Abstract
Sodium-glucose cotransporter 2 (SGLT2), which is specifically expressed on the apical side of proximal tubular cells, is involved in the reabsorption of most of the glucose filtered by the glomeruli, and its inhibitors are gaining publicity as potent antihyperglycemic drugs. In some clinical trials, SGLT2 inhibitors exerted cardiovascular and kidney protective effects, which appeared to be partly independent of the original glucose-lowering effect. SGLT2 inhibitors have both direct and indirect renoprotective effects. The direct effects involve the suppression of hyperplasia/hypertrophy, inflammation, and fibrosis in the proximal tubular cells, utilization of ketone bodies, restored tubuloglomerular feedback, decreased oxygen consumption, improvement in anemia, and preconditioning against ischemia/reperfusion. The indirect effects involve a reduction in insulin levels and resistance, uric acid concentration, body weight, and blood pressure. However, safety concerns remain, including consequences of an enhanced glucose load in the lower nephron, leg amputation, bone fractures, and therapeutic efficacy in patients with advanced chronic kidney disease.
Keywords: sodium-glucose cotransporter 2 (SGLT2) inhibitors, diabetic kidney disease, tubuloglomerular feedback (TGF), final common pathway
Introduction
Diabetes mellitus (DM) is affecting increasing numbers of patients and has become one of the most urgent public health problems in both developed and developing countries. Currently, various antihyperglycemic drugs are available; however, the treatment of diabetic kidney disease - a complication that profoundly affects the morbidity and mortality of DM patients - is mainly limited to renin-angiotensin-aldosterone system (RAAS) inhibitors. Sodium-glucose cotransporter 2 (SGLT2) inhibitors are the first antihyperglycemic drugs to act directly on the kidney. In addition, recent clinical trials have revealed cardiovascular and kidney protective effects that are not necessarily mediated by decreased glucose levels. We herein discuss the pleiotropic effects of SGLT2 inhibitors and their potential as a new therapeutic measure in kidney disease.
Mechanisms of the Antihyperglycemic Effect of SGLT2 Inhibitors
SGLT2 is a cotransporter that is involved in reabsorption of glucose filtered by the glomeruli from the lumen into the cells located in the luminal membrane of the early proximal tubules. SGLT2 utilizes the Na concentration gradient produced by the basal Na-K ATPase and simultaneously transports Na and glucose in a 1:1 ratio (Fig. 1) (1). With normal serum glucose levels and a normal glomerular filtration rate (GFR), approximately 160-180 g of glucose is filtered each day, most of which is reabsorbed; 97% is mediated by SGLT2. SGLT1 is localized in the late proximal tubules and transports Na and the remaining glucose into the cells in a 2:1 ratio (2).
Figure 1.
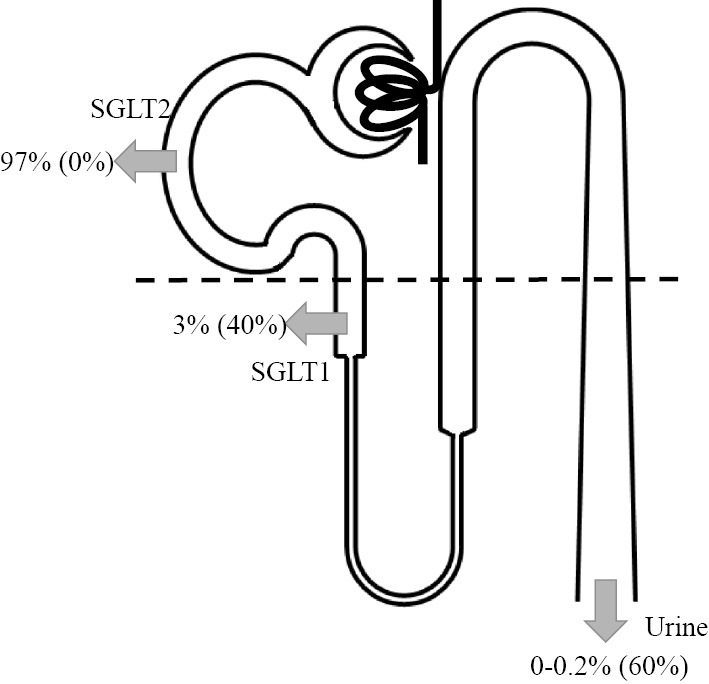
Glucose reabsorption under normoglycemia (modified from 2). Under normoglycemia, -97% of the filtered glucose is reabsorbed by SGLT2 in the early proximal tubules (S1, S2). With the use of SGLT2 inhibitors, SGLT1 in the late proximal tubules (S3) reabsorbs glucose instead. Numbers in parentheses show reabsorption rates with the use of SGLT2 inhibitors. SGLT: sodium-glucose cotransporter
Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. AJP Regul Integr Comp Physiol 300: R1009-R1022, 2011.
SGLT2 inhibitors reach their target from the luminal side, after a large proportion is filtered by the glomeruli, and selectively inhibit SGLT2, leading to the suppression of glucose reabsorption in the proximal tubules and an antihyperglycemic effect. In contrast to SGLT1, which is ubiquitously present in organs such as the brain, heart, and intestine, SGLT2 is specifically present in the renal tubules with the exception of the α cells in the pancreas (1). Because SGLT1 contributes to the reabsorption of water in the intestine and the glucagon secretion triggered by glucose, SGLT inhibitors have only been clinically applied to DM since the acquisition of selectivity for SGLT2.
In type 1 (T1) DM and type 2 (T2) DM patients, the glucose transport maximum of the kidneys is increased by 20%, resulting in total absorption of 600 g of glucose per day. This is thought to be due to hyperplasia and hypertrophy of the proximal tubules and the increased expression of SGLT2.
SGLT2 inhibitors lead to the excretion of only 50-60% of the filtered glucose, which is relatively less than the amount of glucose reabsorbed by SGLT2 (97%). This is because the increased glucose concentration in the late proximal tubules facilitates glucose reabsorption by SGLT1, which is located downstream.
Clinical Trials of SGLT2 Inhibitors
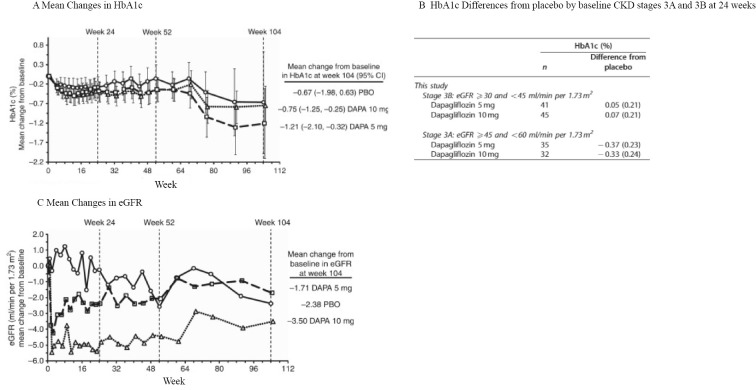
In a double-blind randomized controlled trial (RCT) in which 252 T2DM subjects with chronic kidney disease (CKD) - mainly stage G3 - were assigned to receive a placebo, dapagliflozin (5 mg), or dapagliflozin (10 mg), HbA1c decreased in the dapagliflozin groups in comparison to the placebo group in patients with CKDG3a but not in those with CKDG3b (Fig. 2) (3). Significant reductions in body weight and blood pressure were observed regardless of the renal function. The estimated GFR (eGFR) decreased within 1 week after the initiation of the drug in the dapagliflozin groups, but was maintained thereafter and roughly coincided with the eGFR of the placebo group with a constant decrease over the 104 weeks of the test period. This pattern was similar in both the CKDG3a and CKDG3b groups. In addition, more patients in the dapagliflozin groups shifted to a lower category of albuminuria at the end of the trial, when the patients were classified into three categories: macroalbuminuria, microalbuminuria, and normoalbuminuria.
Figure 2.
Mean changes in HbA1c and eGFR from the baseline in the placebo and dapagliflozin groups (modified from 3). A: Mean changes in HbA1c from the baseline during the trial. Differences between the placebo and dapagliflozin groups were not significant because each group included both CKDG3a and CKDG3b patients. B: HbA1c Differences from placebo by baseline CKD stages 3A and 3B at 24 weeks. C: Mean changes in eGFR from the baseline during the trial. eGFR: estimated glomerular filtration rate, CKD: chronic kidney disease
Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 85: 962-971, 2014.
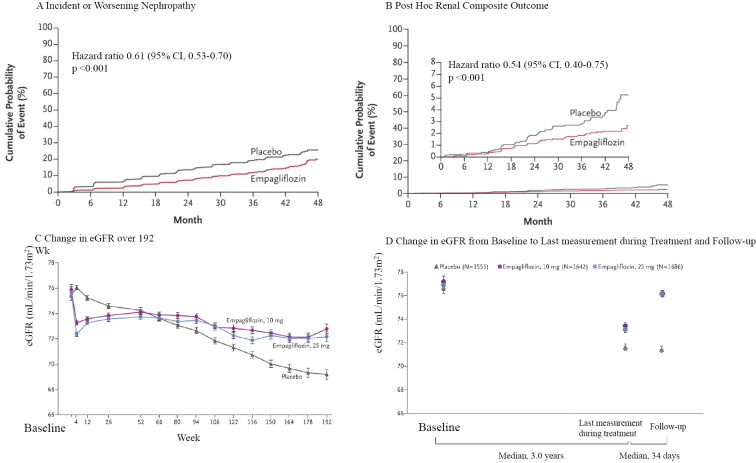
The EMPA-REG OUTCOME trial, a double-blind RCT that demonstrated that the selective SGLT2 inhibitor empagliflozin significantly decreased cardiovascular mortality, showed a significant decrease in incident and worsening nephropathy [progression to macroalbuminuria, a doubling of the serum creatinine level, the initiation of renal-replacement (RRT) therapy, or death from renal disease], a prespecified component of the secondary microvascular outcome of the trial, which was followed by a closer analysis of the renal outcomes (Fig. 3) (4,5). The trial included 7,020 patients with cardiovascular disease and with an eGFR of at least 30 mL/min/1.73 m2; 17.8% had an eGFR of 45-59 mL/min/1.73 m2, and 7.7% had an eGFR of 30-44 mL/min/1.73 m2, and 80.7% had already received RAAS inhibitors.
Figure 3.
Renal outcome and renal function over time in the EMPA-REG OUTCOME trial (modified from 5). A: Kaplan-Meier analysis of a prespecified renal composite outcome (incident and worsening nephropathy). B: Kaplan-Meier analysis of a post-hoc renal composite outcome (doubling of serum creatinine level, initiation of renal-replacement therapy, or death from renal disease). The inset shows the data on an expanded y axis. C: Adjusted mean eGFR over a period of 192 weeks. D: Adjusted mean eGFR at the baseline, the last measurement during treatment, and follow-up. The difference at follow-up was smaller in both the 10 mg and 25 mg empagliflozin groups than in the placebo group by 4.7 mL/min/1.73 m2 (p<0.001). eGFR: estimated glomerular filtration rate
Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323-334, 2016.
Progression to macroalbuminuria occurred in 459 of 4,091 patients (11.2%) in the empagliflozin group; this was significantly less frequent than in the placebo group, in which 330 of the 2,033 (16.2%) patients progressed to macroalbuminuria [number needed to treat (NNT), 20.0]. Doubling of the serum creatinine level occurred in 70 of the 4,645 (1.5%) patients in the empagliflozin group and 60 of the 2,323 (2.6%) patients in the placebo group (NNT, 93), and the initiation of RRT occurred in 13 of the 4,687 (0.3%) patients in the empagliflozin group and 14 of the 2,333 (0.6%) patients in the placebo group (NNT, 310), both of which showed a significant risk reduction; however, the clinical significance was considered to be less credible because of the small numbers of events. The renal function, as measured by the eGFR, demonstrated similar patterns both in patients with an eGFR of >60 mL/min/1.73 m2 and in those with an eGFR of <60 mL/min/1.73 m2. The eGFR of patients in the empagliflozin group decreased within 4 weeks after the initiation of medication, but was maintained in comparison to patients in the placebo group and was completely reversed after the cessation of the study drug.
The results of the CANVAS trial were published most recently (6). This study integrated data from two trials (CANVAS and CANVAS-R) to include 10,142 participants with T2DM and an elevated risk of cardiovascular disease, to investigate the cardiovascular and renal effects and safety outcomes of canagliflozin. The eGFR in all the participants was >30 mL/min/1.73 m2 (mean eGFR, 76.5 mL/min/1.73 m2); the median urinary albumin-to-creatinine ratio was 12.3 mg/gCre.
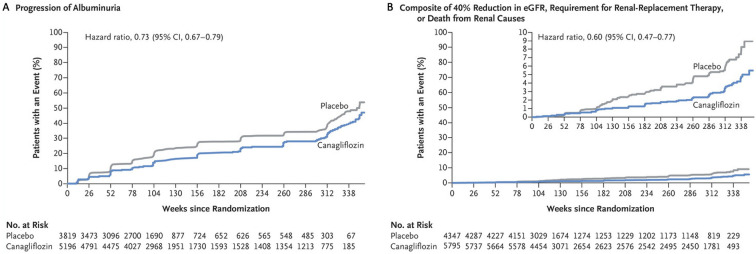
Similar to the EMPA-REG OUTCOME trial, the CANVAS trial showed the cardiovascular and kidney protective effects of canagliflozin. In addition to a significantly lower risk of death from cardiovascular causes, nonfatal myocardial infarction, and nonfatal stroke, the progression of albuminuria was less frequent in patients treated with canagliflozin (89.4 vs. 128.7 patients with an event per 1,000 patient-years; hazard ratio, 0.73; 95% CI, 0.67-0.79; NNT, 25.4) and the regression of albuminuria was more frequent (293.4 vs. 187.5 patients with regression per 1000 patient-years; hazard ratio, 1.70; 95% CI, 1.51-1.91; NNT, 9.443) in comparison to patients who received a placebo. The composite outcome of a sustained 40% reduction in the eGFR, the need for RRT, and death from renal causes occurred less frequently in the canagliflozin group than in the placebo group (5.5 vs. 9.0 patients with the outcome per 1,000 patient-years; hazard ratio, 0.60; 95% CI, 0.47-0.77; NNT, 220) (Fig. 4). Of note, the number of outcome events was considerably small. Moreover, the renal events mostly occurred due to changes in the eGFR. The demonstration of robust effects on the hard renal outcomes requires further investigation, which might be provided by the ongoing Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Patients with Diabetic Nephropathy (CREDENCE: ClinicalTrials.gov identifier NCT02065791) trial, which is recruiting T2DM patients with macroalbuminuria.
Figure 4.
Renal outcomes in the integrated CANVAS program (modified from 6). A: Kaplan-Meier analysis of progression of albuminuria. B: Kaplan-Meier analysis of a renal composite outcome (40% reduction in eGFR, requirement for renal-replacement therapy, or death from renal causes). The inset shows the data on an expanded y axis. eGFR: estimated glomerular filtration rate
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644-657, 2017.
One novel finding of the CANVAS trial, with regard to the safety of SGLT inhibitors, was the higher risk of lower extremity amputation in the canagliflozin group [6.3 vs. 3.4 participants with an event per 1,000 patient-years; hazard ratio, 1.97; 95% CI, 1.41-2.75; number needed to harm (NNH), 340]. This will be discussed in a later section.
The Direct Renal Effects of SGLT2 Inhibitors
Effects mediating decreased glucose reabsorption
Suppression of proximal tubular hyperplasia and hypertrophy
One of the major changes in the earliest stage of diabetic kidney disease is proximal tubular hyperplasia and sequential hypertrophy (2). Renal swelling mainly occurs due to this tubular hypertrophy and is particularly apparent in the cortex and the outer stripe of the outer medulla. Glucose reabsorption by the renal tubules in hyperglycemia induces the expression of growth factors such as TGFβ, VEGF, and IGF, and is considered to be the main cause of tubular hypertrophy. Empagliflozin and phlorizin (non-selective SGLT inhibitors) were shown to ameliorate renal swelling in animal models (7,8).
Suppression of inflammation and fibrosis by a reduction in the intracellular glucose concentration
Proximal tubular cells reabsorb glucose via SGLT2 and are exposed to high intracellular glucose concentrations, which induces the expression of inflammatory cytokines, growth factors, and fibrotic mediators and increases the production of advanced glycation products and reactive oxygen species. SGLT2 inhibitors are assumed to help suppress these events related to the onset and progression of kidney disease. Indeed, the exposure of HK2 cells, a human proximal tubular cell line, to empagliflozin was shown to reduce inflammatory and fibrotic signals, including toll-like receptor 4 (TLR4), NF-κB, IL-6, AP-1, and collagen IV (9). Hypoxia caused by interstitial fibrosis is regarded as a “final common pathway” leading to end-stage kidney disease irrespective of the original cause (10). Thus, the amelioration of hypoxia by SGLT2 inhibitors may have significant treatment implications.
Ketone bodies as an alternative fuel source
SGLT2 inhibitors cannot help but affect the dynamics of energy metabolism and consumption through changes in glucose storage in the body. Consequently, it was hypothesized that cardiomyocytes and renal tubular cells exposed to SGLT2 inhibitors undergo a fuel shift from free fatty acids (FFAs) to β-hydroxybutyrate (β-HB), which produces more energy per oxygen consumption and improves work efficiency (11).
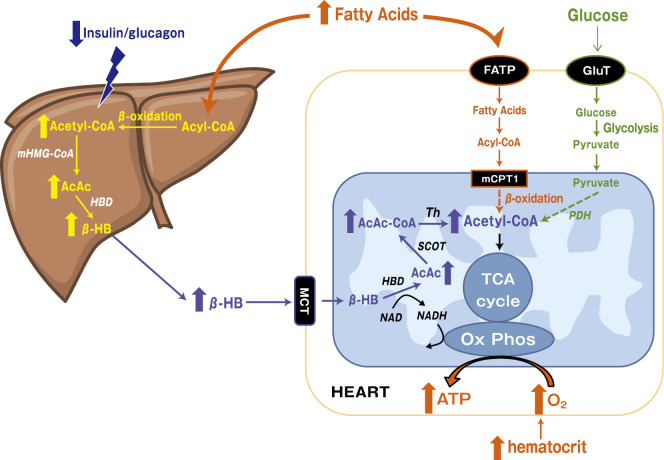
This hypothesis was originally proposed for the heart in an attempt to explain the cardiovascular benefits of empagliflozin in the EMPA-REG OUTCOME trial (11,12). The previously known systemic effects of SGLT2 inhibitors do not adequately explain why the number of cardiovascular deaths was significantly reduced or why this finding was apparent as early as 3 months after the initiation of the drug. SGLT2 inhibitors diminish glucose storage in the body, promote lipolysis, and increase circulating FFAs (Fig. 5). The hepatic production of ketone bodies is accelerated by decreased insulin and increased glucagon, which results in increased β-HB circulation. In the diabetic heart, cardiomyocytes exhibit insulin resistance, which leads to the decreased utilization of glucose and the use of FFAs as fuel, in spite of their limited production of energy per oxygen consumption. The heart and kidney consume more ketone bodies than other organs, with the uptake depending on the concentration in the blood; the consumption efficiency of ketone bodies does not fall, even in cases involving heart failure. The use of β-HB suppresses the use of glucose and FFAs. This fuel shift to β-HB is believed to reduce oxygen consumption.
Figure 5.
Energy metabolism in cardiomyocytes and renal tubular cells. Increased free fatty acids (FFAs) and decreased insulin/glucagon ratio promote production of β-hydroxybutyrate (β-HB) in the liver. Cardiomyocytes and renal tubular cells take up circulating β-HB as a fuel source for ATP, which suppresses the degradation of fatty acids and glucose (broken lines). AcAc: acetoacetate, Ox Phos: oxidative phosphorylation
Ferrannini E, Mark M, Mayoux E. CV protection in the EMPAREG OUTCOME trial: a “Thrifty Substrate” hypothesis. Diabetes Care 39: 1108-1114, 2016.
There are some caveats regarding this hypothesis (13). Without measuring actual ketone oxidation, we cannot be sure that the increased level of circulating β-HB stems from the enhanced production of β-HB rather than the inhibition of energy degradation. Many other factors remain to be clarified, such as the correlation between plasma the ketone body concentration and the cardiac function, the transition of the plasma ketone body concentration after the administration of an SGLT2 inhibitor, the effect of coadministration with insulin-stimulating medication, and possible changes in hypoxia under SGLT2 inhibition.
Effects mediating decreased sodium reabsorption
Restoration of tubuloglomerular feedback
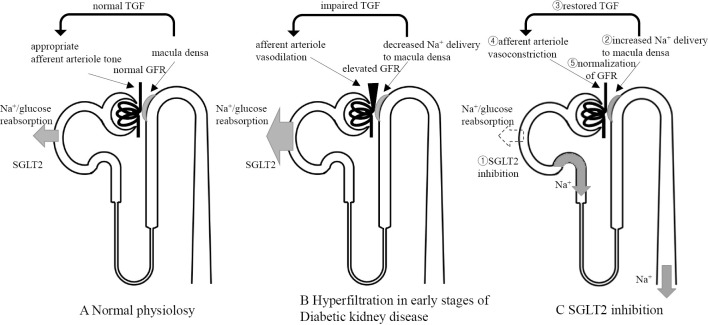
Although the reported prevalence of hyperfiltration has ranged from 13% to 75% in T1DM and from 0% to 40% in T2DM, hyperfiltration is considered to significantly increase the risk of diabetic kidney disease (14). The treatment target has focused on vascular tonus, as represented by RAAS inhibition, but the contribution of tubuloglomerular feedback (TGF) cannot be ignored. TGF is the mechanism by which the GFR is stabilized after its conditional fluctuations. The macula densa within the juxta-glomerular apparatus senses the Na concentration in the distal tubules and adjusts the afferent arteriolar tone by TGF (Fig. 6). In diabetic patients, activated SGLT2 enhances the reabsorption of Na and reduces the distal tubular concentration of Na, which is sensed as a decrease in the effective circulating plasma volume by the macula densa, resulting in afferent arteriolar dilation. It has been proposed that the increase in the urinary concentration of Na that occurs with SGLT2 inhibitor use attenuates TGF, causing afferent arteriolar constriction and a resultant decrease in intraglomerular pressure, with an eventual decrease in the GFR. In a trial investigating the effects of the 8-week administration of empagliflozin on the GFR of T1DM patients, the mean GFR in patients with hyperfiltration (GFR ≥135 mL/min/1.73 m2) at the baseline (n=27) decreased by 33 mL/min/1.73 m2; this was accompanied by a decrease in the plasma NO level and effective renal plasma flow, and an increase in renal vascular resistance (15). The patients with a GFR of 90-134 mL/min/1.73 m2 at the baseline (n=13) did not show these alterations. In addition, RAAS mediators led to significant increases in the patients with hyperfiltration. These findings may be explained by a decrease in effective renal plasma flow due to the natriuretic effect of the SGLT2 inhibitor; in addition, the coadministration with RAAS inhibitors might have induced additive renoprotective effects. SGLT2 inhibitors can correct hyperfiltration in normoalbuminuric T1DM patients with a normal renal function, and they are expected to have a role in the primary prevention of diabetic kidney disease; however, this role remains to be proven.
Figure 6.
Postulated tubuloglomerular feedback (TGF) mechanisms (modified from 15). A: Under physiological conditions, TGF signaling controls afferent arteriole tone to maintain GFR. Transient increases in GFR cause the macula densa to sense the increase in distal tubular Na+ concentration and GFR is eventually decreased via TGF. B: Under chronic hyperglycemia, Na+ and glucose reabsorption by SGLT2 is promoted and TGF is impaired. In other words, in spite of increased GFR, the macula densa detects reduced distal tubular Na+ concentration. By this mechanism, afferent arterioles unsuitably dilate and GFR becomes elevated. C: SGLT2 inhibition results in interrupted Na+ and glucose reabsorption, leading to increased Na+ delivery to the macula densa. This allows afferent arterioles to constrict appropriately, restores TGF, and normalizes GFR. GFR: glomerular filtration rate, SGLT2: sodium-glucose cotransporter 2
Cherney DZI, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587-597, 2014.
Alteration in the distribution of oxygen consumption
The kidney boasts the second largest oxygen consumption (QO2) per gram next to the heart. Approximately 80% of this oxygen is consumed for Na reabsorption by Na-K ATPase. In diabetes, the Na-K ATPase activity and oxygen consumption are enhanced by SGLT2 activation, which is caused by the high intracellular glucose concentration in the proximal tubular cells, as described previously. The QO2 of the proximal tubular cells in diabetic rat models was approximately twice that in controls, and phlorizin was shown to suppress the activation of Na-K ATPase and the increase in QO2 (16). O'Neill et al. demonstrated that the administration of phlorizin resulted in a reduction in total kidney O2 consumption in the diabetic rat kidney in actual experiments, which supported this hypothesis (17). Hyperfiltration and proximal tubular hyperplasia/hypertrophy are believed to contribute to enhanced oxygen consumption, which suggests the possibility that SGLT2 inhibitors might improve interstitial hypoxia by reducing the oxygen consumption that mediates these processes.
However, Na reabsorption beyond the late proximal tubules (S3) increases to compensate for the loss of Na reabsorption in the early proximal tubules (S1 and S2). In a mathematical model predicting the QO2 of a single rat nephron, although the chronic administration of an SGLT inhibitor to diabetic rats led to an approximately 30% decrease in the QO2 in S1 and S2 and overall reduction in the QO2 of the whole kidney, the QO2 in S3, the thick ascending limb, and the collecting tubules in the inner and outer medulla was increased by 26%, 2%, 21%, and 9%, respectively (18). This model did not take the possible fuel shift into account and may contradict the previous fuel shift hypothesis in this respect.
Improvement of anemia
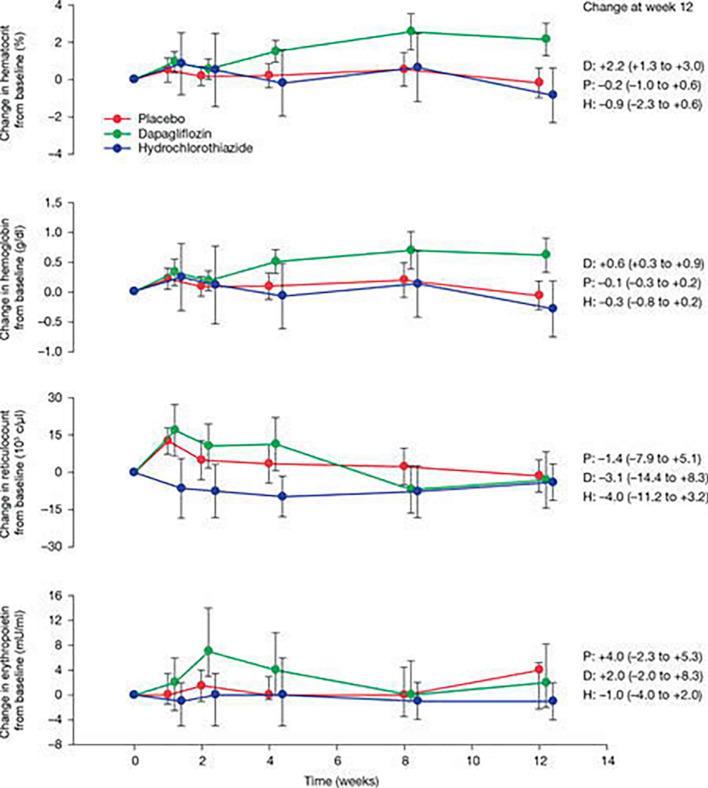
SGLT2 inhibitors have been proven to ameliorate anemia. In a study that randomized 75 DM patients into placebo, hydrochlorothiazide, and dapagliflozin groups, the improvement in anemia was attributed to increased erythropoietin and not to an increased hemoconcentration due to the diuretic effect (Fig. 7) (19). A number of possible mechanisms have been proposed, including the functional recovery of the tubulointerstitial fibroblasts as erythropoietin producing cells after the improvement of hypoxia and injury (20) and the production of erythropoietin resulting from the induction of hypoxia-inducible factor (HIF) 2 by hypoxia around S3, where erythropoietin producing cells are thought to be abundant.
Figure 7.
Mean change in haematocrit, haemoglobin, reticulocyte count and erythropoietin over the course of the study in placebo dapagliflozin, and hydrochlorothiazide groups. Data are reported as mean (±95% CI). Because of an extreme outlier, the median concentrations (25th and 75th percentile) in erythropoietin over time are reported in the figure. Excluding the outlier and calculating the mean value provided a similar picture (data not shown). P: placebo, D: dapagliflozin, H: hydrochlorothiazide. IF THIS IMAGE HAS BEEN PROVIDED BY OR IS OWNED BY A THIRD PARTY, AS INDICATED IN THE CAPTION LINE, THEN FURTHER PERMISSION MAY BE NEEDED BEFORE ANY FURTHER USE. PLEASE CONTACT WILEY'S PERMISSIONS DEPARTMENT ON PERMISSIONS@WILEY.COM OR USE THE RIGHTSLINK SERVICE BY CLICKING ON THE 'REQUEST PERMISSIONS' LINK ACCOMPANYING THIS ARTICLE. WILEY OR AUTHOR OWNED IMAGES MAY BE USED FOR NON-COMMERCIAL PURPOSES, SUBJECT TO PROPER CITATION OF THE ARTICLE, AUTHOR, AND PUBLISHER.
Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 15: 853-862, 2013. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/dom.12127
Renal anemia is one of the few treatable targets in the final common pathway of CKD, and an increase in endogenous erythropoietin could reduce the need for erythropoiesis-stimulating agents.
Preconditioning of ischemia-reperfusion injury in non-diabetic models
Certain effects of SGLT2 inhibition have been investigated in non-diabetic kidney disease models. In experiments using a rat model of renal ischemia-reperfusion (IR) and an HK2 cell model of hypoxia-reoxygenation (HR), pretreatment with dapagliflozin activated HIF1, and conferred protection against IR or HR injury (21). In the mathematical prediction model of a single nephron - in contrast to the diabetic rat model with increased oxygen consumption in S1 and S2 without SGLT2 inhibition - SGLT2 inhibition was associated with an approximately 7% increase in oxygen consumption in the whole kidney of non-diabetic rats; this predominantly reflected an increase in S3 oxygen consumption. This mild hypoxia induced by SGLT2 inhibitors may activate HIF1, which has a renoprotective effect, thereby eventually attenuating injury from more potent hypoxic stimuli.
The Systemic Effects of SGLT2 Inhibitors Probably Lead to Renoprotection
Blood glucose reduction
It has been demonstrated that SGLT2 inhibitors improve blood glucose control, including HbA1c and the fasting and postprandial blood glucose levels. Regardless of the blood glucose-lowering method, accumulated evidence indicates the efficacy of improved blood glucose control in the suppression of new-onset microalbuminuria and progression to macroalbuminuria. The benefit appears to be sustained, even after the subsequent worsening of blood glucose control. However, improvement in the reduced GFR or the doubling of serum creatinine has not been observed (22).
The reduction in insulin levels and the improvement of insulin sensitivity
The uptake of glucose by skeletal muscle in patients taking SGLT2 inhibitors is substantially increased, probably due to the amelioration of glucotoxicity and a reduction in body fat (23). A reduced glucose concentration also leads to a compensatory reduction in insulin secretion. As a result of the amelioration of hyperinsulinemia in T2DM, insult to the microvasculature and eventual damage to many organs, including the kidneys may be alleviated (24).
Serum uric acid reduction
In some clinical trials, SGLT2 inhibitor use led to a modest but significant reduction in the serum uric acid concentration (8). The mechanism of the increased uricosuria may be attributed to the action of SLCA9 (GLUT9) isoform b, which absorbs the luminal glucose - which is increased by SGLT2 inhibition - and which secretes uric acid back into the lumen in exchange (25).
Body weight reduction
In contrast to the current antihyperglycemic medications with insulin-intensifying effects, SGLT2 inhibitors reduce body weight. This may be attributable to the urinary glucose disposition, decreased serum insulin levels (as indicated above), the promotion of lipid degradation, and the increased oxidative metabolism of fat. Obesity is a risk factor for the development of kidney disease and weight reduction is associated with a decrease in macroalbuminuria and microalbuminuria (26).
Blood pressure reduction
In some studies, SGLT2 inhibitor treatment reduced the systolic blood pressure by approximately 5 mmHg. This appears to be due to natriuretic and osmotic diuretic effects and weight reduction.
Areas of Concern in the Use of SGLT2 Inhibitors
The increase in the glucose load of the lower nephron
The main side effects of glucosuria by SGLT2 inhibitors are genital infections and urinary tract infections. However, based on the findings of studies of patients with familial glucosuria, who mainly in whom the expression of SGLT2 is mainly reduced by a genetic mutation, it may be assumed that long-term SGLT2 inhibition is not associated with the deterioration of the renal tubular function (26).
However, caution may be needed. Studies using a rat model of polycystic kidney disease (PCKD) found that the 6-week administration of an SGLT2 inhibitor increased the cyst volume and the total kidney weight by 23% without a decrease in creatinine clearance, a change in cAMP, or an increase in Ki67 (a marker of cellular proliferation) (27). This should be considered when prescribing SGLT2 inhibitors to patients with PCKD.
Leg amputation and bone fracture
Among other relatively tolerable and previously known adverse effects of SGLT2 inhibitors, the increased risk of toe, foot, and leg amputation that was observed in the canagliflozin treatment group of the CANVAS trial was an unexpected finding (6). The most frequent (71%) amputation was at the level of the toe or metatarsal bones. The mechanism is currently unknown; however, the highest absolute risk of amputation occurred among patients with a history of amputation or peripheral vascular disease, indicating the need for special attention when canagliflozin is administered to this group of patients.
An increased risk of bone fracture was observed in the CANVAS trial but not in the CANVAS-R trial. A post-hoc study investigating the effects of canagliflozin on fracture risk in T2DM patients, which used interim data of the CANVAS trial (n=4,327) and a pooled population of 8 non-CANVAS studies (n=5,867), revealed that the risk was increased risk among the patients in the CANVAS trial, but not in the pooled patients from the non-CANVAS studies (28). It was concluded that even though the discrepancy might have been explained by increased bone turnover and reduced BMD due to a small but statistically significant reduction in the bone mineral density (BMD) of the total hip - which was observed over 104 weeks in another study (29) - and an increase in estradiol, the inconsistency of the result might have been due to chance or related to factors that were extrinsic to bone health. The association between SGLT2 inhibitors, including canagliflozin and the risk of fracture, warrants further investigation.
SGLT2 inhibitor use in CKD patients
Most of the clinical trials of SGLT2 inhibitors were conducted in patients with an eGFR of >30 mL/min/1.73 m2, and dehydration and subsequent renal impairment were matters of concern in patients with more advanced CKD. At the very least, the results showed that the blood glucose-lowering effect depends on the serum glucose concentration and GFR, and decreases or disappears with a decrease in the kidney function. Even though an improvement in albuminuria was shown in CKDG3 patients, doctors are instructed to exercise care when administering SGLT2 inhibitors to patients with moderate renal insufficiency and not to use them in cases involving severe renal insufficiency. As we discussed above, considering that glomerular protection can be expected - even in patients with a normal kidney function - it is practical currently to use SGLT2 inhibitors to prevent the onset or progression of diabetic kidney disease in the early stage.
Conclusion
The present study provides an overview of the effects of SGLT2 inhibitors on the kidney and their mechanisms. Because they work directly on the renal tubular cells, their renal effects can be potent and broad. Besides, the alternation in metabolism dynamically affects the whole body and results in various actions in each organ, including the heart and kidneys. Although SGLT2 inhibitors seem to hold great promise as a new therapeutic modality in diabetic kidney disease, much remains to be understood and further studies are warranted to facilitate their safe and effective use.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Škrtić M, Cherney DZI. Sodium-glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr Opin Nephrol Hypertens 24: 96-103, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. AJP Regul Integr Comp Physiol 300: R1009-R1022, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 85: 962-971, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 373: 2117-2128, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323-334, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644-657, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Malatiali S, Francis I, Barac-Nieto M. Phlorizin prevents glomerular hyperfiltration but not hypertrophy in diabetic rats. Exp Diabetes Res 2008: 305403, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallon V, Gerasimova M, Rose MA, et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 306: F194-F204, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panchapakesan U, Pegg K, Gross S, et al. Effects of SGLT2 inhibition in human kidney proximal tubular cells - renoprotection in diabetic nephropathy? PLoS One 8: e54442, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17-25, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care 39: 1115-1122, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “Thrifty Substrate” hypothesis. Diabetes Care 39: 1108-1114, 2016. [DOI] [PubMed] [Google Scholar]
- 13.Lopaschuk GD, Verma S. Empagliflozin's fuel hypothesis: not so soon. Cell Metab 24: 200-202, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Stanton RC. Sodium glucose transport 2 (SGLT2) inhibition decreases glomerular hyperfiltration: is there a role for SGLT2 inhibitors in diabetic kidney disease? Circulation 129: 542-544, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Cherney DZI, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587-597, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Körner A, Eklöf AC, Celsi G, Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes 43: 629-633, 1994. [DOI] [PubMed] [Google Scholar]
- 17.O'Neill J, Fasching A, Pihl L, Patinha D, Franzén S, Palm F. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol 309: F227-F234, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Layton AT, Vallon V, Edwards A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am J Physiol Renal Physiol 310: F1269-F1283, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 15: 853-862, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res 8: 844-847, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang Y-K, Choi H, Jeong JY, et al. Dapagliflozin, SGLT2 inhibitor, attenuates renal ischemia-reperfusion injury. PLoS One 11: e0158810, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ 343: d4169, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 124: 509-514, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groop P-H, Forsblom C, Thomas MC. Mechanisms of disease: pathway-selective insulin resistance and microvascular complications of diabetes. Nat Clin Pract Endocrinol Metab 1: 100-110, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Cheeseman C. Solute carrier family 2, member 9 and uric acid homeostasis. Curr Opin Nephrol Hypertens 18: 428-432, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Thomas MC. Renal effects of dapagliflozin in patients with type 2 diabetes. Ther Adv Endocrinol Metab 5: 53-61, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapoor S, Rodriguez D, Riwanto M, et al. Effect of sodium-glucose cotransport inhibition on polycystic kidney disease progression in PCK rats. PLoS One 10: e0125603, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watts NB, Bilezikian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 101: 157-166, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilezikian JP, Watts NB, Usiskin K, et al. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab 101: 44-51, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]