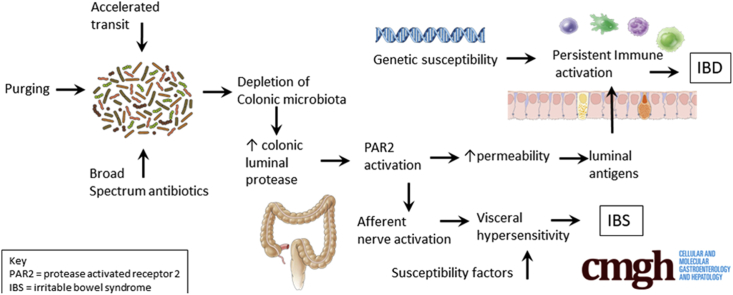
As we learn more about the importance of our microbiome in inflammatory, metabolic, and functional disorders, we are coming to appreciate the risks of disturbing this with broad-spectrum antibiotics. We have known for many years that as antibiotics have dramatically reduced the risk of infectious diseases, the incidence of other diseases such as inflammatory bowel disease, obesity, and type 2 diabetes mellitus have increased. Although the causes undoubtedly are multifactorial, meta-analysis showed that for Crohn’s disease (CD), antibiotic exposure nearly doubles the risk.1 The current article2 provides one possible mechanism where by this might occur. The authors studied 32 patients who provided stool samples before and after taking a range of antibiotics. They found that 8 patients showed a marked increase in fecal protease activity, mostly owing to increased pancreatic proteases. Supernatants from the stools with increased protease activity increased permeability when applied to a polarized monolayer of cultured colonic epithelial cells. This was particularly evident in patients given antibiotics such as levofloxacin and metronidazole, which are known to markedly reduce fecal microbiota, although this was much less obvious after antibiotics such as rifaximin, which have less impact on the microbiota. The pancreas secretes approximately 500 mg of tryptase daily into the gut, yet only approximately 1 mg is excreted and early animal experiments have shown that this degradation largely was prevented by broad-spectrum antibiotics.3
This article takes these ideas forward by examining the impact of antibiotic-induced increased fecal proteases on gut barrier function using cell lines and animal models. They showed that in mice, broad-spectrum antibiotics (vancomycin and metronidazole) increased fecal proteolytic activity, mostly owing to increases in pancreatic serine proteases such as trypsin and chymotrypsin. This lead to an increase in permeability, which in normal wild-type mice lasted approximately 14 days but did not lead to an inflammatory response. However, in genetically susceptible interleukin 10 knock-out mice, repeated antibiotic courses lead to the development of a chronic colitis, which could be blocked by a specific protease inhibitor.
Although CD is important, it also thankfully is rare and the majority of antibiotic courses do not lead to the development of CD. However, irritable bowel syndrome (IBS) is approximately 100 times more common, affecting approximately 1 in 10 of the population. IBS also is associated with antibiotic use, with a 3-fold increase in the risk of developing functional gastrointestinal symptoms in the 4 months after antibiotic consumption.4 IBS with diarrhea (IBS-D), which is characterized by rapid colonic transit and hypersensitivity of the gut to distension, is associated with increased fecal proteases. Furthermore, in animal studies, IBS-D fecal supernatants have been shown to act via protease-activated receptor type 2 to sensitize murine colons to distension, suggesting fecal proteases may account for the visceral hypersensitivity in IBS-D.5 Whether the same is true for CD remains unexplored. Rapid transit, which is a feature of both IBS-D and CD, reduces the time available for protease degradation, providing another mechanism whereby fecal proteases could be increased. Observational studies have shown that fast transit in IBS-D is correlated with increased fecal protease activity, which has been shown to be of pancreatic origin.6 Furthermore, purging the bowel using an osmotic laxative depletes the fecal bacteria and increases fecal protease substantially. These studies in IBS-D patients may be relevant for understanding CD, whose features overlap with inflammatory bowel disease, including increased gut permeability, low-grade immune activation, and increased mast cell activation in some, although not all, IBS patients.
The effect shown in this article was short-lived, so how could it cause chronic diseases? The authors argued that although the increased permeability did not induce an inflammatory response in normal animals, in genetically susceptible individuals it could initiate a self-perpetuating circle of increased permeability, allowing access of microbial antigens, leading to immune activation, which in turn increases permeability. Several studies have found significant alterations in gut microbiota in both CD and IBS-D, which have been linked to mucosal expression of inflammatory genes.7 The effect of antibiotics shown in the current study supports the idea that an altered gut microbiota might be an important part of the pathogenesis of both conditions. However, not all bacteria degrade proteases so specifically examining whether the altered microbiota in these diseases have impaired protease degradation would be a logical next step. Gastroenterologists perhaps are more aware than most doctors through their experience in treating Clostridium difficile of the risks of depleting the microbiota with broad-spectrum antibiotics, and new better-targeted agents are being developed to treat this condition.8, 9 However, most antibiotics are given for nongastrointestinal infections and, until recently, their impact on gut microbiota largely was accepted as inevitable. The current study provides an important reason why we should be developing more specifically targeted antibiotics and thus achieve the benefits of antibiotics without doing harm to long-term health.
Footnotes
Conflicts of interest The author discloses no conflicts.
Supplementary Material
Supplemental Graphical Summary.
References
- 1.Ungaro R., Bernstein C.N., Gearry R., Hviid A., Kolho K.L., Kronman M.P., Shaw S., Van Kruiningen H., Colombel J.F., Atreja A. Antibiotics associated with increased risk of new-onset Crohn's disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol. 2014;109:1728–1738. doi: 10.1038/ajg.2014.246. [DOI] [PubMed] [Google Scholar]
- 2.Yoon H., Schaubeck M., Lagkouvardos I., Blesl A., Heinzlmeir S., Hahne H., Clavel T., Panda S., Ludwig C., Kuster B., Manichanh C., Kump P., Haller D., Hörmannsperger G. Increased pancreatic protease activity in response to antibiotics impairs gut barrier and triggers colitis. Cell Mol Gastroenterol Hepatol. 2018;6:370–388. doi: 10.1016/j.jcmgh.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genell S., Gustafsson B.E. Impaired enteric degradation of pancreatic endopeptidases in antibiotic-treated rats. Scand J Gastroenterol. 1977;12:801–809. doi: 10.3109/00365527709181723. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell P.R., Rink E., Kumar D., Mendall M.A. Antibiotics increase functional abdominal symptoms. Am J Gastroenterol. 2002;97:104–108. doi: 10.1111/j.1572-0241.2002.05428.x. [DOI] [PubMed] [Google Scholar]
- 5.Gecse K., Roka R., Ferrier L., Leveque M., Eutamene H., Cartier C., Ait-Belgnaoui A., Rosztóczy A., Izbéki F., Fioramonti J., Wittmann T., Bueno L. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591–599. doi: 10.1136/gut.2007.140210. [DOI] [PubMed] [Google Scholar]
- 6.Tooth D., Garsed K., Singh G., Marciani L., Lam C., Fordham I., Fields A., Banwait R., Lingaya M., Layfield R., Hastings M., Whorwell P., Spiller R. Characterisation of faecal protease activity in irritable bowel syndrome with diarrhoea: origin and effect of gut transit. Gut. 2014;63:753–760. doi: 10.1136/gutjnl-2012-304042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jalanka-Tuovinen J., Salojarvi J., Salonen A., Immonen O., Garsed K., Kelly F.M., Zaitoun A., Palva A., Spiller R.C., de Vos W.M. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut. 2014;63:1737–1745. doi: 10.1136/gutjnl-2013-305994. [DOI] [PubMed] [Google Scholar]
- 8.Louie T., Nord C.E., Talbot G.H., Wilcox M., Gerding D.N., Buitrago M., Kracker H., Charef P., Cornely O.A. Multicenter, double-blind, randomized, phase 2 study evaluating the novel antibiotic cadazolid in patients with Clostridium difficile infection. Antimicrob Agents Chemother. 2015;59:6266–6273. doi: 10.1128/AAC.00504-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vickers R.J., Tillotson G.S., Nathan R., Hazan S., Pullman J., Lucasti C., Deck K., Yacyshyn B., Maliakkal B., Pesant Y., Tejura B., Roblin D., Gerding D.N., Wilcox M.H., CoDIFy study group Efficacy and safety of ridinilazole compared with vancomycin for the treatment of Clostridium difficile infection: a phase 2, randomised, double-blind, active-controlled, non-inferiority study. Lancet Infect Dis. 2017;17:735–744. doi: 10.1016/S1473-3099(17)30235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]