Abstract
A 68-year-old woman was admitted to our hospital with fever and pleural effusion. Her thoracentesis showed eosinophilic pleural effusion (EPE) without any evidence of malignancy, infection, or trauma. Pleural biopsy revealed pleuritis and intercostal myositis. Characteristic skin manifestations, including Gottron's sign, interstitial lung disease, and pericardial effusion, appeared later in the clinical course. She was finally diagnosed with anti-PL-7 antisynthetase syndrome (ASS) based on the presence of anti-PL-7 antibody, and she fulfilled the diagnostic criteria for dermatomyositis. These clinical manifestations improved with immunosuppressive therapy. EPE might therefore be one of the characteristic features of anti-PL-7 ASS.
Keywords: antisynthetase syndrome (ASS), aminoacyl-tRNA synthetase (ARS), anti-PL-7 antibody, eosinophilic pleural effusion (EPE), dermatomyositis (DM)
Introduction
Antisynthetase syndrome (ASS) is characterized by the presence of antibodies to aminoacyl-transport ribonucleic acid synthetase (ARS), with common clinical features including inflammatory myopathy, interstitial lung disease (MD), inflammatory arthritis, fever, Raynaud's phenomenon and mechanic's hands (1,2). Eight anti-ARS antibodies have been identified so far, with anti-Jo-1 (histidyl-tRNA synthetase) being the most common, occurring in approximately 20% of all idiopathic inflammatory myopathies (IIMs) (1,3). Other anti-ARS antibodies include anti-PL-7, anti-PL-12, anti-OJ, anti-EJ, anti-KS, anti-Zo, and anti-Ha antibodies. Anti-PL-7 antibody recognizes threonyl-tRNA-synthetases and was detected in 5-10% of IIM (1) and in 10-18% of ASS (3-6). Although ASS has common clinical manifestations, its relative frequency varies with the type of anti-ARS antibodies (4-6).
Pleural effusion associated with connective tissue disease (CTD) is frequent in patients with rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), but it is relatively rare in polymyositis/dermatomyositis (PM/DM) or ASS (7). Eosinophilic pleural effusion (EPE), defined as an eosinophil count ≥10% in the pleural fluid (8), is commonly seen in malignancy, infection, trauma and CTD (9,10). Pleural effusion associated with CTD is usually lymphocytic (7); however, this patient, who was diagnosed with anti-PL-7 ASS, had EPE. To the best of our knowledge, there has been no previous report of EPE associated with anti-PL-7 ASS. We herein report a case of anti-PL-7 ASS with EPE in which remission was achieved with a combination of corticosteroids and immunosuppressive agents.
Case Report
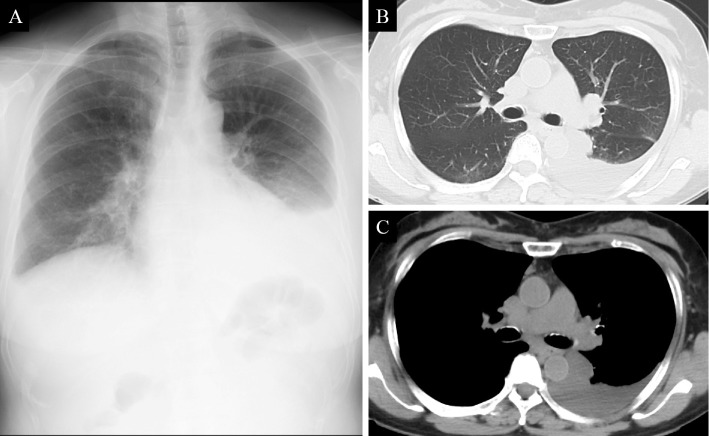
A 68-year-old Japanese woman was admitted to our hospital with fever and left chest pain for the previous two weeks that did not respond to treatment with garenoxacin. She was a never smoker and had no significant past medical history or trauma. Chest X-ray showed left pleural effusion (Fig. 1A). Computed tomography (CT) of the chest confirmed left pleural effusion, while no other abnormality was noted Fig. 1B and C.
Figure 1.
Chest X-ray on admission showing left pleural effusion (A). A computed tomography (CT) scan on admission showing left pleural effusion with no apparent evidence of interstitial lung disease (ILD) (B, C).
Laboratory tests revealed a white blood cell (WBC) count of 16,000 /μL (neutrophils 81.7%, lymphocytes 10.6% and eosinophils 0.2%); total protein (TP), 5.8 g/dL; albumin, 2.4 g/dL; creatine phosphokinase (CPK), 266 U/L; lactate dehydrogenase (LDH), 364 U/L; C-reactive protein (CRP), 3.9 mg/dL; and ferritin, 274.7 ng/mL. The patient was positive for the presence of anti-nuclear antibody (ANA; titer 1:40, speckled pattern) but was negative for the presence of other autoantibodies, including anti-double stranded DNA (anti-dsDNA) antibody and anti-cyclic citrullinated peptide (anti-CCP) antibody. In addition, negative results were obtained for the presence of tumor markers, including carcinoembryonic antigen (CEA), cytokeratin-19 fragments (CYFRA), and pro-gastrin-releasing peptide (ProGRP), and for the interferon-gamma release assay (IGRA).
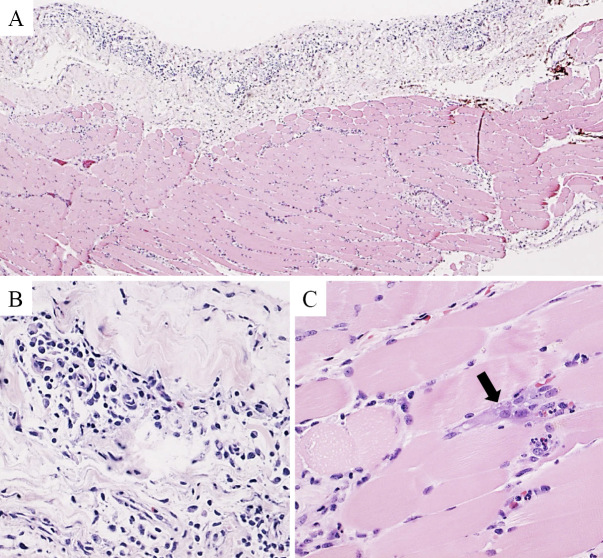
Thoracentesis was performed and revealed the presence of exudative pleural effusion with no evidence of malignancy. A biochemical analysis revealed the following results: TP, 3.1 g/dL; LDH, 232 U/L; WBC, 4,610 /μL; glucose, 108 mg/dL; Adenosine deaminase (ADA), 11.4 U/L; and CEA, 1.8 ng/mL. The differential WBC count showed a high eosinophil count (neutrophils 38.2%, lymphocytes 5.8%, eosinophils 52.6% and monocytes 3.4%). A pleural fluid culture was negative. For a definitive diagnosis, a surgical biopsy of the pleura was performed. The samples contained pleura and intercostal muscle specimens, and the histopathological findings revealed pleuritis and myositis of the intercostal muscle (Fig. 2A). The pleura was predominantly infiltrated by lymphocytes and plasma cells with relatively few eosinophils, suggestive of nonspecific pleuritis (Fig. 2B). The intercostal muscle showed degeneration and infiltration with inflammatory cells, including lymphocytes and plasma cells, suggestive of myositis (Fig. 2C). There were few eosinophils in the pleura and intercostal muscle specimens.
Figure 2.
Histological findings of Hematoxylin and Eosin staining of samples obtained from the pleura and intercostal muscle (A: ×30). Inflammatory cells including lymphocytes and plasma cells were predominant in the pleura (B: ×300). The intercostal muscle tissue specimen showed basophilic degeneration as indicated by the arrow and inflammatory infiltration (C: ×300). There were few eosinophils in the pleura and intercostal muscle specimens.
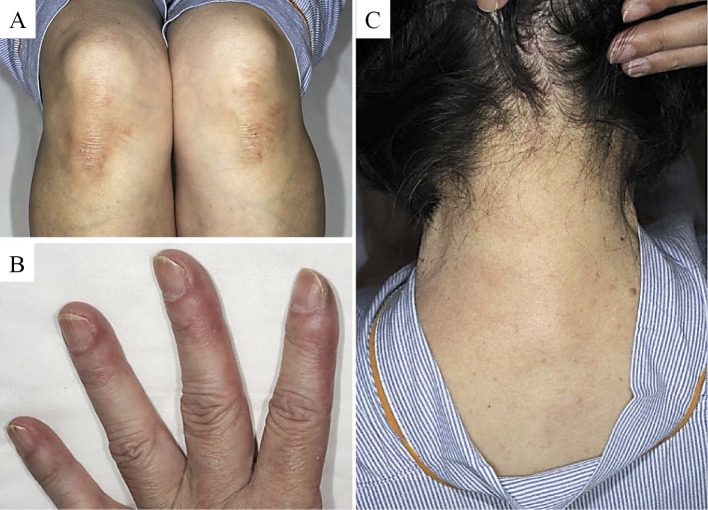
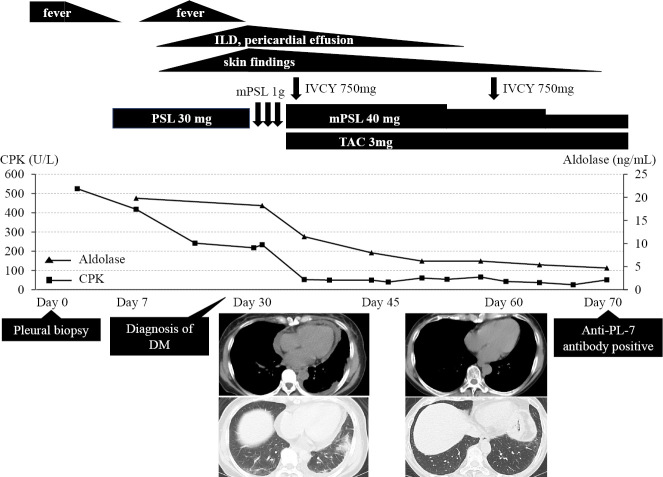
The clinical course after pleural biopsy is shown in Fig. 3. The patient was started on oral prednisolone, 0.5 mg/kg/day, which resulted in a transient remission of fever. However, fever recurred along with new skin manifestations, including Gottron's sign (Fig. 4A), mechanic's hand (Fig. 4B), and Shawl sign (Fig. 4C). In addition, a high-resolution CT (HRCT) scan of the chest revealed new-onset ILD and pericardial effusion (Fig. 3). ILD demonstrated a patchy ground-grass attenuation and consolidation without honeycombing and/or traction bronchiectasis in bilateral basal lungs, suggestive of nonspecific interstitial pneumonia (NSIP) and/or organizing pneumonia (OP)-like pattern, in HRCT. Although she had no muscle symptoms, such as muscle pain or weakness, further laboratory investigations showed a high level of muscle enzymes, including CPK and aldolase; needle electromyogram revealed myopathic changes in the tibialis anterior and biceps brachii muscles. Based on these findings, she was considered to fulfill the diagnostic criteria for DM (Fig. 3).
Figure 4.
Skin findings in our patient, the characteristics of Antisynthetase syndrome (ASS). Gottron’s sign (A), mechanic’s hand (B), and Shawl sign (C).
Figure 3.
Clinical course after the pleural biopsy. Initial treatment with oral prednisolone (0.5 mg/kg) was inadequate.In addition to pleural effusion and inflammatory myopathy, skin findings, interstitial lung disease (ILD), and pericardial effusion appeared later. After the diagnosis of DM, the combination of immunosuppressive therapy including methyl prednisolone (1 g/day intravenously on days 1-3 and 40 mg/day orally from day 4), intravenous cyclophosphamide (750 mg/body on day 4, 28), and tacrolimus (3 mg/day orally from day 4) was commenced. The pleural effusion resolved promptly and the other findings also improved. She was finally diagnosed with anti-PL-7 ASS based on the presence of anti-PL-7 antibody. DM: dermatomyositis, IVCY: intravenous cyclophosphamide, PSL: prednisolone, mPSL: methyl prednisolone, TAC: tacrolimus, CPK: creatine phosphokinase, ASS: antisynthetase syndrome
Since we considered that this case might be a rapidly progressive ILD with anti-melanoma differentiation-associated gene 5 (MDA-5) antibody before obtaining the results of myositis-associated antibodies, she was started on combination immunosuppressive therapy with methyl prednisolone (1 g/day intravenously on days 1-3 and 40 mg/day orally from day 4), cyclophosphamide (750 mg/body intravenously on days 4 and 28), and tacrolimus (3 mg/day orally from day 4) in a sequential manner (Fig. 3). With this treatment, the pleural effusion resolved immediately, while the other clinical manifestations including myositis, skin findings, ILD and pericardial effusion also improved. Lung function tests showed an improvement in the predicted vital capacity (%VC) from 80.5% to 106.4% and in the diffusing capacity for carbon monoxide (%DLco) from 77.4% to 123.2%. Finally, after performing a screening test using an enzyme-linked immunosorbent assay (ELISA) kit for anti-ARS antibodies (MESACUP anti-ARS test; MBL, Nagoya, Japan), including five anti-ARS antibodies (anti-Jo-1, anti-PL-7, anti-PL-12, anti-EJ, and anti-KS antibody) (3), a developed ELISA for each anti-ARS antibody (MBL) revealed the presence of anti-PL-7 antibody, which confirmed the diagnosis of anti-PL-7 ASS (Fig. 3). In addition, anti-MDA-5 antibody was negative. The remarkable improvement noted with immunosuppressive therapy suggested that our patient had several features, including EPE, associated with anti-PL-7 ASS. Although the immunosuppressive therapy was gradually reduced to 8 mg/day of prednisolone and 1.5 mg/day of tacrolimus, she continues to be in remission after 12 months.
Discussion
Interestingly, our patient presented with EPE as one of the early findings of anti-PL-7 ASS. To the best of our knowledge, there has been no previous report of EPE in a patient with anti-PL-7 ASS. In addition, inflammatory myopathy was confirmed in the intercostal muscle according to the histopathological findings. Noguchi et al. studied skeletal muscle involvement in detail in 51 myositis patients with ASS and showed that the respiratory muscle involvement was rare, accounting for only 8% (11). Intercostal myositis, leading to muscle weakness, may have led to respiratory dysfunction in our case. Our patient tested positive for anti-PL-7 antibody and thus was diagnosed with anti-PL-7 ASS; she also fulfilled the diagnostic criteria for DM. Our findings suggest that the clinical features and presence of EPE are associated with anti-PL-7 ASS in this case, as she showed a good improvement with immunosuppressive therapy.
Anti-PL-7 antibody recognizes threonyl-tRNA synthetase and the frequency of anti-PL-7 antibody has been reported to range from 5-10% in IIM (1) and 10-18% of ASS cases (3-6). Several previous reports that studied the clinical manifestations of anti-PL-7 ASS have shown that myositis and ILD are common features (12-15). In a previous report that included a case series from a European multicenter study (12), the most common clinical manifestations of anti-PL-7 ASS were ILD (77.8%), myositis (75%), arthritis/arthralgia (55.6%), Raynaud's phemonenon (40.3%), fever (30.5%), and mechanic's hands (18%). In addition, pericardial effusion was observed in 22.2%, suggesting that pericarditis might be related to anti-PL-7 antibody (12). We consider the pericardial effusion associated with pericarditis in our case to be associated with anti-PL-7 ASS since it resolved with immunosuppressive therapy.
Pleural effusion in CTDs is a frequent manifestation which is most commonly found in patients with RA and SLE (7). However, it is considered rare in other CTDs, including PM/DM or ASS. Fujisawa et al. noted pleural effusion in 9 of 102 patients (8.8%) with myositis-associated ILD (16). Debray et al., in a series of 33 patients with ASS-associated ILD, reported pleural effusion in just one patient (3%) (17). This report included 17 patients with anti-Jo-1, 13 with anti-PL-12, and 3 with anti-PL-7 antibody (17). To date, 10 cases have been reported with pleural effusion associated with PM/DM as summarized in Table (18-25). In patients with pleural effusion associated with PM/DM, there was a male predominance along with a higher incidence of DM and ILD. Four patients were positive for anti-ARS antibody, including 3 with anti-Jo-1 and 1 with anti-PL-7 antibody (our case). Exudative pleural effusion was seen in 8 cases, with either a lymphocytic, neutrophilic, or eosinophilic predominance. Although two patients died from pulmonary insufficiency due to ILD or malignancy, the pleural effusion improved in all cases after treatment with corticosteroids and/or immunosuppressive agents.
Table.
Clinical Characteristics of PM/DM Patients with Pleural Effusion.
| Case | Ref. | Age | Sex | Diagnosis | MSA | ILD | Pleural effusion | Treatment | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | 67 | F | PM | N/A | + | N/A | PSL | Improved | ||||||
| 2 | 19 | 50 | F | PM | Jo-1 (-) | - | Transudative | PSL | Improved | ||||||
| 3 | 19 | 34 | M | DM | Jo-1 (+) | + | Exudative; Lym 60% |
PSL+CyA | Died* | ||||||
| 4 | 20 | 66 | M | PM | Jo-1 (-) | - | Exudative; Lym+, Neu+, Eos- |
PSL | Improved | ||||||
| 5 | 21 | 55 | M | DM+SSc | Jo-1 (-) | + | Exudative; Neu 45%, Lym 25%, Eos 19% |
PSL | Improved | ||||||
| 6 | 22 | 36 | M | DM | Jo-1 (-) | + | Exudative; Lym-, Neu+, Eos- |
PSL | Improved | ||||||
| 7 | 23 | 43 | M | Anti-Jo-1 syndrome | Jo-1 (+) | + | Exudative | PSL+CPA+CyA | Improved | ||||||
| 8 | 24 | 61 | M | DM | Jo-1 (+) SRP (+) | + | Exudative | PSL | Died** | ||||||
| 9 | 25 | 24 | M | CADM | Jo-1 (-) SRP (-) | + | Exudative; Neu 43%, Lym 27% |
PSL+IVIG+MMF | Improved | ||||||
| 10 | This case | 68 | F | ASS/DM | Jo-1 (-) PL-7 (+) | + | Exudative; Neu 38.2%, Lym 5.5%, Eos 52.6% |
PSL+CPA+Tac | Improved |
*pulmonary insufficiency due to interstitial pneumonia, **gastric cancer.
PM: polymyositis, DM: dermatomyositis, Ref.: Reference, MSA: myositis specific antibody, ILD: interstitial lung disease, M: male, F: female, CADM: clinically amyopathic dermatomyositis, N/A: not available, Jo-1: anti-Jo-1 antibody, SRP: anti-signal recognition particle antibody, PL-7: anti-PL7 antibody, Lym: lymphocyte, Neu: neutrophil, Eos: eosinophil, PSL: prednisolone, CyA: cyclosporine A, CPA: cyclophosphamide, IVIG: intravenous immunoglobulin, MMF: mycophenoate, Tac: tacrolimus
EPE is usually defined as an eosinophil count ≥10% in the pleural fluid (8). In a systemic review of 687 cases with EPE, Oba et al. reported that EPE was most often due to malignancy (26%), followed by idiopathic (25%), parapneumonic effusion (13%), pleural air/blood (13%), tuberculosis (7%), transudate (7%), other causes (6%), and CTD (3%) (10). In our case, the etiology of EPE was most likely related to local immune pleuritis associated with myositis and/or ILD due to anti-PL-7 ASS because our case had no other cause for EPE, including malignancy, infection, or trauma, and the pleural effusion resolved with immunosuppressive therapy. The pathogenesis of EPE is still poorly understood, but IL-5 has been suggested to play an important role in the pathogenetic pathway of EPE (8,26). A pleural biopsy should not be routinely performed, but it most commonly shows nonspecific pleuritis without eosinophil infiltration in patients with EPE (8). As in our case, in a previous report, a pleural biopsy demonstrated nonspecific pleuritis and an improvement in EPE with corticosteroid therapy in a patient with EPE associated with PM and systemic sclerosis overlap syndrome (case 5 in Table) (21).
The presence of anti-ARS antibody including anti-PL-7 antibody has been reported to be associated with a favorable response to immunosuppressive therapy and an increased overall survival in PM/DM patients with ILD (27-29). However, it has been suggested that the clinical phenotype and prognosis vary with the type of anti-ARS antibody in patients with ASS. Some reports have demonstrated that ASS patients with anti-PL-7/12 antibodies have a lower survival rate than those with anti-Jo-1 antibody (5,6). Fujisawa et al. reported that the anti-PL-7 antibody is one of the significant predictors of pulmonary worsening during a long-term follow-up of more than a year in patients with PM/DM-ILD (30). Based on these data, a careful long-term follow-up was therefore deemed to be required in our case.
In conclusion, we herein described a case of anti-PL-7 ASS with EPE. Our case showed that EPE can sometimes be associated with anti-PL-7 ASS, and that the symptoms improved after the administration immunosuppressive therapy. EPE might therefore be one of the characteristic features of anti-PL-7 ASS.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Mimori T, Imura Y, Nakashima R, et al. . Autoantibodies in idiopathic inflammatory myopathy: an update on clinical and pathophysiological significance. Curr Opin Rheumatol 19: 523-529, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Mirrakhimov AE. Antisynthetase syndrome: a review of etiopathogenesis, diagnosis and management. Curr Med Chem 22: 1963-1975, 2015. [PubMed] [Google Scholar]
- 3.Nakashima R, Imura Y, Hosono Y, et al. . The multicenter study of a new assay for simultaneous detection of multiple anti-aminoacyl-tRNA synthetases in myositis and interstitial pneumonia. PLoS One 9: e85062, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamaguchi Y, Fujimoto M, Matsushita T, et al. . Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS One 8: e60442, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hervier B, Devilliers H, Stanciu R, et al. . Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun Rev 12: 210-217, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Marie I, Josse S, Decaux O, et al. . Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun Rev 11: 739-745, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Bouros D, Pneumatikos I, Tzouvelekis A. Pleural involvement in systemic autoimmune disorders. Respiration 75: 361-371, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Kalomenidis I, Light RW. Eosinophilic pleural effusions. Curr Opin Pulm Med 9: 254-260, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Krenke R, Nasilowski J, Korczynski P, et al. . Incidence and aetiology of eosinophilic pleural effusion. Eur Respir J 34: 1111-1117, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Oba Y, Abu-Salah T. The prevalence and diagnostic significance of eosinophilic pleural effusions: a meta-analysis and systematic review. Respiration 83: 198-208, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi E, Uruha A, Suzuki S, et al. . Skeletal muscle involvement in antisynthetase syndrome. JAMA Neurol 74: 992-999, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labirua-Iturburu A, Selva-O'Callaghan A, Vincze M, et al. . Anti-PL-7 (anti-threonyl-tRNA synthetase) antisynthetase syndrome: clinical manifestations in a series of patients from a European multicenter study (EUMYONET) and review of the literature. Medicine 91: 206-211, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Marie I, Josse S, Decaux O, et al. . Clinical manifestations and outcome of anti-PL7 positive patients with antisynthetase syndrome. Eur J Intern Med 24: 474-479, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Sato S, Hirakata M, Kuwana M, et al. . Clinical characteristics of Japanese patients with anti-PL-7 (anti-threonyl-tRNA synthetase) autoantibodies. Clin Exp Rheumatol 23: 609-615, 2005. [PubMed] [Google Scholar]
- 15.Yamasaki Y, Yamada H, Nozaki T, et al. . Unusually high frequency of autoantibodies to PL-7 associated with milder muscle disease in Japanese patients with polymyositis/dermatomyositis. Arthritis Rheum 54: 2004-2009, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Fujisawa T, Hozumi H, Kono M, et al. . Prognostic factors for myositis-associated interstitial lung disease. PLoS One 9: e98824, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debray MP, Borie R, Revel MP, et al. . Interstitial lung disease in anti-synthetase syndrome: initial and follow-up CT findings. Eur J Radiol 84: 516-523, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Roach DG, Salter WM. Polymyositis with pulmonary infiltrate and pleural effusion. Minn Med 63: 277-279, 281, 1980. [PubMed] [Google Scholar]
- 19.Miyata M, Fukaya E, Takagi T, et al. . Two patients with polymyositis or dermatomyositis complicated with massive pleural effusion. Intern Med 37: 1058-1063, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Araya J, Nagai T, Oda H, et al. . Polymyositis-induced respiratory failure in the presence of antecedent pleural effusion. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 36: 713-716, 1998(in Japanese). [PubMed] [Google Scholar]
- 21.Maeshima E, Nishimoto T, Yamashita M, Mune M, Yukawa S. Progressive systemic sclerosis-polymyositis overlap syndrome with eosinophilic pleural effusion. Rheumatol Int 23: 252-254, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Iwai H, Koike R, Ogawa J, et al. . Case of dermatomyositis complicated with massive pleural effusion that preceded the myopathy. Nihon Rinsho Meneki Gakkai Kaishi (Jpn J Clin Immunol) 25: 270-276, 2002(in Japanese). [DOI] [PubMed] [Google Scholar]
- 23.Mogulkoc N, Kabasakal Y, Ekren PK, Bishop PW. An unusual presentation of anti-Jo-1 syndrome, mimicking lung metastases, with massive pleural and pericardial effusions. J Clin Rheumatol 12: 90-92, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Sugie K, Tonomura Y, Ueno S. Characterization of dermatomyositis with coexistence of anti-Jo-1 and anti-SRP antibodies. Intern Med 51: 799-802, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Chhaya S, Hurowitz B, Ardiles T, Carlson R. Clinically amyopathic dermatomyositis complicated by pleural effusion case report, literature review, and proposed mechanism. Bull Hosp Jt Dis (2013) 73: 217-220, 2015. [PubMed] [Google Scholar]
- 26.Mohamed KH, Abdelhamid AI, Lee YC, et al. . Pleural fluid levels of interleukin-5 and eosinophils are closely correlated. Chest 122: 576-580, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Yoshifuji H, Fujii T, Kobayashi S, et al. . Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity 39: 233-241, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Hozumi H, Enomoto N, Kono M, et al. . Prognostic significance of anti-aminoacyl-tRNA synthetase antibodies in polymyositis/dermatomyositis-associated interstitial lung disease: a retrospective case control study. PLoS One 10: e0120313, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hozumi H, Fujisawa T, Nakashima R, et al. . Comprehensive assessment of myositis-specific autoantibodies in polymyositis/dermatomyositis-associated interstitial lung disease. Respir Med 121: 91-99, 2016. [DOI] [PubMed] [Google Scholar]
- 30.Fujisawa T, Hozumi H, Kono M, et al. . Predictive factors for long-term outcome in polymyositis/dermatomyositis-associated interstitial lung diseases. Respir Investig 55: 130-137, 2017. [DOI] [PubMed] [Google Scholar]