Abstract
This case report refers to a 31-year-old patient with an 11-year history of Crohn's disease. The patient presented with an edematous elevated lesion in the splenic flexure. Two histological analyses revealed no signs of obvious dysplasia, and the patient subsequently began infliximab treatment. Nine months later, a worsening of the stricture of the edematous elevated lesion was observed in the splenic flexure, and transverse colonic resection was performed. A histological investigation of the lesion in the splenic flexure revealed advanced adenocarcinoma. Six months after the surgery, computed tomography revealed recurrent carcinoma and peritoneal metastases. The patient was administered palliative chemotherapy.
Keywords: Crohn's disease, colonic carcinoma, infliximab, TNFα blockade
Introduction
The efficacy of infliximab, a monoclonal antibody against tumor necrosis factor alpha (TNFα), is well documented for the treatment of Crohn's disease and it is generally well tolerated (1-3). However, there is legitimate concern regarding the impact of TNFα blockade on the incidence of infections, autoimmune disorders, and malignancy (1-5). Generally, a secondary neoplasm is one of the most concerning sequelae of immune system manipulation (6). However, it is unclear whether it is the immune-modulating therapy or the patients' disease that causes such malignancy. We herein report a case of transverse colonic carcinoma that was diagnosed in a patient with Crohn's disease who had previously been treated with infliximab.
Case Report
A 31-year-old man presented with an 11-year history of Crohn's disease manifesting as small bowel stricture. His family history was negative for cancer. The patient was a nonsmoker, and, with the exception of Crohn's disease, his medical history was unremarkable. His clinical course included segmental ileitis and colitis complicated by stenoses. He underwent surgical resection of the terminal ileum with ileoascending anastomosis and strictureplasty in 2009. After surgery, his Crohn's disease activity index (CDAI) score decreased to 164. Thereafter, therapy with mesalazine (3 g/day) was prescribed; however, due to patient noncompliance, he experienced episodic diarrhea and abdominal pain again, for which he did not seek further care.
In 2013, because of an acute exacerbation of symptoms, such as diarrhea and abdominal pain (CDAI score increased to 290), colonoscopy was performed. This revealed edema, friable mucosa, longitudinal ulcers in the left colon, and an edematous elevated lesion with a central reddish depression in the splenic flexure (Fig. 1A) where no ulcer, erosion, or elevated lesion had been observed before. Biopsies from the elevated lesion revealed polymorphic inflammatory infiltration with reactive or regenerative dysplasia. The anastomotic region and the small bowel follow-through showed no abnormality. Radiological enteroclysis revealed no significant findings, such as stricture or scars in the small intestine. Although we recommended immune-modulating therapies, such as infliximab or azathioprine, the patient refused them because of his young age. Furthermore, because the patient had been noncompliant with his drug therapy, we persuaded him to take mesalazine every day and carefully followed him up.
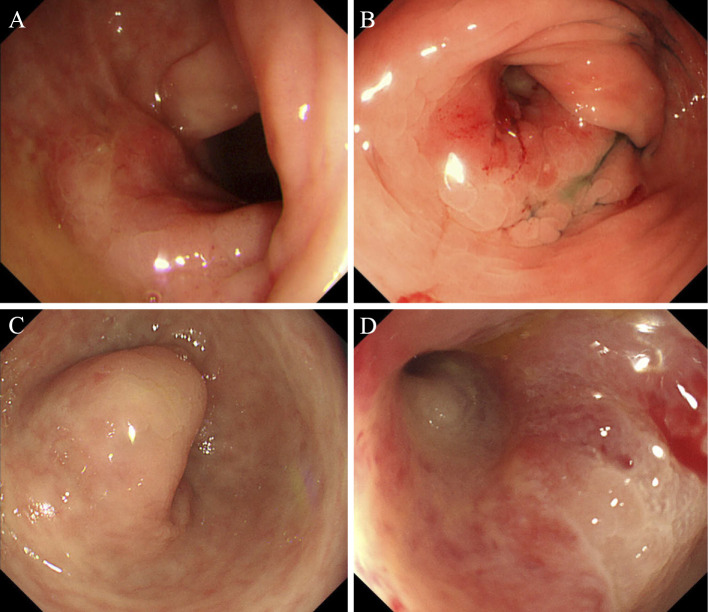
Figure 1.
Endoscopic findings. A: Colonoscopy revealed an edematous elevated lesion with a central reddish depression in the splenic flexure in 2013. B: A worsening of the stricture, caused by the edematous elevated lesion in the splenic flexure, was observed six months later. C, D: Nine months later, after the seventh infusion of infliximab, a further worsening of the stricture of the edematous elevated lesion was observed in the splenic flexure, which prevented the colonoscope from passing through.
Six months later, the patient was admitted to the emergency department with obstructive symptoms consistent with occlusive ileus, which was treated conservatively. Abdominal computed tomography (CT) scan revealed ileus resulting from anastomotic stricture. Colonoscopy showed extensive ulcerations in the left colon interposed with areas of spared mucosa, exacerbation of the stenosis in the anastomotic region, and worsening of the stricture caused by the edematous elevated lesion in the splenic flexure (Fig. 1B). Histology showed inflammatory changes with no obvious sign of dysplasia in the edematous elevated lesion in the splenic flexure and ulceration with no signs of dysplasia. The patient's diarrhea and abdominal pain became frequent (CDAI scores increased to 314) although he continued taking mesalazine every day for six months; therefore, at that time, the patient started induction treatment with infliximab (5 mg/kg intravenous infusion at weeks one, two, and six and every eight weeks thereafter).
Nine months later, after the seventh infusion of infliximab, the obstruction in the anastomotic region recurred. The serum carcinoembryonic antigen (CEA) concentration was 1.10 μg/L, which was within the normal range (<5.0 μg/L). Colonoscopy showed an improvement in edema, friable mucosa, and longitudinal ulcers in the left colon, and his diarrhea and abdominal pain lessened (CDAI score decreased to 263) because of treatment with infliximab; however, a further worsening of the stricture of the edematous elevated lesion was observed in the splenic flexure, which prevented the colonoscope from passing through (Fig. 1C and D). Contrast radiography of the colon revealed indurated stenosis measuring 28 mm in length in the splenic flexure, but no prestenotic dilatation (Fig. 2). Due to suspicions that the lesion might be malignant and because ileus caused by stricture of the lesion was suspected, we performed transverse colon segmental resection and an anastomotic regional resection where ileuses had recurred. Gross examination of the resected specimen showed a well-defined ulcerative type lesion in the splenic flexure, measuring 25×12 mm (Fig. 3). Histological examination of this lesion revealed well-differentiated adenocarcinoma (Fig. 4) with extraserosal invasion. No lymphadenopathy was detected in the resected specimen. The tumor classification was pT4a (SE) N0 M0 L1 V0 Pn0 R0. The margins were clear, and the resection was radical. There was no other obvious dysplasia in the resected specimens. Because of the patient's poorly controlled Crohn's disease without infliximab therapy, adjuvant chemotherapy was deferred. Moreover, as the pathogenesis of the carcinoma contraindicated the use of infliximab, the infliximab treatment was discontinued. After surgery, the patient's diarrhea and abdominal pain remained stable (CDAI score decreased somewhat to 255); therefore, therapy with mesalazine was continued.
Figure 2.
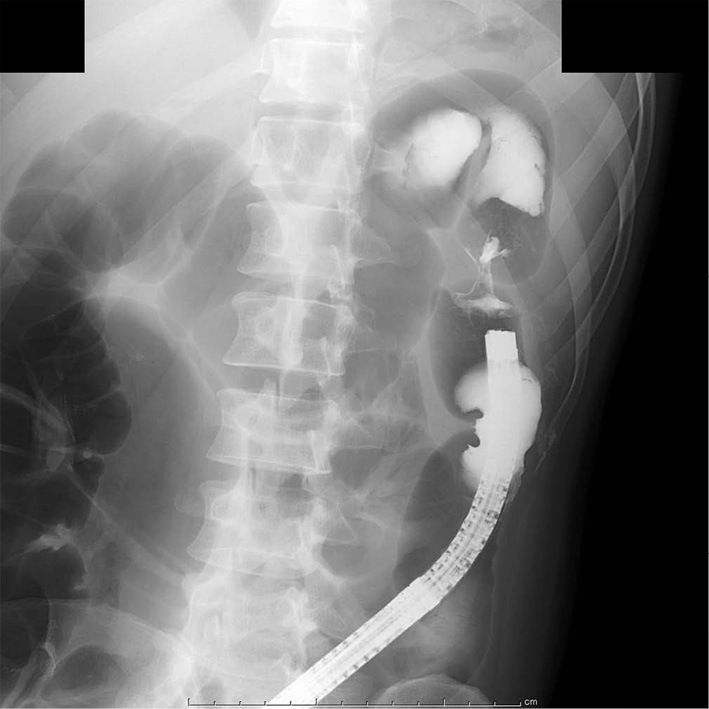
Contrast radiography of the colon. Indurated stenosis in the splenic flexure with no prestenotic dilatation.
Figure 3.
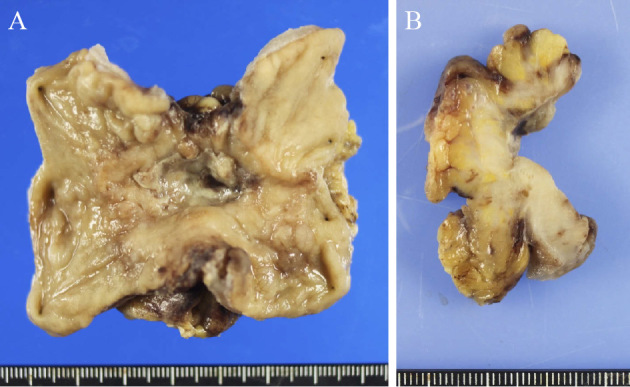
Surgically resected specimen. A: Macroscopic view of the colon adenocarcinoma showed ulceration, and the tumor was considered to be a well-defined ulcerative type lesion. B: The sectioned surface of the lesion showed a tumor with extraserosal invasion.
Figure 4.
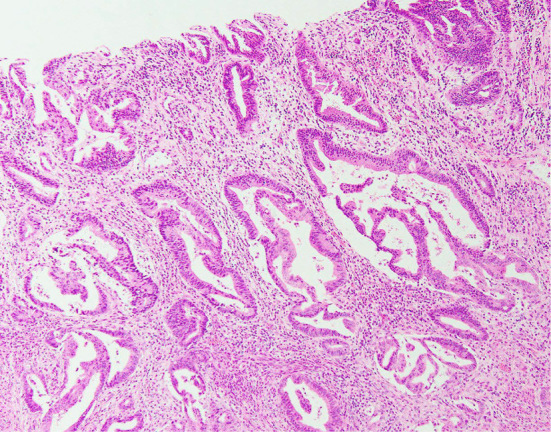
Pathological images of the surgically resected specimen. The pathological evaluation revealed well-differentiated adenocarcinoma infiltrating the colonic wall (Hematoxylin and Eosin staining, ×100).
Six months after the surgery, CT detected two pelvic masses and one mass near the splenic flexure (Fig. 5). Positron emission tomography revealed a tracer uptake in these lesions, while colonoscopy revealed no mass. The serum CEA concentration was 1.17 μg/L. The CT findings indicated recurrent carcinoma. A biopsy of the mass was not indicated because of the risks associated with the procedure. The recurrent carcinoma was considered to be inoperable.
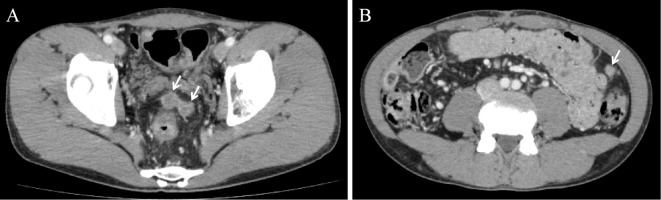
Figure 5.
Contrast-enhanced computed tomography scans of the abdomen. A, B: Two pelvic masses and one mass near the splenic flexure were observed (arrows) six months after the surgery, indicating recurrent carcinoma.
Due to the existence of peritoneal metastasis, the patient was administered palliative chemotherapy. A combination of mFOLFOX6 (oxaliplatin with 5-fluorouracil/leucovorin) and panitumumab was initiated as a first-line therapy, which was well tolerated. After eight cycles of mFOLFOX6 and panitumumab, the patient achieved a partial response. However, after 10 cycles of the chemotherapy, an exacerbation of the patent's peripheral neuropathy, hypocalcemia, and hypomagnesemia occurred; therefore, oxaliplatin and panitumumab were discontinued. A combination of FOLFIRI (irinotecan with 5-fluorouracil/leucovorin) and bevacizumab was initiated as a second-line therapy. This standard frontline regimen was not considered as a first-line therapy for this patient because of the presence of diarrhea and the possibility of restenosis with the anastomotic region; however, after the severe adverse events of the first-line regimen, the irinotecan-based regimen was considered the therapy of last resort, despite the associated risks. The patient received three doses of this chemotherapy; it remained tolerated until the latest follow-up. Even after induction of chemotherapy, the patient's diarrhea did not worsen (CDAI score remained 250-280); therefore, the therapy with mesalazine was still continued.
Discussion
This case report describes a patient with Crohn's disease who had two previous diagnoses of inflammatory changes with reactive or regenerative dysplasia that could not be confirmed as obvious dysplasia. He also developed metastatic adenocarcinoma of the colon nine months after beginning infliximab therapy. The cancer was detected and rapidly progressed, showing a temporal association with the infliximab therapy.
Although there is no definitive proof of a causal association between infliximab therapy and cancer, infliximab could inhibit the natural immune surveillance mechanism, allowing premalignant cells to transform into malignant cells and further enhance their ability to metastasize (7,8). There are only a few reports of tumors in patients treated with infliximab. Colombel et al. (8) reported nine cases of cancer in an institution-based cohort of 500 patients with Crohn's disease treated with infliximab; three of these cases (two lung cancers and one non-Hodgkin's lymphoma) were considered to possibly be related to the infliximab therapy. Furthermore, Brown et al. (9) described eight cases of lymphoma in patients treated with infliximab. Interestingly, tumor regression was observed in one patient after infliximab withdrawal in the absence of any other antitumor therapy.
However, data from the TREAT Registry (10) indicate that the incidence of malignancy per 100 patient-years did not differ significantly between the patients who did and did not receive infliximab (relative risk 1.3, 95% confidence interval 0.36-5.03). This was the case for both all malignancies (0.58 vs. 0.53) and for lymphoma (0.06 vs. 0.05). The authors concluded that, despite having more severe Crohn's disease, patients who received infliximab had similar rates of mortality, neoplasm, and lymphoma as those who received other treatments. Conversely, there is a possibility that infliximab contributes to the progression of tumors that already exist. A previous experimental study (11) indicated that TNFα suppressors, such as infliximab, may enhance tumor growth. In that study, an impaired cytotoxic activity of lymphocytes and an enhanced in vivo tumor growth were observed in TNFα knockout mice.
The endoscopic and clinical findings of worsening stricture are not easily distinguishable between Crohn's exacerbation and malignant cancer; consequently, the diagnosis is often missed or only found during laparotomy for obstructive or perforated lesions, thus explaining why up to 35% of adenocarcinomas in Crohn's disease are diagnosed as stage IV disease with an unfavorable overall survival after diagnosis (12).
In our case, an elevated lesion with a central reddish depression in the splenic flexure was observed six months before the induction of infliximab. This lesion was accompanied by tense mucosa and it was retrospectively found to be different from Crohn's exacerbation. Because we put too much confidence in the two previous histopathological diagnoses of non-malignancy, we assumed that the worsening stricture resulted from a worsening of the edematous stricture associated with Crohn's disease. However, we should have noticed that the stricture progressed rapidly in only six months although there was no active ulceration associated with Crohn's disease, and we should have strongly suspected that the worsening stricture may have been caused by progressive malignant cancer.
In conclusion, infliximab might be associated with cancer, either as a causal factor or by indirectly enhancing the cancer risk in susceptible patients with potential malignancy. While this association is not clear, increased surveillance is warranted in patients treated with infliximab or other TNFα-blocking agents with regard to secondary neoplasms. This is particularly important in patients with a higher risk of cancer, such as those with longstanding, poorly managed Crohn's disease and worsening stricture. Furthermore, based on our experience, in all cases of large bowel progressive stricture associated with Crohn's disease, we recommend that a neoplastic workup should always be part of the differential diagnosis. Resection should also be considered to rule out malignancy, especially in patients who are scheduled for or who are currently receiving infliximab therapy.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Rutgeerts P, D'Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn's disease. Gastroenterology 117: 761-769, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet (London, England) 359: 1541-1549, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis 5: 119-133, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Charles PJ, Smeenk RJ, De Jong J, Feldmann M, Maini RN. Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor alpha: findings in open-label and randomized placebo-controlled trials. Arthritis Rheum 43: 2383-2390, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Baeten D, Kruithof E, Van den, Bosch F, et al. Systematic safety follow up in a cohort of 107 patients with spondyloarthropathy treated with infliximab: a new perspective on the role of host defence in the pathogenesis of the disease? Ann Rheum Dis 62: 829-834, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.First MR, Peddi VR. Malignancies complicating organ transplantation. Transplant proc 30: 2768-2770, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Melichar B, Bures J, Dedic K. Anorectal carcinoma after infliximab therapy in Crohn's disease: report of a case. Dis Colon Rectum 49: 1228-1233, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Colombel JF, Loftus EV Jr, Tremaine WJ, et al. The safety profile of infliximab in patients with Crohn's disease: the Mayo clinic experience in 500 patients. Gastroenterology 126: 19-31, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Brown SL, Greene MH, Gershon SK, Edwards ET, Braun MM. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum 46: 3151-3158, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry. Clin Gastroenterol Hepatol 4: 621-630, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Baxevanis CN, Voutsas IF, Tsitsilonis OE, Tsiatas ML, Gritzapis AD, Papamichail M. Compromised anti-tumor responses in tumor necrosis factor-alpha knockout mice. Eur J Immunol 30: 1957-1966, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Dossett LA, White LM, Welch DC, et al. Small bowel adenocarcinoma complicating Crohn's disease: case series and review of the literature. Am Surg 73: 1181-1187, 2007. [PubMed] [Google Scholar]