactivity is distinct from helodermin- and helospectin-like immunoreactivities in the rat brain. J Chem Neuroanat, 5, 85–94. [DOI] [PubMed] [Google Scholar]
- Koh PO, Noh HS, Kim YS, Cheon EW, Kim HJ, Kang SS, Cho GJ & Choi WS (2003) Cellular localization of pituitary adenylate cyclase-activating polypeptide in the rat testis. Mol Cells, 15, 271–276. [PubMed] [Google Scholar]
- Kovanen L, Saarikoski ST, Haukka J, Pirkola S, Aromaa A, Lonnqvist J & Partonen T (2010) Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol Alcohol, 45, 303–311. [DOI] [PubMed] [Google Scholar]
- Koves K, Arimura A, Gorcs TG & Somogyvari-Vigh A (1991) Comparative distribution of immunoreactive pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in rat forebrain. Neuroendocrinology, 54, 159–169. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Vigh S & Arimura A (1997) Axon terminals containing PACAP- and VIP-immunoreactivity form synapses with CRF-immunoreactive neurons in the dorsolateral division of the bed nucleus of the stria terminalis in the rat. Brain Res, 767, 109–119. [DOI] [PubMed] [Google Scholar]
- Li D, Herault K, Silm K, Evrard A, Wojcik S, Oheim M, Herzog E & Ropert N (2013) Lack of evidence for vesicular glutamate transporter expression in mouse astrocytes. J Neurosci, 33, 4434–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S & Kirouac GJ (2008) Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol, 506, 263–287. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Wei C, Wang H, Sui N & Kirouac GJ (2010) Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology (Berl), 212, 251–265. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Ohtaki T, Masuda Y, Tsuda M & Fujino M (1992) Binding sites for pituitary adenylate cyclase activating polypeptide (PACAP): comparison with vasoactive intestinal polypeptide (VIP) binding site localization in rat brain sections. Brain Res, 575, 113–123. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Suzuki N, Matsumoto H, Tokito F, Matsumoto Y, Tsuda M & Fujino M (1993) Regional distribution of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat central nervous system as determined by sandwich-enzyme immunoassay. Brain Res, 602, 57–63. [DOI] [PubMed] [Google Scholar]
- Matzeu A, Kerr TM, Weiss F & Martin-Fardon R (2016) Orexin-A/Hypocretin-1 Mediates Cocaine-Seeking Behavior in the Posterior Paraventricular Nucleus of the Thalamus via Orexin/Hypocretin Receptor-2. J Pharmacol Exp Ther, 359, 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel S, Itri J, Han JH, Gniotczynski K & Colwell CS (2006) Regulation of glutamatergic signalling by PACAP in the mammalian suprachiasmatic nucleus. BMC Neurosci, 7, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles OW, Thrailkill EA, Linden AK, May V, Bouton ME & Hammack SE (2017) Pituitary Adenylate Cyclase-Activating Peptide in the Bed Nucleus of the Stria Terminalis Mediates Stress-Induced Reinstatement of Cocaine Seeking in Rats. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Ong Z & Mcnally GP (2017) Paraventricular thalamus: Gateway to feeding, appetitive motivation, and drug addiction. Prog Brain Res, 235, 113–137. [DOI] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD & Coy DH (1989) Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun, 164, 567–574. [DOI] [PubMed] [Google Scholar]
- Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, Minamino N & Arimura A (1990) Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem Biophys Res Commun, 170, 643–648. [DOI] [PubMed] [Google Scholar]
- Moga MM, Weis RP & Moore RY (1995) Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol, 359, 221–238. [DOI] [PubMed] [Google Scholar]
- Moore JP Jr. Yang RQ & Winters SJ (2012) Targeted pituitary overexpression of pituitary adenylate-cyclase activating polypeptide alters postnatal sexual maturation in male mice. Endocrinology, 153, 1421–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsimilli L, Farley S, El Khoury MA, Chamot C, Sibarita JB, Racine V, El Mestikawy S, Mathieu F, Dumas S, Giros B & Tzavara ET (2008) Antipsychotics increase vesicular glutamate transporter 2 (VGLUT2) expression in thalamolimbic pathways. Neuropharmacology, 54, 497–508. [DOI] [PubMed] [Google Scholar]
- Mungan Z, Ozmen V, Ertan A & Arimura A (1992) Pituitary adenylate cyclase activating polypeptide-27 (PACAP-27) inhibits pentagastrin-stimulated gastric acid secretion in conscious rats. Regul Pept, 38, 199–206. [DOI] [PubMed] [Google Scholar]
- Murase T, Kondo K, Arima H, Iwasaki Y, Ito M, Miura Y & Oiso Y (1995) The expression of pituitary adenylate cyclase-activating polypeptide (PACAP) mRNA in rat brain: possible role of endogenous PACAP in vasopressin release. Neurosci Lett, 185, 103–106. [DOI] [PubMed] [Google Scholar]
- Nandha KA, Benito-Orfila MA, Smith DM, Ghatei MA & Bloom SR (1991) Action of pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal polypeptide on the rat vascular system: effects on blood pressure and receptor binding. J Endocrinol, 129, 69–73. [DOI] [PubMed] [Google Scholar]
- Pandey S, Badve PS, Curtis GR, Leibowitz SF & Barson JR (2017) Neurotensin in the posterior thalamic paraventricular nucleus: inhibitor of pharmacologically relevant ethanol drinking. Addict Biol, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G & Franklin K 2004. The Mouse Brain in Stereotaic Coordinates (2nd Edition), New York, NY, Elsevier Academic Press. [Google Scholar]
- Paxinos G & Watson C 2005. The Rat Brain in Stereotaxic Coordinates (5th Edition), San Diego, CA, Elsevier Academic Press. [Google Scholar]
- Peng J, Long B, Yuan J, Peng X, Ni H, Li X, Gong H, Luo Q & Li A (2017) A Quantitative Analysis of the Distribution of CRH Neurons in Whole Mouse Brain. Front Neuroanat, 11, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng ZC & Bentivoglio M (2004) The thalamic paraventricular nucleus relays information from the suprachiasmatic nucleus to the amygdala: a combined anterograde and retrograde tracing study in the rat at the light and electron microscopic levels. J Neurocytol, 33, 101–116. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS & Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods, 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Gehlert DR & Collier DA (2013) PACAP and PAC1 receptor in brain development and behavior. Neuropeptides, 47, 421–430. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R & Bartlett SE (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res, 32, 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglosa Y, Takei N & Lindholm D (1999) Distribution of pituitary adenylate cyclase activating polypeptide mRNA in the developing rat brain. Brain Res Mol Brain Res, 65, 1–13. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Kunishige-Yamamoto A, Hashimoto H, Shintani N, Hayata A & Baba A (2010) Increased ethanol preference and serotonin 1A receptor-dependent attenuation of ethanol-induced hypothermia in PACAP-deficient mice. Biochem Biophys Res Commun, 391, 773–777. [DOI] [PubMed] [Google Scholar]
- Van Landeghem FK, Weiss T, Oehmichen M & Von Deimling A (2007) Cellular localization of pituitary adenylate cyclase-activating peptide (PACAP) following traumatic brain injury in humans. Acta Neuropathol, 113, 683–693. [DOI] [PubMed] [Google Scholar]
- Vertes RP & Hoover WB (2008) Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol, 508, 212–237. [DOI] [PubMed] [Google Scholar]
- Vu JP, Goyal D, Luong L, Oh S, Sandhu R, Norris J, Parsons W, Pisegna JR & Germano PM (2015) PACAP intraperitoneal treatment suppresses appetite and food intake via PAC1 receptor in mice by inhibiting ghrelin and increasing GLP-1 and leptin. Am J Physiol Gastrointest Liver Physiol, 309, G816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (1973) Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia, 29, 203–210. [DOI] [PubMed] [Google Scholar]
- Yan L & Silver R (2016) Neuroendocrine underpinnings of sex differences in circadian timing systems. J Steroid Biochem Mol Biol, 160, 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S & Madden TL (2012) Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics, 13, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X & Van Den Pol AN (2017) Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science, 356, 853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]


