Abstract
An 81-year-old man was admitted with bilateral pleural effusion. A clinical examination showed lymphocytic pleura effusion and elevated serum IgG4 levels, so that IgG4-related disease was suggested, whereas tuberculous pleurisy was suspected because of high adenosine deaminase (ADA) levels in the pleural effusion. A surgical pleural biopsy revealed that there were large numbers of IgG4-positive cells and IgG4/IgG positive cell ratio exceeded 40% in several sites. Accordingly, we diagnosed IgG4-related pleuritis and treated with the patient with glucocorticoid therapy. The ADA levels in pleural effusion can increase in IgG4-related pleuritis, and it is therefore important to perform a pleural biopsy.
Keywords: IgG4-related disease with pleuritis, pleural effusion, adenosine deaminase, tuberculous pleurisy, pleural biopsy
Introduction
IgG4-related disease (IgG4-RD) is characterized by elevated serum IgG4 concentration, marked tissue infiltration of IgG4-positive (IgG4+) plasma cells, and fibrosis. IgG4-RD was firstly reported by Hamano et al. (1) and it has been widely recognized that this disease is associated with the presence of various lesions. Although various terms of IgG4-related conditions have been referred to, the research team of Ministry of Health, Labor and Welfare Japan (MHLW Japan) proposed the diagnostic criteria for IgG4-RD in 2011 (2), and the term of IgG4-RD become prevalent in world (3). Since the diagnose criteria was advocated, other criteria for individual organ systems (e.g., pancreas, bile duct, kidney and salivary gland) has been established, and the diagnostic criteria for IgG4-related respiratory disease (IgG4-RRD) (4) were proposed by the Subcommittee of Respiratory Disease of IgG4-related Disease supported by the Health and Labor Sciences Research Grants for the Study of Intractable Diseases from the MHLW Japan in 2014.
In this report, we describe a case of IgG4-RRD with pleuritis accompanied with bilateral pleural effusion. Pleural effusion is a rare finding as a pulmonary involvement of the disease. This case showed exudative effusion with a high level of adenosine deaminase (ADA), and thus it is important to distinguish this case from tuberculous pleuritis. We performed a surgical pleural biopsy and finally diagnosed the patient to have IgG4-related pleuritis.
IgG4-RD is therefore expected to play an important role in the differential diagnosis of exudative pleural effusion.
Case Report
A-81-year old man complained of dyspnea on exertion about one month before admission. The dyspnea gradually became exacerbated and he consulted a doctor at a nearby medical clinic. At that time, bilateral pleural effusion was pointed out on a chest X-ray. He was referred to our hospital to clarify the cause of pleural effusion. He had smoked 30 cigarettes per day for 58 years and taken medication for diabetes, hypertension and dyslipidemia. He had no allergic disease nor any family history of tuberculosis infection. In addition, he was not taking any new drugs during the course.
On physical examination, his temperature was 36.8℃, blood pressure was 114/63 mmHg and pulse was 95 bpm. Oxygen saturation (SpO2) was 94% by providing 4 L/min oxygen. The lung sounds on auscultation were diminished on both sides. According to the laboratory data, a white blood cell count was 5,900/mm3, with an increased percentage of neutrophils (77%). C-reactive protein (CRP) was 0.14 mg/dL, the erythrocyte sedimentation rate (ESR) was elevated to 59 mm/h, the serum IgG level was 2,807 mg/dL including 233 mg/dL of IgG4, and IgE was 5,507 IU/mL. Both rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibody were negative. The findings for tumor markers and the interferon-gamma release test (T-SPOT.TB) were negative (Table 1). Chest X-ray showed massive pleural effusion on both sides (Fig. 1A). In addition to bilateral pleural effusion, contrast-enhanced CT of the neck to pelvis demonstrated subpleural ground-glass opacity in both lungs, consolidation of the left lobe and mild mediastinal lymphadenopathy. No mass lesions or pleural thickening were found (Fig. 1B). The results of echocardiography were within the normal limit as follows: left ventricular ejection fraction was 45% and estimated systolic pulmonary artery pressure was 28.6 mmHg. There were no findings suggesting an elevated left atrial pressure and pericardial effusion. Thoracentesis showed exudative pleural effusion with a high level of ADA and numerous plasma cells, on both sides. Smear, culture and the polymerase chain reaction (PCR) tests for tuberculosis and nontuberculous mycobacteria were all negative (Table 2). The immunohistochemical staining of pleural effusion cell block showed the presence of IgG4+ cells, and the IgG4+/IgG+ cell ratio ranged from 20% to 60%.
Table 1.
Laboratory Findings.
| <CBC> | <Urinalysis> | |||||
| White blood cells | 5,900 | /µL | pH | 5.5 | ||
| Neutrophil | 77 | % | Protein | 1+ | ||
| Eosinophil | 3.0 | % | Glucose | 1+ | ||
| Monocyte | 7.0 | % | Occult blood | ± | ||
| Lymphocyte | 10 | % | Sediment | negative | ||
| Red blood cells | 403×104 | /µL | ||||
| Hemoglobin | 12.7 | g/dL | <Biochemistry> | |||
| Platelet | 31.9×104 | /µL | Total protein | 7.0 | g/dL | |
| Albumin | 2.4 | g/dL | ||||
| <Serology> | Total bilirubin | 0.4 | g/dL | |||
| C-reactive protein | 0.14 | mg/dL | Aspartate aminotransferase | 27 | U/L | |
| Erythrocyte sedimentation rate | 59 | mm/h | Alanine aminotransferase | 21 | U/L | |
| Rheumatoid factor (qualitative test) | negative | Lactate dehydrogenase | 194 | U/L | ||
| Anti-citrullinated protein antibody | 2.4 | U/mL | Alkaline phosphatase | 252 | U/L | |
| IgG | 2,807 | mg/dL | Creatinine kinase | 96 | U/L | |
| IgG4 | 233 | mg/dL | Blood urea nitrogen | 21 | mg/dL | |
| IgA | 369 | mg/dL | Creatinine | 1.22 | mg/dL | |
| IgM | 79 | mg/dL | Ferritin | 136 | ng/mL | |
| IgE | 5,507 | IU/mL | Iron | 45 | µg/dL | |
| C3 | 78 | mg/dL | Sialylated carbohydrate antigen (KL-6) | 719 | U/mL | |
| C4 | 40 | mg/dL | ||||
| CH50 | 58.0 | U/mL | ||||
| Antinuclear antibody | ×40 | |||||
| (Speckled pattern) | ||||||
| SS-A | <1.0 | U/mL | ||||
| SS-B | <1.0 | U/mL | ||||
| sIL-2R | 961 | U/mL | ||||
| <Interferon-gamma release test (T-SPOT)> | negative | |||||
| <Tumor marker> | ||||||
| CEA | 2.4 | ng/mL | ||||
| CA19-9 | 5.1 | U/mL | ||||
| SCC | 18.3 | ng/mL | ||||
| CYFRA | 3.9 | ng/mL | ||||
| proGRP | 44.0 | pg/mL | ||||
| NSE | 13.2 | ng/mL | ||||
CA19-9: carbohydrate antigen 19-9, CEA: carcinoembryonic antigen, proGRP: pro-gastrin-releasing peptide, NSE: neuron specific enolase, SCC: squamous cell carcinoma antigen, sIL-2R: soluble interleukin-2 receptor
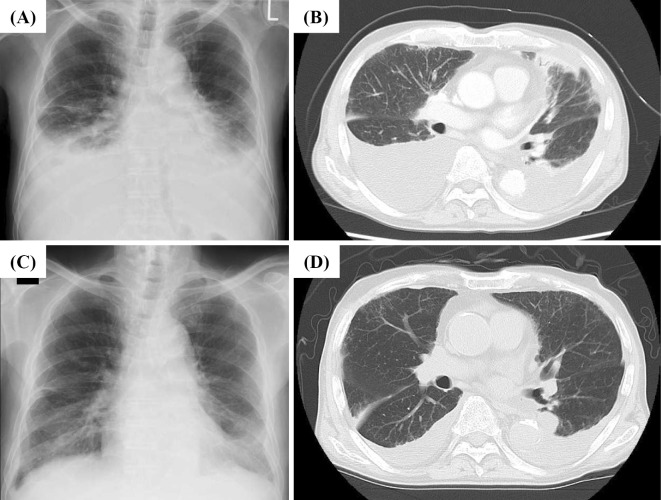
Figure 1.
Changes in the level of pleural effusion after treatment. A chest X-ray (A) and contrast-enhanced CT scan (B) on admission day showed massive bilateral pleural effusion. Contrast-enhanced chest CT of the neck to pelvis also demonstrated subpleural ground-glass opacity in both lungs, consolidation of the left lobe and mild mediastinal lymphadenopathy. No mass lesions or pleural thickening were found. After 4 weeks of treatment with systemic steroids, a chest X-ray (C) and CT scan (D) showed markedly decreased levels of bilateral pleural effusion.
Table 2.
Thoracentesis Findings.
| <Biochemistry> | <Cell counts> | |||||
| Total protein | 5.8 | g/dL | 3,450 /µL | |||
| Albumin | 1.6 | g/dL | Neutrophil | 3 | % | |
| Glucose | 115 | mg/dL | Lymphocyte | 69 | % | |
| Lactate dehydrogenase | 159 | U/L | (consist of plasma cells) | |||
| Amylase | 152 | U/L | Histiocyte | 9 | % | |
| Adenosine deaminase | 85.0 | U/L | Others | 19 | % | |
| CA19-9 | <0.6 | U/mL | ||||
| CEA | 2.1 | mg/mL | ||||
| <Microbiology test> | ||||||
| Smear | negative | |||||
| Culture | negative | |||||
| PCR | ||||||
| Mycobacterium tuber culosis | negative | |||||
| Mycobacterium avium | negative | |||||
| Mycoba cterium intracellular | negative | |||||
CA19-9: carbohydrate antigen 19-9, CEA: carcinoembryonic antigen, PCR: polymerase chain reaction
Based on the clinical course and findings, we excluded rheumatoid pleuritis, heart failure, carcinomatous pleurisy, pyothorax and drug-induced pleurisy as the cause of bilateral pleural effusion. IgG4-RD was serologically suggested because of the high serum IgG4 levels, whereas tuberculous pleurisy was also suspected because of high ADA levels in the pleural effusion. To confirm the diagnosis, we performed a thoracoscopic pleural biopsy. A histological analysis revealed the presence of dense lymphoplasmacytic infiltrate in the specimen accompanied by pronounced stromal hyalinization, the latter representing pleural plaque, which indicated asbestos exposure in the past. We also identified a mild eosinophilic infiltrate in the obtained pleural membrane. Immunohistochemically, large numbers of IgG4+ cells (50 cells or more per HPF) were found in the pleural membranes. The IgG4+/IgG+ cell ratio exceeded 40% in several sites in the pleural membranes, however, the ratio was less than 40% for the whole tissue (Fig. 2). Notably, non-caseating granuloma was observed but with no acid-fast bacteria based on Ziehl-Neelsen staining. According to the diagnostic criteria for IgG4-RRD (4), this case was “possible IgG4-RD”.
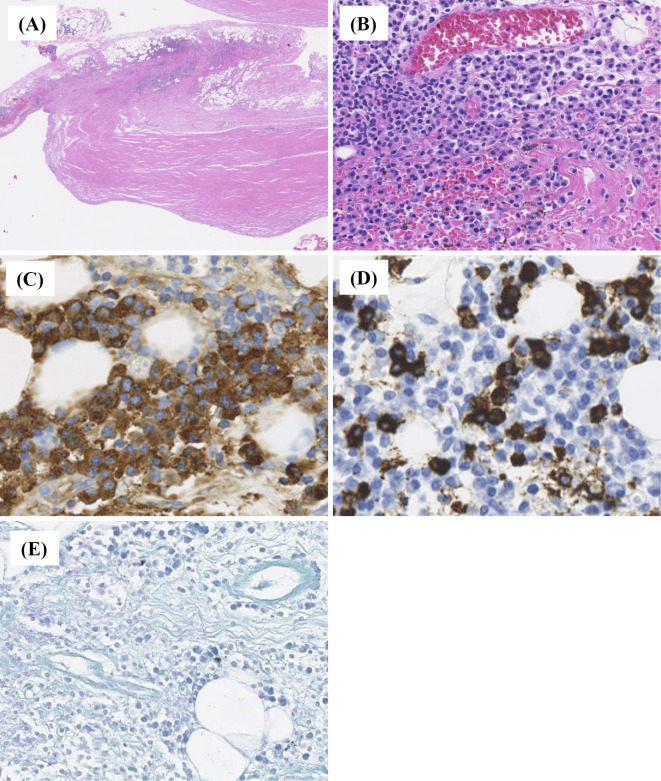
Figure 2.
Histopathological evaluation of the pleura and pleural effusion. A pleural biopsy showed the presence of dense lymphoplasmacytic infiltrate [(A): Hematoxylin and Eosin (H&E) staining, ×40; (B): H&E staining, ×100]. Immunohistochemically, many IgG4-poisitive plasma cells are identified (IgG4-positive plasma cells >50/HPF, IgG4+/IgG+cell ration <40%). [(C): immunohistochemical staining for IgG, ×400; (D): immunohistochemical staining for IgG4, ×400]. (E) Ziehl-Neelsen staining identified no acid-fast bacteria.
We considered that the IgG4+/IgG+ cell ratio did not exceed 40% for the whole tissue since the pleura did not show any tumor formation. Moreover, other diseases, including tuberculosis pleurisy, which was included in the differential diagnosis, were ruled out, and thus we finally diagnosed the patient to have IgG4-related pleuritis.
We started systemic glucocorticoid therapy (prednisolone: PSL, 0.6 mg/kg/day). The administration of PSL resulted in an improvement of dyspnea and bilateral pleural effusion (Fig. 1C and D), with a decreased serum IgG4 level (Fig. 3). Pleural effusion did not increase although we continued to reduce PSL gradually.
Figure 3.
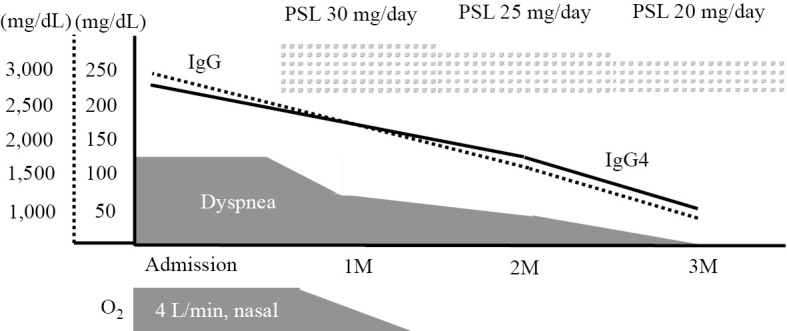
Clinical course. The administration of PSL resulted in an improvement of dyspnea and a decrease in the serum IgG4 level. IgG: immunoglobulin G (solid line), IgG4: immunoglobulin G4 (dotted line). PSL: prednisolone
Discussion
This study describes a case of IgG4-related pleuritis, which needed to be differentiated from tuberculous pleuritis, since bilateral pleural effusion showed a high level of ADA. A thoracoscopic pleural biopsy was performed to make a definitive diagnosis and a significant increase of IgG4-positive cells was confirmed. However, the IgG4+/IgG+ cell ratio was less than 40% for the whole tissue, which did not meet the diagnostic criteria of IgG4-RRD (4). Accordingly, it was necessary to distinguish this case from typical IgG4-RRD
IgG4-RD is generally characterized by a high level of serum IgG4 and a significant IgG4+ cell infiltration in the affected organ. In most cases, a mass lesion is found in the affected organ. However, it is possible that the pleura not associated with mass lesions may have a different ratio of IgG4-positive cells, depending on the biopsy site due to the widespread lesions.
IgG4-RD with pleural effusion (5-14) has been previously described. Although Choi et al. (12) reported a case of IgG4-RRD that presented with massive pleural effusion as the only pulmonary manifestation, IgG4-RRD more commonly has other types of pulmonary involvement (15). In addition, the accurate incidence of pleural effusion is still unknown. Fei et al. (16) noted that, among a prospective cohort of 248 IgG4-RD cases (including probable and possible groups in addition to a definite group), they investigated 87 cases with intrathoracic lesions (35.1%) and found pleural effusion in 4 cases (4.6%). This suggests that pleural effusion is a relatively rare feature of IgG4-RD. All the above-mentioned 87 cases had extrathoracic involvement, however, the patient in our case had only intrathoracic lesions, i.e., pleural effusion and subpleural ground-glass opacity and consolidation of the lung, without any extrathoracic lesions. In this regard, we must be careful to differentiate IgG4-related pleuritis from other diseases yielding pleura effusion, in particular, tuberculous pleuritis due to a high level of ADA in pleural effusion.
One of the differential diseases is rheumatoid arthritis (RA), since rheumatoid pleuritis sometimes presents with pleural effusion and a high level of ADA (17). Moreover, approximately 17% of RA patients have elevated serum IgG4 levels (18). However, arthritis did not appear throughout the clinical course and the serological markers of RA were negative, so that we did not confirm the diagnosis of rheumatoid arthritis. Second, a pleural biopsy revealed the presence of dense lymphoplasmacytic infiltrate with pronounced stromal hyalinization in the specimen. Stromal hyalinization was considered to be pleural plaque indicating asbestos exposure. However, it was reported that pleural plaque is also seen even in cases of low asbestos exposure (19). Our case had never been exposed to asbestos. In addition, neither pleural effusion nor pleural biopsy showed any evidence of malignant mesothelioma.
A diagnosis of tuberculous pleuritis is not always easy. It can be diagnosed if tubercle bacilli are detected from pleural effusion or pleural biopsy samples, however, the frequency of detecting tubercle bacilli from pleural effusion and pleural membranes is as low as approximately 10% (20), even as tuberculous pleuritis is most commonly suspected based on the clinical presentation and findings. ADA in pleural effusion is a biomedical marker most frequently used in the auxiliary diagnosis of tuberculous pleuritis. A report on ADA (21) showed a sensitivity of 100% and a specificity of 93.9% for tuberculous pleuritis if the cut-off value of ADA was set for 40.3 U/L, after which many studies have shown the diagnostic accuracy of tuberculous pleuritis to be high (22,23). To date, it has been considered that a high level of ADA in pleura effusion with lymphocyte-predominant exudate suggests tuberculous pleuritis, however, a few IgG4-RD cases with a high ADA level in pleural effusion have recently been reported (Table 3).
Table 3.
Clinical Features of IgG4-related Pleuritis.
| Reference | Age/ Gender |
Location of pleural effusion | Serum IgG4 level (mg/dL) | Biopsy of pleura | IgG4+/IgG+cell ratio of pleura | ADA in pleural effusion (U/L) |
|---|---|---|---|---|---|---|
| 7 | 78/M | Bilateral | 483 | Performed | 85.4 % | 34.1-46.7 |
| 8 | 85/M | Bilateral | 2,740 | Not performed | - | 122 |
| 9 | 73/M | Right | 1,500 | Not performed | - | 59.8 |
| 25 | 69/M | Right | 2,380 | Performed | About 50 % | 70.6 |
| Present case | 81/M | Bilateral | 233 | Performed | Exceeded 40 % | 85.0 |
The mechanism associated with IgG4-related pleuritis in regard to why the ADA levels in pleural effusion becomes elevated is still unknown. According to Valdés et al. (24), ADA has two isoenzymes: ADA1 and ADA2. ADA1 is widely present in human tissue, while ADA2 is predominantly produced by monocytes or macrophages and then is secreted through the activation of cell-mediated immunity for eradicating the tubercle bacilli at the initial event of intracellular infection. An increase in the ADA level in pleural effusion due to tuberculous pleuritis is mainly caused by an increase in the level of ADA2. As cell-mediated immunity in tuberculosis infection is the main mechanism for ADA production, it is suggested that the interaction of T cells with B cells may induce the activation of cell-mediated immunity in IgG4-RD.
In conclusion, pleural effusion is not a common manifestation of pulmonary involvement associated with IgG4-RD, however, it is necessary to consider it as one of the causes of pleuritis. Although ADA as a biomedical marker is superior in both sensitivity and specificity for the diagnosis of tuberculous pleuritis, increased levels of ADA can also be suggestive of IgG4-related pleuritis. Therefore, it is important to diagnose such cases based on the findings of a pleural biopsy.
Author's disclosure of potential Conflicts of Interest (COI).
Satoshi Kubo: Honoraria, Bristol-Myers, Pfizer and Takeda. Kazuhisa Nakano: Honoraria, UCB, Astellas, Mitsubishi-Tanabe and Eisai. Shingo Nakayamada: Honoraria, Bristol-Myers, UCB, Astellas, Abbvie, Eisai, Pfizer, Takeda, Mitsubishi-Tanabe, Novartis and MSD. Yoshiya Tanaka: Honoraria, Abbvie, Daiichi-Sankyo, Chugai, Takeda, Mitsubishi-Tanabe, Bristol-Myers, Astellas, Eisai, Janssen, Pfizer, Asahi-kasei, Eli Lilly, GlaxoSmithKline, UCB, Teijin, MSD and Santen; Research funding, Mitsubishi-Tanabe, Takeda, Chugai, Astellas, Eisai, Taisho-Toyama, Kyowa Hakko Kirin, Abbvie and Bristol-Myers.
Financial Support
This work was supported in part by a Grant-In-Aid for Scientific Research from the Ministry of Health, Labor and Welfare of Japan, the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the University of Occupational and Environmental Health, Japan, through UOEH Grant for Advanced Research.
References
- 1.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 344: 732-738, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Umehara H, Okazaki K, Masaki Y, et al. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol 22: 1-14, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone JH, Khosroshahi A, Deshpande V, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum 64: 3061-3067, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsui S, Yamamoto H, Minamoto S, et al. Proposed diagnostic criteria for IgG4-related respiratory disease. Respir Investig 54: 130-132, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Miyake K, Moriyama M, Aizawa K, et al. Peripheral CD4+ T cells showing a Th2 phenotype in a patient with Mikulicz's disease associated with lymphadenopathy and pleural effusion. Mod Rheumatol 18: 86-90, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Rossi G, Marchioni A, Guicciardi N, et al. Recurrent pleural and pericardium effusions in a white woman with IgG4-related syndrome. Am J Surg Pathol 33: 802-803, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto H, Suzuki T, Yasuo M, et al. IgG4-related pleural disease diagnosed by a re-evaluation of chronic bilateral pleuritis in a patient who experienced occasional acute left bacterial pleuritis. Intern Med 50: 893-897, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka H, Ichiyasu H, Notsute D, et al. A case of systemic IgG4-related disease with bilateral pleural effusions. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 49: 214-220, 2011(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 9.Suzuki N, Saeki T, Shimaoka Y, et al. Two cases of IgG4-related disease with pleural effusion. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 49: 97-102, 2011(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 10.Kato E, Takayanagi N, Ishiguro T, Kagiyama N, Shimizu Y, Sugita Y. IgG4-related pleuritis with chylothorax. Intern Med 53: 1545-1548, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Ishida M, Hodohara K, Furuya A, et al. Concomitant occurrence of IgG4-related pleuritis and periaortitis: a case report with review of the literature. Int J Clin Exp Pathol 7: 808-814, 2014. [PMC free article] [PubMed] [Google Scholar]
- 12.Choi JH, Sim JK, Oh JY, et al. A case of IgG4-related disease presenting as massive pleural effusion and thrombophlebitis. Tuberc Respir Dis 76: 179-183, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masaki Y, Furuse H, Tsuda T, et al. A case of IgG4-related disease with pleuritis. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 4: 473-477, 2015(in Japanese, Abstract in English). [Google Scholar]
- 14.Ishida A, Furuya N, Nishisaka T, Mineshita M, Miyazawa T. IgG4-related pleural disease presenting as a massive bilateral effusion. J Bronchology Interv Pulmonol 21: 237-241, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Campbell SN, Rubio E, Loschner AL. Clinical review of pulmonary manifestations of IgG4-related disease. Ann Am Thorac Soc 11: 1466-1475, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Fei Y, Shi J, Lin W, et al. Intrathoracic involvements of immunoglobulin G4-related sclerosing disease. Medicine (Baltimore) 94: e2150, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pettersson T, Ojala K, Weber TH. Adenosine deaminase in the diagnosis of pleural effusions. Acta Med Scand 215: 299-304, 1984. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, Tabeya T, Naishiro Y, et al. Value of serum IgG4 in the diagnosis of IgG4-related disease and in differentiation from rheumatic diseases and other diseases. Mod Rheumatol 22: 419-425, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Kishimoto T, Kato K, Arakawa H, Ashizawa K, Inai K, Takeshima Y. Clinical, radiological, and pathological investigation of asbestosis. Int J Environ Res Public Health 8: 899-912, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yew WW, Chan CY, Kwan SY, Cheung SW, French GL. Diagnosis of tuberculous pleural effusion by the detection of tuberculostearic acid in pleural aspirates. Chest 100: 1261-1263, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Krenke R, Safianowska A, Paplinska M, et al. Pleural fluid adenosine deaminase and interferon gamma as diagnostic tools in tuberculosis pleurisy. J Physiol Pharmacol 59(Suppl): 349-360, 2008. [PubMed] [Google Scholar]
- 22.Ocaña I, Martinez-Vazquez JM, Segura RM, Fernandez-De-Sevilla T, Capdevila JA. Adenosine deaminase in pleural fluids. Test for diagnosis of tuberculous pleural effusion. Chest 84: 51-53, 1983. [DOI] [PubMed] [Google Scholar]
- 23.Valdés L, Alvarez D, San José E, et al. Tuberculous pleurisy: a study of 254 patients. Arch Intern Med 158: 2017-2021, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Valdés L, San José E, Alvarez D, Valle JM. Adenosine deaminase (ADA) isoenzyme analysis in pleural effusions: diagnostic role and relevance to the origin of increased ADA in tuberculous pleurisy. Eur Respir J 9: 747-751, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Hara Y, Shinkai H, Yamaguchi N, et al. A case of steroid effective pleuritis and lymphadenopathy needed to distinguish IgG4-related disease from multicentric Castleman's disease. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soci) 2: 544-549, 2013(in Japanese, Abstract in English). [Google Scholar]