Abstract
Objective:
Axillary lymph node dissection (ALND) may be unnecessary in 20%–60% of breast cancer patients with sentinel lymph node (NSLN) metastasis. The aim of the present study was to review the medical records of Chinese patients with early-stage breast cancer and positive NSLN metastasis to identify clinicopathological characteristics as risk factors for non-NSLN metastasis.
Methods:
The medical records of 2008 early-stage breast cancer patients who received intraoperative sentinel lymph node biopsy (SLNB) between 2006 and 2016 were retrospectively reviewed. These patients were clinically and radiologically lymph node-negative and had no prior history of receiving neoadjuvant chemotherapy or endocrinotherapy. The clinicopathological characteristics of patients with positive NSLN metastasis who underwent ALND were investigated.
Results:
In the present study, 296 patients with positive NSLN metastases underwent ALND. Positive non-NSLN metastases were confirmed in 95 patients (32.1%). On univariate analysis, ≥ 3 positive NSLN metastases (P <0.01), NSLN macrometastases ( P = 0.023), and lymphovascular invasion (P = 0.04) were associated with non-NSLN metastasis (P <0.05). In multivariate analysis, the number of positive SLNs was the most significant predictor of non-SLN metastasis. For patients with 0, 1, 2, or 3 associated risk factors, the non-SLN metastatic rates were 11.5%, 22.5%, 35.2%, and 73.1%, respectively.
Conclusions:
The number of positive NSLNs, NSLN macrometastases, and lymphovascular invasion were correlated with non-SLN metastasis. The number of positive SLNs was an independent predictor for non-NSLN metastasis. When 2 or 3 risk factors were present in one patient, the probability of non-NSLN was higher than that in the American College of Surgeons Oncology Group Z0011 trial (27.3%); thus, avoiding ALND should be considered carefully.
Keywords: Breast cancer, sentinel lymph node metastasis, axillary lymph node dissection, non-sentinel lymph node metastasis
Introduction
Breast cancer is the most common cancer diagnosed in women. In the Asia-Pacific region, breast cancer accounts for 15.1% of cancer diagnoses and 6.9% of cancer-related mortality in women1. Early-stage operable breast cancer that is clinically node-negative is usually treated surgically with breast-conserving surgery or mastectomy, accompanied by the removal and biopsy of one or more sentinel lymph nodes (NSLNs), and the choice of further locoregional treatment, as well as systematic adjuvant therapy guided by sentinel lymph node biopsy (SLNB).
Completion axillary lymph node dissection (ALND) is often performed in patients with NSLN metastasis2. However, the optimal management of breast cancer patients with positive NSLN metastasis remains controversial, since 20%–60% of breast cancer patients with NSLN metastasis do not have further involvement of the axillary lymph nodes3-5. For these patients, undergoing ALND has no therapeutic benefit, provides little prognostic information, and can be associated with unnecessary postoperative arm morbidity, such as lymphedema, restriction of shoulder joint motions, seroma formation in the armpit, and numbness.
The American Society of Clinical Oncology (ASCO) guidelines6 indicate that ALND is not recommended for patients who undergo breast-conserving surgery with total breast radiation therapy and have 1–2 NSLNs containing metastases. In China, because the rate of administering breast-conserving therapy remains low, at approximately 20%7, the application of ASCO guidelines for avoiding ALND in patients with positive SLNs remains challenging. Therefore, predicting non-NSLN metastasis has become significantly necessary to screen out extremely low-risk or high-risk patients. The aim of the present study was to retrospectively analyze the medical records of Chinese patients with early-stage breast cancer and positive NSLN metastasis to identify clinicopathological characteristics as risk factors for non-NSLN metastasis.
Materials and methods
Patients
The medical records of early-stage breast cancer patients treated at the First Hospital of Jilin University between January 2006 and December 2016 were retrospectively reviewed. This study was approved by the Ethics Committee of The First Hospital of Jilin University. The inclusion criteria were (1) early-stage breast cancer diagnosed based on preoperative core needle biopsy or intraoperative frozen section analysis, (2) clinically and radiologically lymph node-negative, (3) no prior use of neoadjuvant chemotherapy or endocrinotherapy, and (4) successful SLNB. NSLN-positive patients who did not undergo ALND were excluded.
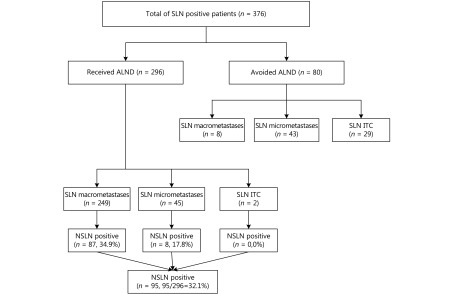
SLNB was successfully performed in 2008 patients, and positive SLNs were identified in 376 patients (Figure 1). A total of 296 patients who met the inclusion and exclusion criteria were enrolled in our study. Among these 296 patients, 249 patients had NSLN macrometastases, 45 patients had NSLN micrometastases, and 2 patients had isolated tumor cells (ITCs) in SLNs. A total of 80 patients with positive NSLNs did not undergo ALND. Among these patients, 8 patients had NSLN macrometastases, 43 patients had NSLN micrometastases, and 29 patients had ITCs in NSLNs. The numbers of NSLN and non-NSLN metastases detected or tumor involved are displayed in Table 1.
1.
Schematic representation of nodal status. NSLN transfer ratio: number of NSLN involved/number of NSLN detected.
1.
Numbers of NSLN and non-NSLN metastases detected or tumor involved
| Characteristics | Number of lymph nodes |
| Detected SLN | |
| Median | 3 |
| Range | 1–11 |
| Positive SLN | |
| Median | 1 |
| Range | 1–7 |
| Positive NSLN | |
| Median | 2 |
| Range | 1–14 |
The demographic and clinical characteristics of patients in the study group were recorded, including gender, age, location of the primary mass, multifocal or multicentric disease, tumor size, molecular classification [luminal A, luminal B, triple-negative, or human epidermal receptor-2 (HER-2)-positive; these were according to estrogen receptor (ER), progesterone receptor (PR), and HER-2 statuses, and the Ki-67 index], histologic grade, clinicopathologic index, lymphovascular invasion, operative procedure, tracer technique, number of SLNs, number of positive NSLNs, and type of NSLN metastasis. All patients included in the study underwent systematic adjuvant therapy, according to National Comprehensive Cancer Network (NCCN) guideline recommendations. Informed consent was obtained from all patients.
SLNB
SLNB was performed intraoperatively in patients who underwent mastectomy or breast-conserving surgery using one of the following tracer techniques: blue dye, combined blue dye–radioactive tracer, or combined blue dye–fluorescence. These techniques have comparable success and false-negative rates8. Positive SLNs were examined by intraoperative frozen section analysis and postoperative hematoxylin and eosin staining. Immunohistochemistry was not routinely used for the SLN diagnoses unless it was required. NSLN metastases were defined as macrometastases (pN1, metastasis size >2 mm), micrometastases (pN1mi, metastasis size from >0.2 mm to ≤2 mm), or ITCs (pN0[i+], metastasis size ≤0.2 mm), according to the American Joint Committee on Cancer Staging System (7 th edition)9. Additional axillary lymph node (non-NSLN) metastases were examined by postoperative hematoxylin and eosin staining.
Statistical analysis
Statistical analyses were conducted using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Univariate analysis was performed using the
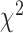 test and Fisher’s exact test. Multivariate analysis used stepwise logistic regression, and all variables with a P value <0.05 were included in the univariate analysis.
test and Fisher’s exact test. Multivariate analysis used stepwise logistic regression, and all variables with a P value <0.05 were included in the univariate analysis.
Results
Negative non-NSLN metastases were detected in 201 patients (67.9%, 201/296), while positive non-NSLN metastases were detected in 95 patients (32.1%, 95/296) (Figure 1).
Results of the univariate analysis of clinicopathological characteristics that have the potential to predict non-SLN status are summarized in Table 2. In univariate analysis, ≥3 positive NSLN metastases (P<0.01), NSLN macrometastases (P=0.023), and lymphovascular invasion (P=0.04) were associated with non-SLN metastasis (P<0.05). In multivariate analysis, the number of positive SLNs was the most significant predictor of non-NSLN metastasis. The odds ratios of 1 and 2 positive SLNsvs. ≥3 positive SLNs were 0.269 [95% confidence interval (CI): 0.079–0.912] and 0.125 (95% CI: 0.031–0.510), respectively.
2.
Associations between clinicopathological characteristics and non-SLN metastasis
| Clinicopathological characteristics | Positive non-SLNs, n (%) | Negative non-SLNs, n | Total |
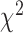
|
P |
| Gender | - | - | |||
| Male | 0 | 1 | 1 | ||
| Female | 95 (32.2) | 200 | 295 | ||
| Age (years) | 0.147 | 0.702 | |||
| <50 | 45 (31.3) | 100 | 145 | ||
| ≥50 | 50 (33.1) | 101 | 151 | ||
| Mammary gland | 0.190 | 0.663 | |||
| Left | 47 (33.3) | 94 | 141 | ||
| Right | 48 (31.0) | 107 | 155 | ||
| Location of primary mass | 2.739 | 0.602 | |||
| Outer upper | 42 (37.2) | 71 | 113 | ||
| Outer under | 9 (34.6) | 17 | 26 | ||
| Upper inner | 18 (26.5) | 50 | 68 | ||
| Lower inner | 5 (33.3) | 10 | 15 | ||
| Other | 24 (30.4) | 55 | 79 | ||
| Multicentric, multifocal | 3.842 | 0.050 | |||
| Yes | 12 (50) | 12 | 24 | ||
| No | 83 (30.5) | 189 | 272 | ||
| Clinical tumor size | 0.528 | 0.468 | |||
| T1 | 52 (30.4) | 119 | 171 | ||
| ≥T2 | 43 (34.4) | 82 | 125 | ||
| Surgery | 0.956 | 0.328 | |||
| Mastectomy | 81 (33.3) | 162 | 243 | ||
| Breast conversing surgery | 14 (26.4) | 39 | 53 | ||
| Tracer method | 1.113 | 0.573 | |||
| Blue dye alone | 23 (35.4) | 42 | 65 | ||
| Blue dye + radioisotope | 28 (28.0) | 72 | 100 | ||
| Blue dye + fluorescence | 44 (33.6) | 87 | 131 | ||
| Number of SLN detected | 1.917 | 0.166 | |||
| 1–2 | 42 (36.8) | 72 | 114 | ||
| ≥3 | 53 (29.1) | 129 | 182 | ||
| Metastatic SLN | 20.207 | <0.001 | |||
| 1 | 56 (28.1) | 143 | 199 | ||
| 2 | 18 (27.3) | 48 | 66 | ||
| ≥3 | 21 (67.7) | 10 | 31 | ||
| Continued | |||||
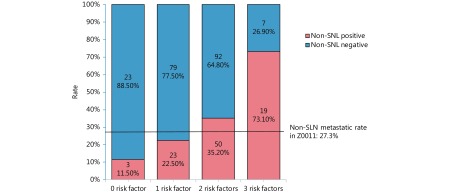
The proportion of non-NSLN metastases was calculated respectively when 0, 1, 2, or 3 associated risk factors existed at the same time in one patient, which is shown in a histogram (Figure 2). In patients with none of these three risk factors, the non-NSLN transfer rate was 11.5%; when only 1 risk factor existed, the non-NSLN transfer rate was 22.5%. Meanwhile, the probabilities of non-NSLN metastasis were relatively high when a patient had 2 or all 3 of these risk factors, which were 35.2% and 73.1%, respectively.
2.
Non-SLN rates when there are 0 to 3 risk factors. Risk factors include: (1) number of positive SLN ≥3; (2) SLN macrometastases; (3) lymphovascular invasion.
Discussion
Breast cancer treatment is constantly evolving4. The St. Gallen Consensus Conference on early breast cancer treatment standards recommended the de-escalation of some surgical aspects of tumor resection and escalation of adjuvant systematic therapy10. It is widely accepted that SLNB can replace ALND for axillary treatment in patients with negative lymph nodes because of the equivalent disease-free survival (DFS), overall survival (OS), or loco-regional recurrence (LRR)11. Traditionally, breast cancer patients with positive SLNs routinely underwent ALND. However, non-SLNs may only contain metastases in 20%–60% of these patients4-5. In fact, the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial revealed similar survival rates in patients with clinical T1–T2 invasive breast cancer and 1 or 2 SLN metastases who were treated with breast conservation and systemic therapy and underwent SLNB alone vs. ALND (10-year OS rates were 80.2% vs. 78.2%)12-13. Accordingly, in the present study, there was no axillary recurrence during the median 24-month follow-up in 80 patients who met the ACOSOG Z0011 inclusion criteria and did not undergo ALND. In the International Breast Cancer Study Group (IBCSG) 23-01 trial14, the 5-year DFS was similar in patients with breast cancer with one or more micrometastatic foci in SLNs who were treated with breast conservation and systemic therapy and underwent axillary dissection to that in those who did not undergo axillary dissection (84.4% vs. 87.8%). However, all patients in the ACOSOG Z0011 trial underwent breast-conserving therapy, and the majority of patients in the IBCSG 23-01 trial had a low disease burden with <2-cm tumor size, positive ERs, and 1-mm NSLN micrometastases. Thus, the results of these trials were still not useful in all patients who were NSLN-positive. These findings highlight the need to identify factors that predict low-risk positive non-SLNs in patients with breast cancer and NSLN metastases 15.
In the present study, 32.1% of patients with early-stage breast cancer and positive NSLN(s) had non-NSLN metastasis, and this finding is comparable with that of previous studies3. Notably, 34.9% of patients with NSLN macrometastases and 17.8% of patients with NSLN micrometastases had positive non-SLNs in our data. In a previous study, 35% of patients with NSLN micrometastases and 10% of patients with ITCs in NSLNs had positive non-NSLN metastases16.
The presence of ≥3 positive NSLNs conferred a very high risk in our patients, in which 75%, 27.3%, and 28.5% of patients with 3, 2, and 1 positive SLNs, respectively, had non-NSLN metastases. We identified the number of positive NSLNs as an independent predictor of non-NSLN metastasis in multivariate analysis. Lymphovascular invasion was correlated with non-NSLN involvement in the univariate analysis, and this was consistent with most other research17-21. The metastatic tumor sizes of NSLNs have been reported in several studies as a characteristic related to non-NSLN involvement; in our study, we also found that when a macrometastasis existed, the possibility of further nodal involvement was doubled. However, neither lymphovascular invasion nor NSLN metastatic tumor size showed significant predictive value in multivariate analysis.
In contrast to the findings in some other studies, in the present study, the size of the primary tumor, pathological type, multifocality, ER or PR status, and HER-2 status were not associated with non-SLN metastasis4,22-23. Cancer cell differentiation levels are associated with non-SLN, according to some research24-25. It has been demonstrated that well-differentiated tumors are less likely to have non-NSLN metastasis. Besides, some studies have suggested that molecular subtype classifications of breast cancer are a strong predictive factor of non-NSLN metastasis, but different studies have not come to a unified conclusion about which subtype is more likely to have non-NSLN metastasis26-28. However, in the present study, we did not discover statistical differences between different cancer cell differentiation levels (histological grade) or biological subtypes with non-NSLN metastatic rates.
In the NCCN guidelines for breast cancer, ALND is recommended for patients who do not meet the criteria of the Z0011 or 23-01 trials29. However, in China, most NSLN-positive patients do not meet those criteria because the ratio of mastectomy is high7. We aimed to predict non-SLN metastasis effectively, to reduce the overtreatment ratio in non-SLN-negative patients. As shown in the histogram (Figure 2), the results of our study can be a helpful reference for surgeons’ decisions when they prepare to avoid ALND in patients. When there is 0 or 1 risk factor, alternative systematic adjuvant therapy may be considered, but further evaluation of these patients is needed to determine the value of ALND, while if 2 or especially 3 of those risk factors exist, exemption of ALND should be considered carefully.
Several nomograms that predict non-NSLN metastasis have been proposed, including those developed at the Memorial Sloan Kettering Cancer Center, Tenon Hospital, Cancer Research UK Cambridge Center, Stanford Cancer Center The University of Texas MD Anderson Cancer Center, and Helsinki University Central Hospital30-34,5. However, presently available tools that predict non-SLN involvement in patients with breast cancer and positive SLNs remain inconsistent, which is possibly due to variations in the demographics of the patient populations studied and their locations. More widespread research that involves multiple centers in different regions of the world may provide a more comprehensive strategy for predicting non-NSLN metastasis. We considered that the 10-year data that we analyzed from breast cancer patients in the Jilin province may contribute to the formation of a unitive prediction system for non-NSLN patients nationwide or even worldwide.
The present study has some limitations. First, the majority of patients with ITCs in NSLNs did not undergo ALND. Therefore, non-NSLN involvement could not be accurately evaluated in these patients. Second, the influence of total tumor burden and pathological tumor stage were not investigated, although these have been previously identified as useful predictors of non-NSLN metastasis35-36.
In conclusion, ≥3 positive NSLN metastases,NSLN macrometastases, and lymphovascular invasion were related to non-NSLN metastasis.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Zuo TT, Zheng RS, Zeng HM, Zhang SW, Chen WQ. Female breast cancer incidence and mortality in China, 2013. Thorac Cancer. 2017;8:214–8. doi: 10.1111/1759-7714.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–33. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meretoja T, Leidenius M. Response. JNCI J Natl Cancer Inst. 2013;105:1514. doi: 10.1093/jnci/djt231. [DOI] [PubMed] [Google Scholar]
- 4.Dingemans SA, de Rooij PD, van der Vuurst de Vries RM, Budel LM, Contant CM, van der Pool AEM. Validation of six nomograms for predicting non-sentinel lymph node metastases in a Dutch breast cancer population. Ann Surg Oncol. 2016;23:477–81. doi: 10.1245/s10434-015-4858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittendorf EA, Hunt KK, Boughey JC, Bassett R, Degnim AC, Harrell R, et al. Incorporation of sentinel lymph node metastasis size into a nomogram predicting nonsentinel lymph node involvement in breast cancer patients with a positive sentinel lymph node. Ann Surg. 2012;255:109–15. doi: 10.1097/SLA.0b013e318238f461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2014;32:1365–83. doi: 10.1200/JCO.2013.54.1177. [DOI] [PubMed] [Google Scholar]
- 7.Liu XY, Gou ZC, Cao ZG, Jiang YZ, Shao ZM. Surgical management of breast cancer in China: The Fudan University Shanghai Cancer Center experience. Transl Cancer Res. 2017;6:588–98. [Google Scholar]
- 8.Lorek A, Boratyn-Nowicka A, Szlachta-Światkowska E. Sentinel lymph node (sln) in breast cancer. Review of current identification methods. Wiad Lek. 2017;70:85–91. [PubMed] [Google Scholar]
- 9.Edge SB, Compton C C. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM . Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 10.Gnant M, Harbeck N, Thomssen C. St. Gallen/Vienna 2017: a brief summary of the consensus discussion about escalation and de-escalation of primary breast cancer treatment. Breast Care. 2017;12:102–7. doi: 10.1159/000475698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bromham N, Schmidt-Hansen M, Astin M, Hasler E, Reed MW. Axillary treatment for operable primary breast cancer. Cochrane Database Syst Rev. 2017;1:CD004561. doi: 10.1002/14651858.CD004561.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–75. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatzemeier W, Mann G B. Which sentinel lymph-node (SLN) positive breast cancer patient needs an axillary lymph-node dissection (ALND) – ACOSOG Z0011 results and beyond. Breast. 2013;22:211–6. doi: 10.1016/j.breast.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzal F, Galimberti V, Kühn T, Rutgers EJ, Untch M. Lymph node staging in invasive breast cancer. Breast Care. 2014;9:211–4. doi: 10.1159/000365315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mccready DR, Yong WS, Ng AKT, Miller N, Done S, Youngson B. Influence of the new AJCC breast cancer staging system on sentinel lymph node positivity and false-negative rates. J Natl Cancer Inst. 2004;96:873–5. doi: 10.1093/jnci/djh142. [DOI] [PubMed] [Google Scholar]
- 17.Fougo JL, Afonso M, Senra FS, Dias T, Leal C, Araújo C, et al. Predictive factors for non-sentinel lymph node involvement in breast cancer patients with a positive sentinel node: should we consider sentinel node-related factors? Clin Transl Oncol. 2009;11:165–71. doi: 10.1007/s12094-009-0333-y. [DOI] [PubMed] [Google Scholar]
- 18.Gurleyik G, Aker F, Aktekin A, Saglam A. Tumor characteristics influencing non-sentinel lymph node involvement in clinically node negative patients with breast cancer. J Breast Cancer. 2011;14:124–8. doi: 10.4048/jbc.2011.14.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klevesath MB, Pantel K, Agbaje O, Provenzano E, Wishart GC, Gough P, et al. Patterns of metastatic spread in early breast cancer. Breast. 2013;22:449–54. doi: 10.1016/j.breast.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Shigematsu H, Taguchi K, Koui H, Ohno S. Clinical significance of extracapsular invasion at sentinel lymph nodes in breast cancer patients with sentinel lymph node involvement. Ann Surg Oncol. 2015;22:2365–71. doi: 10.1245/s10434-014-4269-2. [DOI] [PubMed] [Google Scholar]
- 21.Koyama Y, Ichikawa H, Sakata J, Sakata E, Tatsuda K, Hasegawa M, et al. The association between sentinel lymph node metastasis and Ki-67 labeling index. Adv Breast Cancer Res. 2013;2:60–5. [Google Scholar]
- 22.Boler DE, Uras C, Ince U, Cabioglu N. Factors predicting the non-sentinel lymph node involvement in breast cancer patients with sentinel lymph node metastases. Breast. 2012;21:518–23. doi: 10.1016/j.breast.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Turner RR, Chu KU, Qi K, Botnick LE, Hansen NM, Glass EC, et al. Pathologic features associated with nonsentinel lymph node metastases in patients with metastatic breast carcinoma in a sentinel lymph node. Cancer. 2000;89:574–81. doi: 10.1002/1097-0142(20000801)89:3<574::aid-cncr12>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 24.Canavese G, Bruzzi P, Catturich A, Vecchio C, Tomei D, Del Mastro L, et al. A risk score model predictive of the presence of additional disease in the axilla in early-breast cancer patients with one or two metastatic sentinel lymph nodes. Eur J Surg Oncol. 2014;40:835–42. doi: 10.1016/j.ejso.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Friedman D, Gipponi M, Murelli F, Meszaros P, Solari N, Massa M, et al. Predictive factors of non-sentinel lymph node involvement in patients with invasive breast cancer and sentinel node micrometastases. Anticancer Res. 2013;33:4509–14. [PubMed] [Google Scholar]
- 26.Gülben K, Berberoğlu U, Aydoğan O, Kınaş V. Subtype is a predictive factor of nonsentinel lymph node involvement in sentinel node-positive breast cancer patients. J Breast Cancer. 2014;17:370–5. doi: 10.4048/jbc.2014.17.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou WB, He ZY, Xue JL, Wang MH, Zha XM, Ling LJ, et al. Molecular subtype classification is a determinant of non-sentinel lymph node metastasis in breast cancer patients with positive sentinel lymph nodes. PLoS One. 2012;7:e35881. doi: 10.1371/journal.pone.0035881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reyal F, Belichard C, Rouzier R, de Gournay E, Senechal C, Bidard FC, et al. Non-sentinel lymph node metastasis prediction in breast cancer with metastatic sentinel lymph node: impact of molecular subtypes classification. PLoS One. 2012;7:e47390. doi: 10.1371/journal.pone.0047390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daly MB, Pilarski R, Berry M, Buys SS, Farmer M, Friedman S, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, Version 2.2017. J Natl Compr Canc Netw. 2017;15:9–20. doi: 10.6004/jnccn.2017.0003. [DOI] [PubMed] [Google Scholar]
- 30.Van Zee KJ, Manasseh DME, Bevilacqua JLB, Boolbol SK, Fey JV, Tan LK, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–51. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Barranger E, Coutant C, Flahault A, Delpech Y, Darai E, Uzan S. An axilla scoring system to predict non-sentinel lymph node status in breast cancer patients with sentinel lymph node involvement. Breast Cancer Res Treat. 2005;91:113–9. doi: 10.1007/s10549-004-5781-z. [DOI] [PubMed] [Google Scholar]
- 32.Pal A, Provenzano E, Duffy SW, Pinder SE, Purushotham AD. A model for predicting non-sentinel lymph node metastatic disease when the sentinel lymph node is positive. Br J Surg. 2008;95:302–9. doi: 10.1002/bjs.5943. [DOI] [PubMed] [Google Scholar]
- 33.Kohrt HE, Olshen RA, Bermas HR, Goodson WH, Wood DJ, Henry S, et al. New models and online calculator for predicting non-sentinel lymph node status in sentinel lymph node positive breast cancer patients. BMC Cancer. 2008;8:66. doi: 10.1186/1471-2407-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meretoja TJ, Leidenius MH, Heikkilä PS, Boross G, Sejben I, Regitnig P, et al. International multicenter tool to predict the risk of nonsentinel node metastases in breast cancer. J Natl Cancer Inst. 2012;104:1888–96. doi: 10.1093/jnci/djs455. [DOI] [PubMed] [Google Scholar]
- 35.Nabais C, Figueiredo J, Lopes P, Martins M, Araújo A. Total tumor load assessed by one-step nucleic acid amplification assay as an intraoperative predictor for non-sentinel lymph node metastasis in breast cancer. Breast. 2017;32:33–6. doi: 10.1016/j.breast.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Pohlodek K, Bozikova S, Meciarova I, Mucha V, Bartova M, Ondrias F. Prediction of additional lymph node involvement in breast cancer patients with positive sentinel lymph nodes. Neoplasma. 2016;63:427–34. doi: 10.4149/312_150922N497. [DOI] [PubMed] [Google Scholar]