Abstract
Objective:
To compare the effects and safety of conventional transarterial chemoembolization (cTACE) and yttrium-90 transarterial radioembolization [TARE (90Y)] for hepatocellular carcinoma (HCC)
Methods:
Nine high-quality observational studies, one low bias-risk randomized controlled trial (RCT), and one moderate bias-risk RCT included 1,652 patients [cTACE, 1,124; TARE (90Y), 528], from whom data were extracted for this systematic review and meta-analysis.
Results:
The extracted study outcomes included 1-year and 2-year overall survival (OS) rates, objective responses (ORs), and serious adverse events (AEs). 1-year OS rates: OR = 0.939, 95 % CI: 0.705-1.251, P = 0.66. 2-year OS rates: overall pooled OR = 0.641, 95% CI: 0.382-1.075, P = 0.092; observational study subgroup OR = 0.575, 95% CI: 0.336-0.984, P = 0.043; RCT subgroup OR* = 0.641, 95% CI: 0.382-1.075, P = 0.346. OR: overall pooled OR = 0.781, 95% CI: 0.454-1.343, P = 0.371; mRECIST subgroup OR = 0.584, 95 % CI: 0.349-0.976, P = 0.040; WHO subgroup OR = 1.065; 95% CI: 0.500-2.268, P = 0.870. Serious AEs: overall pooled RR = 1.477, 95% CI: 0.864-2.526, P = 0.154; RCT subgroup RR = 0.680, 95% CI: 0.325-1.423, P = 0.306; observational study subgroup RR = 1.925; 95 % CI: 0.978-3.788, P = 0.058.
Conclusions:
TARE (90Y) increased 2-year OS rates in the observational subgroup and resulted in better OR rates, according to mRECIST criteria, in comparison with cTACE. Furthermore, a lower risk of AEs was observed for TARE (90Y) than for cTACE.
Keywords: Hepatocellular carcinoma, conventional transarterial chemoembolization, transarterial radioembolization, yttrium-90
Introduction
Hepatocellular carcinoma (HCC) is the sixth most widespread type of cancer. More than half of all patients with HCC are diagnosed in China, and cancer-related death from cirrhosis is a major cause of HCC1,2. Surgical resection is the primary treatment for HCC3, and most patients with HCC are diagnosed at intermediate or advanced stages, when approximately 70% of cases lose the window for ablation and surgical resection4,5. In recent decades, with the rapid development of transcatheter interventional therapy, conventional transarterial chemoembolization (cTACE) has been recommended as a first-line treatment for patients with unresectable Barcelona Clinic Liver Cancer (BCLC) stage B HCC, and cTACE is also an effective treatment for BCLC stage C HCC6,7. Yttrium-90 (90Y) transarterial radioembolization (TARE), TARE (90Y), is also considered a valuable alternative treatment for HCC. Similar scientific rationales have been adopted in both cTACE and TARE (90Y), as locoregional therapies. cTACE involves the infusion of high, focused chemical drug dosages, which exceed systemic tolerability, directly into the hepatic tumor-feeding blood vessels (hepatic arteries). This generates strong cytotoxic, microembolic, and ischemic effects in tumors that are cotreated with conventional iodinated oil, and has shown positive impacts on disease progression and overall survival (OS)8. TARE (90Y) involves a transarterial brachytherapy, where small resin or glass microspheres that are loaded with β-emitting yttrium-90 are selectively delivered into the hepatic arteries. This results in radiation-induced tumor necrosis, minimizes damages to corresponding non-tumor normal tissues, reduces tumor burden, helps HCC downstage for surgical resection, and functions as a bridging therapy, prior to liver transplantation9,10.
Although several studies have recently compared the two locoregional therapies for HCC, whether one therapy offers obvious advantages over the other has not been sufficiently elucidated. Therefore, we conducted a systematic review and meta-analysis, to compare the effect and safety of cTACE and TARE (90Y) for patients with HCC. Clinical outcomes including 1- and 2-year OS rates, objective responses (ORs), and serious adverse events (AEs) were evaluated. We anticipate that this article will assist clinicians in making treatment strategies for patients with HCC (especially those with intermediate or advanced stage disease).
Materials and methods
Search strategy and selection criteria
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed, to prepare our search strategy and selection criteria11. We systematically searched PubMed, Embase, EBSCO, Cochrane Library, Web of Science, and MedLine databases for studies that were published between January 2009 and July 2017 and evaluated the effects and safety of cTACE and TARE (90Y) regimens for HCC, with no language restrictions. We selected human studies and human trials, to obtain useful data and information. Meanwhile, we searched ClinicalTrials.gov to obtain available outcomes of ongoing studies.
We used the following MeSH terms and combined texts: “Carcinoma, Hepatocellular”, “HCC”, “Chemoembolization, Therapeutic”, “TACE”, “transcatheter arterial chemoembolization”, “transarterial chemoembolization”, “TARE”, “transcatheter arterial radioembolization”, “transarterial radioembolization”, “90Y”, and “Yttrium-90”. The following strategy was used for our PubMed search: (Carcinomas, Hepatocellular [Text Word] OR Hepatocellular Carcinomas [Text Word] OR “Liver Cell Carcinoma, Adult” [Text Word] OR “Liver Cancer, Adult” [Text Word] OR Adult Liver Cancer [Text Word] OR Adult Liver Cancers [Text Word] OR “Cancer, Adult Liver” [Text Word] OR “Cancers, Adult Liver” [Text Word] OR “Liver Cancers, Adult” [Text Word] OR Liver Cell Carcinoma [Text Word] OR “Carcinoma, Liver Cell” [Text Word] OR “Carcinomas, Liver Cell” [Text Word] OR “Cell Carcinoma, Liver” [Text Word] OR “Cell Carcinomas, Liver” [Text Word] OR Liver Cell Carcinomas [Text Word] OR Hepatocellular Carcinoma [Text Word] OR Hepatoma [Text Word] OR Hepatomas [Text Word] OR “Carcinoma, Hepatocellular” [Mesh Terms]) AND (TACE [Text Word] OR transcatheter arterial chemoembolization [Text Word] OR transarterial chemoembolization [Text Word] OR transarterial chemoembolization [Text Word] ) AND (Therapeutic Chemoembolization [Text Word] OR “Chemoembolizations, Therapeutic” [Text Word] OR Therapeutic Chemoembolizations [Text Word] OR “Chemoembolization, Therapeutic”[Mesh Terms]) AND (90Y [Text Word] OR Yttrium-90 [Text Word] OR TARE [Text Word] OR transcatheter arterial radioembolization [Text Word] OR transarterial radioembolization [Text Word] OR radioembolization [Text Word]).
The inclusion criteria were as follows:
1) Randomized controlled trials (RCT), observational studies, and clinical studies.
2) Patients were diagnosed with HCC.
3) cTACE or TARE (90Y) as monotherapy.
4) Showed the effects and/or safety after treatment with cTACE or TARE (90Y).
The exclusion criteria were as follows:
1) Reviews, commentaries, case reports, meeting abstracts, experimental studies, systematic reviews, and meta-analyses.
2) No comparison between cTACE and TARE (90Y) therapies.
3) cTACE combined with TARE (90Y).
4) Drug eluting bead-TACE(DEB-TACE) as monotherapy.
5) Lacked key data from outcomes after treatment with cTACE or TARE (90Y).
All titles and abstracts were independently screened by both authors. Meanwhile, we performed a manual search of the relevant references, to retrieve additional articles. The automatic elimination of duplicates was carried out by EndnoteTM (Version X8.1.0.2).
Data extraction and quality assessment
All selected full-texts were screened independently by both authors to extract data as follows:
1) The extracted general data and study characteristics included the authors, year of publication, country, study design, treatment method, samples size, sex of patients, age of patients.
2) The collected data of the state of HCC and liver function included Child-Pugh class, BCLC stage, and etiology (HBV, HCV, and alcohol use).
3) The key data that was used for our meta-analysis of the effects and safety were 1- and 2-year OS rates, Kaplan-Meier curves, serious AEs, and ORs.
OR rates were analyzed in this meta-analysis following World Health Organization (WHO) and modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria, and we performed subgroup analyses. Only 1- and 2-year OS rates were collected if Kaplan-Meier curves were also present. Adobe Reader XI (Version 11.0.20) was used to obtain 1- and 2-year OS rates from Kaplan-Meier curves, by using the measuring tool in the edit menu, if these were not specifically provided in the text.
A quality assessment of the extracted studies was performed according to the Newcastle-Ottawa Scale (NOS), which grades the quality of observational studies on a 9-point scale. The risk of bias for RCTs was assessed using the Cochrane Collaboration tool of RevMan (Version 5.3).
Statistical analysis
All statistical analyses were performed using Stata (Version 12.0) and SPSS (Version 22). The effect sizes for 1- and 2-year OS rates were evaluated with the odds ratio* (OR*). The effect sizes for serious AEs were evaluated with the risk ratio (RR). We used the Q- and I2-tests to evaluate data heterogeneity, where P < 0.1, for the Q-test, or I2 > 50% represented significant heterogeneity. Sensitivity analysis was conducted by limiting the quality of the studies. Only studies that were determined to be of high quality, or with moderate/low risk of bias, were extracted. We applied a 95% confidence interval (CI) to reveal the accuracy of the overall effect size of interest. Random effects models (Mantel-Haenszel test) were adopted to calculate the pooled effects, when significant heterogeneity existed in the data ( I2 > 50%). Otherwise, fixed effects models (Mantel-Haenszel test) were adopted ( I2 ≤ 50%)12. The results of the OR* and RR analyses were presented by forest plots. The potential publication biases were demonstrated by funnel plots.
Results
Study selection
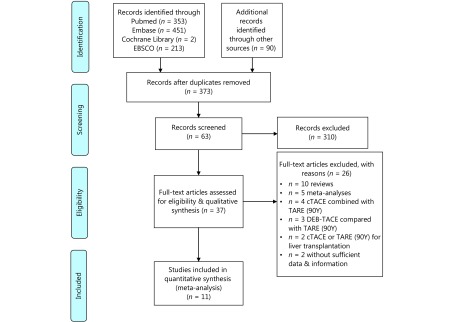
One thousand and nineteen potentially related citations were identified through our search strategy. Studies were excluded due to insufficient data and information (n=2), cTACE combined with TARE (90Y) (n=4), treatment either cTACE or TARE (90Y; n=2), DEB-TACE as treatment (n=3), and non-clinical studies (n=15). Finally, 11 articles met the inclusion criteria for our meta-analysis13-23.
Study characteristics
All of the articles were published between 2009 and 2016 and included with nine observational studies13,16-23 and two RCTs14,15. The locations of the studies included Germany, Spain, China, Egypt, Turkey, and the USA. Characteristics of the 1, 652 included patients [1, 124 in cTACE group, 528 in TARE (90Y) group] across the 11 studies are summarized inTable 1. The states of HCC and liver functions of all patients, with respect to each Child-Pugh class and BCLC stage, are shown in Table 2. We firmly believe that all of the patients had been diagnosed to satisfy the inclusion criteria. No statistical difference in ratios of the following variables: % males (78.0% vs. 78.0%; P = 1.00), % HBV (14.1% vs. 12.9%; P > 0.05), % HCV (32.1% vs. 40.7%; P > 0.05), % alcohol (26.8% vs. 24.3%; P > 0.05), % BCLC A (61.2% vs. 65.5%; P = 0.16), % BCLC B (36.0% vs. 32.7%; P = 0.22), % BCLC C (2.8% vs. 1.8%; P = 0.51), % Child-Pugh class A (62.2% vs. 65.1%; P = 0.40), % Child-Pugh class B (36.3% vs. 34.2%; P = 0.53), or% Child-Pugh class C (1.5% vs. 1.0%; P = 0.32) were detected among the patient characteristics with respect to sex, etiology, state of HCC, and liver function. Heterogeneity was observed in the 2-year OS rate group, the OR group, and the serious AEs group. Random effects models were adopted to investigate heterogeneity, while subgroup analyses were performed to reduce heterogeneity, where results were sorted by study design (observational study and RCT) and OR criteria (WHO criteria and mRECIST criteria). After reviewing all studies and rechecking the data, no other variables that could cause heterogeneity were detected.
1.
Study characteristics
| Author (year) | Country | Study design | Treatment | Sample size (n) | Age (years) | Gender | |||||||
| Total | cTACE | TARE
(90Y) |
cTACE | TARE
(90Y) |
cTACE | TARE (90Y) | |||||||
| Male | Female | Male | Female | ||||||||||
| RCT: Randomized controlled trial; NA: Negative available | |||||||||||||
| Soydal et al.13 | Turkey | Observational studies | cTACE /
TARE (90Y) |
80 | 40 | 40 | 66.15±7.81 | 62.28±9.73 | 34 | 6 | 33 | 7 | |
| Salem et al.14 | USA | RCT | cTACE /
TARE (90Y) |
45 | 21 | 24 | 64
(62–70) |
62
(58–65) |
16 | 5 | 17 | 7 | |
| Kolligs et al.15 | Germany+
Spain |
RCT | cTACE /
TARE (90Y) |
28 | 15 | 13 | 66.7±9.04 | 65.8±6.73 | 13 | 2 | 11 | 2 | |
| El Fouly
et al.16 |
Germany+
Eygpt |
Observational studies | cTACE /
TARE (90Y) |
86 | 42 | 44 | 58.3±6.7 | 66.1±8.9 | 38 | 4 | 36 | 8 | |
| She et al.17 | China | Observational studies | cTACE /
TARE (90Y) |
32 | 16 | 16 | 62.5
(48–78) |
55
(37–73) |
13 | 3 | 15 | 1 | |
| Moreno-Luna
et al.18 |
USA | Observational studies | cTACE /
TARE (90Y) |
116 | 55 | 61 | 66
(46–84) |
64
(29–88) |
43 | 12 | 49 | 12 | |
| Salem et al.19 | USA | Observational studies | cTACE /
TARE (90Y) |
245 | 122 | 123 | 61
(33–38) |
66
(30–38) |
102 | 20 | 87 | 36 | |
| Lance et al.20 | USA | Observational studies | cTACE /
TARE (90Y) |
73 | 35 | 38 | 61
(51–84) |
63
(44–85) |
28 | 7 | 33 | 5 | |
| Kooby et al.21 | USA | Observational studies | cTACE /
TARE (90Y) |
790 | 691 | 99 | NA | NA | 518 | 173 | 70 | 29 | |
| Carr et al.22 | USA | Observational studies | cTACE /
TARE (90Y) |
71 | 44 | 27 | 61.0±9.9 | 58.7±10.8 | 36 | 8 | 23 | 4 | |
| Lewandowski
et al.23 |
USA | Observational studies | cTACE /
TARE (90Y) |
86 | 43 | 43 | 65
(58.9–67.8) |
68
(62.8–75) |
36 | 7 | 38 | 5 | |
2.
State of HCC and liver function
| Author (year) | Child-Pugh class | BCLC stage | Etiology | |||||||||||||||||||||||
| cTACE | TARE (90Y) | cTACE | TARE (90Y) | cTACE | TARE (90Y) | cTACE | TARE (90Y) | |||||||||||||||||||
| A | B | C | A | B | C | A | B | C | D | A | B | C | D | HBV | HCV | HBV | HCV | Alcohol | ||||||||
| Soydal et al.13 | NA | NA | 0 | 34 | 6 | 0 | 0 | 33 | 7 | 0 | NA | NA | NA | |||||||||||||
| Salem et al.14 | 15 | 6 | 0 | 12 | 12 | 0 | 17 | 4 | 0 | 0 | 18 | 6 | 0 | 0 | 2 | 10 | 3 | 12 | 1 | 4 | ||||||
| Kolligs et al.15 | 9 | 4 | 2 | 9 | 3 | 1 | 4 | 8 | 3 | 0 | 5 | 5 | 3 | 0 | NA | NA | NA | NA | ||||||||
| El Fouly et al.16 | 33 | 9 | 0 | 37 | 7 | 0 | NA | NA | 1 | 36 | 6 | 8 | 0 | 10 | ||||||||||||
| She et al.17 | 14 | 2 | 0 | 15 | 1 | 0 | NA | NA | 13 | 3 | 12 | 0 | NA | NA | ||||||||||||
| Moreno-Luna et al.18 | 44 | 11 | 0 | 53 | 8 | 0 | 23 | 13 | 19 | 0 | 12 | 34 | 14 | NA | NA | 7 | NA | 8 | 13 | 12 | ||||||
| Salem et al.19 | 67 | 53 | 2 | 67 | 54 | 2 | 47 | 61 | 12 | 2 | 43 | 65 | 13 | 2 | 12 | 56 | 13 | 42 | 21 | 20 | ||||||
| Lance et al.20 | 24 | 11 | 0 | 31 | 7 | 0 | NA | NA | NA | 11 | NA | 13 | 3 | 8 | ||||||||||||
| Kooby et al.21 | NA | NA | NA | NA | 97 | 132 | 9 | 30 | 217 | 37 | ||||||||||||||||
| Carr et al.22 | 22 | 22 | 0 | 13 | 14 | 0 | NA | NA | NA | 25 | NA | 10 | NA | NA | ||||||||||||
| Lewandowski
et al.23 |
23 | 18 | 2 | 24 | 19 | 0 | 0 | 37 | 4 | 2 | 0 | 34 | 9 | 0 | 6 | 16 | 2 | 14 | 10 | 9 | ||||||
Quality of the included studies and risk of bias
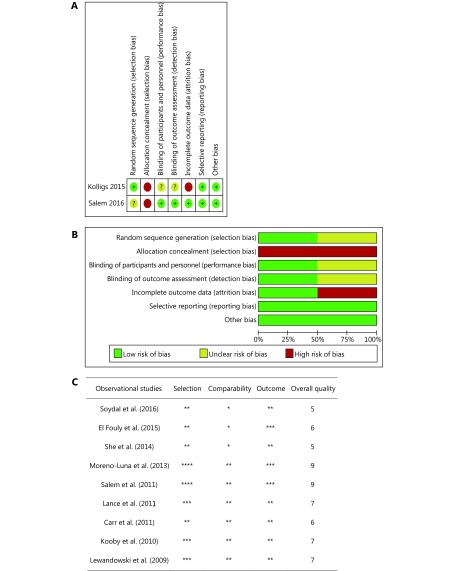
All nine observational studies were judged as high quality, based on the NOS13,16-23 (Figure 2). The risk of bias was assessed using the Cochrane Collaboration tool for two RCTs (Figure 2). One RCT, with more than two high-risk components, was considered to have a moderate risk of bias15, and another RCT was determined to have a low risk of bias14.
2.
Cochrane Collaboration’s tool for assessing the risk of bias: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting; and (7) other bias. (A) Risk of bias summary. (B) Risk of bias graph. (C) Quality assessment of extracted studies was performed based on the NOS. (*Represents the one criterion of each subsection was satisfied).
1.
PRISMA flow diagram of the process for study selection.
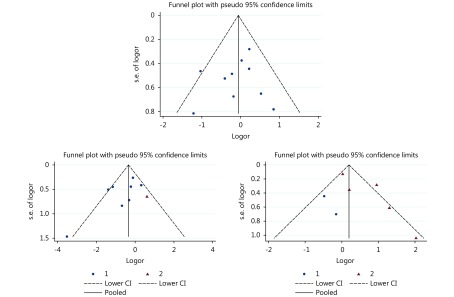
No significant publication bias was found using funnel plots (Figure 3). Egger’s test: 1-year OS rate group, P = 0.605; 2-year OS rate group, P = 0.591; serious AEs group, P = 0.797. We did not perform funnel plot-publication bias tests for the mRECIST and WHO subgroups within the OR group, due to the insufficient power of test.
3.
Funnel plots for publication bias of cTACE vs. TARE (90Y) including 1-year overall survival rate group, 2-year overall survival rate group, and serious adverse events group.
4.
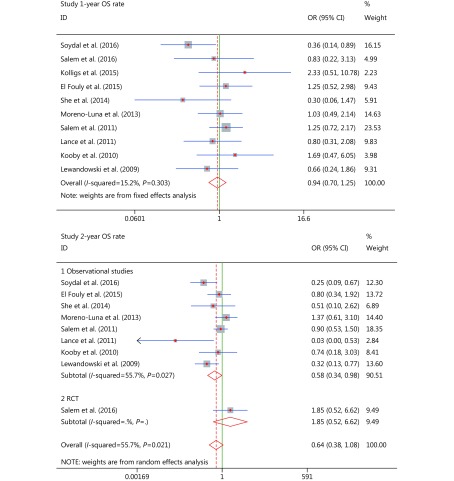
OR analyses and forest plots for 1-year OS rate group and 2-year OS rate group.
1-year and 2-year OS rates
Ten studies reported 1-year OS rates13-21,23 and nine studies reported 2-year OS rates13,14,16-21,23. The fixed effects model was used to analyze 1-year OS rates (P = 0.303, I2 = 15.2%), and random effects models were adopted to analyze 2-year OS rates (overall P = 0.021, I2 = 55.7%), since heterogeneity was present in this study. A subgroup analysis produced a reliable solution for reducing heterogeneity, through the classification of reports by study design (observational study subgroup P = 0.027, I2 = 55.7%; RCT subgroup P = 0.000, I2 = 0.0%).
The meta-analysis showed that there were no significant differences in 1-year OS rates (OR* = 0.939, 95% CI: 0.705–1.251, P = 0.66), which indicated that the patients who were treated with cTACE or TARE (90Y) had similar 1-year OS rates. Significant improvements were found, in most of the reports of the observational study subgroup, in 2-year OS rates (OR* = 0.575, 95% CI: 0.336–0.984, P = 0.043), which demonstrated that the TARE (90Y) group had a significantly higher 2-year OS rate than the cTACE group and signified the improved long-term survival benefits of patients who underwent TARE (90Y). No significant differences were found for pooled 2-year OS rates (overall pooled OR* = 0.641, 95% CI: 0.382–1.075, P = 0.092), which slightly favored the outcomes of the RCT subgroup (OR* = 0.641, 95% CI: 0.382–1.075, P = 0.346). Therefore, the OR* of the RCT subgroup had influenced our overall pooled OR* results.
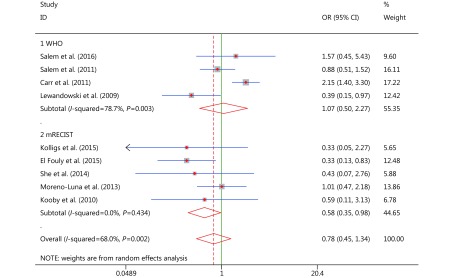
Objective response
Tumor responses were assessed with the assistance of radiologic techniques and medical imaging and were classified into four categories: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Our meta-analysis included nine studies14-19,21-23 that contained tumor response data, with four studies14,19,21,23 depending on WHO criteria24 (Group 1 in Figure 5) and five studies15-18,21 depending on mRECIST criteria25 (Group 2 in Figure 5). OR was defined as CR + PR, and in the OR analysis, subgroups were classified by mRECIST and WHO criteria. A random effects analysis was used to solve heterogeneity (WHO subgroup P = 0.003, I2 = 78.7%; mRECIST subgroup P = 0.434, I2 = 0.0%; Figure 5). Significant differences were found in the mRECIST subgroup (OR* = 0.584, 95% CI: 0.349–0.976, P = 0.040), which revealed that patients who were treated with TARE (90Y) had stronger ORs, than those treated with cTACE, and may obtain better clinical benefits. No significant differences were noted in the WHO subgroup analysis (OR* = 1.065; 95% CI: 0.500–2.268, P = 0.870). Since multiple tumor response criteria were used, the overall pooled analysis (overall pooled OR* = 0.781, 95% CI: 0.454–1.343, P = 0.371) was considered to have statistics that were less constructive than those of the other analyses and poorly reliable.
5.
OR analyses and forest plots for OR.
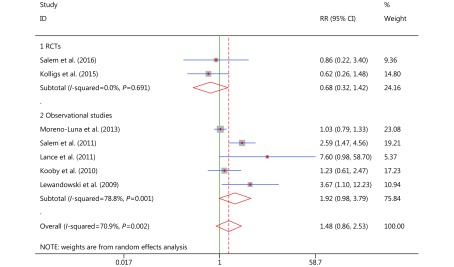
Serious adverse events
Nine studies reported relevant data of AEs, which included pain, hemorrhage, fatigue, nausea, vomiting, anorexia, diarrhea, headache, confusion, fever, rash, chest pain, gastric ulceration, and hepatic abscess. Serious AEs were defined as AEs of grades 3/4, based on the Common Terminology Criteria for Adverse Events Version 3.0 (CTCAE V3.0)26 Treatment-related deaths were rare in the extracted studies. Significant heterogeneity in the serious AEs data was observed (overall P = 0.002, I2 = 70.9%; Figure 6), and both random effects and subgroup analyses were employed to solve the heterogeneity (RCT subgroup P = 0.691, I2 = 0.0%; observational study subgroup P = 0.02, I2 = 70.9%; Figure 6).
6.
RR analyses and forest plots for serious adverse events group.
Subgroup and overall pooled analyses demonstrated that there was no significant difference among modalities in serious AEs (RCT subgroup RR = 0.680; 95% CI: 0.325–1.423, P = 0.306, group 1 in Figure 6; observational study subgroup RR = 1.925; 95% CI: 0.978–3.788, P = 0.058, group 2 in Figure 6; overall pooled RR = 1.477, 95% CI: 0.864–2.526, P = 0.154), which demonstrates that the risks of AEs, among patients who were treated with either cTACE or TARE (90Y), are similar. However, the RR analysis and forest plot of the observational study subgroup showed a non-significant trend that slightly favored cTACE, which may be associated with higher risks of AEs, although the heterogeneity is significant. From these results, it is probable that serious AEs occurred less after TARE (90Y) than after cTACE.
Discussion
cTACE is advocated as the first-line therapy for intermediate HCC27-29 and is a beneficial treatment that prolongs the OS of patients who cannot receive surgery30,31. However, cTACE still requires amelioration, due to the many negative AEs, such as portal vein thrombosis (PVT), tumor metastasis, chemical drug resistance, hepatic dysfunction, and postembolization syndrome. Although DEB-TACE offers survival improvements over cTACE32, only three observational studies33-35 have compared DEB-TACE with TARE (90Y). In this study, we conducted a meta-analysis of TARE (90Y) vs. cTACE, due to insufficient evidence of DEB-TACE results.
TARE (90Y) has been reported to overcome the typical contraindications of cTACE-PVT, with the advantages of no significant occlusions, less damage to the liver vasculature36-38, and good tolerance and safety37,39. Three comparative studies23,40,41 and one retrospective study42 have shown semblable OS, response rates, and safety profiles between cTACE and TARE (90Y), although better qualities of life after TARE (90Y), in comparison with cTACE, have also been reported43.
A reliable analysis is needed to clarify the superiority of one therapy, so that the therapy could be considered as a better treatment strategy for HCC. The purpose of our meta-analysis was to compare these two locoregional therapies in terms of 1- and 2-year OS rates, OR, and serious AEs in patients with HCC.
The meta-analysis for 1-year OS rates (OR* = 0.939, 95% CI: 0.705–1.251, P = 0.66) and 2-year OS rates (overall pooled OR* = 0.641, 95% CI: 0.382–1.075, P = 0.092; observational study subgroup OR* = 0.575, 95% CI: 0.336–0.984, P = 0.043; RCT subgroup OR* = 0.641, 95% CI: 0.382–1.075, P = 0.346) revealed that patients who were treated with cTACE or TARE (90Y) had similar 1-year OS rates. For the 2-year OS rate analysis, TARE (90Y) resulted in better long-term survival benefits for patients with HCC in the observational subgroup. However, no survival benefits were observed in the RCT subgroup and pooled group. It is remarkable that one RCT influenced the positive outcomes of TARE (90Y) in the observational subgroup. Therefore, we could not definitively conclude that TARE (90Y) generated a better 2-year survival benefit, because of the high level of evidence for the RCT. We hypothesize that TARE (90Y) might have better long-term survival benefits, which is supported by most of the included studies.
The meta-analysis for OR (overall pooled OR* = 0.781, 95% CI: 0.454–1.343, P = 0.371; mRECIST subgroup OR* = 0.584, 95% CI: 0.349–0.976, P = 0.040; WHO subgroup OR* = 1.065; 95% CI: 0.500–2.268, P = 0.870) suggested that TARE (90Y) was associated with stronger ORs than cTACE, according to mRECIST criteria. However, no distinct superiority was observed according to WHO criteria. The overall pooled analysis was regarded as meaningless and unreliable. The EASL-EORTC Clinical Practice Guidelines recommended mRECIST criteria for assessing tumor response27. Therefore, it might be more scientific and credible to conclude that TARE (90Y) generates a stronger OR than cTACE, according to mRECIST criteria, using the same statistical approach for the two subgroup analyses.
The meta-analysis for serious AEs (overall pooled RR = 1.477, 95% CI: 0.864–2.526, P = 0.154; RCT subgroup RR = 0.680, 95% CI: 0.325–1.423, P = 0.306; observational study subgroup RR = 1.925; 95% CI: 0.978–3.788, P = 0.058) showed similar risks of AEs among patients, regardless of whether cTACE or TARE (90Y) was used for treatment. Although there were no statistical differences between the two therapies, RR and forest plot analyses suggested that lower risks of AEs existed in the observational study subgroup of patients who were treated with TARE (90Y). Additional detailed AE subgroup analyses will be included in our next meta-analysis.
There are several potential limitations to our meta-analysis.
1) Great heterogeneity was observed because both observational studies and RCTs were included in our meta-analysis.
2) Only two RCTs were included in our meta-analysis.
3) The different investigators are likely to make selection biases, although we have chosen studies with high quality or low/moderate risk of bias.
4) Standards of operations varied in different countries and in different medical centers, and technology details differed among the extracted studies. For example, variations were observed in the radiological dosimetry of TARE (90Y) therapies, in the doses and types of chemical drugs that were used in cTACE, and the measurement conditions and time points, all of which may influence on our findings.
5) Patients were from different countries, and the majority were from the USA. The diversity of human ethnicities may bias our results.
The most current studies that had explored comparisons between cTACE and TARE (90Y) were observational studies, which could not provide sufficient convincing evidence. We guarantee that our study is the most comprehensive analysis that has explored the superiorities of cTACE and TARE(90Y). More multi-center RCTs with large samples are warranted to further confirm which therapy is better for patients with HCC. The latest studies will be followed-up and treatment upgrades will be made in the future. More refined subgroups analyses will be conducted, and additional evaluation parameters, such as time to progression (TTP), progression-free survival (PFS), and disease control rate (DCR), should be analyzed in future studies. Moreover, we urge physicians to objectively and carefully interpret our conclusions when practicing in the clinic.
In summary, our above subgroup analyses, overall pooled analyses, and forest plots demonstrate that that TARE (90Y) shows similar 1- and 2-year OS rates and AEs, in comparison with cTACE. However, TARE (90Y) showed better 2-year OS rates in the observational subgroup and greater OR, according mRECIST criteria.
Conclusions
Although additional studies are urgently needed to establish clinical trials and RCTs, our findings generally support the application of TARE (90Y) for patients with HCC (especially intermediate or advanced stages) as a therapy that might be superior to cTACE, in 2-year OS rates and OR rates, according mRECIST criteria.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60:1767–75. doi: 10.1002/hep.27222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulin M, Hillon P, Cercueil JP, Bonnetain F, Dabakuyo S, Minello A, et al. Idarubicin-loaded beads for chemoembolisation of hepatocellular carcinoma: results of the IDASPHERE phase I trial. Aliment Pharmacol Ther. 2014;39:1301–13. doi: 10.1111/apt.12746. [DOI] [PubMed] [Google Scholar]
- 5.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 6.Meza-Junco J, Montano-Loza AJ, Liu DM, Sawyer MB, Sawyer VG, Ma M, et al. Locoregional radiological treatment for hepatocellular carcinoma; Which, when and how? Cancer Treat Rev. 2012;38:54–62. doi: 10.1016/j.ctrv.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Fong ZV, Tanabe KK. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer. 2014;120:2824–38. doi: 10.1002/cncr.28730. [DOI] [PubMed] [Google Scholar]
- 8.Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348–59. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 9.Vente MAD, Wondergem M, Van Der Tweel I, Van Den Bosch MAAJ, Zonnenberg BA, Lam MGEH, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol. 2009;19:951–9. doi: 10.1007/s00330-008-1211-7. [DOI] [PubMed] [Google Scholar]
- 10.Ettorre GM, Santoro R, Puoti C, Sciuto R, Carpanese L, Antonini M, et al. Short-term follow-Up of radioembolization with yttrium-90 microspheres before liver transplantation: new perspectives in advanced hepatocellular carcinoma. Transplantation. 2010;90:930–1. doi: 10.1097/TP.0b013e3181f10f04. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soydal C, Arslan MF, Kucuk ON, Idilman R, Bilgic S. Comparison of survival, safety, and efficacy after transarterial chemoembolization and radioembolization of Barcelona Clinic Liver Cancer stage B-C hepatocellular cancer patients. Nucl Med Commun. 2016;37:646–9. doi: 10.1097/MNM.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 14.Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151:1155–63.e2. doi: 10.1053/j.gastro.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolligs FT, Bilbao JI, Jakobs T, Iñarrairaegui M, Nagel JM, Rodriguez M, et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. 2015;35:1715–21. doi: 10.1111/liv.12750. [DOI] [PubMed] [Google Scholar]
- 16.El Fouly A, Ertle J, El Dorry A, Shaker MK, Dechêne A, Abdella H, et al. In intermediate stage hepatocellular carcinoma: radioembolization with yttrium 90 or chemoembolization? Liver Int. 2015;35:627–35. doi: 10.1111/liv.12637. [DOI] [PubMed] [Google Scholar]
- 17.She WH, Cheung TT, Yau TCC, Chan ACY, Chok KSH, Chu FCK, et al. Survival analysis of transarterial radioembolization with yttrium-90 for hepatocellular carcinoma patients with HBV infection. Hepatobiliary Surg Nutr. 2014;3:185–93. doi: 10.3978/j.issn.2304-3881.2014.07.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36:714–23. doi: 10.1007/s00270-012-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu R, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497–507.e2. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lance C, McLennan G, Obuchowski N, Cheah G, Levitin A, Sands M, et al. Comparative analysis of the safety and efficacy of transcatheter arterial chemoembolization and yttrium-90 radioembolization in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1697–705. doi: 10.1016/j.jvir.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Kooby DA, Egnatashvili V, Srinivasan S, Chamsuddin A, Delman KA, Kauh J, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:224–30. doi: 10.1016/j.jvir.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer. 2010;116:1305–14. doi: 10.1002/cncr.24884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–8. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 24.Miller AB, Hoogstraten B, Hoogstraten M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 26.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 27.European Association For the Study of The Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20–37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Facciorusso A, Licinio R, Muscatiello N, Di Leo A, Barone M. Transarterial chemoembolization: evidences from the literature and applications in hepatocellular carcinoma patients. World J Hepatol. 2015;7:2009–19. doi: 10.4254/wjh.v7.i16.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–9. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 31.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 32.Ludwig JM, Zhang D, Xing M, Kim HS. Meta-analysis: adjusted indirect comparison of drug-eluting bead transarterial chemoembolization versus (90)Y-radioembolization for hepatocellular carcinoma. Eur radiol. 2017;27:2031–41. doi: 10.1007/s00330-016-4548-3. [DOI] [PubMed] [Google Scholar]
- 33.Padia SA, Johnson GE, Horton KJ, Ingraham CR, Kogut MJ, Kwan S, et al. Segmental Yttrium-90 radioembolization versus segmental chemoembolization for localized hepatocellular carcinoma: results of a single-center, retrospective, propensity score-matched study. J Vasc Interv Radiol. 2017;28:777–85.e1. doi: 10.1016/j.jvir.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Pitton MB, Kloeckner R, Ruckes C, Wirth GM, Eichhorn W, Wörns MA, et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. CardioVasc Intervent Radiol. 2015;38:352–60. doi: 10.1007/s00270-014-1012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akinwande O, Kim D, Edwards J, Brown R, Philips P, Scoggins C, et al. Is radioembolization (90Y) better than doxorubicin drug eluting beads (DEBDOX) for hepatocellular carcinoma with portal vein thrombosis? A retrospective analysis . Surg Oncol. 2015;24:270–5. doi: 10.1016/j.suronc.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Salem R, Mazzaferro V, Sangro B. Yttrium 90 radioembolization for the treatment of hepatocellular carcinoma: biological lessons, current challenges, and clinical perspectives. Hepatology. 2013;58:2188–97. doi: 10.1002/hep.26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012;56:464–73. doi: 10.1016/j.jhep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Memon K, Kulik L, Lewandowski RJ, Mulcahy MF, Benson AB, Ganger D, et al. Radioembolization for hepatocellular carcinoma with portal vein thrombosis: impact of liver function on systemic treatment options at disease progression. J Hepatol. 2013;58:73–80. doi: 10.1016/j.jhep.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826–37. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 40.Xing MZ, Kokabi N, Camacho JC, Kooby DA, El-Rayes BF, Kim HS. 90Y radioembolization versus chemoembolization in the treatment of hepatocellular carcinoma: an analysis of comparative effectiveness . J Comp Eff Res. 2013;2:435–44. doi: 10.2217/cer.13.37. [DOI] [PubMed] [Google Scholar]
- 41.Facciorusso A, Serviddio G, Muscatiello N. Transarterial radioembolization vs chemoembolization for hepatocarcinoma patients: a systematic review and meta-analysis. World J Hepatol. 2016;8:770–8. doi: 10.4254/wjh.v8.i18.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geschwind JF, Salem R, Carr BI, Soulen MC, Thurston KG, Goin KA, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127:S194–205. doi: 10.1053/j.gastro.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 43.Salem R, Gilbertsen M, Butt Z, Memon K, Vouche M, Hickey R, et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol. 2013;11:1358–65.e1. doi: 10.1016/j.cgh.2013.04.028. [DOI] [PubMed] [Google Scholar]