Abstract
Background
Adverse drug reactions (ADRs) can be divided into pharmacological ADRs (type A) and hypersensitivity reactions (type B). Type B reactions can be further subdivided into immediate (<1 h, urticaria, anaphylaxis) and delayed reactions (>1 h, variable manifestation like exanthema, hepatitis, cytopenias). Prevention of hypersensitivity is often still a challenge.
Methods
Selective literature search in Medline and Google Scholar as well as research in ADR databases like OpenVigil or SIDER.
Results
Laboratory tests ([specific] IgE, lymphocyte transformation test), histological examination, dermatological tests (prick tests, epicutaneous testing) and—under certain circumstances—provocation tests can be used for diagnostics. There are only a few pharmacogenetic biomarkers to predict hypersensitivity reactions. Currently, testing for defined HLA genes is mandatory before prescription of abacavir and before the use of carbamazepine in Han Chinese or Thai patients. Immediate discontinuation of the trigger is essential in all allergic hypersensitivity reactions. Immediate reactions are treated with antihistamines, glucocorticoids and occasionally with epinephrine. Delayed reactions are usually treated with glucocorticoids.
Conclusions
Careful, structured diagnostics in case of suspected hypersensitivity together with adequate documentation (allergy passport) is necessary in order to avoid incidents in patients receiving subsequent treatment. Consistent use of existing resources (diagnostics and documentation) can help to avoid hypersensitivity reactions or to rapidly recognize and treat them, respectively.
Drug treatment often leads to adverse events (AE). Some of these are so-called medication errors which occur due to the handling of the drug, rather than due to the drug itself (e1). Adverse drug reactions (ADR), colloquially called “side effects,” are adverse events that are due to the inherent biological effects of the drug. These, in turn, are divided into pharmacologically mediated ADR (type A) and hypersensitivity reactions (type B) (1). Type A reactions can occasionally be therapeutically useful or even lead to new indications: for example, minoxidil causes hair growth, and sildenafil has a beneficial effect on erectile dysfunction. Drug-induced liver damage is a well-known kind of type A reaction that can be caused, e.g., by an overdose of acetaminophen, whereas flucloxacillin-associated liver damage is an HLA-associated type B reaction (2). Type A reactions are generally dose-dependent, while type B reactions are generally considered to be independent of the dose once a low threshold dose has been exceeded (3).
Definition.
Adverse drug reactions, colloquially called “side effects,” are adverse events due to the inherent biological effects of the drug. These, in turn, are divided into pharmacologically mediated ADR (type A) and hypersensitivity reactions (type B)—mnemonically, A for “augmented” and B for “bizarre.”
Both classic immunological (allergic) and non-allergic hypersensitivity reactions involve activation of the immune system or of its effector pathways, such as inflammatory reactions (Table 1, Figure 1). Hypersensitivity reactions are clinically categorized as either immediate (arising less than one hour after exposure) or late (arising more than one hour after exposure). The classic allergic reactions are divided into four types, in the scheme of Coombs and Gell; types I and IV are the ones most commonly encountered.
Table 1. The classification, frequencies, mechanisms, and manifestations of undesired events, with examples and treatment options (frequencies in relation to the overall number of undesired events).
| Group | Type |
Frequency (Reference) |
Mechanism | Example |
Treatment options aside from discontinuation of the offending substance |
| Medication error | 20% (e1) | Medical appropriateness index too high, e.g., double prescription |
Prescription of the same drug with generic name and trade name |
– regular checking (computer-assisted if possible) of medications and of the patient‘s adherence to treatment (e25, e26) | |
| ADR | pharmacological (type A) |
72% (39) | PK: pharmacogenetic variants or PK-DI |
Irinotecan in carriers of the UGT1A1 variant |
– regular checking (computer-assisted if possible) of DI – therapeutic drug monitoring (TDM) |
| PD: multidimensional effects |
Cutaneous reaction to EGFR antagonists such as cetuximab |
– immune modulation with doxycycline (e29) | |||
| hypersensitivity (type B) |
6% (6) | Not allergic (pseudoallergy) |
Red man syndrome in response to vancomycin |
– H1 blockers (e.g., dimenhydrinate 62 mg i. v.) – H2 blockers (e.g., ranitidine 150 mg i. v.) – glucocorticoids (e.g., prednisolone 500 mg i. v.) – volume/norepinephrine as indicated – epinephrine (e.g., 0.5 mg i. m.) as indicated – ventilation/coniotomy as indicated |
|
| 0.4% | Type I (IgE) | Anaphylaxis in response to penicillins |
|||
| rare | Type II (IgG/IgM) | Hemolytic anemia or thrombocytopenia in response to penicillins |
– substitution of blood components | ||
| rare | Type III (IgG/IgM) | Nephritis in response to penicillins |
– glucocorticoids or other anti-inflammatory substances/immune modulators – volume |
||
| 1.6% | Type IV | DIA | – reverse isolation (protection of the patient from micro-organisms) – prophylactic antibiotic and antimycotic coverage (e.g., ampicillin + sulbactam 4 g/d + 0.5 g/d, ciprofloxacin 750 mg/d, fluconazole 200 mg/d) – growth factors such as filgrastim |
||
| DILI | – H1 blockers for pruritus | ||||
| DRESS (type IVb) | – antipyretic drugs for fever – H1 blockers for pruritus – glucocorticoids, plasmapheresis and/or high-dose intravenous immunoglobulins |
||||
| SJS/TEN (type IVc) | – reverse isolation as indicated – local treatment as an artifical cutaneous barrier, ‧possibly with the addition of glucocorticoids and antimicrobial drugs – systemic glucocorticoids, cyclosporine, intravenous ‧immunoglobulins – antibiotics if there is any evidence of infection – wound treatment analogous to that of burns (no early debridement!) – electrolyte and volume substitution – analgesia |
||||
| AGEP (type IVd), MPR | – H1 blockers for pruritus – in the early phase, glucocorticoids |
||||
ADR: adverse drug reactions, AGEP: acute generalized exanthematous pustulosis, DI: drug interactions, DIA: drug-induced agranulocytosis, DILI: drug-induced liver injury,
DIRI: drug-induced renal injury, DRESS: drug reaction with eosinophilia and systemic symptoms, EGFR: epidermal growth factor receptor,
IgG: immunglobulin G, IgM: immunglobulin M, i.m.: intramuscular; i.v.: intravenous, MPR: makulopapular rash, PD: pharmacodynamics, PK: pharmakokinetics,
SJS: Stevens-Johnson syndrome, TEN: toxic epidermal necrolysis, UGT: UDP-glucuronyltransferase
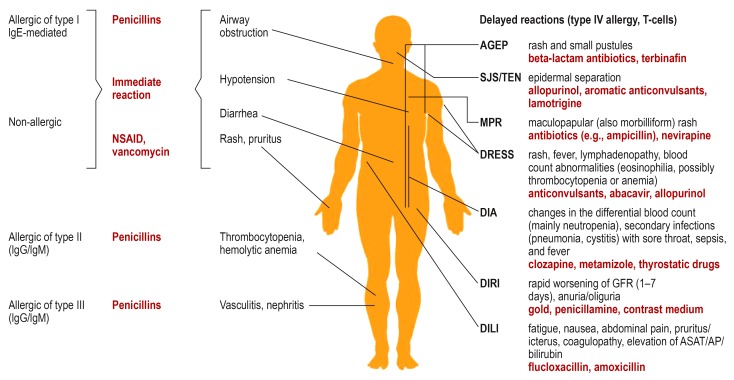
Figure 1.
Hypersensitivity reactions with their immunological classification, classical clinical entities, and examples of precipitating drugs (in red).
AGEP: acute generalized exanthematous pustulosis, AP: alkaline phosphatase, ASAT: aspartate aminotransferase, DIA: drug-induced agranulocytosis,
DILI: drug-induced liver injury, DIRI: drug-induced renal injury, DRESS: drug reaction with eosinophilia and systemic symptoms, GFR: glomerular filtration rate, IgE: immunglobulin E, IgG: immunglobulin G, IgM:immunglobulin M, MPR: maculopapular rash, NSAID: nonsteroidal anti-inflammatory drugs, SJS: Stevens-Johnson syndrome, TEN: toxic epidermal necrolysis.
Type A and type B side effects.
Drug-induced liver damage is a well-known kind of type A reaction that can be caused, e.g., by an overdose of acetaminophen, whereas flucloxacillin-associated liver damage is an HLA-associated type B reaction.
A drug may trigger very different kinds of hypersensitivity reactions across individuals, or even in the same individual (4).
The relevance of type B adverse drug reactions.
Type B adverse drug reactions comprise only a small minority of adverse events but are of high clinical relevance because of their apparent unpredictability.
Common type B adverse drug reactions.
Immediate reactions (reactions that arise within one hour) are the most common type B adverse drug reaction.
Penicillins, for example, may induce non-allergic hypersensitivity, as well as allergies of types I–IV. These different kinds of reaction can also arise simultaneously. Topical penicillin preparations are no longer on the market because of the high risk of contact allergy (10%).
Learning objectives
Immediate reactions.
Immediate reactions have variable manifestations, ranging from pruritus to edema, urticaria, and anaphylactic shock.
This article is intended to impart knowledge of:
the triggers and course of common kinds of hypersensitivity reaction;
the appropriate treatment of hypersensitivity reactions; and
strategies for the avoidance of such reactions, with the aid of phenotypic testing (laboratory tests, skin tests), pharmacogenetic testing, and desensitization.
Method
This review is based on publications retrieved by a search in Medline and other databases that contain relevant information on adverse drug reactions (eBox 1).
eBOX. Methods and search terms.
We carried out a selective literature search in MEDLINE and Google Scholar employing the following terms: “hypersensitivity,” “exanthem,” “AGEP,” “DRESS,” “SJS,” “DILI,” “MPE” combined with “symptoms,” “score,” “mortality,” “HLA,” “drug,” “etiology.”
For a search in the OpenVigil ADR database, standard search terms (standard MedDRA queries, SMQ) of the Medical Dictionary for Regulatory Activities were used: “drug reaction with eosinophilia and systemic symptoms syndrome,” “hypersensitivity,” “severe cutaneous adverse reactions,” “hepatic failure, fibrosis and cirrhosis and other liver damage-related conditions.”
For a search in SIDER, the following terms were used: “hypersensitivity,” “Stevens-Johnson syndrome,” “rash,” “anaphylactic shock.”
The classification and etiology of hypersensitivity reactions
The etiology of type I allergic reactions.
Type I allergy involves the IgE-mediated elaboration of inflammatory mediators such as histamine, heparin, tryptase, platelet-activating factor, and prostaglandins, which give rise to an inflammatory reaction.
Hypersensitivity reactions were once thought to be unpredictable, but an improved understanding of the immune system, along with data from cohort studies and pharmacovigilance, have made it possible to identify the drugs and mechanisms that are mainly responsible for such reactions, and to delineate distinct clinical syndromes (5, e2).
Immediate reactions
Immediate reactions have variable manifestations, ranging from pruritus to edema, urticaria, and anaphylactic shock.
The etiology of type I allergic reactions
Type I allergy involves the IgE-mediated elaboration of inflammatory mediators such as histamine, heparin, tryptase, platelet-activating factor (PAF), and prostaglandins, which give rise to an inflammatory reaction. Reactions of this type are typically induced by penicillins, for example (figure 1).
The etiology of non-allergic hypersensitivity reactions
Non-allergic hypersensitivity reactions account for approximately 77% of all hypersensitivity reactions (6) and can be induced by substances of many kinds, including penicillins and nonsteroidal anti-inflammatory drugs (NSAID) (figure 1) (4, e3). The triggers may induce the release of histamine from storage vesicles (vancomycin, for example) or lead to activation of the complement system (e.g., radiologic contrast dye). The number needed to harm (NNH) describes the number of persons to be exposed to a certain trigger until a reaction occurs (1/incidence). The NNH is high (>1000) for vancomycin, but lower for NSAID and morphine (NNH ˜100). Hypersensitivity reactions are much more common in response to food and food additives such as benzoates (NNH 11 in persons with allergic rhinitis) (e4) or sulfites (NNH 14–58) (e5).
The pharmacogenetics of non-allergic hypersensitivity reactions
Hypersensitivity reactions may be provoked by variants in genes being involved in the synthesis or degradation of inflammatory mediators such as bradykinin, histamine, prostaglandins, or leukotrienes, or in the activity of the corresponding receptors. The most prominent example is an asthma attack induced by a nonsteroidal anti-inflammatory drug such as diclofenac (7). Another, potentially dangerous reaction of this type is angioedema induced by ACE inhibitors. The latter reaction is associated with a genetic variant of plasma aminopeptidase (8).
Delayed reactions
Delayed reactions, too, may be due to immunologic or other reactions (efigure).
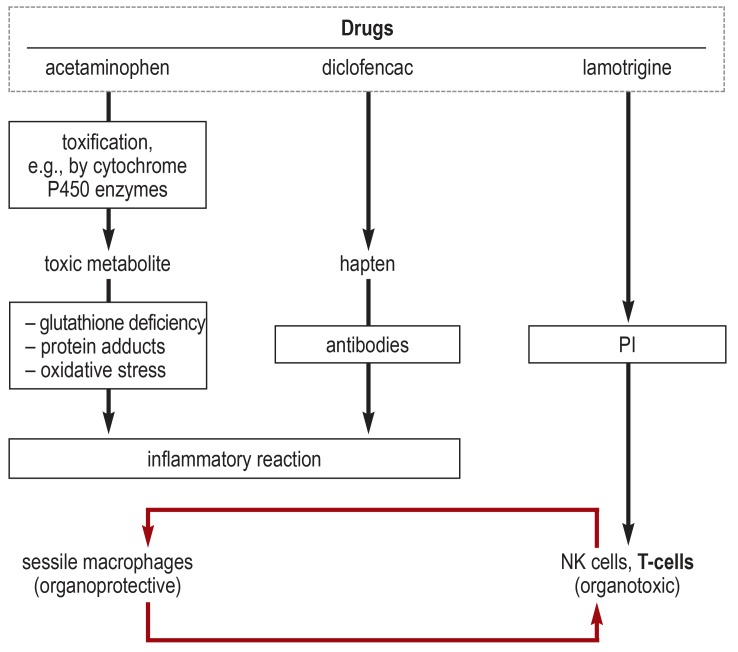
eFigure.
Mechanisms of organ damage (after [e27]).
Drugs can induce adverse drug reactions (ADR) in a variety of ways.
For example, liver damage can be caused directly by the oxidation of hepatic proteins by the toxic acetaminophen metabolite
N-acetyl-p-benzoquinonimine (NAPQI) (type A ADR). The extent of NAPQI production depends mainly on clinical factors. Cell death secondarily activates the immune system.
Diclofenac, togther with hepatic proteins, can form haptens that are recognized by antibodies (type B ADR). There is subsequent cell destruction, with an immune reaction.
Finally, some drugs can also directly activate T-cell receptors or killer-cell-immunoglobulin-like receptors (the so-called PI concept, i.e., the pharmacological interaction of drugs with immune receptors [e28]).
The pharmacogenetics of non-allergic hypersensitivity reactions.
Hypersensitivity reactions are associated with variants in the genes that play a role in the synthesis or degradation of inflammatory mediators such as bradykinin, histamine, prostaglandins, or leukotrienes or in the activity of their receptors.
The etiology of type II and III allergic reactions
In type II allergic reactions, antibodies bind to the active substance when it is bound to blood cells, thereby leading either to hemolysis or to thrombocytopenia. In type III allergic reactions, antibodies bind to the free active substance in the blood, forming immune complexes which, in turn, damage the vascular walls and glomeruli (4).
The etiology of type IV allergic reactions
Type IV allergic reactions are mediated by T-cells (figure 1). These reactions belong to subtypes a through d, depending on the participating subgroups of T cells (table 1) (9). Common syndromes include:
drug-induced agranulocytosis (DIA)
-
drug-induced skin disorders (DISI) such as:
contact allergy
fixed drug eruption (FDE)
acute, generalized exanthematic pustulosis (AGEP)
maculopapular rash (MPR), also called morbilliform rash
drug reaction with eosinophilia and systemic symptoms (DRESS)
Stevens-Johnson syndrome / Lyell syndrome (synonym: toxic epidermal necrolysis) (SJS/TEN)
drug-induced liver injury (DILI)
drug-induced renal injury (DIRI)
Contact allergies of the skin, usually consisting of contact eczema, are also type IV allergies; these can be induced, for example, by topically applied neomycin. This classic allergic reaction after obligate prior sensitization is also, to some extent, dose-dependent (3). It depends on the HLA type as well (10).
These reactions can be hard to distinguish from type A side effects. For example, glutathione deficiency may be cytotoxic, paracetamol is indirectly hepatotoxic, and clozapine can cause agranulocytosis. Even DRESS has a relevant metabolic component (efigure).
Mortality
The etiology of type IV allergic reactions.
Type IV allergic reactions are mediated by T-cells. These reactions belong to subtypes a through d, depending on the participating subgroups of T-cells.
Although delayed reactions make up only a small percentage of all undesired events, they are highly important because of their severity. Acute generalized exanthematic pustulosis, Stevens-Johnson / Lyell syndrome (synonym: toxic epidermal necrolysis) and DRESS carry a high mortality (>1%) are are therefore also called severe cutaneous reactions. The mortality of drug-induced agranulocytosis is approximately 5% (11), that of DRESS 2–10% (12, e6), that of Stevens-Johnson / Lyell syndrome approximately 34% (13), and that of drug-induced liver damage in a range from 0% to over 10% (14).
The high metabolic activity of the skin and liver presumably accounts for their vulnerability to such reactions. The skin, in particular, is constantly immunologically stimulated by pathogens and noxious substances because of its exposed position. The same can be said of the gastrointestinal mucosa, which is another preferred site for hypersensitivity reactions (cramping, diarrhea).
Pharmacogenetic biomarkers
Biomarkers (mostly human leukocyte antigens, HLA) have been identified for a number of delayed reactions. HLA genes code for proteins of the major histocompatibility complex (MHC). HLA-A, HLA-B, and HLA-C code for MHC class I proteins, while HLA-DM to HLA-DR code for MHC class II proteins that interact with T-cells. The nomenclature includes at least the following: <HLA gene>*<group>:<allele>, e.g., HLA-B*57:01.
Temporal course
Delayed reactions that take place within the body, rather than on the skin, may remain unrecognized. In patients who were not sensitized to the inducing drug at the beginning of their treatment, delayed reactions can arise after a delay of days to weeks—sometimes even after the drug has been discontinued—without causing any symptoms until then.
Triggering drugs
Antibiotics (particularly beta-lactams) and anticonvulsants are the most common triggering drugs, accounting for three-quarters of all cases of hypersensitivity (e7). Further triggers, e.g., NSAID, antiretroviral drugs, sulfonamides, and allopurinol, are listed in Figure 1 classic examples), in eTable 1 (spontaneous reports), and eTable 2 (manufacturers’ summaries of product characteristics, via SIDER).
eTable 1. Drugs that most commonly cause reported hypersensitivity reactions (Proportional Reporting Ratios)*.
| Drug | DIA | DILI | Hypersensitivity | Anaphylaxis | SCAR | DRESS |
| Abacavir | 9.6 | |||||
| Acetaminophen | 3.3 | |||||
| Allopurinol | 4.7 | 2.4 | 2.1 | 9.9 | 26.8 | |
| Amiodarone | 2.7 | |||||
| Amoxicillin | 3.3 | 3.8 | 5.6 | 10.0 | 17.2 | |
| Azathioprine | 2.2 | |||||
| Azithromycin | 2.5 | 2.5 | 3.8 | |||
| Bevacizumab | 3.1 | |||||
| Bortezomib | 5.7 | |||||
| Carbamazepine | 2.0 | 9.4 | 24.7 | |||
| Carboplatin | 11.6 | 2.5 | ||||
| Cefazoline | 13.4 | |||||
| Cefotaxime | 65.9 | |||||
| Ceftriaxone | 3.7 | 8.5 | 11.4 | 22.2 | ||
| Cefuroxime | 11.6 | |||||
| Cetuximab | 4.7 | 2.5 | 3.8 | |||
| Cyclosporine | 4.2 | 2.4 | ||||
| Ciprofloxacin | 2.5 | 2.1 | 5.0 | 7.4 | ||
| Cisplatin | 14.9 | |||||
| Clarithromycin | 2.5 | 4.1 | ||||
| Clavulanic acid | 3.8 | 7.1 | 8.9 | 9.9 | ||
| Clindamycin | 3.7 | 7.0 | 9.7 | |||
| Clobazam | 15.6 | |||||
| Clozapine | 2.1 | |||||
| Codeine | 2.3 | |||||
| Cyclophosphamide | 16.0 | 2.5 | ||||
| Cytarabine | 22.1 | 2.9 | ||||
| Diclofenac | 4.0 | 2.7 | 2.6 | |||
| Didanosine | 10.3 | |||||
| Docetaxel | 12.4 | |||||
| Doxorubicin | 14.5 | 2.1 | ||||
| Doxycycline | 2.2 | 6.8 | ||||
| Efavirenz | 4.9 | |||||
| Emtricitabine | 3.2 | 2.5 | 3.6 | |||
| Enoxaparin | 2.3 | |||||
| Epirubicin | 14.9 | |||||
| Ethambutol | 14.2 | 62.1 | ||||
| Etoposide | 20.5 | |||||
| Fluconazole | 3.3 | 4.8 | 5.4 | |||
| Fludarabine | 13.4 | |||||
| Fluorouracil | 8.6 | |||||
| Furosemide | 2.4 | |||||
| Gadolinium | 3.2 | 10.0 | ||||
| Gemcitabine | 7.1 | 3.0 | ||||
| Glatiramer acetate | 2.2 | 4.4 | ||||
| Ibuprofen | 2.3 | |||||
| Ifosfamide | 22.5 | |||||
| Imatinib | 3.2 | |||||
| Iopromide | 7.8 | 15.4 | ||||
| Irinotecan | 7.5 | |||||
| Isoniazide | 8.4 | 9.0 | 31.8 | |||
| Lamivudine | 4.2 | 2.7 | 4.1 | |||
| Lamotrigine | 2.7 | 8.7 | 12.9 | |||
| Lenalidomide | 4.1 | |||||
| Levetiracetam | 3.2 | 7.7 | ||||
| Levofloxacin | 3.3 | 3.2 | ||||
| Lidocaine | 2.1 | 7.7 | ||||
| Lopinavir | 3.1 | |||||
| Methotrexate | 3.1 | |||||
| Methylprednisolone | 2.3 | |||||
| Metronidazole | 2.4 | 2.7 | 4.1 | 8.8 | ||
| Midazolam | 7.8 | |||||
| Minocycline | 8.5 | 36.6 | ||||
| Moxifloxacin | 3.9 | 15.0 | ||||
| Mycophenolate mofetil | 2.4 | |||||
| Naproxen | 2.1 | |||||
| Nevirapine | 5.7 | 6.0 | ||||
| Nicotine | 2.0 | |||||
| Octreotide | 2.4 | |||||
| Omalizumab | 2.5 | 8.6 | ||||
| Ondansetrone | 2.2 | |||||
| Oxaliplatin | 4.7 | 2.4 | 2.6 | |||
| Paclitaxel | 7.7 | 2.3 | ||||
| Pantoprazole | 2.7 | |||||
| Peginterferon alfa-2a | 2.4 | 2.6 | 2.1 | 3.8 | ||
| Peginterferon alfa-2b | 3.1 | |||||
| Phenobarbital | 26.9 | |||||
| Phenytoin | 2.3 | 14.5 | 16.7 | |||
| Piperacillin | 2.9 | 10.1 | 17.1 | |||
| Prednisolone | 4.0 | 2.1 | 2.1 | |||
| Prednisone | 3.6 | |||||
| Propofol | 2.2 | 12.9 | ||||
| Propranolol | 2.4 | |||||
| Pyrazinamide | 78.5 | |||||
| Raltegravir | 12.6 | |||||
| Ranitidine | 3.3 | |||||
| Ribavirin | 2.1 | 2.4 | 3.6 | |||
| Rifampicin | 7.1 | 13.1 | 50.1 | |||
| Ritonavir | 2.9 | 3.4 | ||||
| Rituximab | 9.6 | |||||
| Rocuronium | 22.8 | |||||
| Sorafenib | 5.5 | |||||
| Spironolactone | 4.7 | 2.1 | ||||
| Stavudine | 8.4 | |||||
| Sulfamethoxazole | 7.6 | 3.2 | 2.8 | 7.5 | 7.1 | |
| Sulfasalazine | 2.0 | 6.5 | 24.3 | |||
| Tacrolimus | 3.0 | 2.9 | ||||
| Tazobactam | 10.2 | 18.1 | ||||
| Telaprevir | 3.3 | 3.8 | 9.2 | |||
| Temozolomide | 12.4 | |||||
| Tenofovir | 4.3 | |||||
| Terbinafin | 2.6 | 7.8 | ||||
| Topiramate | 2.2 | |||||
| Trastuzumab | 5.4 | |||||
| Trimethoprim | 7.7 | 3.3 | 2.5 | 7.1 | 7.8 | |
| Valaciclovir | 3.3 | |||||
| Valdecoxib | 2.8 | 21.2 | ||||
| Valproate | 3.9 | 7.7 | ||||
| Vancomycin | 3.4 | 2.8 | 12.5 | 35.0 | ||
| Verapamil | 2.4 | |||||
| Vincristine | 17.0 | 3.4 | ||||
| Zidovudine | 3.1 | |||||
| Zonisamide | 14.9 | 38.5 |
* Data extracted from OpenVigil 2.1-MedDRA on 17 October 2017; U.S. pharmacovigilance data, 2004–2014; first 50 events sorted by frequency; active substance and trade names combined; confounders such as adrenaline, antihistamines, and glucocorticoids have been removed. The heading DIA also includes precipitants of type A ADR, such as cytotoxic substances (e.g., carboplatin). The figures are Proportional Reporting Ratios (PRR), indicating the relative risk compared to all other drugs in the database. A PRR of 2 indicates that the reporting of this combination is twice as frequent as expected (i.e., a 100% elevation of the frequency).
DIA: drug- induced agranulocytosis; DILI: drug-induced liver injury; DRESS: drug reaction with eosinophilia and systemic symptoms, SCAR: severe cutaneus adverse drug reaction; dark red fields indicate PRR = 10, i.e., reporting of this event for this drug is at least 10 times more frequent than expected; lightly colored fields indicate PRR = 3 and <10 (a three- to ?tenfold elevation above the expected risk).
eTable 2. Drugs that may cause Stevens-Johnson syndrome, according to manufacturers’ summaries of product characteristics*.
| Drug | Frequency of Stevens-Johnson syndrome |
| Aliskiren | postmarketing, uncommon |
| Amprenavir | rare |
| Ciprofloxacin | very rare, postmarketing, rare |
| Cladribine | rare |
| Efavirenz | postmarketing, uncommon, 0–3.5% |
| Felbamate | rare |
| Fluconazole | postmarketing, rare |
| Fosamprenavir | rare |
| Imatinib | rare |
| Nevirapine | postmarketing, uncommon, 0.3% |
| Omeprazol | postmarketing, rare |
| Paclitaxel | very rare, postmarketing, uncommon |
| Pregabalin | rare |
| Saquinavir | uncommon |
| Vemurafenib | postmarketing, common |
| Voriconazole | uncommon |
* extracted from SIDER 4.1 on 24 October 2017; all entries in which a frequency is given and the frequency is higher than “very rare.”
An overview of pharmacogenetic biomarkers can be seen in the HLADR database (15).
Other factors
Certain diseases alter the probability of hypersensitivity reactions: HIV patients react more commonly to sulfonamides, while persons with mastocytosis react variably to a wide range of substances (9).
The skin and the liver.
The high metabolic activity of the skin and liver presumably accounts for their vulnerability to such reactions. The skin, in particular, is constantly immunologically stimulated by pathogens and noxious substances because of its exposed position.
Diagnostics
The measures needed to securely establish the diagnosis of a hypersensitivity reaction and to document it adequately (table 2) are often not carried out in routine clinical practice, either to save time and money, or else because of physicians’ inadequate experience with hypersensitivity reactions. For example, the detection of abacavir-induced cutaneous reactions was jeopardized at first by inadequate documentation of the phenotype (e8). Standardized questionnaires (16) and photographic documentation markedly improved the documentation of hypersensitivity reactions.
Table 2. Recommended diagnostic measures for suspected hypersensitivity reactions*.
| Diagnostic measure | Significance / Example |
| Determination of the interval from drug intake to onset of reaction |
– distinguishes immediate (non-allergic or type I) from delayed reactions; delayed reactions generally arise a few days to six months after intake, depending on the triggering drug |
| Dechallenge? | – discontinuation of the triggering drug for therapeutic purposes and to confirm that it was responsible |
| Determination of concomitantly taken medication |
– evaluation of which drug was the triggering one – consideration of the contributory effect of drug interactions |
| Determination of comorbidities and other special circumstances |
– infections and other inflammatory conditions can either elevate or lower the risk of hypersensitivity reactions |
| First exposure? | – non-allergic versus allergic |
| Type of reaction? | – cf. Figures – erythema: non-allergic or type I – Could a known pharmacological adverse drug reaction be responsible ? |
| IgE and other lab tests (basophil activation test, leukotriene release test) |
– causal demonstration of type I, but of little clinical specificity |
| Genetic testing | – HLA testing for type IV reactions |
| Reexposure (provocative test)? |
– Systemic provocative testing only makes sense if there is a clear need for treatment and alternative treatments or testing methods are unavailable or have already been exhausted – Dermatological tests (prick test,epicutaneous testing) are less risky, but also less informative. The patient must be monitored, and emergency treatment (e.g., intubation) must be available in case of need. |
Common precipitating drugs.
Antibiotics (particularly beta-lactams) and anticonvulsants are the most common precipitating drugs, accounting for three-quarters of all cases of hypersensitivity.
The diagnostic evaluation of hypersensitivity reactions consists of thorough history-taking, in vitro laboratory testing, and in vivo cutaneous tests and provocative tests (17).
History
The clinical history must include documentation of the time from drug exposure to the adverse event, a precise description of the event (including gastrointestinal and respiratory symptoms), and an account of the accompanying circumstances (concomitant medication, viral infections, underlying disease).
Dechallenge
Dechallenge.
A dechallenge-rechallenge test, i.e., the regression of symptoms after discontinuation of the presumed offending drug and their re-emergence after it is reintroduced, either deliberately (provocative testing) or unintentionally (inadvertent reexposure), is the most convincing proof of causality.
A dechallenge-rechallenge test, i.e., the regression of symptoms after discontinuation of the presumed triggering drug and their re-emergence after it is reintroduced, either deliberately (drug challenging) or unintentionally (inadvertent reexposure), is the most convincing proof of causality. Before a dechallenge can take place, a hypothesis must be formulated as to which drug (possibly one of a long list of drugs) is the trigger. Clues in this matter can be obtained from manufacturers’ summaries of product characteristics or from searches in adverse drug reaction databases such as SIDER or OpenVigil (18, 19). The interval of time from drug exposure to symptom emergence is of paramount importance: unless a delayed reaction has taken place, the last drug added is usually the one responsible for the adverse drug reaction.
Laboratory testing
In vitro testing comprises tests for specific IgE (type I allergy) and for the release of leukotrienes or histamine. Specific IgEs can be detected and semiquantitatively analyzed through their binding to an allergen-containing cellulose sponge (CAP) followed by testing with either radioactivity (RAST) or fluorescence (FEIA). Type I reactions can also be detected by the basophil activation test. The lymphocyte transformation test (LTT) also provides information about type IV allergies, but it is not standardized. All testing methods are of limited sensitivity and specificity. Not every positive finding is correlated with clinically relevant symptoms, and vice versa.
Laboratory testing.
In vitro testing comprises tests for specific IgE (type I allergy) and for the release of leukotrienes or histamine.
Many genetic markers (variants in, e.g., 5´-lipooxygenase, the histamine receptor, cysteinyl leukotriene synthetase, arylamine-N-acetyltransferase, aminopeptidase P, platelet-activating-factor-acetylhydrolase, and HLA) have been found to be associated with hypersensitivity reactions, but predictive testing is currently clinically relevant only with respect to HLA status when certain specific drugs are taken. Many markers are of little predictive value (9).
Dermatologic testing
Dermatologic testing includes the prick test and the intracutaneous test when type I allergy is suspected (immediate response, can be read 20 minutes after application) and the epicutaneous patch test or the intracutaneous test with delayed readout when type IV allergy is suspected (delayed reaction, readout in 24–72 hours). Unlike laboratory tests, these tests may pose a risk to the patient (e.g., an anaphylactic reaction in type I allergy or sensitization in type IV allergy).
As drug metabolites often cause hypersensitivity reactions, the results of testing on the skin, which has a different liver metabolic profile, cannot simply be extrapolated to other modes of application of the presumed triggering substance. Moreover, cutaneous irritation can occur.
Skin biopsy
In drug-induced cutaneous reactions, skin biopsies can be taken to prove the diagnosis of type III (vasculitis) and type IV reactions, especially because a number of serious drug-induced cutaneous reactions cannot be detected by epi- or intracutaneous testing.
Drug challenging
Drug challenging, i.e., systemic reexposure to the presumably triggering drug (by the intravenous, oral, or other route), may be contraindicated in cases of severe hypersensitivity. For example, reactions to reexposure with abacavir are markedly faster (occurring within a few hours) and carry a higher mortality (20).
The clinical features of selected delayed reactions
Drug-induced agranulocytosis
Aside from toxic (type A) effects of drugs on granulocytes (e9), it is mainly the HLA-dependent activation of T-cells that leads to drug-induced agranulocytosis (21).
Dermatological testing.
This includes the prick test and the intracutaneous test when type I allergy is suspected (immediate response, can be read 20 minutes after application) and the epicutaneous patch test or the intracutaneous test with delayed readout when type IV allergy is suspected (delayed reaction, readout in 24–72 hours).
The diagnosis is made by a peripheral blood count with differential (<500 granulocytes per µL of blood). An unexpectedly rapid and severe course of a usually trivial infection is often the first clinical sign. Sepsis with uncommon pathogens (e.g., mycoses, Brucella, Helicobacter) may be another sign. The classic manifestation is severe inflammation at the typical portals of pathogen entry—the rectum, bladder, and pharynx. If the condition is untreated, sepsis and death ensue. The presumed triggering drug should be discontinued, the patient should be isolated, and prophylactic antibiotics should be given to cover both Pseudomonas aeruginosa and fungal infections.
The pharmacogenetics of clozapine-induced agranulocytosis
Clozapine-induced agranulocytosis has a frequency of 0.8% (e10) and is due to an interaction of this atypical antipsychotic drug with HLA-DQB1 and an HLA-B variant (158T) in which the drug itself acts as a hapten. The frequencies of these genetic traits are 12% and 17%, respectively, with a 4% frequency of joint occurrence in the study population (21). For example, individuals carrying the HLA-DQB1 trait are 2.6 times as likely to develop agranulocytosis after taking clozapine (22).
Severe cutaneous reactions
Drug reaction with eosinophilia and systemic symptoms (DRESS)
DRESS has variable manifestations, generally a maculopapular rash initially, followed later by lymphadenopathy, hepatitis, and eosinophilia. Abacavir-induced hypersensitivity differs from hypersensitivity reactions to other drugs only in that eosinophilia is rarer (e11, e12); the abacavir reaction is nonetheless considered a type of DRESS (e13). Scoring systems enable objective diagnostic evaluation (23). In these cases, too, discontinuation of the trigger is the only available causal treatment.
Acute, generalized exanthematous pustulosis
This condition manifests with erythema and numerous pinhead-sized pustules on the face, skin folds, and trunk. A scoring system is available as an aid for diagnostic evaluation (24).
Stevens-Johnson syndrome / toxic epidermal necrolysis
Drug-induced agranulocytosis.
Aside from toxic (type A) effects of drugs on granulocytes, it is mainly the HLA-dependent activation of T-cells that leads to drug-induced agranulocytosis
Stevens-Johnson syndrome and toxic epidermal necrolysis, which are considered to be variants of a single condition, manifest themselves with blisters and erosions occupying large areas of the skin (mainly on the trunk and face) and mucous membranes, progressing in a cranial-to-caudal direction. The histologic findings include mainly subepidermal cleavage and epidermal necrosis. The differential diagnosis includes erythema exsudativum multiforme, which must be ruled out; this entity is not a hypersensitivity reaction and generally arises after an infection, but it bears some clinical resemblance to Stevens-Johnson syndrome / toxic epidermal necrolysis. It is distinct from them in presenting with raised, target-shaped lesions (called bull’s-eye lesions or cockades). A generalized bullous fixed drug eruption is a further, rare element of the differential diagnosis.
The assessment of rashes
The following can be warning signs of a serious reaction carrying an elevated mortality: a bullous skin reaction, facial and mucosal involvement, eosinophilia, elevated liver enzymes, dyspnea, and systemic symptoms such as fever above 38.5 °C and lymphadenopathy (figure 2). Infectious rashes should be excluded in the differential diagnosis (e.g., Epstein-Barr virus, Staphylococcus exotoxin). Viruses are the most common cause of rash in children, drugs in adults. A preceding sore throat and skin involvement beginning on the face are indications of a probably viral rash.
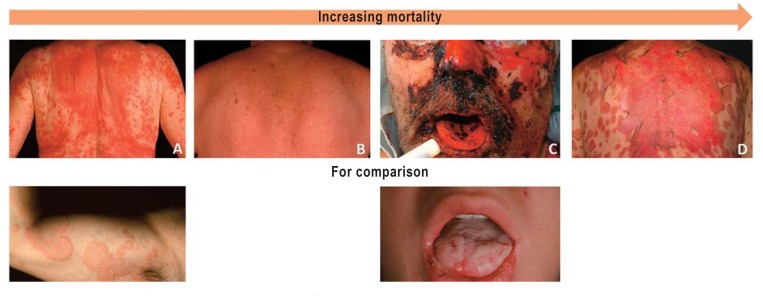
Figure 2.
Cutaneous manifestations of type IV hypersensitivity reactions, in order of increasing mortality:
A) maculopapular rash (MPR): macule and several papules, markedly confluent, without any further systemic manifestations
B) drug reaction with eosinophilia and systemic symptoms (DRESS): variable clinical picture, predominantly papules over the entire body, systemic manifestations including eosinophilia and fever
C) Stevens-Johnson syndrome (SJS): blisters and epidermal separation (erosions) that typically start on the face and are later seen mainly on the trunk
D) Reactions with skin separation over larger areas are designated as toxic epidermal necrolysis (TEN) or Lyell syndrome.
E) Urticaria in a type I reaction for comparison: hives (wide area,raised), pruritus
F) Oral mucosal involvement in erythema exsudativum multiforme (Fuchs syndrome) for comparison: less mucosal involvement than in SJS, skin lesions often slightly raised.
Stevens-Johnson syndrome/toxic epidermal necrolysis.
Stevens-Johnson syndrome and toxic epidermal necrolysis manifest themselves with blisters and erosions occupying large areas of the skin (mainly on the trunk and face) and mucous membranes, progressing in a cranial-to-caudal direction.
The pharmacogenetics of severe drug-induced cutaneous reactions
The assessment of rashes.
The following can be warning signs of a serious reaction carrying an elevated mortality: a bullous skin reaction, facial and mucosal involvement, eosinophilia, elevated liver enzymes, dyspnea, and systemic symptoms such as fever above 38.5 °C and lymphadenopathy.
The finding of HLA-B*57:01 before the administration of the antiretroviral drug abacavir has a 50% positive predictive value for severe cutaneous reactions, while the absence of this finding has a negative predictive value above 99% (25). The documentation of HLA status is therefore mandatory in Europe before this drug can be given, as the drug may not be prescribed to to carriers of HLA-B*57:01 (70% probability of a reaction in a median time of 11 days), while the risk of a cutaneous reaction is much lower (ca. 2%) in non-carriers (25, e14). Cutaneous hypersensitivity reactions to carbamazepine are also associated with certain HLA alleles (HLA-A31:01, HLA-B*15:02), whose prevalence is markedly dependent on the patient’s ethnic origin (table 3) (26, e15). The risk of a severe cutaneous reaction to carbamazepine a few days to approximately one month after the onset of treatment is ca. 3% in general, but 100% among carriers of the biomarker HLA-B*15:02, when it is found in persons of Han Chinese or Thai ethnicity (27). Likewise, HLA-B*15:02 is associated with severe cutaneous reactions to lamotrigine, another anticonvulsant (28).
Table 3. Examples of drugs that induce type IV allergic hypersensitivity reactions, with potential predictive tests, number needed to screen (NNS), and number needed to harm (NNH).
| Drug | Biomarker (prevalence) | Reaction | NNS (reference) | NNH (according to ‧manufacturers’ ‧summaries of product characteristic) | Is testing required in ‧Germany? |
| Abacavir | HLA-B*57:01 (7% in Caucasians) |
DRESS | 13 –16 (5) | 1–10 | yes |
| Allopurinol | HLA-B*58:01 in Han Chinese, Thais, and Southeast Asians (10%) |
DRESS | 250 (5) | <3000 | no |
| HLA-B*58:01 in other ethnic groups (3%) |
DRESS | 825 | 10 000 | no | |
| Carbamazepine | HLA-B*15:02 in Han Chinese, Thais, and Southeast Asians (15%) |
SJS/TEN | 1000 (5) | <1600 | yes for patients of Han Chinese or Thai ethnicity |
| HLA-B*15:02 in other ethnic groups (< 1%) |
SJS/TEN | >1000 | >10 000 | no | |
| HLA-A*31:01 in Japanese (10%) |
DRESS | 67 (40) | 33 | no | |
| HLA-A*31:01 in other ethnic groups (3%) |
DRESS | 47 (40) | 4 | no | |
| Flupirtine*1 | HLA-DRB1*16:01 and DQB1*05:02 |
DILI | 8000 (9) | >10 000 | no |
| Flucloxacillin | HLA-B*57:01 | DILI | 13 000 (9) | >1000 | no |
*1It was recommended in February 2018 that the approval of flupirtine be revoked because of its hepatotoxicity. The manufacturers of drugs containing flupirtine thereupon withdrew them voluntarily from the market.
DILI:drug-induced liver injury, DRESS: drug reaction with eosinophilia and systemic symptoms, SJS: Stevens-Johnson syndrome, TEN: toxic epidermal necrolysis.
Drug-induced liver damage
Typical externally evident signs of severe liver damage include fatigue, weakness, abdominal pain, nausea, dark urine, jaundice, pruritus, and fever. Laboratory testing reveals elevated concentrations of the hepatic aminotransferases (ALT, AST) and alkaline phosphatase (AP). The ratio of ALT/AP enables further differentiation of the hepatobiliary damage. Isolated ALT elevation, or an ALT elevation that is five times higher than the AP elevation (when the measured concentration of each drug is compared to the upper limit of its normal range), indicates hepatocellular damage (e.g., due to acetaminophen). Conversely, predominant elevation of AP may reflect cholestasis (induced, for example, by an ACE inhibitor) or fibrosis (induced, for example, by methotrexate) (29). The degree of severity can also be estimated (30). Reexposure usually leads to a renewed hypersensitivity reaction whose course is faster (days, not weeks) and more severe than the original one (31).
Viral hepatitis is the main differential diagnosis to be ruled out. Aside from the antibiotics listed in Figure 1 and the substances mentioned above, further triggers can be found in the LiverTox database (32). A history of consumption of certain botanical extracts and food supplements is relevant; the so-called natural anxiolytic Kava kava, for example, was forbidden at one time and is now available only by prescription because of its hepatotoxicity, which is associated with variants of UDP-glucuronosyltransferase 1A1 (UGT1A1) (e16).
Pharmacogenetics
Certain types of drug-related hepatotoxicity are associated with HLA markers, e.g., hepatotoxicity due to the beta-lactam antibiotics flucloxacillin (2) and amoxicillin/clavulanic acid (33) in carriers of HLA-B*57:01. Moreover, HLA-A*33:01 is associated with hepatotoxicity due to enalapril, erythromycin, fenofibrate, methyldopa, sertraline, terbonafine, and ticlopidine (30), while HLA-DRB1*16:01-DQB1*05:02 is associated with hepatotoxicity due to flupirtine (34).
Drug-induced liver damage.
Typical externally evident signs of severe liver damage include fatigue, weakness, abdominal pain, nausea, dark urine, jaundice, pruritus, and fever.
Documentation
The diagnosis of drug hypersensitivity must be properly documented. Hospital information systems now enable the deposition of such information in the patient’s record so that it will be available when the patient undergoes further treatment or is readmitted. Such information must also be noted in hospital discharge summaries to prevent the readministration of the provoking drug later on. Unfortunately, this is estimated to occur within six months in 27% of all patients who have suffered hypersensitivity reactions, solely because of inadequate communication (35).
If a drug reaction is documented on the basis of information provided by the patient, the reliability of this information should be proven and documented as well. The patient should be provided with an allergy passport in which the triggering substance and examples of drugs containing it are explicitly mentioned. A common type of inadequate documentation is that of a so-called penicillin allergy; in many such cases, a type A side effect (e.g., gastrointestinal discomfort) has been misinterpreted as a hypersensitivity reaction. Physicians are also occasionally confronted with vague information dating back to the patient’s childhood that the patient cannot remember at all, or, if so, then only incompletely. The uncritical acceptance and documentation of such “allergies” leads to the unnecessary avoidance of effective treatments in favor of others that may be less effective or more costly. No more than 20% of so-called penicillin allergies are really allergies in the strict, classic sense (36).
Drugs of second choice can also be tested and documented in the allergy passport so that valid options will be available later if treatment is needed. It must be kept in mind, however, that such tests cannot be anything more than snapshots of the current situation, and that “prophetic” tests, such as patients often request, are not possible. HLA genotyping is the method of choice for the prevention of certain type IV reactions (e2).
Several further types of genetic testing are available but are of relatively low predictive value and are fraught with a high number needed to screen (NNS) and a high cost/benefit ratio (table 3). Such genetic markers could rather be used for the scientific explanation of hypersensitivity reactions that have already occurred, with an eye toward strategies of preventing reexposure.
The documentation of drug hypersensitivity.
Information about drug hypersensitivity reactions must be noted in hospital discharge summaries to prevent the readministration of the triggering drug later on.
Treatment
When a hypersensitivity reaction arises, the immediate discontinuation of the triggering drug is the safest option. The reaction itself can only be managed with supportive care, as there is no causally directed treatment (table 1). Drug rashes have traditionally been treated with glucocorticoids, despite their questionable efficacy (e17, e18). Stevens-Johnson syndrome and toxic epidermal necrolysis seem not to respond reliably to either glucocorticoids or anti-inflammatory drugs (e19, e20). Cyclosporine A might lower mortality (37). High-dose intravenous immunoglobulins are given to treat DRESS, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Their efficacy in this situation is thought be mediated by antibodies directed against the apoptosis-associated molecules Fas (first apoptosis signal receptor) and FasL (Fas-ligand L) (e21).
The avoidance of hypersensitivity reactions
Considering the estimated mean cost of €2700 for an undesired event in Germany (e22), the avoidance of such events is not just an ethical imperative, but an economic one as well. Many of these events could, indeed, be avoided (table 1).
If a patient reports having suffered from an “allergy” in the past, this should prompt further allergological testing, unless precise documentation (an allergy passport) is already available. Often, multiple testing methods must be used to confirm or refute the suspected diagnosis.
In case reexposure is possible or medically necessary, patients who have sustained immediate-type reactions could undergo desensitization therapy (e23).
Economic aspects
The avoidance of undesired events seems economically meaningful. In particular, pharmacogenetic testing (as it is now established in modern oncology, for example, in the form of companion diagnostic testing) can help prevent serious drug reactions. Genetic testing before carbamazepine treatment, for example, has been found to be cost-effective (e24).
Treatment.
When a hypersensitivity reaction arises, the immediate discontinuation of the triggering drug is the safest option. The reaction itself can only be managed with supportive care, e.g., with glucocorticoids.
Data from the Hong Kong health-care system have revealed, however, that physicians generally did not perform the required genetic testing for HLA-B*15:02 before using carbamazepine, but rather went ahead and directly prescribed the more expensive alternative drugs (38). This approach prevents the use of drugs that are known to be highly effective in favor of others of less certain efficacy, while burdening the health-care system with unnecessary costs and, furthermore, complicating the evaluation of the current guidelines, because recent data are inevitably distorted by this kind of evasive behavior.
The avoidance of hypersensitivity reactions.
Considering the high cost of undesired events in Germany, their avoidance is not just an ethical imperative, but an economic one as well.
Further information on CME.
Participation in the CME certification program is possible only over the Internet: cme.aerzteblatt.de. This unit can be accessed until 14 October 2018. Submissions by letter, e-mail or fax cannot be considered.
-
The following CME units can still be accessed for credit:
“Hints on Diagnosing and Treating Headache” (issue 17/2018) until 22 July 2018
“The Treatment of Gliomas in Adulthood” (issue 21/2018) until 12 August 2018
“Helicobacter pylori infection” (issue 25/2018) until 16 September 2018
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN), which is found on the CME card (8027XXXXXXXXXXX). The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or else entered in “Meine Daten,” and the participant must agree to communication of the results.
CME credit for this unit can be obtained via cme.aerzteblatt.de until 14 October 2018. Only one answer is possible per question. Please choose the most appropriate answer.
Question 1
Which type B adverse drug reactions are the most common?
non-allergic hypersensitivity reactions
type I allergies
type II allergies
type III allergies
type IV allergies
Question 2
Which type B adverse drug reactions are T-cell-mediated?
non-allergic hypersensitivity reactions
type I allergies
type II allergies
type III allergies
type IV allergies
Question 3
Which drug classes most commonly induce hypersensitivity reactions?
glucocorticoids and nonsteroidal anti-inflammatory drugs
proton-pump inhibitors and tetracyclines
virostatic drugs and anti-estrogen drugs
beta-lactam antibiotics and anticonvulsants
antihypertensive drugs and antimycotic drugs
Question 4
Which drug can induce an asthma attack in a genetically predisposed patient?
dexamethasone
diclofenac
epinephrine
L-dopamine
L-thyroxine
Question 5
Which genotype must be excluded before the initiation of treatment with abacavir?
HLA-A*24:02
HLA-B*27
HLA-B*57:01
HLA-DRB1*16:01
HLA-DQB1*05:02
Question 6
What genotype must be excluded before the initiation of treatment with carbamazepine in a patient of Han Chinese or Thai ethnicity?
HLA-A*31:01
HLA-B*15:02
HLA-B*58:01
HLA-C*01:02
HLA-C*14:03
Question 7
Which method of evaluating a hypersensitivity reaction carries the highest risk of inducing a life-threatening reaction?
systemic provocative testing
prick test
epicutaneous patch test
lymphocyte transformation test
genotyping
Question 8
Which of the following measures should be taken when a drug reaction with eosinophilia and systemic symptoms (DRESS) is diagnosed?
discontinuation of the presumed triggering drug
treatment with inhaled glucocorticoids
topical administration of glucocorticoids
reverse isolation precautions
volume substitution and catecholamine infusion
Question 9
A patient with gout is admitted to the hospital because of the sudden onset of fever, lymphadenopathy, and a macular rash. His medications include ramipril, celiprolol, and allopurinol. What is the most likely diagnosis?
an acute exacerbation of gout
influenza
a non-allergic hypersensitivity reaction to ramipril
peripheral hyperemia due to celiprolol
a drug reaction with eosinophilia and systemic symptoms (DRESS) due to allopurinol
Question 10
A woman with rheumatoid arthritis is admitted to the hospital because of high fever and an edematous rash with pinhead-sized white papules in the groin, axillae, and elbow creases, of three days’ duration. She chronically takes prednisolone. Three months ago, she was given cefpodoxime (a beta-lactam antibiotic) for one week to treat a urinary tract infection. What is the most likely diagnosis?
acute, generalized exanthematous pustulosis due to cefpodoxime
Stevens-Johnson syndrome due to prednisolone
fungal infection due to cefpodoxime
exacerbation of rheumatoid arthritis with pustular psoriasis
steroid acne due to prednisolone
► Participation is possible only via the Internet: cme.aerzteblatt.de
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
The authors state that they have no conflicts of interest.
References
- 1.Doña I, Barrionuevo E, Blanca-Lopez N, et al. Trends in hypersensitivity drug reactions: more drugs, more response patterns, more heterogeneity. J Investig Allergol Clin Immunol. 2014;24:143–153. [PubMed] [Google Scholar]
- 2.Daly AK, Donaldson PT, Bhatnagar P, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 3.Jensen CS, Menné T, Lisby S, Kristiansen J, Veien NK. Experimental systemic contact dermatitis from nickel: a dose-response study. Contact Dermatitis. 2003;49:124–132. doi: 10.1111/j.0105-1873.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 4.Dona I, Blanca-Lopez N, Torres MJ, et al. Drug hypersensitivity reactions: response patterns, drug involved, and temporal variations in a large series of patients. J Investig Allergol Clin Immunol. 2012;22:363–371. [PubMed] [Google Scholar]
- 5.Pavlos R, Mallal S, Ostrov D, et al. T cell-mediated hypersensitivity reactions to drugs. Annu Rev Med. 2015;66:439–454. doi: 10.1146/annurev-med-050913-022745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demoly P, Lebel B, Messaad D, et al. Predictive capacity of histamine release for the diagnosis of drug allergy. Allergy. 1999;54:500–506. doi: 10.1034/j.1398-9995.1999.00020.x. [DOI] [PubMed] [Google Scholar]
- 7.Park SM, Park JS, Park HS, Park CS. Unraveling the genetic basis of aspirin hypersensitivity in asthma beyond arachidonate pathways. Allergy Asthma Immunol Res. 2013;5:258–276. doi: 10.4168/aair.2013.5.5.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cilia La Corte AL, Carter AM, Rice GI, et al. A functional XPNPEP2 promoter haplotype leads to reduced plasma aminopeptidase P and increased risk of ACE inhibitor-induced angioedema. Hum Mutat. 2011;32:1326–1331. doi: 10.1002/humu.21579. [DOI] [PubMed] [Google Scholar]
- 9.Böhm R, Cascorbi I. Pharmacogenetics and predictive testing of drug hypersensitivity reactions. Front Pharmacol. 2016;7 doi: 10.3389/fphar.2016.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liden S, Beckman L, Cedergren B, Göransson K, Nyquist H. HLA antigens in allergic contact dermatitis. Acta derm-vener Suppl. 1977;58:53–56. [PubMed] [Google Scholar]
- 11.Andres E, Maloisel F. Idiosyncratic drug-induced agranulocytosis or acute neutropenia. Curr Opin Hematol. 2008;15:15–21. doi: 10.1097/MOH.0b013e3282f15fb9. [DOI] [PubMed] [Google Scholar]
- 12.Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–1080. doi: 10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 13.Sekula P, Dunant A, Mockenhaupt M, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013;133:1197–1204. doi: 10.1038/jid.2012.510. [DOI] [PubMed] [Google Scholar]
- 14.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterol. 2008;135:1924–1934. doi: 10.1053/j.gastro.2008.09.011. 34 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du T, Yang L, Luo H, et al. HLADR: a database system for enhancing the discovery of biomarkers for predicting human leukocyte antigen-mediated idiosyncratic adverse drug reactions. Biomark Med. 2015;9:1079–1093. doi: 10.2217/bmm.15.98. [DOI] [PubMed] [Google Scholar]
- 16.Wedi B. Fragebogen Medikamentenüberempfindlichkeit. wwweaaci.org/attachments/669_German-ENDA-Questionnaire.pdf (last accessed on 9 October 2017) [Google Scholar]
- 17.Brockow K, Przybilla B, Aberer W, et al. Leitlinie allergologische Diagnostik von Überempfindlichkeitsreaktionen auf Arzneimittel. Allergo J Int. 2015;24:44–57. [Google Scholar]
- 18.Böhm R, von Hehn L, Herdegen T, et al. OpenVigil FDA - inspection of U.S. American adverse drug events pharmacovigilance data and novel clinical applications. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157753. e0157753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn M, Letunic I, Jensen LJ, Bork P. The SIDER database of drugs and side effects. Nucleic Acids Research. 2015 doi: 10.1093/nar/gkv1075. gkv1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escaut L, Liotier JY, Albengres E, Cheminot N, Vittecoq D. Abacavir rechallenge has to be avoided in case of hypersensitivity reaction. Aids. 1999;13:1419–1420. doi: 10.1097/00002030-199907300-00026. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein JI, Jarskog LF, Hilliard C, et al. Clozapine-induced agranulocytosis is associated with rare HLA-DQB1 and HLA-B alleles. Nat Commun. 2014;5 doi: 10.1038/ncomms5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism in HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72:458–463. doi: 10.4088/JCP.09m05527yel. [DOI] [PubMed] [Google Scholar]
- 23.Chen YC, Cho YT, Chang CY, Chu CY. Drug reaction with eosinophilia and systemic symptoms: A drug-induced hypersensitivity syndrome with variable clinical features. Dermatologica Sinica. 2013;31:196–204. [Google Scholar]
- 24.Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)–a clinical reaction pattern. J Cutan Pathol. 2001;28:113–119. doi: 10.1034/j.1600-0560.2001.028003113.x. [DOI] [PubMed] [Google Scholar]
- 25.Mallal S, Nolan D, Witt C, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359:727–732. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- 26.Amstutz U, Shear NH, Rieder MJ, et al. Recommendations for HLA-B*15:02 and HLA-A*31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions. Epilepsia. 2014;55:496–506. doi: 10.1111/epi.12564. [DOI] [PubMed] [Google Scholar]
- 27.Chung WH, Hung SI, Hong HS, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428 doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- 28.Kazeem GR, Cox C, Aponte J, et al. High-resolution HLA genotyping and severe cutaneous adverse reactions in lamotrigine-treated patients. Pharmacogenet Genom. 2009;19:661–665. doi: 10.1097/FPC.0b013e32832c347d. [DOI] [PubMed] [Google Scholar]
- 29.Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J Clin Pathol. 2009;62:481–492. doi: 10.1136/jcp.2008.058248. [DOI] [PubMed] [Google Scholar]
- 30.Nicoletti P, Aithal GP, Bjornsson ES, et al. Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Format: Gastroenterology. 20171;52:1078–1089. doi: 10.1053/j.gastro.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papay JI, Clines D, Rafi R, et al. Drug-induced liver injury following positive drug rechallenge. Regul Toxicol Pharmacol. 2009;54:84–90. doi: 10.1016/j.yrtph.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Hoofnagle JH, Serrano J, Knoben JE, Navarro VJ. LiverTox: A website on drug-induced liver injury. Hepatology. 2013;57:873–874. doi: 10.1002/hep.26175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucena MI, Molokhia M, Shen Y, et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141:338–347. doi: 10.1053/j.gastro.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicoletti P, Werk AN, Sawle A, et al. HLA-DRB1*16: 01-DQB1*05: 02 is a novel genetic risk factor for flupirtine-induced liver injury. Pharmacogenet Genom. 2016;26:218–224. doi: 10.1097/FPC.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 35.van der Linden CM, Kerskes MC, Bijl AM, Maas HA, Egberts AC, Jansen PA. Represcription after adverse drug reaction in the elderly: a descriptive study. JAMA. 2006;166:1666–1667. doi: 10.1001/archinte.166.15.1666. [DOI] [PubMed] [Google Scholar]
- 36.Salkind AR, Cuddy PG, Foxworth JW. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498–2505. doi: 10.1001/jama.285.19.2498. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann S, Sekula P, Venhoff M, et al. Systemic immunomodulating therapies for Stevens-Johnson Syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2017;153:514–522. doi: 10.1001/jamadermatol.2016.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z, Liew D, Kwan P. Real-world cost-effectiveness of pharmacogenetic screening for epilepsy treatment. Neurology. 2016;86:1086–1094. doi: 10.1212/WNL.0000000000002484. [DOI] [PubMed] [Google Scholar]
- 39.Mjorndal T, Boman MD, Hagg S, et al. Adverse drug reactions as a cause for admissions to a department of internal medicine. Pharmacoepidemio Drug Saf. 2002;11:65–72. doi: 10.1002/pds.667. [DOI] [PubMed] [Google Scholar]
- 40.Yip VL, Pirmohamed M. The HLA-A*31:01 allele: influence on carbamazepine treatment. Pharmgenomics Pers Med. 2017;10:29–38. doi: 10.2147/PGPM.S108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Kaumanns K, Kayser C, Paeschke N, et al. Medikationsfehler im Fokus der Forschung und Pharmakovigilanz. Bulletin zur Arzneimittelsicherheit. 2015;6:27–35. [Google Scholar]
- E2.Rive CM, Bourke J, Phillips EJ. Testing for drug hypersensitivity syndromes. Clin Biochem Rev. 2013;34:15–38. [PMC free article] [PubMed] [Google Scholar]
- E3.Canto MG, Andreu I, Fernandez J, Blanca M. Selective immediate hypersensitivity reactions to NSAIDs. Curr Opin Allergy Clin Immunol. 2009;9:293–297. doi: 10.1097/ACI.0b013e32832db943. [DOI] [PubMed] [Google Scholar]
- E4.Pacor ML, Di Lorenzo G, Martinelli N, Mansueto P, Rini GB, Corrocher R. Monosodium benzoate hypersensitivity in subjects with persistent rhinitis. Allergy. 2004;59:192–197. doi: 10.1046/j.1398-9995.2003.00380.x. [DOI] [PubMed] [Google Scholar]
- E5.Wigand P, Blettner M, Saloga J, Decker H. Prevalence of wine intolerance: results of a survey from Mainz, Germany. Dtsch Arztebl Int. 2012;109:437–444. doi: 10.3238/arztebl.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Walsh S, Creamer D. Drug reaction with eosinophilia and systemic symptoms (DRESS): a clinical update and review of current thinking. Clin Exp Dermatol. 2011;36:6–11. doi: 10.1111/j.1365-2230.2010.03967.x. [DOI] [PubMed] [Google Scholar]
- E7.Gomes ER, Demoly P. Epidemiology of hypersensitivity drug reactions. Curr Opin Allergy Clin Immunol. 2005;5:309–316. doi: 10.1097/01.all.0000173785.81024.33. [DOI] [PubMed] [Google Scholar]
- E8.Phillips EJ, Chung WH, Mockenhaupt M, Roujeau JC, Mallal SA. Drug hypersensitivity: pharmacogenetics and clinical syndromes. J Allergy Clin Immunol. 2011;127:S60–S66. doi: 10.1016/j.jaci.2010.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Tesfa D, Keisu M, Palmblad J. Idiosyncratic drug-induced agranulocytosis: possible mechanisms and management. Am J Hematol. 2009;84:428–434. doi: 10.1002/ajh.21433. [DOI] [PubMed] [Google Scholar]
- E10.Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA. Clozapine-induced agranulocytosis Incidence and risk factors in the United States. N Engl J Med. 1993;329:162–167. doi: 10.1056/NEJM199307153290303. [DOI] [PubMed] [Google Scholar]
- E11.Peyriere H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155:422–428. doi: 10.1111/j.1365-2133.2006.07284.x. [DOI] [PubMed] [Google Scholar]
- E12.Pirmohamed M, Friedmann PS, Molokhia M, et al. Phenotype standardization for immune-mediated drug-induced skin injury. Clin Pharmacol Ther. 2011;89:896–901. doi: 10.1038/clpt.2011.79. [DOI] [PubMed] [Google Scholar]
- E13.Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609–611. doi: 10.1111/j.1365-2133.2006.07704.x. [DOI] [PubMed] [Google Scholar]
- E14.Gelbe Liste. Fachinformationen Ziagen® 300 mg Filmtabletten. www.gelbe-liste.de/produkte/Ziagen-300mg-Filmtabletten_353455/fachinformation (last accessed on 26 June 2018) [Google Scholar]
- E15.McCormack M, Alfirevic A, Bourgeois S, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364:1134–1143. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E16.Aghdassi AA, Kraft M, Domschke W, Lerch MM, Weiss FU. Genetic polymorphisms in the UDP-glucuronosyltransferase UGT1A7 gene in patients with acute liver failure after kava-kava consumption. Arch Toxicol. 2015;89:2173–2174. doi: 10.1007/s00204-015-1578-6. [DOI] [PubMed] [Google Scholar]
- E17.Barreiro P, Soriano V, Casas E, et al. Prevention of nevirapine-associated exanthema using slow dose escalation and/or corticosteroids. Aids. 2000;14:2153–2157. doi: 10.1097/00002030-200009290-00012. [DOI] [PubMed] [Google Scholar]
- E18.Eshki M, Allanore L, Musette P, et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: a cause of unpredictable multiorgan failure. Arch Dermatol. 2009;145:67–72. doi: 10.1001/archderm.145.1.67. [DOI] [PubMed] [Google Scholar]
- E19.Paradisi A, Abeni D, Bergamo F, Ricci F, Didona D, Didona B. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278–283. doi: 10.1016/j.jaad.2014.04.044. [DOI] [PubMed] [Google Scholar]
- E20.Kardaun SH, Jonkman MF. Dexamethasone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol. 2007;87:144–148. doi: 10.2340/00015555-0214. [DOI] [PubMed] [Google Scholar]
- E21.Cho YT, Chu CY. Treatments for severe cutaneous adverse reactions. J Immunol Res. 2017;2017 doi: 10.1155/2017/1503709. 1503709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E22.Meier F, Maas R, Sonst A, et al. Adverse drug events in patients admitted to an emergency department: an analysis of direct costs. Pharmacoepidemiol Drug Saf. 2015;24:176–186. doi: 10.1002/pds.3663. [DOI] [PubMed] [Google Scholar]
- E23.Thien FC. 3. Drug hypersensitivity. Med J Aust. 2006;185:333–338. doi: 10.5694/j.1326-5377.2006.tb00591.x. [DOI] [PubMed] [Google Scholar]
- E24.Plumpton CO, Yip VL, Alfirevic A, Marson AG, Pirmohamed M, Hughes DA. Cost-effectiveness of screening for HLA-A* 31: 01 prior to initiation of carbamazepine in epilepsy. Epilepsia. 2015;56:556–563. doi: 10.1111/epi.12937. [DOI] [PubMed] [Google Scholar]
- E25.Moßhammer D, Haumann H, Mörike K, Joos S. Polypharmacy—an upward trend with unpredictable effects. Dtsch Arztebl Int. 2016;113:627–633. doi: 10.3238/arztebl.2016.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E26.Holt S, Schmiedl S, Thurmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int. 2010;107:543–551. doi: 10.3238/arztebl.2010.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E27.Holt MP, Ju C. Mechanisms of drug-induced liver injury. The AAPS J. 2006;8:E48–E54. doi: 10.1208/aapsj080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E28.Chen CB, Abe R, Pan RY, et al. An updated review of the molecular mechanisms in drug hypersensitivity. J Immunol Res. 2018 doi: 10.1155/2018/6431694. 6431694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E29.Deplanque G, Gervais R, Vergnenegre A, et al. Doxycycline for prevention of erlotinib-induced rash in patients with non-small-cell lung cancer (NSCLC) after failure of first-line chemotherapy: a randomized, open-label trial. J Am Acad Dermatol. 2016;74:1077–1085. doi: 10.1016/j.jaad.2016.01.019. [DOI] [PubMed] [Google Scholar]