Abstract
Objective:
In remote, Alaska Native communities, traditional foods remain a significant source of essential nutrients and appear to protect against the development of chronic diseases. Relatively low intake of traditional foods among Alaska Native children is therefore of concern. The aim of this study was to identify household and parental predictors of child traditional food (TF) consumption and weight in Yup’ik remote communities of Alaska.
Design:
Children (10–18 years old) and parents in two communities (populations <500) were invited to participate in this cross-sectional study. Intake of traditional foods among children and parents was estimated from two-24 hour recalls using NDS-R. Weight and height were measured and BMI calculated. Sociodemographic factors, including income and education, were collected from parents. A partial least square path modeling analysis and bootstrapping were performed to identify predictors of child TF consumption and weight.
Results:
Parental intake of traditional foods, Yup’ik identity and income were positively associated with child intake of traditional foods. Further, parental intake of traditional foods predicted lower child BMI. Parental education was negatively associated with child traditional food intake and positively associated with child BMI.
Conclusions:
Findings suggest that interventions targeting parents may be an effective strategy to increase intake of traditional foods and improve diet quality among Alaska Native youth.
Keywords: Yup’ik culture, traditional food, child, diet, socioeconomic factors, dyads
Introduction
In remote, Yup’ik communities of the Yukon-Kuskowkim Delta in southwestern Alaska, traditional foods remain a significant source of essential nutrients (Erber et al. 2010; Bersamin et al. 2007; Bersamin et al. 2006). The Yup’ik traditional diet is high in fats from fish and marine mammals, which are hypothesized to contribute to a favorable lipid profile (Boyer et al. 2007; Bersamin et al. 2008; Ebbesson et al. 2015), and could also play a protective role against type 2 diabetes and cardiovascular disease (Zhang, Picard-Deland, and Marette 2013). However, as in other Alaska Native communities, residents are transitioning to a more market-based diet that tends to be highly processed and nutrient poor. This is especially true among children, raising the concern that they will be at particularly elevated risk of chronic diseases as they age (Bersamin et al. 2006; Boyer et al. 2007). Further, Alaskan Native (AN) children appear more vulnerable to childhood overweight and obesity than their non-native counterparts (Everett Jones et al. 2011). As a result, identifying predictors of the traditional food intake and adiposity in children appears critical for implementing effective prevention programs in remote, Yup’ik communities.
Numerous socio-environmental factors can affect child diet behaviors including intake of traditional foods. First, parental behaviors predict child eating behaviors in other populations (Dickens and Ogden 2014), due to role modeling. For this reason, understanding how parental intake of traditional foods affects child intake appears critical. Furthermore, the importance of the complex system of traditional values, knowledge and practices that historically sustained the well-being of AN communities to overcome chronic health issues such as substance abuse is well-recognized (Bersamin et al. 2014; Ayunerak et al. 2014). Data suggest that parent education and income levels may play a role in decreasing traditional food harvest and consumption (Bersamin et al. 2007), possibly by decreasing the time available for engaging in traditional food related activities. Finally, food availability and accessibility have been shown to shape child food preferences and consumption (Birch and Marlin 1982). The goal of this study, therefore, was to provide insights on how parents influence child intake of traditional food to help guide intervention efforts. In particular, we investigated socio-economic and cultural correlates of child intake of traditional foods.
Methods
Sample
This cross-sectional study was conducted in two communities located in the Yukon-Kuskowkim Delta, a region that is home to approximately 22,216 people living in small communities (Boedeker and Foster 2011). Children aged 10–18 years old and one of their parents were recruited via flyers, word-of-mouth, and VHF ratio to participate. When children shared the same parent, only the younger sibling of each couple was included in the analysis in order to assess the parental predictors of traditional food consumption early in pre-adolescent and adolescent life.
Questionnaires
Questionnaires were administered in person to the parents. Income was assessed both per household in a year and per household per individual in a year (Table 1). Education was assessed by the total number of education’s years completed in school and college, and the declaration of a professional training (college or technical). Two items, about main language spoken at home and about native way of living, measured Yup’ik culture participation. Traditional food availability was assessed via an item on fish availability at home, the main source of energy from traditional food in Yup’ik coastal communities (Bersamin et al. 2006).
Table 1.
Descriptive statistics of a parent-child dyad study in two Yup’ik communities of the Yukon-Kuskowkim Delta in southwestern Alaska (n=28).
| Mean ± SD / n (%) | Range | |
|---|---|---|
| Child variables | ||
| Gender | ||
| Female | 14 (50) | |
| Male | 14 (50) | |
| Age | 12.8±2.1 | 10–18 |
| Child intake of TF (% kcal.)a | ||
| Community 1 | 21.04±15.57 | |
| Community 2 | 13.80±20.17 | |
| Total | 17.94±17.71 | 00.00–63.61 |
| Weight Status category | ||
| Underweight | 1 (3.6) | |
| Normal | 21 (75) | |
| Overweight | 2 (7.1) | |
| Obese | 4 (14.3) | |
| Body fat (%) | 22.04±7.44 | 8.40–34.10 |
| Parental variables | ||
| Gender | ||
| Female | 26 (92.9) | |
| Male | 2 (7.1) | |
| Age | 45.6±8.2 | 31–63 |
| Number of education years (median) | 12 | 8–17 |
| College and/or technical training | ||
| Yes | 11 (39.3) | |
| No | 17 (60.7) | |
| Annual income in the household | ||
| Less than $10,000 | 6 (21.4) | |
| $10,000 - $14,999 | 3 (10.7) | |
| $15,000 - $24,999 | 5 (17.9) | |
| $25,000 - $34,999 | 4 (14.3) | |
| $35,000 - $49,999 | 3 (10.7) | |
| $50,000 - $74,999 | 2 (7.1) | |
| $75,000 - $99,999 | 0 | |
| $100,000 or more | 0 | |
| Income per individual per year in the household | 4784.3±4772.6 | 500–20,830 |
| Yup’ik as the main language at home | ||
| Yes | 20 (71.4) | |
| No | 8 (28.6) | |
| Follow the traditional Yup’ik way of living | ||
| A lot | 15 (53.6) | |
| Some | 12 (42.9) | |
| Not at all | 1 (3.6) | |
| Frequency of fish at home | ||
| Always | 16 (57.1) | |
| Most of the time | 5 (17.9) | |
| Sometimes | 6 (21.4) | |
| Rarely | 1 (3.6) | |
| Never | 0 | |
| Parental intake of TF (% kcal.)a | ||
| Community 1 | 41.14±17.64 | |
| Community 2 | 22.47±28.18 | |
| Total | 33.13±24.19 | 00.00–88.11 |
| Body Mass Index | 31.94±8.37 | 20.50–51.70 |
| Body fat (%) | 38.26±8.73 | 17.40–54.50 |
Percentage of calories consumed from traditional foods, including the foods harvested from the local environment (fish, marine mammals, game animals, berries and wild green). There was a significant difference in the TF consumption between children and parents (Wilcoxon matched-pairs signed-rank test, V=38, P<.001).
Dietary Data
Participants were asked to recall all food and beverages consumed over a 24-hour period using a multiple pass approach to minimize recall bias. Nutrient calculations for the 24-hour recall were performed using the NDS-R Food and Nutrient Database 33, released July 2003, which includes many Alaska Native foods. Both Alaska Native and Western foods that were missing from the database were either substituted for similar food items when appropriate or the food was added to the database by request. Traditional foods were defined as those foods harvested from the local environment, and included berries, marine mammals, fish, game animals, and wild greens. The contribution of traditional foods to mean energy was calculated. Mixed foods were disaggregated, so only traditional ingredients were included in the calculation.
Diet data were collected from each participant on two days by certified interviewers using a computer assisted 24-hour recall (Nutrition Data System for Research (NDS-R) software version 2010, University of Minnesota, Minneapolis). In order to take into account the effect of school lunch, only the recalls of weekdays in the community 1 and week-end days for the community 2 were retained. The mean of the records was then computed for each individual. No week-end records were available for one individual from Community 2, who was thus excluded (final sample size n=28).
Anthropometric measures
Weight to the nearest 0.1 kg and percent body fat were assessed once by bioelectrical impedance (Tanita® TBF-200, Tanita Corporation, Tokyo, Japan). Repeated standing height measurements were taken to the nearest 1/8 inch using a portable stadiometer. Participants removed their shoes and wore paper gowns while measurements were taken by trained technicians. Body mass index (BMI) was calculated as kg/m2. For children, Body Mass Index (BMI) for age percentiles were calculated using the CDC SAS program (CDC 2000) and used to create a categorical variable with the weight status categories of the CDC: <5th: Underweight; Normal: ≥ 5th and <85th; Overweight: 85th ≥ and <95th; Obese: ≥95th (Barlow 2007).
Statistical analysis
Partial least square Path modeling (PLS-PM)
PLS-PM is a component-based estimation method that tests complex relationships among latent variables (LV) assessed from manifest variables (MV, ie. measuring a concept indirectly) (Sanchez 2013). In PLS-PM, an outer model estimates the relationship between the LVs and their corresponding MVs, and an inner model estimates the relationship among LVs (Vinzi, Trinchera, and Amato 2010). PLS-PM does not require distributions assumptions nor does it require a minimum sample size (Tenenhaus 2008). We used Bootstrapping to determine interval confidence of the estimations (1500 repetitions, α<.1).
Variables used in PLS-PM
We tested if Yup’ik identity, income, fish availability, parental education, parental intake of traditional foods (TF) and parental adiposity predicted child intake of TF and adiposity. In addition, the model included the following paths: Parental education to Income; Yup’ik identity and socioeconomic variables to Fish availability; Yup’ik identity, socioeconomic variables and Fish availability to Parental intake of TF and Parental adiposity; Parental intake of TF to Parental adiposity; Child intake of TF to Child adiposity. Paths from the variable Community to all LVs were included to account for differences between the two samples (environmental specificities, day of 24h-recall).
Data were complete, except income for which five values were missing. We replaced them using the multivariate imputation by chained equations technique (MICE) with polytomous regression (for categorical variables) and realizing 30 imputations (White, Royston, and Wood 2011). The median of the 30 values obtained for each of the missing values was then used to complete the dataset. Both income/household and income/person in the household were used in the path model. All statistical analyses were performed using R software, version 3.1.3 (R Core Team 2013) and the plspm (Sanchez 2013) and mice (van Buuren 2015) packages.
Results
Descriptive statistics
Twenty-eight parent-child dyads completed the study. Mean age for children and parents was 12.8 years (± 2.1) and 45.6 years (± 8.2) respectively (Table 1). Equal numbers of boys (n=14) and girls (n=14) participated. The majority of parents were women (n=26/28) and had completed at least the 12th grade (n=24/28). The majority of children had a BMI that fell within the healthy range (n=21/28). The majority of parents were overweight or obese (n=23/28).
On average, children consumed 17.94% (±17.71) of their calories from traditional foods, which was significantly lower than the estimate among parents (33.13±24.19) (Wilcoxon matched-pairs signed-rank test, V=38, P<.001). Parents in community 1 consumed significantly more traditional foods than in community 2 (Wilcoxon rank sum test, W=143, P=.03) while child consumption was not significantly different between communities (W=136.5, P=.06) (Table 1).
PLS-PM
Figure 1 presents the significant total effects of the inner model. A predictor’s total effect is the sum of its direct and indirect effects, the latter being obtained by multiplying the paths coefficients of indirect pathways. Table S1 provides the complete direct path and total effects, their coefficients, standards errors and confidence intervals (α<.1).
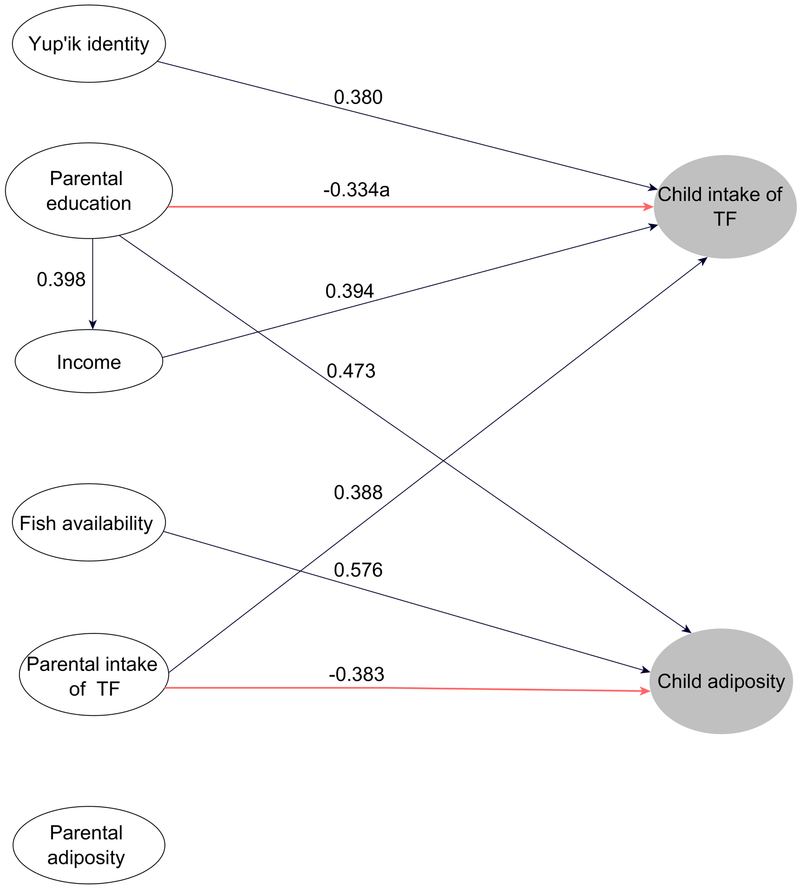
Figure 1.
Partial Least Square Path Model with parental predictors (white ellipses) of child consumption of traditional food (TF) and adiposity (grey ellipses) among two samples of South-West Alaskan Yup’ik communities (n=28). Arrows represent the significant total effects among latent variables (α<.1). a: for the effect of Parental education on Child intake of TF, the significant direct path coefficient is indicated, as total effect was not significant. The variable of adjustment “Community” is not shown here.
Parental intake of TF (r=0.388, α<.1), income (r=0.394, α<.1) and Yup’ik identity (r=0.380, α<.1) all had a significant total effect on child intake of TF. Parental education did not have a significant total effect (r=−0.172, ns), but had a significant negative direct effect (r=−0.334, α<.1) (Table S1).
For child adiposity, parental education (r=0.473, α<.1) and fish availability (r=0.576, α<.1) had a significant positive total effect. In contrast, parental intake of TF had a significant negative total effect (r=−0.383, α<.1).
The community variable had only significant direct and total effects on parental intake of TF (r=0.416 and 0.389, α<.1), consistent with the fish seasonal abundance and community locations.
The overall model R2= .68 for child adiposity, .52 for child intake of traditional food had a high effect sizes, while R2=.33 for Income, .36 for Fish availability and .23 for Parental intake of TF were moderate effect sizes and R2=.19 for parental adiposity was a small effect size, based on the categories of <0.20 , 0.20–0.50, and >0.50 for small, medium, and large effect size (Sanchez 2013). The model Goodness of Fit was 0.48 %, above the cut-point of 0.36 suggested by Wetzels et al. (2009) for large effect sizes of R2.
Table 2 presents the outer model with weight, loading, and communalities of the manifest variables (Sanchez 2013). In PLS-PM, latent variables are computed as the weighted sum of their manifest variables. Weights of manifest variables represent their contribution to the score of their latent variable. Most of the weights of the MVs were balanced, except for Child weight, for which weight status category had a higher contribution than body fat percentage. Loading of a manifest variable represents its correlation with its latent variable, and communality is the squared loading and assesses the amount of variability of a manifest variable explained by its latent variable. MVs had all loadings above or very close to the recommended value of 0.70 (Sanchez 2013), meaning that half or more of their variability (0.72=50%) was integrated into the latent variable that they assessed. Each MV was more correlated to its own LV than to the other LVs (cross-loading computed, data not shown), meaning that all indicators were more correlated to the latent construct to which they were assigned.
Table 2.
Outer model and blocks’ unidimensionality of the Partial Least Square path model in a parent-child dyad study in two Yup’ik communities of the Yukon-Kuskowkim Delta in southwestern Alaska (n=28). Weight represents the weight of a manifest variable in the score of a latent variable; Loading is the correlation between a latent variable and one of its manifest variables; Communality is the amount of variability of a manifest variable explained by its latent variable; α: Cronbach’s alpha; ρ: Dillon-Goldstein rho; 1st eig.: First eigenvalue; 2nd eig.: Second eigenvalue.aTF: Traditional foods.
| Outer model | Unidimensionality | |||||||
|---|---|---|---|---|---|---|---|---|
| Latent variables | Manifest variables | Weight | Loading | Communality | α | ρ | 1st eig. | 2nd eig. |
| Community | Community | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Parental education | College and/or technical training | 0.502 | 0.899 | 0.808 | 0.803 | 0.910 | 1.671 | 0.329 |
| Number of education years | 0.592 | 0.928 | 0.862 | |||||
| Yup’ik identity | Yup’ik way of living | 0.652 | 0.875 | 0.766 | 0.590 | 0.830 | 1.418 | 0.582 |
| Yup’ik as main language | 0.532 | 0.806 | 0.650 | |||||
| Income | Income per individual per year in the household | 0.438 | 0.852 | 0.726 | 0.858 | 0.934 | 1.751 | 0.249 |
| Annual income in the household | 0.667 | 0.939 | 0.882 | |||||
| Fish availability at home | Frequency of fish at home | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Parental intake of TFa | Parental intake of TF (% kcal.) | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Parental adiposity | Parental Body Mass Index | 0.608 | 0.977 | 0.955 | 0.929 | 0.966 | 1.867 | 0.133 |
| Parental body fat percentage | 0.425 | 0.953 | 0.908 | |||||
| Child intake of TF | Child intake of TF (% kcal.) | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Child adiposity | Weight status category | 0.791 | 0.940 | 0.883 | 0.598 | 0.833 | 1.427 | 0.573 |
| Child body fat percentage | 0.373 | 0.688 | 0.474 | |||||
The internal consistencies of the latent variables is assessed by Cronbach’s α, Dillon-Goldstein rho, considered good when >0.7, and first and second eigenvalues which should be respectively <1 and >1.
Discussion
Our results provide new insights on the eating patterns of Yup’ik children in remote, Alaska Native communities. To our knowledge, this is the first study conducted among Yup’ik people (Ayunerak et al. 2014) to estimate the average difference in traditional food consumption at the intergenerational level, i.e. between parents and their children. Moreover, it provides indications on which environmental factors may potentially be critical to promote a healthy weight and intake of traditional foods among children. Specifically, we found that parental intake of traditional foods, Yup’ik identity and income were positively associated with child intake of traditional foods. Further, parental intake of traditional foods predicted lower child BMI. Interestingly, parental education was negatively associated with traditional food intake and positively associated with child BMI.
Our finding that parental education was inversely associated with child intake of traditional foods, is consistent with the observations at the individual level among Inuit of Nunavut (Canada) (Hopping et al. 2010) and Yukon-Kuskowkim inhabitants (Bersamin et al. 2007). In Nunavut, despite being more likely to have higher knowledge of nutritional recommendations (Mead et al. 2010) and vegetable and fruit consumption (Hopping et al. 2010), individuals with more education did not necessarily show more self-efficacy to engage in other healthy diet behaviors (Mead et al. 2010). In addition, our study reveals that the effect of parental education on child traditional food intake remains significant after adjustment for parental intake of traditional foods, underlying a potential specific effect of education on the next generation. These results may have many explanations. It is possible that parents with more education are more likely to have a job and less likely to have time to subsist. It is also possible that they have less traditional knowledge, particularly if they spent more time outside the community for their education. In combination, these factors could catalyze an acculturation process that results in their child adopting a less traditional diet.
Parental education was also a positive predictor of child adiposity after controlling for parental adiposity. Because income is not correlated to child adiposity, this relation could not be explained solely by the ability of parents to afford more grocery food, and thus higher child food intake. It appears more plausible that parental education promotes a less traditional lifestyle, meaning lower physical activity levels (Bersamin et al. 2014), as well as grocery choices that lead to higher calorie intake among children.
Our finding that household income predicted higher child intake of TF is consistent with those found at the individual level in Inuvialuit of Northwest Territories and Nunavut Inuit (Canada) communities, where individuals with higher socioeconomic status were more likely to adopt healthier dietary behaviors, acquire both more unhealthy and healthy foods (including TF) (Mead et al. 2010), and consume traditional foods, fruits and vegetables (Erber et al. 2010). Income was described as critical in today’s traditional Inuit activities, allowing the acquisition of hunting and fishing gear including fuel. It may also facilitate traditional food access through food sharing. Dombrowski et al. (2013) showed that in the dominantly Inuit remote community of Nain (Labrador), individuals with greater income and employment tended to be more central in traditional food sharing networks. If this is true for Yup’ik of remote communities, income could then counteract effects of parental education on child traditional food intake.
Interestingly, fish availability did not significantly predict child intake of traditional foods, but contributed to the total effect of Yup’ik identity on child intake of TF (Appendix 1). This suggests that fish availability alone is not the strongest determinant of child diet balance between traditional and non-traditional foods, but instead that it mediates the effect of Yup’ik culture. Fish availability was however a predictor of higher weight among children. Because few children were overweight or obese, this might indicate that having more fish at home simply predicts more adequate food availability.
Our sample size, its convenient-base constitution and the cross-sectional nature of the data call for prudence in the interpretation of our results. However, considering the geographic isolation and low density of the Yup’ik rural communities, these results remain unique in documenting one of the most recent nutrition transitions occurring in arctic populations. We examined the impact of parent socio-economic characteristics, eating behavior, and culture identity on child traditional food intake. Fully understanding determinants of child intake of traditional foods requires measuring other facets of the child’s environment. In future studies, because Yup’ik communities have strong social interdependency, considering a larger social circle, including school and peers, may reveal other important predictors of child traditional food intake. Furthermore, TF availability is dependent upon seasonality. To address this annual variation, a Food Frequency Questionnaire in combination with a 24-hour recall measurement may provide better estimates of TF average intakes over the year.
This study provides indications that both parental Yup’ik culture participation and socioeconomic levels may be essential in understanding the dietary patterns observed among children in Yup’ik remote communities. It highlights the importance of prevention approaches that revitalize underlying cultural factors compatible with the contemporary communities’ constraints by promoting the key role of parents as actor of transmission of favorable eating patterns among children. This is particularly important considering that mixed economies in rural Alaska seem to be persisting, with individuals particularly engaged in wage economy also notably productive in terms of subsistence activities (BurnSilver et al. 2016). Also, an ethnographic study analyzing specifically how parental wage economy participation influences TF access and child intake of TF would be of particular interest.
Acknowledgements:
We gratefully acknowledge our participants in the YK Delta and the CANHR field team including Kathryn Cessnun, Janne Maier, and Marjie Richards. We also thank Dr. Christine Tichit, INRA Unité ALISS-1303, for her valuable advice on the analyses.
This work was supported by the National Institute of General Medical Sciences under Grants P20 RR16430 (AB) 5U54 GM104944 and 1P30GM103325 (JP); by the University of Paris-Saclay under Grant “Bourse de mobilité sortante 2015” (ACM).
Appendix 1.
Table S1.
Inner model with the path coefficients, Standard Error (SE) and Confidence Intervals at 90% (CI Inf., CI Sup.) of the bootstrapping (1500 Repetitions); (n=28).a TF: traditional foods.
| Direct Path |
Total Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | CI Low | CI High | Coefficient | SE | CI Low | CI High | ||
| Community → Parental education | 0.012 | 0.195 | −0.308 | 0.322 | 0.012 | 0.195 | −0.308 | 0.322 | |
| Community → Yupik identity | −0.038 | 0.207 | −0.355 | 0.320 | −0.038 | 0.207 | −0.355 | 0.320 | |
| Community → Income | 0.196 | 0.183 | −0.135 | 0.474 | 0.201 | 0.194 | −0.147 | 0.495 | |
| Community → Fish availability | 0.217 | 0.176 | −0.052 | 0.525 | 0.269 | 0.194 | −0.050 | 0.593 | |
| Community → Parental intake of TFa | 0.416* | 0.189 | 0.090 | 0.709 | 0.389* | 0.196 | 0.057 | 0.708 | |
| Community → Parental weight | 0.179 | 0.271 | −0.208 | 0.669 | 0.113 | 0.190 | −0.210 | 0.403 | |
| Community → Child TFC | −0.075 | 0.219 | −0.461 | 0.231 | 0.206 | 0.206 | −0.129 | 0.532 | |
| Community → Child weight | 0.025 | 0.229 | −0.338 | 0.373 | 0.019 | 0.209 | −0.332 | 0.346 | |
| Parental education → Income | 0.398* | 0.166 | 0.119 | 0.630 | 0.398* | 0.166 | 0.119 | 0.630 | |
| Parental education → Fish availability | 0.037 | 0.159 | −0.199 | 0.302 | 0.165 | 0.158 | −0.092 | 0.425 | |
| Parental education → Parental intake of TF | −0.020 | 0.182 | −0.316 | 0.281 | −0.020 | 0.162 | −0.278 | 0.246 | |
| Parental education → Parental adiposity | 0.056 | 0.213 | −0.309 | 0.370 | 0.032 | 0.181 | −0.274 | 0.311 | |
| Parental education → Child intake of TF | −0.334* | 0.175 | −0.630 | −0.059 | −0.172 | 0.145 | −0.404 | 0.075 | |
| Parental education → Child adiposity | 0.430* | 0.179 | 0.156 | 0.698 | 0.473* | 0.176 | 0.174 | 0.723 | |
| Yupik identity → Fish availability | 0.350 | 0.261 | −0.128 | 0.699 | 0.350 | 0.261 | −0.128 | 0.699 | |
| Yupik identity → Parental intake of TF | 0.303 | 0.252 | −0.207 | 0.617 | 0.274 | 0.215 | −0.138 | 0.533 | |
| Yupik identity → Parental adiposity | −0.218 | 0.283 | −0.603 | 0.305 | −0.211 | 0.216 | −0.517 | 0.186 | |
| Yupik identity → Child intake of TF | 0.222 | 0.219 | −0.148 | 0.542 | 0.380* | 0.174 | 0.056 | 0.599 | |
| Yupik identity → Child adiposity | −0.058 | 0.230 | −0.456 | 0.286 | −0.051 | 0.232 | −0.470 | 0.309 | |
| Income → Fish availability | 0.323 | 0.218 | −0.032 | 0.655 | 0.323 | 0.218 | −0.032 | 0.655 | |
| Income → Parental intake of TF | 0.033 | 0.212 | −0.312 | 0.362 | 0.006 | 0.202 | −0.313 | 0.340 | |
| Income → Parental adiposity | −0.166 | 0.245 | −0.611 | 0.188 | −0.096 | 0.255 | −0.551 | 0.306 | |
| Income → Child intake of TF | 0.340* | 0.234 | 0.009 | 0.762 | 0.394* | 0.202 | 0.026 | 0.702 | |
| Income → Child adiposity | −0.216 | 0.217 | −0.511 | 0.155 | −0.132 | 0.202 | −0.449 | 0.210 | |
| Fish availability → Parental intake of TF | −0.083 | 0.231 | −0.441 | 0.308 | −0.083 | 0.231 | −0.441 | 0.308 | |
| Fish availability → Parental adiposity | 0.223 | 0.269 | −0.242 | 0.618 | 0.245 | 0.257 | −0.193 | 0.618 | |
| Fish availability → Child intake of TF | 0.188 | 0.222 | −0.211 | 0.500 | 0.180 | 0.239 | −0.252 | 0.513 | |
| Fish availability → Child adiposity | 0.532* | 0.241 | 0.171 | 0.892 | 0.576* | 0.209 | 0.248 | 0.871 | |
| Parental intake of TF → Parental adiposity | −0.261 | 0.232 | −0.679 | 0.074 | −0.261 | 0.232 | −0.679 | 0.074 | |
| Parental intake of TF → Child intake of TF | 0.416* | 0.223 | 0.110 | 0.834 | 0.388* | 0.189 | 0.116 | 0.716 | |
| Parental intake of TF → Child adiposity | −0.263 | 0.255 | −0.646 | 0.116 | −0.383* | 0.188 | −0.745 | −0.143 | |
| Parental adiposity → Child intake of TF | 0.107 | 0.201 | −0.193 | 0.467 | 0.107 | 0.201 | −0.193 | 0.467 | |
| Parental adiposity → Child adiposity | 0.213 | 0.174 | −0.059 | 0.505 | 0.196 | 0.149 | −0.048 | 0.431 | |
| Child intake of TF → Child adiposity | −0.164 | 0.286 | −0.683 | 0.214 | −0.164 | 0.286 | −0.683 | 0.214 | |
Notes:
Significant path coefficients. A coefficient is considered statistically significant when its confidence interval does not contain the zero value. Goodness of fit (GoF) for model is 0.478. R2 for Income = 0.328, Fish availability = 0.362, Parental intake of TF = 0.229, Parental adiposity = 0.192, Child intake of TF = 0.517, Child adiposity = 0.679. Cohen f2 (R2/(1-R2)) for Income = 0.489, Fish availability = 0.567, Parental intake of TF = 0.298, Parental adiposity = 0.237, Child intake of TF = 1.070, Child adiposity = 2.115.
Footnotes
The authors declared having no conflicts of interest.
Contributor Information
Anne-Claire Maurice, Center for Alaska Native Health Research, University of Alaska Fairbanks.
Jacques Philip, Center for Alaska Native Health Research, Institute of Arctic Biology, University of Alaska Fairbanks, jphilip@alaska.edu.
Andrea Bersamin, Center for Alaska Native Health Research, Institute of Arctic Biology, University of Alaska Fairbanks, abersamin@alaska.edu.
References :
- Ayunerak Paula, Alstrom Deborah, Moses Charles, Charlie James, and Rasmus Stacy M.. 2014. “Yup’ik Culture and Context in Southwest Alaska: Community Member Perspectives of Tradition, Social Change, and Prevention.” American Journal of Community Psychology 54 (1–2): 91–99. doi: 10.1007/s10464-014-9652-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow Sarah E. 2007. “Expert Committee Recommendations Regarding the Prevention, Assessment, and Treatment of Child and Adolescent Overweight and Obesity: Summary Report.” Pediatrics 120 (Supplement 4): S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- Bersamin Andrea, Luick Bret R., King Irena B., Stern Judith S., and Zidenberg-Cherr Sheri. 2008. “Westernizing Diets Influence Fat Intake, Red Blood Cell Fatty Acid Composition, and Health in Remote Alaskan Native Communities in the Center for Alaska Native Health Study.” Journal of the American Dietetic Association 108 (2): 266–273. doi: 10.1016/j.jada.2007.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersamin Andrea, Luick Bret R., Ruppert Elizabeth, Stern Judith S., and Zidenberg-Cherr Sheri. 2006. “Diet Quality among Yup’ik Eskimos Living in Rural Communities Is Low: The Center for Alaska Native Health Research Pilot Study.” Journal of the American Dietetic Association 106 (7): 1055–1063. doi: 10.1016/j.jada.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Bersamin Andrea, Wolsko Christopher, Luick Bret R., Boyer Bert B., Lardon Cecile, Hopkins Scarlett E., Stern Judith S., and Zidenberg-Cherr Sheri. 2014. “Enculturation, Perceived Stress, and Physical Activity: Implications for Metabolic Risk among the Yup’ik-the Center for Alaska Native Health Research Study.” Ethnicity & Health 19 (3): 255–269. doi: 10.1080/13557858.2012.758691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersamin Andrea, Sheri Zidenberg-Cherr Judith S. Stern, and Luick Bret R.. 2007. “Nutrient Intakes Are Associated with Adherence to a Traditional Diet among Yup’ik Eskimos Living in Remote Alaska Native Communities: The CANHR Study.” International Journal of Circumpolar Health 66 (1): 62–70. [DOI] [PubMed] [Google Scholar]
- Birch Lean Lipps, and Wolfe Marlin Diane. 1982. “I Don’t like It; I Never Tried It: Effects of Exposure on Two-Year-Old Children’s Food Preferences.” Appetite 3 (4): 353–360. doi: 10.1016/S0195-6663(82)80053-6. [DOI] [PubMed] [Google Scholar]
- Boedeker Bonnie, and Foster Sherry. 2011. 2010 Census Counts - American Indians/Alaska Natives Alone or in Combination With One or More Other Races - Alaska. Anchorage, Alaska: Alaska Area: Native Health Service; https://www.ihs.gov/alaska/includes/themes/newihstheme/display_objects/documents/hf/area.pdf. [Google Scholar]
- Boyer Bert B., Mohatt Gerald V., Plaetke Rosemarie, Herron Johanna, Stanhope Kimber L., Stephensen Charles, Havel Peter J., and CANHR Project Team. 2007. “Metabolic Syndrome in Yup’ik Eskimos: The Center for Alaska Native Health Research (CANHR) Study.” Obesity (Silver Spring, Md.) 15 (11): 2535–2540. doi: 10.1038/oby.2007.302. [DOI] [PubMed] [Google Scholar]
- BurnSilver Shauna, Magdanz James, Stotts Rhian, Berman Matthew, and Kofinas Gary. 2016. “Are Mixed Economies Persistent or Transitional? Evidence Using Social Networks from Arctic Alaska.” American Anthropologist 118 (1): 121–129. doi: 10.1111/aman.12447. [DOI] [Google Scholar]
- CDC. 2000. “SAS Program ( Ages 0 to < 20 Years ) | Resources | Growth Chart Training | Nutrition | DNPAO | CDC.” http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm.
- Dickens Emma, and Ogden Jane. 2014. “The Role of Parental Control and Modelling in Predicting a Child’s Diet and Relationship with Food after They Leave Home. A Prospective Study.” Appetite 76 (May): 23–29. doi: 10.1016/j.appet.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Dombrowski Kirk, Khan Bilal, Channell Emily, Moses Joshua, McLean Kate, and Misshula Evan. 2013. “Kinship, Family, and Exchange in a Labrador Inuit Community.” Arctic Anthropology 50 (1): 89–104. doi: 10.3368/aa.50.1.89. [DOI] [Google Scholar]
- Ebbesson Sven O. E., Voruganti Venkata S., Higgins Paul B., Fabsitz Richard R., Ebbesson Lars O., Laston Sandra, Harris William S., et al. 2015. “Fatty Acids Linked to Cardiovascular Mortality Are Associated with Risk Factors.” International Journal of Circumpolar Health 74: 28055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erber E, Beck L, Hopping BN, Sheehy T, De Roose E, and Sharma S. 2010. “Food Patterns and Socioeconomic Indicators of Food Consumption amongst Inuvialuit in the Canadian Arctic.” Journal of Human Nutrition and Dietetics: The Official Journal of the British Dietetic Association 23 Suppl 1 (October): 59–66. doi: 10.1111/j.1365-277X.2010.01097.x. [DOI] [PubMed] [Google Scholar]
- Jones Everett, Sherry Khadija Anderson, Lowry Richard, and Conner Holly. 2011. “Risks to Health Among American Indian/Alaska Native High School Students in the United States.” Preventing Chronic Disease 8 (4). [PMC free article] [PubMed] [Google Scholar]
- Hopping BN, Erber E, Mead E, Sheehy T, Roache C, and Sharma S. 2010. “Socioeconomic Indicators and Frequency of Traditional Food, Junk Food, and Fruit and Vegetable Consumption amongst Inuit Adults in the Canadian Arctic.” Journal of Human Nutrition and Dietetics 23 (October): 51–58. doi: 10.1111/j.1365-277X.2010.01100.x. [DOI] [PubMed] [Google Scholar]
- Mead Erin L., Gittelsohn Joel, Roache Cindy, and Sharma Sangita. 2010. “Healthy Food Intentions and Higher Socioeconomic Status Are Associated with Healthier Food Choices in an Inuit Population.” Journal of Human Nutrition and Dietetics 23 (October): 83–91. doi: 10.1111/j.1365-277X.2010.01094.x. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2013. “R: A Language and Environment for Statistical Computing.” http://cran.fiocruz.br/web/packages/dplR/vignettes/timeseries-dplR.pdf.
- Sanchez Gaston. 2013. PLS Path Modeling with R. http://gastonsanchez.com/PLS_Path_Modeling_with_R.pdf.
- Tenenhaus Michel. 2008. “Structural Equation Modelling for Small Samples.” https://hal-hec.archives-ouvertes.fr/hal-00580148. [Google Scholar]
- van Buuren Stef. 2015. “Package ‘Mice.’” https://cran.r-project.org/web/packages/mice/mice.pdf. [Google Scholar]
- Vinzi Vincenzo E., Trinchera Laura, and Amato Silvano. 2010. “Chapter 2 PLS Path Modeling: From Foundations to Recent Developments and Open Issues for Model Assessment and Improvement” In Handbook of Partial Least Squares, Springer Handbooks of Computational Statistics, Springer-Verlag, 47–82. Berlin: Heidelberg. [Google Scholar]
- Wetzels Martin, Odekerken-Schroder Gaby, and Van Oppen Claudia. 2009. “Using PLS Path Modeling for Assessing Hierarchical Construct Models: Guidelines and Empirical Illustration.” Management Information Systems Quarterly 33 (1): 177–195. [Google Scholar]
- White Ian R., Royston Patrick, and Wood Angela M.. 2011. “Multiple Imputation Using Chained Equations: Issues and Guidance for Practice.” Statistics in Medicine 30 (4): 377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- Zhang Ming, Picard-Deland Eliane, and Marette André. 2013. “Fish and Marine Omega-3 Polyunsatured Fatty Acid Consumption and Incidence of Type 2 Diabetes: A Systematic Review and Meta-Analysis.” International Journal of Endocrinology 2013: 501015. doi: 10.1155/2013/501015. [DOI] [PMC free article] [PubMed] [Google Scholar]