Abstract
The development of immunotherapies for lymphoma has undergone a revolutionary evolution over the past decades. Since the advent of rituximab as the first successful immunotherapy for B-cell non-Hodgkin lymphoma over two decades ago, a plethora of new immunotherapeutic approaches to treat lymphoma has ensued. Four of the most exciting classes of immunotherapies include: chimeric antigen receptor T-cells, bispecific antibodies, immune checkpoint inhibitors, and vaccines. However, with addition of these novel therapies the appropriate timing of treatment, optimal patient population, duration of therapy, toxicity, and cost must be considered. In this review, we describe the most-promising immunotherapeutic approaches for the treatment of lymphoma in clinical development, specifically focusing on clinical trials performed to date and strategies for improvement.
Keywords: CAR-T cells, immune checkpoint blockade, bispecific antibodies
Introduction
The treatment for lymphomas has advanced significantly over the past two decades as multiple new targeted and immunotherapeutic approaches have been developed. However, despite significant improvements in outcomes, 35% of patients with aggressive B-cell non-Hodgkin lymphomas (NHL), 15% of patients with Hodgkin lymphoma (HL), and 60% of patients with aggressive T-cell NHL will have progression of disease after standard frontline therapy1-3. For patients with aggressive lymphomas who relapse after initial therapy, a durable response is difficult to achieve with standard salvage therapies alone, due in part to the emergence of drug resistant clones and inability to provide fully planned doses of therapy due to toxicities. As such, new approaches are of continued interest to researchers and clinicians alike4,5. Treatment methods using less toxic therapies either as a single agent or in combination, may improve outcomes, with decreased adverse events (AEs) for this needy patient population.
The successful development of immunotherapy for the treatment of lymphomas began with the use of rituximab over 20 years ago. Rituximab is a human/murine, chimeric IgG1 anti-CD20 monoclonal antibody6. It revolutionized the treatment of B-cell CD20+ hematologic malignancies, improving overall response rates (ORRs) and survival in both indolent and aggressive B-cell NHL7-10. In addition to improving outcomes, the toxicity profile was also found to be very favorable. Rituximab works through antibody cell cytotoxicity (ADCC). Rituximab binds to CD20 on the cell surface and allows for the activation of the complement cascade, direct B-cell lysis, and phagocytosis by macrophages via recognition of complement and Fc receptors. Furthermore, interactions with the FcR also activate natural killer (NK) cells, through ADCC6. Given the success of rituximab-based treatments for B-cell NHL, significant efforts toward developing other novel immunotherapies for patients with lymphoma are underway. In this review we will focus on the most recent advances in lymphoma immunotherapy. This will include CAR T cells, bispecific antibodies, immune checkpoint inhibitors, and lymphoma vaccines.
CAR T-cell therapy
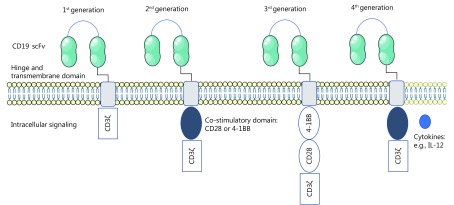
CAR T cells are autologous T lymphocytes that have been engineered to express the antigen binding region of an antibody directed against tumor-associated antigens11. CAR T cells were originally developed by Zelig Eshhar, when he introduced a CAR into a T cell, repurposing the T cell with new antigen specificity12. CAR T cells consist of a recombinant molecule comprising 3 parts: 1) single-chain variable domain of an antibody (scFv), 2) a transmembrane domain, and 3) a signal transduction domain of the T-cell receptor (TCR)13. The scFv allows for antigen specificity (Figure 1). It is created by cloning the variable regions of an antigen specific monoclonal antibody. Cloned DNA plasmids containing either gamma retroviral or lentiviral recombinant vectors are then transfected into target cells14-16. Thus far, CD19 has been the most extensively studied target of CAR T-cell therapy for the treatment of lymphoma17,18.
1.
CD19 CAR T-cell structure.
Upon engaging with a specific antigen, the T cell is activated through the signal transduction domain of the TCR, allowing for T-cell expansion and direct cell cytotoxicity. First-generation CAR T cells used a CD3ζ as the signal transduction domain of the TCR. Thus, T-cell activation was solely dependent on interleukin (IL)-2 production. While this produced excellent tumor-specific killing in vitro, there was poor T-cell expansion and anti-tumor activity in vivo19,20. Inadequate in vivo efficacy for first-generation CAR T cells occurred because under physiologic conditions, T cells require interaction with their TCR and multiple co-stimulatory receptors, such as CD28 and 4-1BB21. Thus, first generation CAR T cells were limited by a lack of co-stimulation. To improve upon first-generation CAR T cells, second-generation CAR T cells contained a co-stimulatory domain, either CD28 or 4-1BB. With the addition of a co-stimulatory domain, second-generation CAR T cells demonstrated significantly improved in vivo cytotoxicity, tumor killing, expansion, and persistence18,22. Interestingly the choice of co-stimulatory domains leads to a different functional T-cell subset. In CAR T cells with a CD28 co-stimulatory domain, T-cell expansion and activations is characteristic of effector T cells. While in those designed with a 4-1BB co-stimulatory domain, expanded T cells exhibited characteristics of memory T cells22-24. Third-generation CAR T cells were designed with two co-stimulatory domains. The first domain was either CD28 or 4-1BB, and the second domain was CD28, 4-1BB, or OXO4025-27. The efficacy and utility of third-generation CAR T cells are currently under investigation. More recently, a fourth-generation of “armored CAR T cells” has been designed to protect T cells from the immunosuppressive tumor microenvironment28,29. Armored CAR T cells have been engineered to express cytokines or costimulatory ligands, to help promote T-cell expansion and longevity within the tumor microenvironment29. Lastly, CAR T cells have also been generated to recognize multiple antigens. This can either be used to enhance specificity of the target tissue and improve safety; or produce synergistic enhancement of effector functions when both antigens are simultaneously encountered30,31.
Clinical application of CAR T cells for the treatment of lymphoma
Thus far, the majority of clinical studies in lymphoid malignancies have used second-generation CAR T cells32. To produce clinical-grade CAR T cells, patients must first undergo apheresis of their peripheral blood, where peripheral blood mononuclear cells (PBMC’s) are extracted. PBMCs are then transferred to a cell processing facility, where T cells undergo ex vivo stimulation and expansion in the presence of CD3 and CD28 magnetic beads33. Activated T cells are subsequently transfected using lentiviral or retroviral vectors carrying the CAR construct. The clone is then expanded using CD3/CD28 stimulation. Manufacturing takes approximately 2 weeks33. Prior to the infusion of the CAR-T cell product, patients typically receive a preconditioning regimen consisting of cyclophosphamide and fludarabine. This serves to deplete lymphocytes, specifically regulatory T cells, as well as decrease tumor burden, allowing for CAR-T cell expansion11. Patients usually require hospital admission for CAR T cell infusions in order to closely monitor for toxicities, especially cytokine release syndrome (CRS) and central nervous system (CNS) toxicity11.
There have been several collaborations between academic investigators and pharmaceutical companies in the development of CAR T-cell therapies for lymphoma. Investigators at the University of Pennsylvania have collaborated with Novartis to develop a second generation CD19 CAR T-cell product named, CTL019. This construct involves a murine anti-CD19 scFV; a CD8 transmembrane domain, a 4-1BB costimulatory domain, and CD3ζ signal transduction domain34. Schuster et al.34 recently reported the results of initial case series of patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) or follicular lymphoma (FL). In total, 28 of the 38 patients enrolled in the study were treated with CTL019, 14 with FL and 14 with DLBCL (Table 1). Fifty-six percent of the patients with FL were double refractory to treatment, and 86% of the patients with DLBCL were also refractory. At 3 months, 64% of the patient had a response. Among patients with DLBCL, ORR was 50%, and FL ORR was 79%. At 6 months, 57% of patients had a complete response (CR):43% for patients with DLBCL, and 71% for patients with FL. Interestingly, 3 patients with FL who had a partial response (PR) at 3 months also had a CR by 6 months. One patient with DLBCL who had a PR at 3 months, had a CR by 6 months34. All patients in CR at 6 months remained in remission. After a median follow-up of 28.6 months, 57% of all patients remained progression-free. Among patients with DLBCL, median progression-free survival (PFS) was 3.2 months. Among patients with FL, median PFS was not reached34. There was no reported difference in response rate based on DLBCL subtype34. Median peak expansion of CTL019 cells in the blood occurred at 8 days in patients who had a response and at 10 days for those who did not. Treatment was overall well tolerated, and toxicities were typically self-limiting. Eighteen percent of patients suffered from grade 3/4 CRS, with only one requiring administration of the anti-IL-6 antibody, tocilizumab. Eleven percent of patients suffered from grade 3/4 CNS toxicity, resulting in one death34.
1.
Summary of key CD19 CAR T-cell clinical trials for the treatment of lymphoma
| Study | Phase | Lymphoma subtype | Patients (n) | CAR-T product | Efficacy (%) | Safety (%) |
| Schuster et al.34 | 1/2 | R/R | 28 | CTL019 | ORR | 3/4 CNS: 11 |
| DLBCL & FL | 2nd generation; 41BB | DLBCL: 43 | 3/4 CRS: 18 | |||
| Lentiviral vector | FL: 71 | |||||
| Schuster et al.35 | 2 | R/R DLBCL | 81 | CTL019 | ORR: 53.1 | 3/4 CNS: 12 |
| 2nd generation; 41BB | CRR: 39.5 | 3/4 CRS: 23 | ||||
| Lentiviral vector | ||||||
| ZUMA-140 | 1/2 | R/R DLBCL | 101 | Axi-cel | ORR: 82 | 3/4 CNS: 28 |
| PMBCL | 2nd generation; CD28 | CRR: 58 | 3/4 CRS: 14 | |||
| TFL | Retroviral vector | |||||
| Turtle et al.47 | 1 | R/R B-cell NHL | 32 | JCAR014 | ORR | 3/4 CNS: 28 |
| 2nd generation; 41BB | Cy/Etop conditioning: 50 | 3/4 CRS: 12.5 | ||||
| 1:1 ratio of CD4+and
CD8+T-cells |
||||||
| Retroviral vector | Cy/Flu conditioning: 72 | |||||
| Abramson et al.49 | 1 | R/R DLBCL | 74 | JCAR017 | DL1 (5*107CAR-T cells) | 3/4 CNS: 14 |
| PMBCL | 2nd generation; 41BB | ORR: 40 | 3/4 CRS: 1 | |||
| MCL | Defined ratio of CD4+and
CD8+T-cells |
CRR: 27 | ||||
| FL | Retroviral vector | DL2 (1*108CAR-T cells) | ||||
| ORR: 63 | ||||||
| CRR: 58 |
In the multi-center, single-arm, open-label, phase 2 JULIET (NCT02445248) trial, patients with R/R DLBCL were treated with CTL019. A recent interim analysis was presented at the 59th annual meeting of the American Society of Hematology. Patients were eligible for the trial if they had progressed after receiving ≥2 lines of chemotherapy, or were ineligible for or had failed autologous stem cell transplant (ASCT)35. Of the 81 patients analyzed, the best ORR was 53.1% (95% CI, 42% to 64%; P<0.0001), with 39.5% CR and 13.6% PR. At month 3, the CR rate (CRR) was 32% and the PR rate (PRR) was 6%. Forty-six patients were evaluable for at least 6 months with a durable CRR of 30% and PRR of 7%. Median duration of response (DOR) was not reached; the 6-month probability of being relapse-free was 73.5% (95% CI, 52.0%–86.6%). Median overall survival (OS) was not reached; the 6-month probability of OS was 64.5% (95% CI, 51.5%–74.8%). No patient who achieved a response (CR or PR) proceeded to ASCT or allogeneic stem cell transplant (Allo-SCT). CTL019 was detected in peripheral blood by quantitative PCR for up to 367 days in responders. Overall, 86% of patients had grade 3 or 4 AEs. CRS occurred in 58% of patients, with 23% having grade 3/4 toxicity. Twelve percent of patients suffered from grade 3/4 neurologic toxicity. Fifteen percent of patients required the administration of tocilizumab, and 11% required corticosteroids. Three patients died due to disease progression, while, no deaths were secondary to treatment toxicity35.
Investigators at the National Cancer Institute (NCI) and KITE Pharmaceuticals developed a second generation CD19 CAR T-cell product named, Axicabtagene Ciloleucel (axi-cel). This construct consists of a single-chain variable fragment extracellular domain targeting CD19 proteins with CD3ζ and a CD28 co-stimulatory domain. In initial early phase studies performed at the NCI, 15 patients with R/R DLBCL, indolent lymphoma, or CLL were treated with a conditioning chemotherapy regimen of cyclophosphamide and fludarabine followed by a single infusion of anti-CD19 CAR T cells. Of the 15 patients, 8 achieved CR, 4 achieved PRs, 1 had stable disease, and 2 were not evaluable. Responses were durable, ranging from 9 to 22 months. Toxicities including fever, hypotension, and neurologic toxicities occurred, requiring two patients to receive tocilizumab36-38. Long term follow-up of responders demonstrated that in 4 of the 5 CRs, the durations of remission were 56, 51, 44, and 38 months; without evidence of relapse. Furthermore, CRs continued after recovery of non-malignant polyclonal B cells in 3 of 4 patients with long-term complete remissions. Lastly, patients had a low incidence of severe infections despite B-cell depletion and hypogammaglobulinemia38.
ZUMA-1 was a multi-center phase 1 safety, single arm, open-label trial in patients with refractory DLBCL treated with axi-cel. In total, 7 patients were treated. Patients received cyclophosphamide and fludarabine conditioning, followed by axi-cel at a target dose of 2 × 106 CAR T cells/kg. Only 1 patient developed grade 4 CRS/neurotoxicity, with a grade 3/4 CRS incidence of 14% and a grade 3/4 neurotoxicity incidence of 57%. The ORR was 71%, with a CRR of 57%. CAR T cells demonstrated peak expansion within 2 weeks and continued to be detected at greater than twelve months in patients with ongoing CR39. Subsequently, a phase 2 treatment portion of ZUMA-1 was opened for patients with refractory DLBCL, primary mediastinal B-cell lymphoma, and transformed follicular lymphoma40. The primary analysis of ZUMA-1 was initially presented at the 2017 American Association of Cancer Research annual meeting41. One-hundred and eleven patients were enrolled in the study, with 101 patients receiving axi-cel product. Seventy-seven of the patients had DLBCL, with 24 having either primary mediastinal B-cell lymphoma or transformed follicular lymphoma40. At a minimum of 6 months follow-up, the ORR was 82% (95% CI, 73–89), with a 54% CRR. The median time to response was 1 month, with a median DOR of 8.1 months. Response rates were consistent among all clinical and disease specific variables, including IPI score, cell-of-origin subtype, presence of bulky disease, patients with primary refractory disease, those who had relapsed after auto-transplant, or were treated with either glucocorticoids or tocilizumab40. Nine of the 52 patients who had disease progression were retreated, of which 5 had a response. At an updated one-year follow-up analysis of both the phase 1 and 2 portions of ZUMA-1, the objective response rate was 82%, including a CRR of 58%. Interestingly, in 23 of the patients who did not have an initial CR, at a longer follow-up as late as 15 months, they were found to have a CR in the absence of additional treatment. The median DOR was 11.1 months (95% CI, 3.9-could not be estimated). The median duration of PFS was 5.8 months (95% CI, 3.3-could not be estimated), with PFS survival rates of 49% (95% CI, 39–58) at 6 months, 44% (95% CI, 34–53) at 12 months, and 41% (95% CI, 31–50) at 15 months. The median overall survival was not yet reached (95% CI, 12.0 months-could not be estimated), with OS rates of 78% (95% CI, 69–85) at 6 months, 59% (95% CI, 49–68) at 12 months, and 52% (95% CI, 41–62) at 18 months40.
CAR-T levels peaked in the peripheral blood within 14 days after infusion of axi-cel product and were detectable in most patients at 180 days after infusion, and even up to 24 months. Patients who responded had 5.4 times more expansion of CAR T cells compared to those who did not respond. Peak expansion was significantly associated with neurologic events, but interestingly not with CRS. Elevated levels of IL-6, -10, -15, -2Rα, and granzyme B, were significantly associated with grade 3 or higher neurologic toxicity and CRS. However, elevated levels of IL-2, granulocyte–macrophage colony-stimulating factor, and ferritin, were only associated with neurologic toxicity40.
The most common grade 3 or higher AEs were neutropenia (in 78%), anemia (in 43%), and thrombocytopenia (in 38%). The incidence of grade 3 or higher febrile neutropenia was 31%. Grade 3 or higher CRS occurred in 13% of patients, while grade 3 or higher CNS toxicity occurred in 28% of patients. 43% of patients received tocilizumab and 27% received glucocorticoids for the management of CRS or CNS toxicity40. Results from the ZUMA-1 trial led to the approval of axi-cel by the US Food and Drug Administration (FDA) for treatment of adults with relapsed or refractory DLBCL after 2 or more lines of systemic therapy42.
Recently, both CD19 and programmed death ligand one (PD-L1) status in baseline and post-progression biopsies of patients who had progressed after treatment with axi-cel were assessed. Twenty-seven percent of patients with CD19-positive lymphoma cells at baseline developed CD19-negative disease at time of disease progression. Furthermore, in ten patients evaluable at the time of disease progression, 80% were PD-L1–positive43. These findings, as well as that increased levels of PD-L1 are frequently found in patients with refractory DLBCL, led to the development of the ZUMA-6 trial (NCT02926833); a phase 1/2 single arm trial of axi-cel in combination with atezolizumab (PD-L1 ab) for the treatment of patients with refractory DLBCL44,45. Preliminary results from this trial were presented at the 59th annual meeting of the American Society of Hematology. Six patients have thus far been treated without any DLT’s observed thus far. The addition of atezolizumab did not result in an increase in CRS, CNS toxicity, or other CAR T cell-related toxicities. The most common grade 3 or higher AE were anemia (67%), encephalopathy (67%), and hyponatremia (50%). The incidences of grade 3 or higher CRS and CNS toxicity was 33% and 67%, respectively. Thus far, five patients have been evaluable for response; with an ORR of 100%, with 1 CR. Interestingly, all patients had a least a 2-fold greater expansion of CAR T cells than those observed in patients enrolled in ZUMA-145. Other ongoing clinical trials exploring axi-cel for the treatment of lymphoma are ZUMA-2 (NCT02601313), a phase 2 multicenter study of axi-cel in subjects with R/R mantle cell lymphoma, and ZUMA-5 (NCT03105336), a phase 2 multicenter study of axi-cel in patients with R/R indolent NHL.
Investigators at Memorial Sloan Kettering Cancer Center (MSKCC), Seattle Children’s Research Institute, and Fred Hutchinson Cancer Research Center (FHCRC) formed a venture, Juno Therapeutics, to investigate their CAR T-cell products JCAR014-017. JCAR014 is a CAR T-cell product consisting of a murine anti-CD19 scFV; an IgG4 hinge, a CD28 transmembrane domain; a 4-1BB costimulatory domain; and a CD3ζ signal transduction domain46. It is also transfected with a truncated from of the epidermal growth factor receptor, allowing for eradication of the CAR T-cell product46. JCAR014 is derived from CD8+ and CD4+ central memory T-cell subsets in a 1:1 ratio47. This is in contrast to other CD19 CAR T-cell products which are either derived from unselected T-cells, or one subset alone47. In preclinical studies, JCAR014 has been demonstrated to produce enhanced antitumor activity and increased lysis of CD19+ tumors48. Researchers at FHCRC performed a phase 1 clinical trial of JCAR014 in patients with R/R B-cell NHL. Thirty-two patients were studied, all receiving cyclophosphamide based lymphodepleting chemotherapy47. Eleven patients had DLBCL, 11 patients had histologically transformed DLBCL, 4 patients had mantle cell lymphoma (MCL), and 6 patients had follicular lymphoma (FL). The investigators found that the CAR T-cell expansion and response rates varied greatly depending on the type of lymphodepleting chemotherapy used. In the 12 patients who received cyclophosphamide (Cy) and etoposide (Etop) lymphodepleting chemotherapy, 90% of patients lost CAR T cells by day 100, with recovery from B-cell aplasia. The ORR for these patients was 50%, with 8 patients developing progressive disease (PD). The remaining 20 patients received Cy and fludarabine (Flu) lymphodepleting chemotherapy. Compared to the patients who received the Cy/Etop lymphodepleting regimen, patients who received Cy/Flu had markedly greater expansion of CD4+ and CD8+ CAR T-cells in the blood in the first 10 days after infusion, and higher numbers of CAR T-cells in the blood 1 and 3 months later. This correlated with an improved ORR of 72%, with enhanced durability of remission in patients who had a CR47. The researchers also found that patients who received 2 × 106/kg cell dose had the best response rates, with an ORR of 83%. Severe CRS developed in 12.5% patients, all of whom had received Cy/Flu conditioning. Severe neurotoxicity occurred in 28% of patients and was more frequently seen in patients who received the Cy/Flu preparatory regimen. The highest incidence of severe CRS and neurotoxicity occurred in patients who received 2 × 107 CAR T-cells/kg and Cy/Flu lymphodepleting chemotherapy47.
Currently, Juno Therapeutics is conducting a multicenter phase 1 trial of JCAR017 in R/R B-cell NHL (NCT02631044). JCAR017 is a CD19-directed CAR T-cell product, with a 4-1BB co-stimulatory domain administered in a defined composition at a precise dose of CD8+ and CD4+ CAR T cells. An interim analysis of the study was recently reported at the 59th annual meeting of the American Society of Hematology. Patients received Cy/Flu lymphodepleting chemotherapy, followed by an infusion of JCAR017 at either of the two dose levels: DL 1, 5 × 107 CAR T-cells; DL 2, 1 × 108 CAR T-cells. Thus far 74 patients have been treated, 69 with DLBCL, 1 FL grade 3B, 1 primary mediastinal B-cell lymphoma (PMBCL), and 5 with MCL. Of the 69 patients in the DLBCL cohort evaluable for safety, 30% had CRS, with 1% grade 4. Twenty percent had neurotoxicity, with 14% having grade 3/4 neurotoxicity. Nineteen percent of patients were treated with tocilizumab, steroids, or both. Sixty-eight patients thus far have been evaluable for response. The best overall, 3-month, and 6-month response rates were 75%, 49%, and 40%, respectively. The best overall, 3-month, and 6-month CRRs were 56%, 40%, and 37% respectively. It appears there are improved response rates at 3 months in patients treated at DL 2 compared to DL1, with ORR of 63% (95% CI; 38–84) vs. 40% (95% CI; 23–59); P = 0.148, and a CRR of 58% (95% CI; 34–80) vs. 27% (95% CI; 12–46); P = 0.0385. In subgroup analysis among patients with double or triple hit lymphoma, the best ORR was 81%, and 3-month CRR was 60%49.
Correlative analysis from pre- and post-treatment biopsy samples were analyzed to assess for clinical efficacy. Patients who had a CR or PR at 3 months appeared to have a higher percentage of endogenous CD4+ cells in pretreatment tumors than those with PD (CR, PR median: 7.9%; PD median: 0.38%; P<0.0001). The level of CAR T-cell tumor infiltration trended higher in patients achieving a CR (median: 3.9%) or PR (median: 1.1%) compared to those with a SD or PD (median: 0.51). Patients who achieved a CR had a higher ratio of CAR T-cells that were CD8+ compared to CD4+, than those with SD or PD (CR median: 0.83; SD/PD median: 0.14; P = 0.0097). Similarly, achieving a CR or PR trended toward having an increased infiltration of CD8+ T-cells in tumors as compared to patients achieving SD or PD (CR, PR median change: +5.3%; SD, PD median change: +0.06%; P = 0.1225). Lastly, increases in CD8+ T-cell infiltration was associated with increases in indoleamine-pyrrole 2, 3-dioxygenase and PD-L1 expression50.
Investigators also found that pre-CAR T-cell infusion biomarkers associated with the occurrence of neurotoxicity were higher serum LDH, ferritin, CRP, IL-6, IL-8, IL-10, TNF-α, IFN-α2, MCP-1, and MIP-1β (P<0.05 for each). Higher pre-CAR T-cell infusion plasma IL-8, IL-10, and CXCL10 were specifically associated with increased grade 3/4 neurotoxicity (P<0.05). Furthermore, investigators discovered that higher ECOG scores and transformed DLBCL correlated with lower durable response at 3 months (P = 0.02). Pre-CAR T-cell infusion correlates associated with best ORR included lower ferritin, LDH, CXCL10, G-CSF, and IL-10. Correlates associated with durable response at 3 months included lower ferritin, CRP, LDH, CXCL10, IL-8, IL-10, IL-15, MCP-1, MIP-1β, TNF-α, and higher pre-CAR T-cell infusion, hemoglobin and albumin (P<0.05)51.
CAR T-cell therapies have shown great promise and are now starting to be used to treat patients with lymphoma outside of clinical trials. However, questions remain in optimizing their use. First, determining the mechanisms of resistance and how they can be overcome are yet to be fully elucidated. Furthermore, optimization of conditioning regimens, as well as potentially combining CAR T-cell therapies with other immunotherapeutic approaches (i.e. checkpoint inhibitors), will likely improve efficacy. The optimal timing for CAR-T cell therapy will need to be explored as well. Currently, CAR T-cell therapies have only been studied in patients with R/R disease, typically after failing ASCT. However, studies will need to be performed to determine the optimal sequence of treatment. If CAR T-cell therapy is found to be more efficacious than ASCT, this will have significant implications when it comes to the cost of patient care. Lastly, how to design targets specific for other lymphoma subtypes is still currently in development. Targets that are actively being studied include CD20, CD22, CD30, and CD79a.
Bispecific antibodies
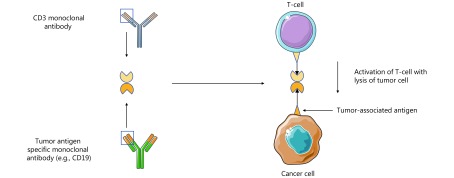
Bispecific antibodies (BsAb) are rationally designed antibodies that specifically redirect T-cells to a target antigen52. In the setting of tumor immunotherapy, T-cells are directed against a specific tumor antigen. The first BsAb was generated in the 1986 by Staerz et al.53,54. Since then there has been significant progress in the development of BsAbs, and now several different classes have been designed. BsAbs are engineered by combining scFv domains of two different antibodies with a polypeptide linker chain. One scFv domain recognizes CD3ζ on T-cells, while the other scFv binds tumor associated antigens52. This allows the antibody to target surrounding T cells to specific antigen-expressing tumor cells. Thus, T-cell activation is exclusively dependent on the interaction between tumor-associated antigens and CD3ζ, and independent of MHC complex and antigen specificity required to activate naive T-cells55.
The design of BsAbs can be divided into whether an Fc domain has been incorporated. We will focus on those without Fc domains, as they are the most developed and currently in clinical practice. One design that has been successful in both preclinical and clinical investigation are bispecific T-cell engagers (BiTes). BiTes are comprised of one single-chain variable fragments specific for CD3, and one for a tumor specific antigen (Figure 2)52. Blinatumomab, is a CD19 specific BiTe, that has demonstrated notable clinical benefit in CD19+ B-cell malignancies, specifically ALL, resulting in its FDA approval in 2014. Blinatumomab has been studied in a phase 1 open label dose escalation clinical trial in patients with R/R NHL56. Seventy-six patients were enrolled, 24 with FL, 28 with MCL, and 14 with DLBCL. The maximum tolerated dose (MTD) of blinatumomab was found to be 60 μg/m2/day on day 1. At the MTD, the ORR was 69%, with 37% CRR. The ORR was higher in indolent lymphoma (FL, ORR 80%; MCL, ORR 71%; DLBCL, ORR 55%). The median DOR at the MTD, was 13.5 months. The most common grade 3/4 AEs were hematologic, with 79% developing lymphopenia, 17% neutropenia, and 12% thrombocytopenia. Twenty-two percent of patients developed grade 3 neurotoxicity56. In a phase 2 study of patients with R/R DLBCL, the efficacy and safety of blinatumomab was assessed using different dosing schemes57. The investigators used a Simon 2-stage design, where patients either received continuous blinatumomab as a dose escalation to a target dose of 112 μg/d, or a flat fixed dose of 112 μg/d. They found that when using the flat dosing scheme, the incidence of neurotoxicity was too high. Subsequently, all but two patients were treated with a dose escalation strategy. The ORR was 43%, with a CRR of 19%. The median DOR was 11.6 months (95% CI; 0.9-not estimable). The median PFS was 3.7 months (95% CI; 1.4–7.7), and the median OS was 5.0 months (95% CI; 2.3-not estimable). The most common grade 3/4 hematologic AEs were leukopenia (17%) and thrombocytopenia (17%). Twenty-two percent of patients developed grade 3/4 neurotoxicity57. There are currently several clinical trials investigating blinatumomab for patients with lymphoma. These studies include combining blinatumomab with chemotherapy for relapsed lymphoma (NCT02568553), assessing the efficacy of blinatumomab in patients with DLBCL who have minimal residual disease status after auto-transplant, (NCT03298412), and investigating a subcutaneous formulation in patients with indolent lymphoma (NCT02961881).
2.
Bispecific T-cell engager design (BiTE).
FBTA05 (Lymphomun) is a trifunctional heterodimeric BsAb that recognized both CD20 and CD3. Ten pediatric patients with R/R B-cell malignancies were treated with escalating dosing of FBTA05. Of these 10 patients, 3 had DLBCL. The patients with DLBCL, received daily FBTA05 after receiving debulking chemotherapy. One patient developed a CR, 1 had a PR, and 1 had SD58. A chemically crosslinked CD20/CD3 BsAb has also been evaluated in clinical trials59,60. In two separate clinical trials, patients with R/R NHL were treated with CD3/CD20 BsAb in combination with an infusion of activated T-cells following ASCT. In one of the trials patients also received concomitant IL-2 infusions. While efficacy was difficult to assess in the setting of ASCT, the infusions were found to be safe, and produced endogenous cytotoxic responses against lymphoma targets59,60.
For BsABs, studies are just starting to assess their efficacy for the treatment of patients with lymphoma. Unlike CAR T cells, the immediate availability of the drugs, the ability to stop the drug if side effects arise, and decreased likelihood of developing long-term side effects make treatment with BsABs very appealing. Future studies will need to focus on determining the best treatment setting for their use, either as part of induction vs. consolidation vs. relapse. Other potential targets are also being investigated including CD20 and CD3052. Furthermore, continued improvements in antibody structure, as well as combination with other immunotherapies may improve efficacy. One such approach has been to genetically modify tumor or effector immune cells to express BsABs or cytokines, to help overcome the immunosuppressive tumor microenvironment52.
Checkpoint inhibitors
Immune checkpoints refer to the inhibitory pathways of the immune system that are critical for maintaining self-tolerance. Thus, they help regulate the duration and magnitude of physiological immune responses to promote immune tolerance and minimize tissue damage61. Over the past two decades research has demonstrated that tumors are able to exploit immune checkpoints, as a mechanism of immune resistance and tumor escape61. Specifically, expression of immune checkpoint ligands and receptors has been found to be potent inhibitors of T-cell tumor immune surveillance and immunity62. T-cell activation requires two steps. The primary signal is between the TCR and antigen bound to the MHC complex of the antigen presenting cell (APC). The second signal is co-stimulation, whereby a co-stimulatory receptor on the T-cell will interact with a ligand on the APC. However, co-inhibitory signaling also occurs, where the receptor-ligand interaction will result in inhibition of the T-cell response. The most well studied stimulatory ligands-receptors include CD28 with B7 ligands CD80 and CD8663. The most well studied co-inhibitory ligands-receptors include cytotoxic T-lymphocyte associated protein 4 (CTLA-4), programmed cell death 1 (PD-1), and its ligand PD-L163,64. In general, inhibitory receptors and ligands that regulate T-cell effector functions are frequently overexpressed on tumor cells within the tumor microenvironment, as opposed to activating receptors and ligands which are generally not overexpressed on tumor cells. CTLA-4 expression primarily occurs in lymph nodes. Conversely, PD-1 overexpression primarily occurs within the tumor microenvironment, inhibiting T-cell anti-tumor immunity61.
CTLA-4 was the first immune-checkpoint receptor to be clinically targeted. It is expressed primarily on T cells where it controls the initial stages of T-cell activation. Principally, CTLA-4 opposes the action of the T-cell co-stimulatory receptor, CD2865. Once the TCR engages with antigen, CD28 amplifies TCR signaling to activate T-cells. CD28 and CTLA-4 share ligands: CD80 and CD8666,67. CTLA-4 appears to have an increased binding for both ligands, and thus decreases the activation of T-cells by outcompeting CD28 for the binding of CD80 and CD86. This leads to active inhibition of T-cells, with the delivery of inhibitory intracellular signaling68,69. The critical role of CTLA-4 as a negative regulator of T-cells was demonstrated in a CTLA-4 knockout mouse model, in which mice developed lethal systemic immune hyperactivation70,71. While CTLA-4 is expressed on CD8+ T-cells, the major physiological role appears to occur through its effects on CD4+ T-cells. Specifically, CTLA-4 blockade results in an augmented immune response that is dependent on helper T-cells, while CTLA-4 interaction with regulatory T-cells (Treg) boosts their suppressive function72. Therefore, clinical blockade of CTLA-4 results in both the enhancement of CD4+ helper T-cell activity, and inhibition of Treg cell immunosuppression72.
The primary function of the PD-1/PD-L1 co-inhibitory axis is to limit the activation of T- cells in peripheral tissues in response to infection, and to limit autoimmunity73,74. PD-1 expression is induced on the surface of activated T-cells. The two ligands for PD-1 are PD-L1 and programmed cell death ligand 2 (PD-L2)75,76. Similar to CTLA-4, PD-1 is highly expressed on Tregs, allowing for enhanced proliferation upon engagement with PD-L177. Tumors are highly infiltrated with Tregs that suppress effector immune response. Thus, within the tumor microenvironment this has allowed for a major mechanism of immune escape and resistance78. Blockade of the PD-1/PD-L1 axis may also augment antitumor immune responses by decreasing the activity of intra-tumoral Tregs.
PD-1 is also induced on other activated non-T-cell lymphocytes, including NK cells and B-cells. Overexpression of PD-1 on these lymphocytes also inhibits their anti-tumor activity79. Thus, inhibition of the PD-1/PD-L1 axis may also enhance NK cell tumor immunity and may improve antibody production against tumors by B-cells. Lastly, chronic antigen exposure, either during chronic viral infection or malignancy, can lead to high levels of persistent PD-1 expression, inducing a state of exhaustion among antigen-specific T-cells. T-cell exhaustion appears to be partially reversed by PD-1 blockade80.
Clinical application of immune checkpoint inhibitors in the treatment of lymphoma
Mutations affecting the PD-1 and PD-L1 pathways have been found in both HL and NHL. However, the response to immune checkpoint blockade varies greatly between different subtypes of lymphoma, likely reflecting the variable expression and resistance pattern of immune checkpoints44. Furthermore, lymphomas associated with EBV infection are associated with very high levels of PD-L1 expression, as well as high expression of PD-1 on tumor infiltrating lymphocytes81. This is consistent in both HL and DLBCL, where EBV infection appears to increase PD-L1 promotor activity and subsequent expression on tumor cells82,83. Moreover, several lymphomas have been found to have a high presence of genetic aberrations at the 9p24 gene locus, the location of the PD-L1 and PD-L2 genes. Specifically, in PMBCL studies have demonstrated gains or amplifications at the 9p24 locus in up to 70% of cases84,85. While in classical HL (cHL) up to 97% of cases will have alterations at the 9p24 locus86. In EBV- primary CNS lymphoma and primary testicular lymphoma, greater than 50% of cases will have 9p24.1 copy gain and increased expression87. Lastly, in EBV driven natural killer/T-cell lymphomas (NKTCL), expression of PD-L1 is high on tumor cells secondary to upregulation of the MAPK/NF-κB pathway. High serum soluble PD-L1 (≥3.4 ng/mL) or a high percentage of PD-L1 expression in tumor specimens (≥38%) was associated with poor CRR and OS88. In the next sections we will review the results of clinical trials for checkpoint inhibitors in patients with lymphoma.
HL
HL is thought to be the ideal disease subtype for checkpoint inhibitors given its underlying pathophysiology. As previously mentioned, there are almost uniform alterations present at the 9p24 locus85. However, the tumor microenvironment, which is rich in inflammatory tumor infiltrating lymphocytes with rare lymphoma cells, also makes it an attractive disease target for checkpoint blockade89. Nivolumab, a human IgG4 anti-PD-1 monoclonal antibody, was first studied in heavily pretreated HL patients in the phase 1 CheckMate-039 trial (Table 2). Twenty-three patients were enrolled in the study; 78% had already underwent ASCT or had received salvage treatment with brentuximab vedotin (BV). The MTD was not determined, but the highest dose cohort at 3 mg/kg was well tolerated. Grade 3 drug related AEs occurred in 22% of patients. The ORR was 87% (95% CI; 66–97), including 17% with CR and 70% with PR. The PFS at 24 weeks was 86%90. This was followed by CheckMate-205, a multicenter phase 2 prospective study of nivolumab dosed (3 mg/kg every two weeks) for patients with R/R HL after treatment with both ASCT and BV. Eighty patients were enrolled in the study, and at a median follow-up of 8.9 months, the ORR was 66.3% (95% CI; 54.8–76.4), with a CRR of 7%. The most common drug-related grade 3/4 AEs were neutropenia and elevated lipase in 5% of patients each. Correlative analysis of pretreatment biopsies in 10 patients revealed overexpression or increases in copy number in PD-L1 and PD-L2 in all 10 sampled biopsies91. Based on this study, nivolumab was granted accelerated approval by FDA in May 2016 for the treatment of patients with HL who had relapsed or progressed despite ASCT and BV92.
2.
Summary of key checkpoint inhibitor clinical trials for the treatment of lymphoma
| Study | Phase | Lymphoma subtype | Study agent | Patients (n) | Efficacy (%) |
| HL | |||||
| Checkmate-03990 | 1 | R/R HL | Nivolumab | 23 | ORR: 87 |
| CRR: 17 | |||||
| PFS at 24 weeks: 80 | |||||
| Checkmate-20591 | 2 | R/R HL | Nivolumab | 80 | ORR: 66.3 |
| CRR: 7 | |||||
| Keynote-01393 | 1 | R/R HL | Pembrolizumab | 31 | ORR: 65 |
| CRR: 16 | |||||
| PFS at 24 weeks: 69 | |||||
| Keynote-08794 | 2 | R/R HL | Pembrolizumab | 210 | ORR: 69 |
| CRR: 22.4 | |||||
| PFS at 6 months 72.4 | |||||
| Herrera et al.95 | 1/2 | R/R HL | Nivolumab +
brentuximab vedotin |
62 | ORR: 85 |
| CRR: 62 | |||||
| Diefenbach et al.96 | 1 | R/R HL | Ipilumumab +
brentuximab vedotin |
12 | ORR: 67 |
| CRR: 42 | |||||
| B-cell NHL | |||||
| Keynote-01398 | 1b | R/R PMBCL | Pembrolizumab | 21 | ORR: 50 |
| CRR: 25 | |||||
| Keynote-17099 | 2 | R/R PMBCL | Pembrolizumab | 49 | ORR: 41 |
| CRR: 14 | |||||
| Checkmate-039100 | 1 | DLBCL, FL, and other
B-cell NHLs |
Nivolumab | 31 | DLBCL |
| ORR: 36 | |||||
| CRR: 18 | |||||
| FL | |||||
| ORR: 40 | |||||
| CRR: 10 | |||||
| B-cell NHL | |||||
| ORR: 0 | |||||
| Checkmate-039101 | 1 | DLBCL and FL | Ipilimumab +
nivolumab |
15 | ORR: 9 |
| CRR: 0 | |||||
| T-cell NHL | |||||
| Checkmate-039100 | 1 | MF, | Nivolumab | 23 | MF |
| PTCL, sézary syndrome CTCL, and other CTCL | ORR: 15 | ||||
| Continued | |||||
Pembrolizumab is a humanized IgG4 anti-PD-1 monoclonal antibody. In the phase 1b keynote-013 trial in patients with relapsed hematologic malignancies, pembrolizumab was administered intravenously at a dose of 10 mg/kg every 2 weeks. Of this study population 31 patients had R/R HL. All patients had previously received BV, and 71% had undergone ASCT. Sixteen percent of patients developed grade 3 treatment-related colitis, increased ALT and AST levels, nephrotic syndrome, joint swelling, back pain, and axillary pain. The ORR for the cohort was 65% (90% CI; 48–79), with 16% of patients achieving a CR. Responses were durable, with 70% of patients who achieved a response, having a DOR ≥ 24 week (range, 0.14 to 74 weeks). The PFS and OS rates at 24 weeks were 69% and 100%, respectively. Correlative studies demonstrated increased levels of PD-L1 and PD-L2 in Reed-Sternberg cells by immunohistochemistry (IHC) in biopsied samples. Furthermore, treatment with pembrolizumab resulted in a significant increase in the absolute number of T cells, CD4+ and CD8+ T-cell subsets, and NK cells in patients’ peripheral blood samples. Lastly, treatment with pembrolizumab led to increased activation of expanded immune-related signaling pathways93. This lead to the phase 2 keynote-087, a single-arm study of pembrolizumab in 3 patient cohorts: (1) those who had received ASCT and BV; (2) those who were treated with salvage chemotherapy and BV, and who were ineligible for ASCT; and (3) patients who received ASCT, without BV. Pembrolizumab was administered at 200 mg intravenously every 3 weeks. In total, 210 patients were enrolled in the trial, with close to equal distribution between cohorts. The ORR across all cohorts was 69.0% (95% CI; 62.3–75.2) and the CRR was 22.4% (95% CI; 16.9–28.6). The ORR was 73.9% (95% CI; 61.9–83.7) for cohort 1, 64.2% (95% CI; 52.8–74.6) for cohort 2, and 70.0% (95% CI; 56.8–81.2) for cohort 3, which was not statistically different from one another. ORR was similar for patients who had received less than vs. greater than 3 previous lines of therapy: 71.4% vs. 68.7%. At 6 months, the PFS was 72.4%, while the OS was 99.5%. Furthermore, 75.6% of patients had a DOR greater than 6 months. Over 90% of patients had high intensity staining for PD-L1 in Reed-Sternberg cells94. This lead to accelerated FDA approval to pembrolizumab for the treatment of patients with refractory cHL, or those who have relapsed after 3 or more prior lines of therapy in March of 2017.
Recently, the novel combination of nivolumab plus BV was studied in patients with R/R cHL. The study was designed in 21-day cycles for up to 4 cycles. BV was administered at a dose of 1.8 mg/kg on cycle 1, day 1, and nivolumab was administered at a dose of 3 mg/kg on cycle 1, day 8, and day 1 of cycles 2–4. Sixty-two patients were enrolled in the study, with 58 completing all 4 cycles of therapy. The incidence of grade 3/4 immune-related AEs (IRAE’s) was 13%, with 5% of patients requiring the use of corticosteroids. Infusion-related reactions occurred in 41% of patients. The ORR was 85% with a CRR of 62%. Sixty-five percent of patients initiated ASCT, and all patients were able to have stem cells collected. Peripheral blood immunophenotyping revealed an increase in pro-inflammatory cytokines and chemokines after treatment with BV. There was a reduction in Tregs after administration of BV, with a subsequent increase in T-cell subsets after combination treatment. Lastly, T-cell expansion was also observed after combination therapy95.
Ipilimumab is a human IgG1 anti-CTLA-4 monoclonal antibody that was investigated in combination with BV in patients with R/R cHL. In the phase 1 E4412 Eastern Cooperative Oncology Group-Acrin study, patients were initially treated with BV (1.8 mg/kg) and two escalating doses of ipilimumab (1 mg/kg or 3 mg/kg). The 3 mg/kg dose was deemed safe and therefore a subsequent cohort was treated at the higher dose level. Patients received BV every 3 weeks for up to 16 cycles and ipilimumab every 3 weeks × 4 doses and then every 3 months for up to a year. The incidence of grade 3/4 toxicity was low, with one patient each developing rash, vomiting, peripheral sensory neuropathy, and thrombocytopenia. At the interim analysis of the 12 evaluable patients, the ORR was 67% with a CRR of 42%. With a median follow-up of 0.66 years, the median PFS was 0.74 years96.
Currently there are several trials investigating the role of checkpoint inhibitors as part of front line therapy either as a single agent (NCT03331731) or in combination with conventional chemotherapy (NCT03331341 & NCT03004833). It is also being studied as consolidation after front line treatment for high risk patients (NCT03033914). In the relapsed setting, checkpoint inhibitors are being compared head to head against BV (NCT02684292), as well as in combination with targeted agents such as ibrutinib (NCT02940301).
B-cell NHL
While efficacy and response rates to checkpoint inhibitors in HL have been very encouraging, in NHL clinical efficacy has been more modest. Ipilimumab was studied in 18 patients with R/R B-cell NHL. Patients were treated with ipilimumab at 3 mg/kg once and then monthly at either at 1 mg/kg × 3 months, with subsequent escalation to 3 mg/kg monthly × 4 months. Only two patients had responses; one CR in a patient with DLBCL and one PR in a patient with FL. However, responses were durable97. Given the frequent alterations at 9p24 locus in patients with PMBCL, keynote-013, a phase 1b trial in patients with R/R PMBCL, was initiated where patients were first treated with pembrolizumab 10 mg/kg every 2 weeks, but later changed to 200 mg every 3 weeks for up to 2 years. The most recent interim analysis was presented at the 14th International Conference on Malignant Lymphoma Palazzo dei Congress98. Twenty-one patients thus far have been treated. At a median follow-up duration of 14.3 months, only 4 patients experienced grade 3/4 treatment-related AEs, with neutropenia being most common (3/4 patients). Nineteen of the 21 patients were evaluable for response, with an ORR of 50% and CRR of 25%. Median DOR was not reached (range, 1.4 to 28.9 months); but DOR in patients with CR ranged from 1.4 to 27.1 months98. This has led to a multicenter phase 2 trial (Keynote-170) of pembrolizumab in patients with R/R PMBCL. At the most recent interim analysis, 49 patients have been treated. Presently, the ORR has been 41%, with a CRR of 14%. Of the patients who responded, 76% were positive for PD-L1, 3% were PD-L1 negative, and 21% had unknown PD-L1 status. At 12 months, 62% of patients were alive99.
Nivolumab has also been studied in patients with B-Cell NHL as part of the Checkmate-039 trial100. In this phase 1, open-label, dose-escalation trial, 31/81 patients had B-cell NHL (11 DLBCL, 10 FL, 10 other). Patients received nivolumab at doses of 1 or 3 mg/kg every 2 weeks for up to two years. Twenty-six percent of patients with B-cell NHL suffered grade 3/4 AEs, with the most common being pneumonitis. For patients with DLBCL the ORR was 36%, with a CRR of 18%. Median PFS was 7 weeks (95% CI; 6–29). For FL, the ORR was 40%, with a CRR of 10%. The median PFS was NR (95% CI; 7–NR). For other B-cell NHLs, there was no objective responses. Correlative studies revealed that PD-L1 expression was present on nonmalignant cells within the tumor microenvironment, and less frequently on tumor cells100. As part of the Checkmate-039 trial, a cohort of patients received combination of ipilimumab + nivolumab. In total, 65 patients with R/R HL, B-cell NHL, T-cell NHL, and multiple myeloma were treated. Overall, the combination was well tolerated, however there was not a significant improvement in response rates with combination therapy compared to single agent nivolumab101.
Currently, there are several trials investigating the role of checkpoint inhibitors in combination with rituximab (NCT02446457 & NCT03245021), in combination with chemoimmunotherapy (NCT02541565, NCT03259529, and NCT03366272), or in combination with lenalidomide (NCT03015896 and NCT02631577). Preliminary results from these novel treatment approaches are encouraging, demonstrating improved response rates 102,103.
T-cell NHL
Pembrolizumab has been studied in patients with R/R cutaneous T-cell lymphoma (CTCL). In a phase 2 trial, 24 patients received pembrolizumab (2 mg/kg every 3 weeks) for up to 2 years. With a median follow-up time of 40 weeks, the ORR was 38% with 1 CR and 8 PRs. Six of the responding patients had greater than 90% improvement in cutaneous disease. Responses were durable with 89% ongoing at a median duration of 32 weeks (4-46). The one-year PFS was 69%. Treatment was well tolerated, except 25% of patients experienced skin flares upon treatment initiation; all of whom had Sézary syndrome. Interestingly, in patients who experienced a skin flare, increases in IFN-γ, IL-12p40, IL-15, LIF, G-CSF, and CCL4 occurred following treatment104.
As previously mentioned, NKTCLs appear to facilitate immune escape via upregulation of the PD-1/PD-L1 axis88. A recent retrospective analysis of 7 patients with R/R NKTCLs who had failed asparaginase containing chemotherapy regimens were treated with salvage pembrolizumab. This resulted in objective responses for each patient, with 5 patients achieving durable CRs. PD-L1 status was available in 5 of the patients, 4 of whom had high PD-L1 expression and 1 with weak expression by IHC. Of the patients with high PD-L1 expression, 3 achieved a CR105. Similar results have been observed following treatment with nivolumab106.
Nivolumab has also been studied in patients with T-cell NHL as part of the Checkmate-039 trial100. In this phase 1, open-label, dose-escalation trial, 23/81 patients had T-cell NHL [13 patients with mycosis fungoides (MF), 5 with peripheral T-cell lymphoma (PTCL), 2 with Sézary syndrome CTCL, and 3 with other non-CTCL]. Twenty-two percent of patients with T-cell NHL suffered grade 3/4 AEs, with the most common being pneumonitis. For patients with MF, the ORR was 15%, with a CRR of 0%. Median PFS was 10 weeks (95% CI; 7–35). For patients with PTCL, the ORR was 40%, with a CRR of 0%. Median PFS was 14 weeks (95% CI; 3-NR). For patients with Sézary syndrome CTCL and other non-CTCL, the ORR was 0%100.
Currently, there are several trials investigating the role of checkpoint inhibitors in combination with approved agents for T-cell lymphoma. This includes pembrolizumab with romidepsin (NCT03278782), pembrolizumab with decitabine and pralatrexate (NCT03240211), durvalumab with lenalidomide (NCT03011814), or checkpoint inhibitors with radiotherapy (NCT03385226 & NCT03235869).
The introduction of immune checkpoint inhibitors over the past decade has revolutionized the treatment of cancer. This has also been seen for the treatment of lymphoma. Future studies will need to focus on which lymphoma subtypes best respond to checkpoint inhibitors and the best indication for them. Outside of cHL, data for other subtypes of lymphomas is still small and further studies will need to be performed. Reliable biomarkers indicating which patients may respond to treatment in other subtypes of lymphoma outside of cHL need to be developed. Furthermore, when treating patients with aggressive lymphomas, historically responses are fast after the use of conventional chemo-immunotherapy. With immune checkpoint inhibitors, it may take weeks to months before a response is seen, and at that time a patient may be have worsening symptoms or be mislabeled as having progressive disease. Thus, for patients with aggressive lymphomas, future strategies that focus on combining immune checkpoint inhibitors with known or novel therapies may more effective. Also, the potential of combining checkpoint inhibitors in the setting of ASCT or Allo-SCT will also need to be evaluated, as they may be able to improve eradication of MRD in these settings.
Vaccines
One of the major advantages of treatment with immunotherapy vs. targeted therapies, is the ability to induce a durable adaptive immune response against the tumor107. While treatment with targeted therapies over time can lead to the development of resistant clones, yielding the therapy ineffective. B-cell lymphomas are clonal, forming from a single B-cell, with each cancer cell’s B-cell receptor (BCR) expressing the same immunoglobulin heavy-chain variable region. This immunoglobulin idiotype (Id) is tumor specific and is a particularly attractive target for vaccine therapy108. The interaction between the tumor microenvironment and lymphoma cells in indolent B-cell lymphomas is attractive for vaccine therapy, as indolent lymphomas are particularly dependent on signaling by tumor-infiltrating immune cells for survival and growth. For example, follicular lymphomas have been found to be dependent on anti-apoptotic signaling from CD40+ dendritic cells (DCs)109. Thus, vaccination strategies that can augment signaling of tumor-infiltrating immune cells within the tumor microenvironment have the potential to provide durable responses. Moreover, after completing initial frontline therapy for indolent lymphomas, most patients will have a long enough remission that their immune system will have time to recover. It is at this point while burden of disease is low, and immune reconstitution has occurred, that vaccination strategies would be ideal.
Thus far most vaccine approaches for the treatment of lymphoma have been directed against Id antigens. Id peptides or whole Id determinants have been used to vaccinate patients as solely DNA or protein-based vaccines, or alternatively as DNA or proteins loaded into DC vaccines110. The difference between the two approaches, is that DNA or protein-based vaccines will illicit DC responses in vivo after vaccination, while DC loaded vaccines ex vivo allow for the generation of tumor antigen specific DCs, that activate the immune system upon injection in vivo110.
DNA-based vaccines
DNA vaccines offer several advantages because multiple tumor antigens can be included into one vaccine, overall low cost to construct, and straight-forward production. For Id DNA-based vaccines, the immunoglobulin chains are clones and inserted into a plasmid vector for injection. The specific antigen is presented on local APCs, and DCs are recruited, helping generate an adaptive immune response. While initial clinical trials with DNA-based vaccines demonstrated them to be safe, feasible, with modest anti-tumor immune responses, there was no clinical benefit attained111. Currently, approaches have focused on conjugating the Id sequence to immunostimulatory sequences to enhance DC chemotaxis and antigen presentation. This has included fusing it with the tetanus toxin, cytokine producing gene, chemokines, and viral proteins112.
Protein-based vaccines
Initially, protein-based vaccines were challenging to make because they were patient specific, thus requiring a personalized vaccine product. However, advances in hybridoma technology, where the Id protein is generated by fusing lymphoma cells with mouse myeloma cells to create Id-producing hybridomas helped overcome these challenges. Alternative strategies have also included cloning tumor specific Id genes into cell lines that will subsequently produce the protein108. Since the Id protein is weakly immunogenic by itself, investigators have chemically coupled it with keyhole limpet hemocyanin (KLH) and co-administered it with granulocyte-macrophage colony-stimulating-factor (GM-CSF) to enhance immunogenicity. This strategy has been found to be successful in producing tumor-specific adaptive immune responses in both preclinical and early phase clinical trials113. These early promising results lead to three phase 3 randomized control trials of Id-KLH peptide-based vaccines in combination with GM-CSF for patients with FL after treatment with initial frontline chemo-immunotherapy.
Levy et al.114 studied a recombinant Id peptide-based vaccine, MyVax, in 287 patients with advanced stage FL after receiving 8 cycles of CHOP. Patients achieving a PR or CR were randomized 2:1 to 7 months of MyVax, where the primary end of point of the study was to assess PFS. At a median follow-up of 58 months, median PFS was 19.1 months and 23.3 months (HR 0.98; 95% CI, 0.72–1.33) in the experimental vs. the control arms respectively. However, in patients with humoral immune responses observed (41%), the median PFS was significantly longer (40 months) (Table 3). Freedman et al.115 studied a recombinant Id-KLH peptide-based vaccine, mitumprotimut-T, in 349 patients with either treatment-naive or relapsed/refractory disease achieving a complete response (CR), partial response (PR), or stable disease (SD) after receiving four weekly rituximab infusions. Patients were then randomized to either receive mitumprotimut-T + GM-CSF or placebo + GM-CSF until disease progression. The primary end point was TTP. The median TTP was 9 months for mitumprotimut-T/GM-CSF and 12.6 months for placebo/GM-CSF (HR = 1.384; P = 0.019). Lastly, Schuster et al.116 studied a hybridoma derived Id-KLH peptide-based vaccine in 177 patients with advanced stage FL who achieved a CR after receiving frontline prednisone, doxorubicin, cyclophosphamide, and etoposide (PACE) chemotherapy.
3.
Summary of key vaccine therapy clinical trials for treatment of lymphoma
| Study | Lymphoma subtype | Vaccine type | Induction therapy | Patients (n) | Immune response | Efficacy |
| Levy et al.114 | Untreated FL | Protein-based; Id-KLH + GM-CSF vs. KLH + GM-CSF | CVP | 287 | Anti-Id humoral responses in 41% of treated patients. | Median PFS 19.1 months (experimental) vs. 23.3 months (control) |
| Freedman
et al.115 |
Untreated FL | Protein-based; Id-KLH + GM-CSF vs. placebo + GM-CSF | Rituximab | 349 | N/A | Median TTP 9 months (experimental) vs. 12.6 months (control) (HR = 1.384; P = 0.019) |
| Schuster et al.116 | Untreated FL | Protein-based; Id-KLH + GM-CSF vs. KLH + GM-CSF | PACE | 177 | IgM isotype vaccines compared to IgG isotypes improves survival (52.9 vs.28.7 months, P = 0.001) | Median DFS 44.2 months (experimental), vs. 30.6 months (control) (HR, 0.62; P = 0.047) |
| Di NiCola et al.119 | R/R indolent B-cell NHL | DC loaded killed autologous tumor cell vaccine | N/A | 18 | Humoral responses against lymphoma antigens. Decrease in regulatory T-cells and increase in cytotoxic NK cells | ORR 33%, with 3 CRs |
| Brody et al.126 | R/R indolent B-cell NHL | In situ vaccination with CpG DNA + XRT | N/A | 15 | Vaccination resulted in the development of tumor-reactive memory CD8+ T-cells | ORR 27%, with 1 CR |
| Kim et al.127 | R/R CTCL | In situ vaccination with CpG DNA + XRT | N/A | 15 | Immunized sites appeared to demonstrate a reduction in infiltration of regulatory T-cells | 5 clinical responses noted |
Patients were randomized 2:1 to receive either vaccination with Id-KLH/GM-CSF or control (KLH/GM-CSF). The primary endpoint was disease free survival (DFS). At a median follow-up of 56.6 months, median DFS arm for vaccine was 44.2 months and 30.6 months for the control arm (HR, 0.62; 95% CI, 0.39 to 0.99; P = 0.047). Patients who were treated with IgM isotype vaccines compared to IgG isotypes had better outcomes (52.9 vs. 28.7 months; P = 0.001). Of the three trials, only Schuster et al.116 found a survival benefit with vaccination. This might be because patients who were vaccinated in this trial were in a CR prior to vaccination. Thus, significant tumor burden may interfere with developing an adequate adaptive immune response after vaccination, and thus treatment strategies to employ Id-KLH peptide-based vaccines to treat MRD instead of active disease may have better success. Furthermore, using hybridomas and IgM isotypes to construct a peptide-based vaccine may also be important, as this may produce more immunogenic vaccines. Preclinical data has supported this approach as Id IgM isotypes are more immunogenic, while class switching to IgG can yield tolerance116.
DC-based vaccines
To improve protein-based vaccines, researchers began investigating methods to load antigen presenting DCs ex vivo with TAA’s, then stimulate them, and subsequently inoculate hosts. DCs are isolated from peripheral blood monocytes and differentiated by a combination of cytokines. The source of TAAs has varied, and has included tumor derived peptides, proteins, whole tumor lysates, or apoptotic bodies110. Initial studies using Id protein-based and tumor lysate pulsed dendritic cell vaccination were able to illicit significant T- and B-cell adaptive immune responses with durable clinical benefit for patients with B- and T-cell NHL117-119. Di Nicola et al.119 treated 18 patients with relapsed indolent B-cell NHL with a DC loaded killed autologous tumor cell vaccine. The ORR was 33%, with 3 CRs, and only 4 patients with disease progression. Interestingly, specific humoral responses against lymphoma antigens were detected in responding patients, with an overall decrease in regulatory T-cells and increase in cytotoxic NK cells. Approaches to improve upon DC-based vaccines include fusion of DCs with tumor cells, loading DCs with tumor mRNA, as well as enhanced antigen presenting tumor cell vaccination120-122.
In situ vaccination
While DC loaded ex vivo vaccines appear to be more immunogenic with promising response rates, they are still incumbered by the fact that they require cell processing. Toll-like-receptor (TLR) agonists are known to stimulate innate immunity, enhancing antigen presentation by DCs, that can subsequently lead to the activation of the adaptive immune system123. CpG oligodeoxynucleotides (CpG DNA) is a TLR-9 agonist, that has been demonstrated to have robust anti-lymphoma activity in preclinical models both in combination with DC tumor-antigen loaded vaccines and in combination with chemotherapy with injected intra-tumorally. (in situ vaccination)124,125. In situ vaccination has the advantage that it provides tumor antigens to antigen-presenting cells, in vivo, allowing APCs to subsequently present to endogenous T-cells. Furthermore, it does not require patient specific cell processing, which can be costly and time-intensive. Brody et al.126 treated 15 patients with relapsed indolent B-cell NHL with local radiation followed by a single intra-tumoral injection of CpG DNA. Overall, treatment was well tolerated. The ORR was 27%, with one CR. Correlative analysis demonstrated that vaccination resulted in the development of tumor-reactive memory CD8+ T-cells. Similarly, Kim et al.127 used the same approach to study in situ vaccination in 15 patients with relapsed MF. Five clinical responses were noted, with responses both at the primary and at distant sites. Interestingly, immunized sites appeared to demonstrate a reduction in infiltration of regulatory T-cells. More recently, in situ vaccination strategies using The Stimulator of Interferon Gene (STING) agonists, in combination with ibrutinib, and in combination with inhibitors of PD-1 and OXO-40 have been found to have significant anti-lymphoma activity in preclinical models128-131.
In situ vaccination strategies have also been used for the treatment of MRD during transplantation. Chu et al.132 studied the use of immunotransplant in patients with MCL. In this approach prior to treatment, patient’s lymphoma cells are collected, a patient-specific vaccine is created by incubating fresh tumor cells with CpG DNA, and then cryopreserved. Patients then receive standard chemoimmunotherapy, and those that at least achieve a PR subsequently receive 3 sequential subcutaneous autologous tumor vaccinations mixed with CpG DNA. Vaccine primed T-cells are then harvested. After ASCT, patients receive primed T-cells and a 4th vaccination, followed by a 5th vaccination at 3 months after ASCT. In the interim analysis of the initial phase 1/2 clinical trial, 24 patients had been treated, with a 1-year after ASCT freedom from MRD of 90.5%. The 3-year PFS and OS at interim analysis are 54.5% and 63.6%, respectively. Investigators found that higher expression of co-stimulatory molecules on patient’s lymphoma cells after treatment with CpG DNA was associated with improved freedom from MRD (P =0.02).
The safety and feasibility of vaccine therapy for the treatment of lymphoma has been demonstrated over the past 1–2 decades. Research have thus far demonstrated indolent lymphomas as the ideal subtype, with a focus of improving eradication of MRD in patients with CRs. The optimal vaccination strategy has not yet been identified, although in situ vaccination strategies that do not require ex vivo cell processing are demonstrating promising results. Combining vaccine therapies with immune checkpoint inhibitors to enhance anti-tumor response is a promising approach. This has been successfully studied in preclinical models, and is now being explored in early phase clinic trials (NCT03121677)108. Furthermore, enhancing immunogenicity of vaccines by either targeting tumor-draining lymph nodes, or using nano-particle vaccine delivery systems is also currently being investigated133.
Conclusions
Currently, the treatment for patients with lymphoma is undergoing a revolutionary shift. As more immunotherapeutic approaches become available, the use and need for conventional chemotherapy will continue to decline. The introduction of CD19-targeted CAR T-cell therapies, bispecific antibodies, and immune checkpoint inhibitors for R/R lymphomas have improved outcomes for chemotherapy refractory patients. However, even with treatment with CD19 CAR T-cells, only about half of patients will develop a CR. In contrast to treatment with chemotherapy however, patients who have PR or SD with immunotherapy can have meaningful and durable clinical benefit. Future studies will need to focus on novel targets, as well as combining current immunotherapeutic approaches to optimize treatment for patients with lymphoma. How to sequence treatment with the various immunotherapies will also need to be elucidated. Lastly, a focus on genetic and molecular biomarkers of a patient’s lymphoma will likely help guide future clinical trials and treatment choices.
Acknowledgements
This work was supported by NIH grants (Grant No. R01 CA136934, and R01 CA193167).
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Zhou Z, Sehn LH, Rademaker AW, Gordon LI, LaCasce AS, Crosby-Thompson A, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837–42. doi: 10.1182/blood-2013-09-524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moccia AA, Donaldson J, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, et al. International Prognostic Score in advanced-stage Hodgkin's lymphoma: altered utility in the modern era. J Clin Oncol. 2012;30:3383–8. doi: 10.1200/JCO.2011.41.0910. [DOI] [PubMed] [Google Scholar]
- 3.Beaven AW, Diehl LF. Peripheral T-cell lymphoma, NOS, and anaplastic large cell lymphoma. Hematology Am Soc Hematol Educ Program. 2015;2015:550–8. doi: 10.1182/asheducation-2015.1.550. [DOI] [PubMed] [Google Scholar]
- 4.Gisselbrecht C, Glass B, Mounier N, Gill DS, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–90. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–8. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salles G, Barrett M, FoàR , Maurer J, O’Brien S, Valente N, et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34:2232–73. doi: 10.1007/s12325-017-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feugier P, Van Hoof A, Sebban C, Solal-Celign P, Bouabdallah R, Fermé C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23:4117–26. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 8.Pfreundschuh M, Trümper L, Österborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–91. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 9.Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–32. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 10.Schulz H, Bohlius J, Skoetz N, Trelle S, Kober T, Reiser M, et al. Chemotherapy plus Rituximab versus chemotherapy alone for B-cell non-Hodgkin's lymphoma. Cochrane Database Syst Rev. 2007;17:CD003805. doi: 10.1002/14651858.CD003805.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batlevi CL, Matsuki E, Brentjens RJ, Younes A. Novel immunotherapies in lymphoid malignancies. Nat Rev Clin Oncol. 2016;13:25–40. doi: 10.1038/nrclinonc.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90:720–4. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol. 2018;15:31–46. doi: 10.1038/nrclinonc.2017.128. [DOI] [PubMed] [Google Scholar]
- 14.Hollyman D, Stefanski J, Przybylowski M, Bartido S, Borquez-Ojeda O, Taylor C, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother. 2009;32:169–80. doi: 10.1097/CJI.0b013e318194a6e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–86. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 16.Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13:5426–35. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 17.Kochenderfer JN, Feldman SA, Zhao YB, Xu H, Black MA, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savoldo B, Ramos CA, Liu EL, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–6. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brocker T, Karjalainen K. Signals through T cell receptor-zeta chain alone are insufficient to prime resting T lymphocytes. J Exp Med. 1995;181:1653–9. doi: 10.1084/jem.181.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brocker T. Chimeric Fv-zeta or Fv-epsilon receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood. 2000;96:1999–2001. [PubMed] [Google Scholar]
- 21.Chen LP, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–42. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–90. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawalekar OU, O'Connor RS, Fraietta JA, Guo LL, McGettigan SE, Posey Jr AD, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44:380–90. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Zhao ZG, Condomines M, Van Der Stegen SJC, Perna F, Kloss CC, Gunset G, et al. structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28:415–28. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication . Mol Ther. 2010;18:413–20. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Till BG, Jensen MC, Wang JJ, Qian XJ, Gopal AK, Maloney DG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940–50. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hombach AA, Rappl G, Abken H. Arming cytokine-induced killer cells with chimeric antigen receptors: CD28 outperforms combined CD28-OX40 "super-stimulation". Mol Ther. 2013;21:2268–77. doi: 10.1038/mt.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeku OO, Brentjens RJ. Armored CAR T-cells: utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumour efficacy. Biochem Soc Trans. 2016;44:412–8. doi: 10.1042/BST20150291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71:5697–706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 30.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–5. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley AS, et al. TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol Ther Nucleic Acids. 2013;2:e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain MD, Davila ML. Concise review: emerging principles from the clinical application of chimeric antigen receptor T Cell therapies for B Cell malignancies. Stem Cells. 2018;36:3644. doi: 10.1002/stem.2715. [DOI] [PubMed] [Google Scholar]
- 33.Wang XY, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 2016;3:16015. doi: 10.1038/mto.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, et al. Chimeric antigen receptor T Cells in refractory B-Cell lymphomas. N Engl J Med. 2017;377:2545–54. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Primary analysis of juliet: a global, pivotal, phase 2 trial of CTL019 in adult patients with relapsed or refractory diffuse large B-Cell lymphoma. Blood. 2017;130:577. [Google Scholar]
- 36.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RPT, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–9. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kochenderfer JN, Somerville RPT, Lu TY, Yang JC, Sherry RM, Feldman SA, et al. Long-duration complete remissions of diffuse large B Cell lymphoma after anti-CD19 chimeric antigen receptor T Cell therapy. Mol Ther. 2017;25:2245–53. doi: 10.1016/j.ymthe.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC, Hosing CM, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 Anti-CD19 CAR T Cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25:285–95. doi: 10.1016/j.ymthe.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-Cell Therapy in refractory large B-Cell lymphoma. N Engl J Med. 2017;377:2531–44. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Locke f, Neelapu S, Bartlett N, et al. Primary results from ZUMA-1: a pivotal trial of axicabtagene ciloleucel (axicel; KTE-C19) in patients with refractory aggressive non-Hodgkin lymphoma (NHL)//Proceedings of the American Association for Cancer Research Annual Meeting 2017. Washington, DC: AACR. 2017.
- 42.https://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM581226.pdf.
- 43.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobsen ED, et al. Long-term follow-up ZUMA-1: a pivotal trial of axicabtagene ciloleucel (Axi-Cel; KTE-C19) in patients with refractory aggressive non-Hodgkin lymphoma (NHL) Blood. 2017;130:577. [Google Scholar]
- 44.Vranic S, Ghosh N, Kimbrough J, Bilalovic N, Bender R, Arguello D, et al. PD-L1 status in refractory lymphomas. PLoS One. 2016;11:e0166266. doi: 10.1371/journal.pone.0166266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Locke FL, Westin JR, Miklos DB, Herrara AF, Jacobson CA, Lee J, et al. Phase 1 results from ZUMA-6: axicabtagene ciloleucel (axi-cel; KTE-C19) in combination with atezolizumab for the treatment of patients with refractory diffuse large B Cell lymphoma (DLBCL) Blood. 2017;130:2826. [Google Scholar]
- 46.Makita S, Yoshimura K, Tobinai K. Clinical development of anti-CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. Cancer Sci. 2017;108:1109–18. doi: 10.1111/cas.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, et al. Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells . Sci Transl Med. 2016;8:355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo . Leukemia. 2016;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Arnason JE, Wang M, et al. High durable CR rates in Relapsed/Refractory (R/R) aggressive B-NHL treated with the CD19-Directed CAR T Cell Product JCAR017 (TRANSCEND NHL 001): defined composition allows for dose-finding and definition of pivotal cohort. Blood. 2017;130:581. [Google Scholar]
- 50.Swanson C, Do T, Merrigan S, et al. Predicting clinical response and safety of JCAR017 in B-NHL patients: potential importance of tumor microenvironment biomarkers and CAR T-Cell tumor infiltration//JUNO Therapeutics at the 59th Ash Annual Meeting and Exposition. Atlanta, GA: JUNO. 2017.
- 51.Siddiqi T, Abramson JS, Li D, et al. Patient characteristics and Pre-Infusion biomarkers of inflammation correlate with clinical outcomes after treatment with the defined composition, CD19-Targeted CAR T Cell Product, JCAR017//JUNO Therapeutics at the 59th Ash Annual Meeting and Exposition. Atlanta, GA: JUNO. 2017.
- 52.Velasquez MP, Bonifant CL, Gottschalk S. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood. 2018;131:30–8. doi: 10.1182/blood-2017-06-741058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staerz UD, Bevan MJ. Activation of resting T lymphocytes by a monoclonal antibody directed against an allotypic determinant on the T cell receptor. Eur J Immunol. 1986;16:263–70. doi: 10.1002/eji.1830160310. [DOI] [PubMed] [Google Scholar]
- 54.Staerz UD, Bevan MJ. Hybrid hybridoma producing a bispecific monoclonal antibody that can focus effector T-cell activity. Proc Natl Acad Sci USA. 1986;83:1453–7. doi: 10.1073/pnas.83.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol. 2015;93:290–6. doi: 10.1038/icb.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goebeler ME, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, et al. Bispecific T-Cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with Relapsed/Refractory non-hodgkin lymphoma: final results from a phase I study. J Clin Oncol. 2016;34:1104–11. doi: 10.1200/JCO.2014.59.1586. [DOI] [PubMed] [Google Scholar]
- 57.Viardot A, Goebeler ME, Hess G, Neumann S, Pfreundschuh M, Adrian N, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127:1410–6. doi: 10.1182/blood-2015-06-651380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schuster FR, Stanglmaier M, Woessmann W, Winkler B, Siepermann M, Meisel R, et al. Immunotherapy with the trifunctional anti-CD20 x anti-CD3 antibody FBTA05 (Lymphomun) in paediatric high-risk patients with recurrent CD20-positive B cell malignancies. Br J Haematol. 2015;169:90–102. doi: 10.1111/bjh.13242. [DOI] [PubMed] [Google Scholar]
- 59.Lum LG, Thakur A, Liu Q, Deol A, Al-Kadhimi Z, Ayash L, et al. CD20-targeted T cells after stem cell transplantation for high risk and refractory non-Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2013;19:925–33. doi: 10.1016/j.bbmt.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lum LG, Thakur A, Pray C, Kouttab C, Kouttab N, Abedi M, et al. Multiple infusions of CD20-targeted T cells and low-dose IL-2 after SCT for high-risk non-Hodgkin's lymphoma: a pilot study. Bone Marrow Transplant. 2014;49:73–9. doi: 10.1038/bmt.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ilcus C, Bagacean C, Tempescul A, Popescu C, Parvu A, Cenariu M, et al. Immune checkpoint blockade: the role of PD-1-PD-L axis in lymphoid malignancies. Onco Targets Ther. 2017;10:2349–63. doi: 10.2147/OTT.S133385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dunn-Pirio AM, Vlahovic G. Immunotherapy approaches in the treatment of malignant brain tumors. Cancer. 2017;123:734–50. doi: 10.1002/cncr.30371. [DOI] [PubMed] [Google Scholar]
- 64.McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med. 2013;2:662–73. doi: 10.1002/cam4.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–8. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 66.Azuma M, Ito D, Yagita H, Okumura K, Phillips JH, Lanier LJ, et al. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366:76–9. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 67.Freeman GJ, Gribben JG, Boussiotis VA, Ng JW, Restivo Jr VA, Lombard LA, et al. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993;262:909–11. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 68.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- 69.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 71.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4 . Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 72.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–25. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor . Immunity. 1999;11:141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 75.Dong HD, Zhu GF, Tamada K, Chen LP. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 76.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 77.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–5. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 79.Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71:5393–9. doi: 10.1158/0008-5472.CAN-11-0993. [DOI] [PubMed] [Google Scholar]
- 80.Barber DL, Wherry EJ, Masopust D, Zhu BG, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 81.Kwon D, Kim S, Kim PJ, Go H, Nam SJ, Paik JH, et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology. 2016;68:1079–89. doi: 10.1111/his.12882. [DOI] [PubMed] [Google Scholar]
- 82.Green MR, Rodig S, Juszczynski P, Ouyang J, Sinha P, O'Donnell E, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18:1611–8. doi: 10.1158/1078-0432.CCR-11-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quan LN, Chen X, Liu AC, Zhang Y, Guo XC, Yan SJ, et al. PD-1 blockade can restore functions of T-Cells in epstein-barr virus-positive diffuse large B-Cell lymphoma in vitro . PLoS One. 2015;10:e0136476. doi: 10.1371/journal.pone.0136476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bentz M, Barth TFE, Brüderlein S, Bock D, Schwerer MJ, Baudis M, et al. Gain of chromosome arm 9p is characteristic of primary mediastinal B-cell lymphoma (MBL): comprehensive molecular cytogenetic analysis and presentation of a novel MBL cell line. Genes Chromosomes Cancer. 2001;30:393–401. doi: 10.1002/1098-2264(2001)9999:9999<::aid-gcc1105>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 85.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–77. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, Connelly CF, et al. PD-L1 and PD-L2 genetic alterations define classical hodgkin lymphoma and predict outcome . J Clin Oncol. 2016;34:2690–7. doi: 10.1200/JCO.2016.66.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chapuy B, Roemer MG, Stewart C, Tan YX, Abo RP, Zhang LY, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127:869–81. doi: 10.1182/blood-2015-10-673236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bi XW, Wang H, Zhang WW, Wang JH, Liu WJ, Xia ZJ, et al. PD-L1 is upregulated by EBV-driven LMP1 through NF-κB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J Hematol Oncol. 2016;9:109. doi: 10.1186/s13045-016-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dada R. Program death inhibitors in classical Hodgkin's lymphoma: a comprehensive review. Ann Hematol. 2018;97:555–61. doi: 10.1007/s00277-017-3226-0. [DOI] [PubMed] [Google Scholar]
- 90.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–94. doi: 10.1016/S1470-2045(16)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kasamon YL, De Claro RA, Wang YP, Shen YL, Farrell AT, Pazdur R. FDA approval summary: nivolumab for the treatment of relapsed or progressive classical hodgkin lymphoma. Oncologist. 2017;22:585–91. doi: 10.1634/theoncologist.2017-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Armand P, Shipp MA, Ribrag V, Michot JM, Zinzani PL, Kuruvilla J, et al. Programmed Death-1 blockade with pembrolizumab in patients with classical hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol. 2016;34:3733–9. doi: 10.1200/JCO.2016.67.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic hodgkin lymphoma. J Clin Oncol. 2017;35:2125–32. doi: 10.1200/JCO.2016.72.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Herrera AF, Moskowitz A, Bartlett N, Vose JM, Ramchandren R, Feldman TA, et al. Interim results from a phase 1/2 study of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory hodgkin lymphoma. Blood. 2017;35:85–6. doi: 10.1182/blood-2017-10-811224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Diefenbach CS, Hong FX, Cohen J, Robertson MJ, Ambinder RF, Fenske TS, et al. Preliminary safety and efficacy of the combination of brentuximab vedotin and ipilimumab in relapsed/refractory hodgkin lymphoma: a trial of the ECOG-ACRIN cancer research group (E4412) Blood. 2015;126:585. [Google Scholar]
- 97.Ansell SM, Hurvitz SA, Koenig PA, LaPlant BR, Kabat BF, Fernando D, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15:6446–53. doi: 10.1158/1078-0432.CCR-09-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zinzani P, Ribrag V, Moskowitz CH, Michot J, Kuruvilla J, Bartlett N, et al. Phase 1B study of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-Cell lymphoma (rrPMBCL): updated results from the keynote-013 trial. Hematol Oncol. 2017;35:189–90. [Google Scholar]
- 99.Zinzani PL, Thieblemont C, Melnichenko V, et al. Efficacy and safety of pembrolizumab in relapsed/refractory primary mediastinal large B-Cell Lymphoma (rrPMBCL): updated analysis of the keynote-170 phase 2 trial//Proceedings from the 59th Annual ASH Meeting and Exposition. Atlanta, Georgia. Blood. 2017; 130: 2833.
- 100.Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34:2698–704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ansell S, Gutierrez ME, Shipp MA, Gladstone D, Moskowitz A, Borello I, et al. A phase 1 study of nivolumab in combination with ipilimumab for relapsed or refractory hematologic malignancies (CheckMate 039) Blood. 2016;128:183. [Google Scholar]
- 102.Nastoupil LJ, Westin JR, Fowler NH, Fanale MA, Samaniego F, Oki Y, et al. Response rates with pembrolizumab in combination with rituximab in patients with relapsed follicular lymphoma: interim results of an on open-label, phase II study. J Clin Oncol. 2017;35:7519. [Google Scholar]
- 103.Younes A, Burke JM, Diefenbach CS, Ferrari S, Sharman JP, Tani M, et al. Safety and efficacy of atezolizumab in combination with obinutuzumab and bendamustine in patients with previously untreated follicular lymphoma: an interim analysis//Proceedings of 2017 ASH Annual Meeting. Atlanta, GA: McKesson Specialty Health. 2017.
- 104.Khodadoust M, Rook AH, Porcu P, Foss FM, Moskowitz AJ, Shustov AR, et al. Pembrolizumab for treatment of relapsed/refractory mycosis fungoides and sezary syndrome: clinical efficacy in a citn multicenter phase 2 study. Blood. 2016;128:181. [Google Scholar]
- 105.Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing L-asparaginase. Blood. 2017;129:2437–42. doi: 10.1182/blood-2016-12-756841. [DOI] [PubMed] [Google Scholar]
- 106.Chan TSY, Li J, Loong F, Khong PL, Tse E, Kwong YL. PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing L-asparaginase: efficacy and safety. Ann Hematol. 2018;97:193–6. doi: 10.1007/s00277-017-3127-2. [DOI] [PubMed] [Google Scholar]
- 107.Zappasodi R, De Braud F, Di Nicola M. Lymphoma immunotherapy: current status. Front Immunol. 2015;6:448. doi: 10.3389/fimmu.2015.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Allegra A, Russo S, Gerace D, Calabrò L, Maisano V, Innao V, et al. Vaccination strategies in lymphoproliferative disorders: failures and successes. Leuk Res. 2015;39:1006–19. doi: 10.1016/j.leukres.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 109.Goval JJ, Thielen C, Bourguignon C, Greimers R, Dejardin E, Choi YS, et al. The prevention of spontaneous apoptosis of follicular lymphoma B cells by a follicular dendritic cell line: involvement of caspase-3, caspase-8 and c-FLIP. Haematologica. 2008;93:1169–77. doi: 10.3324/haematol.12127. [DOI] [PubMed] [Google Scholar]
- 110.Brody J, Kohrt H, Marabelle A, Levy R. Active and passive immunotherapy for lymphoma: proving principles and improving results. J Clin Oncol. 2011;29:1864–75. doi: 10.1200/JCO.2010.33.4623. [DOI] [PubMed] [Google Scholar]
- 111.Timmerman JM, Singh G, Hermanson G, Hobart P, Czerwinski DK, Taidi B, et al. Immunogenicity of a plasmid DNA vaccine encoding chimeric idiotype in patients with B-cell lymphoma. Cancer Res. 2002;62:5845–52. [PubMed] [Google Scholar]
- 112.Meleshko AN, Petrovskaya NA, Savelyeva N, Vashkevich KP, Doronina SN, Sachivko NV. Phase I clinical trial of idiotypic DNA vaccine administered as a complex with polyethylenimine to patients with B-cell lymphoma. Hum Vaccin Immunother. 2017;13:1398–403. doi: 10.1080/21645515.2017.1285477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park HJ, Neelapu SS. Developing idiotype vaccines for lymphoma: from preclinical studies to phase III clinical trials. Br J Haematol. 2008;142:179–91. doi: 10.1111/j.1365-2141.2008.07143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Levy R, Ganjoo KN, Leonard JP, Vose JM, Flinn IW, Ambinder RF, et al. Active idiotypic vaccination versus control immunotherapy for follicular lymphoma. J Clin Oncol. 2014;32:1797–803. doi: 10.1200/JCO.2012.43.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Freedman A, Neelapu SS, Nichols C, Robertson MJ, Djulbegovic B, Winter JN, et al. Placebo-controlled phase III trial of patient-specific immunotherapy with mitumprotimut-T and granulocyte-macrophage colony-stimulating factor after rituximab in patients with follicular lymphoma. J Clin Oncol. 2009;27:3036–43. doi: 10.1200/JCO.2008.19.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, et al. Vaccination With Patient-Specific Tumor-Derived Antigen in First Remission Improves Disease-Free Survival in Follicular Lymphoma. J Clin Oncol. 2011;29:2787–94. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maier T, Tun-Kyi A, Tassis A, Jungius KP, Burg G, Dummer R, et al. Vaccination of patients with cutaneous T-cell lymphoma using intranodal injection of autologous tumor-lysate-pulsed dendritic cells. Blood. 2003;102:2338–44. doi: 10.1182/blood-2002-08-2455. [DOI] [PubMed] [Google Scholar]
- 118.Timmerman JM, Czerwinski DK, Davis TA, Hsu FJ, Benike C, Hao ZM, et al. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99:1517–26. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- 119.Di Nicola M, Zappasodi R, Carlo-Stella C, Mortarini R, Pupa SM, Magni M, et al. Vaccination with autologous tumor-loaded dendritic cells induces clinical and immunologic responses in indolent B-cell lymphoma patients with relapsed and measurable disease: a pilot study. Blood. 2009;113:18–27. doi: 10.1182/blood-2008-06-165654. [DOI] [PubMed] [Google Scholar]
- 120.Wang JL, Saffold S, Cao XT, Krauss J, Chen Wei. Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. J Immunol. 1998;161:5516–24. [PubMed] [Google Scholar]
- 121.Ni X, Richmond HM, Liao XM, Decker WK, Shiue LH, Shpall EJ, et al. Induction of T-cell responses against cutaneous T-cell lymphomas ex vivo by autologous dendritic cells transfected with amplified tumor mRNA. J Invest Dermatol. 2008;128:2631–9. doi: 10.1038/jid.2008.125. [DOI] [PubMed] [Google Scholar]
- 122.Biagi E, Rousseau R, Yvon E, Schwartz M, Dotti G, Foster A, et al. Responses to human CD40 ligand/human interleukin-2 autologous cell vaccine in patients with B-cell chronic lymphocytic leukemia. Clin Cancer Res. 2005;11:6916–23. doi: 10.1158/1078-0432.CCR-05-0484. [DOI] [PubMed] [Google Scholar]
- 123.Holtick U, Scheulen ME, Von Bergwelt-Baildon MS, Weihrauch MR. Toll-like receptor 9 agonists as cancer therapeutics. Expert Opin Investig Drugs. 2011;20:361–72. doi: 10.1517/13543784.2011.553187. [DOI] [PubMed] [Google Scholar]
- 124.Horkheimer I, Quigley M, Zhu JG, Huang XP, Chao NJ, Yang YP. Induction of type I IFN is required for overcoming tumor-specific T-cell tolerance after stem cell transplantation. Blood. 2009;113:5330–9. doi: 10.1182/blood-2008-05-155150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li JL, Song WR, Czerwinski DK, Varghese B, Uematsu S, Akira S, et al. Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. J Immunol. 2007;179:2493–500. doi: 10.4049/jimmunol.179.4.2493. [DOI] [PubMed] [Google Scholar]
- 126.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324–32. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim YH, Gratzinger D, Harrison C, Brody JD, Czerwinski DK, Ai WZ, et al. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood. 2012;119:355–63. doi: 10.1182/blood-2011-05-355222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sallets A, Kardosh A, Robinson A, et al. In-situ vaccination using sting agonists combined with immune-modulating antibodies to treat lymphoma. Blood. 2017;130:4102. [Google Scholar]
- 129.Hammerich L, Dhainaut M, Keler T, et al. <italic>In situ</italic> vaccination improves efficacy of PD-1 blockade in unresponsive lymphoma tumors through induction of a highly efficient cross-presenting dendritic cell subset expressing TLR3//Immunotherapy-Radiotherapy Conference. New York, NY: Stich Radiation Oncology Center. 2017.
- 130.Barfi IS, Czerwinski DK, Levy R. Eradication of systemic lymphoma by local immunotherapy. Blood. 2017;130:113. [Google Scholar]
- 131.Sagiv-Barfi I, Kohrt HE, Burckhardt L, Czerwinski DK, Levy R. Ibrutinib enhances the antitumor immune response induced by intratumoral injection of a TLR9 ligand in mouse lymphoma. Blood. 2015;125:2079–86. doi: 10.1182/blood-2014-08-593137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chu MP, Brody J, Kohrt HE, Frank MJ, Khodadoust M, Reddy S, et al. Phase I/II clinical trial of CpG-activated whole cell vaccine in mantle cell lymphoma (MCL): results in safety and efficacy from planned interim analysis. Blood. 2015;126:1536. [Google Scholar]
- 133.Jeanbart L, Ballester M, De Titta A, Corthésy P, Romero P, Hubbell JA, et al. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol Res. 2014;2:436–47. doi: 10.1158/2326-6066.CIR-14-0019-T. [DOI] [PubMed] [Google Scholar]