Abstract
Background
Routine urine culture testing is not recommended for uncomplicated urinary tract infections (UTIs). As a result, the antibiotic resistance patterns or the organisms causing UTIs are not adequately reflected in routine data. We studied the sensitivity of Escherichia coli (E. coli) to trimethoprim (TMP) and to cotrimoxazole (i.e., trimethoprim/sulfamethoxazole, TMP/SMX) in community-acquired UTI and compared the findings with the resistance data of the Antimicrobial Resistance Surveillance System (ARS).
Methods
General practitioners and internists in private practice prospectively recruited all of their adult patients with symptoms of a urinary tract infection from May 2015 to February 2016. Urine specimens from all patients were tested (including urine culture testing and antibiotic susceptibility) and infections were defined as uncomplicated or complicated UTIs.
Results
1245 participants from 58 medical practices were enrolled in the study. Pathogenic organisms were found in the urine of 877 patients, of whom 74.5% had E. coli infections. Among the E.-coli-positive UTIs, 52.4% were classified as uncomplicated and 47.6% as complicated. The prevalence of E. coli that was resistant to TMP and to TMP/SMX in uncomplicated UTIs was 15.2% and 13.0%, respectively, compared to 25.3% and 24.4%, respectively, from all UTIs in ARS in 2015. Study participants who had previously taken antibiotics had the highest prevalence of E. coli resistance (30.9%), followed by those who had two or more UTIs within the past six months (28.9%).
Conclusion
E. coli with resistance to TMP was significantly less prevalent among the study patients with uncomplicated UTIs than in the routine data of the ARS. Accordingly, TMP should still be considered as an option for the treatment of uncomplicated UTIs. TMP/SMX is considered the agent of second choice because of its side effects. Surveillance systems based on routine data do not yield a representative sample for the evaluation of the resistance situation in patients with uncomplicated UTIs.
Urinary tract infection (UTI) is one of the most common bacterial infections seen in primary care and thus one of the most common indications for which antibiotics are precribed. (1– 3). In 2013, the prevalence of the diagnoses “Urinary Tract Infection” (N39.0) or “Acute Cystitis” (N30.0) was 7.3% and 1.7%, respectively, among all females aged 12 years or older insured with the German statutory health insurer Barmer GEK (4). As the majority of the community-acquired UTIs manifested as an acute cystitis, in this study the term uncomplicated UTI primarily refers to acute uncomplicated cystitis (5). Among other studies, E. coli has been found to be one of the main causative agents (70–80%) of uncomplicated UTI (6– 8). Complicated urinary tract infections are defined as UTIs occurring in patients with risk factors for severe disease, sequelae or treatment failure. Microbiological testing of the urine prior to initiation of treatment is recommended for patients with complicated UTIs, pregnant women and patients with recurrent urinary tract infections. Uncomplicated urinary tract infections, most commonly affecting young healthy women, are caused by a narrow spectrum of causative agents. The antibiotic susceptibility of these bacteria can be predicted with high probability (9). Thus, routine urine culture testing is not recommended for practical and economic reasons; instead, uncomplicated UTIs are treated with empiric antibiotic therapy (6, 10).
Treatment recommendations for empiric antibiotic therapy are based on antimicrobial resistance data from epidemiological studies or from surveillance systems such as the Antimicrobial Resistance Surveillance System (ARS). ARS is a laboratory-based surveillance system established at the Robert Koch Institute with the aim of providing reference data on the antimicrobial resistance situation in the community-based generalist/internist and inpatient hospital care settings (11, 12).
Patterns of antibiotic resistance can show time and regional differences (13). ARS provides regular up-to-date data and is an essential source of information for the selection of appropriate empiric antibiotic therapy. However, since microbiological testing is not carried out on a routine basis for patients with uncomplicated UTIs, results in ARS for uncomplicated UTIs are underrepresented. In contrast, ARS data likely reflects the situation of complicated UTIs where urine cultures are obtained in the community-based medical practice setting. A wider bacterial spectrum and higher proportions of resistance can be expected in cases of complicated UTIs, so the occurence of antibiotic resistance in patients with uncomplicated UTIs is likely overestimated (14– 17). This could lead to the misconception that actually appropriate antibiotics are no longer recommended as first-line agents for uncomplicated UTI and that reserve antibiotics with a broader spectrum are used instead.
Trimethoprim (TMP) and co-trimoxazole (trimethoprim/sulfamethoxazole, TMP/SMX) are generally well-tolerated and cost-effective drugs, used in the past as first-line agents in the empirical management of uncomplicated urinary tract infections. Proportions of resistance should be below 20% to ensure optimum benefits from these antibiotics (6, 13, 18). Because proportions of resistance significantly greater than 20% had been found, TMP und TMP/SMX were no longer considered first-line treatments for uncomplicated UTIs in the recommendations of the German clinical practice (S3) guideline published in 2010 (6, 8, 19). Nevertheless, these agents were still among the antibiotics most frequently prescribed for the treatment of urinary tract infections (4). In the revised version of this clinical practice guideline published in 2017, TMP was once again recommended as the first-line agent (6, 20).
Our study aimed to provide up-to-date data on the antibiotic susceptibility of E. coli in community-acquired uncomplicated UTIs. Another aim was to determine if and to what extent the proportions of antibiotic resistance in uncomplicated UTIs are overestimated in ARS data. The results of this study will contribute to answering the question of how ARS routine data from urine cultures can be used to guide the management of community-acquired uncomplicated UTI in the future.
Methods
In the SARHA study (Surveillance der Antibiotikaresistenz von Harnwegsinfektionen, die ambulant erworben wurden [Surveillance of antibiotic resistance in community-acquired urinary tract infections]), the current proportions of resistance of E. coli to TMP, TMP/SMX and other antibiotics in community-acquired uncomplicated urinary tract infections were assessed. These results were compared with the resistance situation in complicated UTIs, in the participating practices in the year prior to the study period, and with the ARS resistance data from urine cultures requested by community-based general/internal medicine practices.
Study population
Among 40 microbiological laboratories participating in ARS in 2015, 4 laboratories were recruited for this study based on the following criteria:
regular transfer of outpatient care data on organisms and resistances detected in urine specimens from various regions in Germany
interest in study participation.
From May 2015 to February 2016, community-based internists and general practitioners who were clients of the participating laboratories were recruited. All patients aged 18 years or older presenting in these practices with clinical signs and symptoms of urinary tract infection (dysuria, frequent urge to urinate) were included in this study after giving their informed consent. Each participant provided one urine sample. Microbiological testing was performed on all urine samples, regardless of the recommendation of the clinical practice guideline.
Antimicrobial susceptibility testing
Pathogen identification and resistance testing was performed using automated systems. A bacterial count = 10³ colony-forming units (CFU)/mL was considered a positive urine culture (21, 22). In addition to TMP and TMP/SMX, the following antibiotics were used in the antimicrobial susceptibility testing: fosfomycin, nitrofurantoin, ampicillin, amoxicillin/clavulanic acid, and ciprofloxacin. The interpretation of the results was based on the guidelines of the European Committee on Antimicrobial Susceptibility Testing (EUCAST).
Questionnaire
The questionnaire collected information about year and month of birth, sex and the following parameters:
Pregnancy
Diabetes mellitus
Indwelling urinary catheter
Immunosuppression
Functional/anatomical abnormalities
Urologic/renal diseases
Antibiotic therapy within the last 2 weeks
Frequency of occurrence of urinary tract infections within the last 6 months.
In addition, the treating physicians stated whether they would have ordered a urine culture under routine conditions, rather than within the context of this study alone.
Statistical methods
R 3.3.1 was used for data analysis (23). Confidence intervals (95%) for proportions were calculated using the Clopper-Pearson-method (24).
In a univariable and multivariable analysis, the association of various factors with the resistance to TMP or TMP/SMX among patients with UTIs and with E.coli detected was assessed using logistic regression. “pcorr” is used to identify p-values corrected for multiple comparisons. Uncorrected p-values are to be considered as descriptive only.
For a comprehensive description of the surveillance system ARS, the comparison groups and further details on the statistical methods used, please refer to the eMethods section.
Ethics committee approval application
A study approval of the ethics committee of the Charité Universitätsmedizin Berlin is available (number EA2/008/15).
Results
Sample
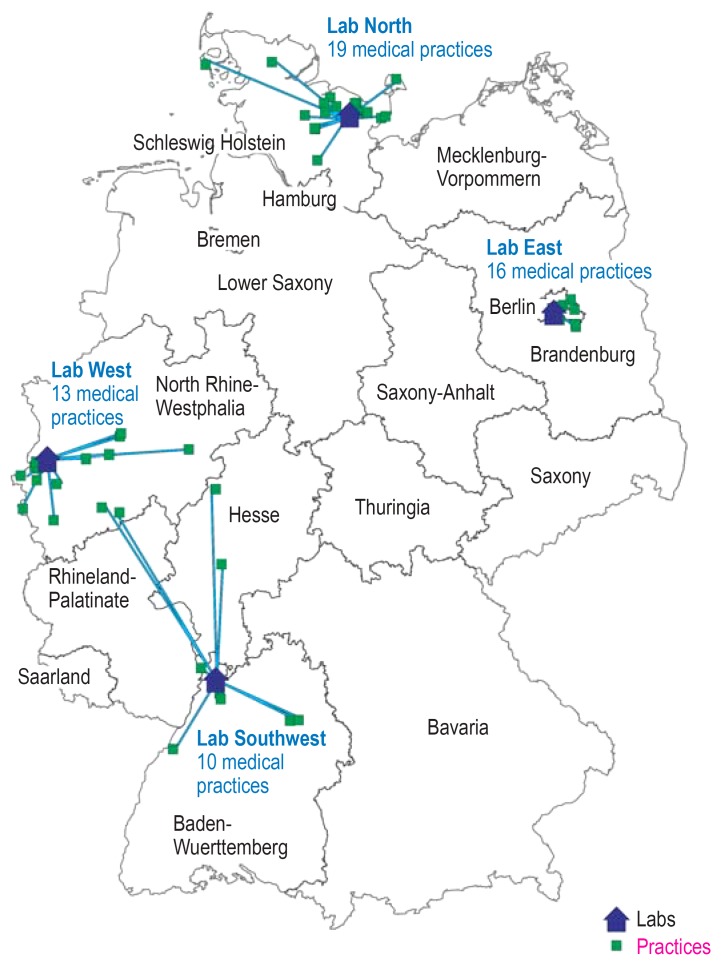
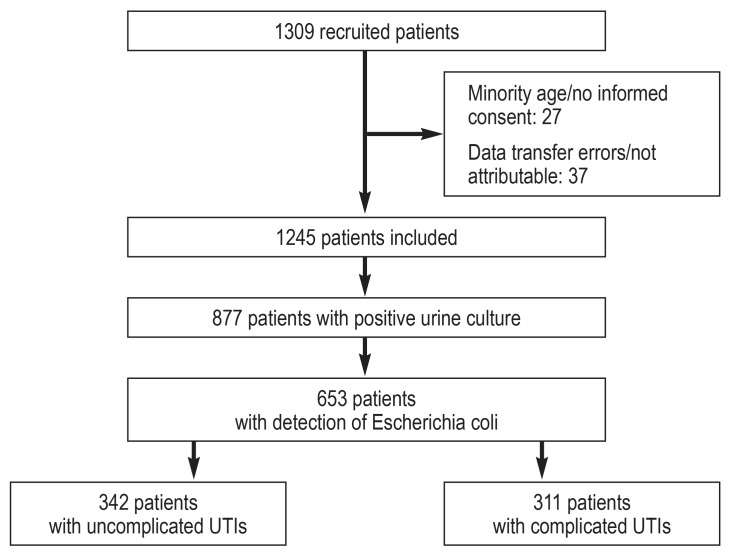
Four laboratories from various regions of Germany (north, east, west, southwest) participated in this study (efigure 1). Among 58 practices, a total of 1309 patients were recruited. Of these, 1245 patients were included in this study and their data were analyzed. Reasons for patient exclusions were missing informed consent, age <18 years (minority) or inconsistencies between sample and patient data.
eFigure 1.
Geographic distribution
of the participating laboratories and medical practices
In 877 (70.4% of 1245) patients, positive urine culture results were available (800 [91.2%] female; 77 [8.8%] male). The average age of women diagnosed with UTI was 57.5 years (standard deviation [SD] 16.1; range 18–95) and the average age of men was 68.3 years (SD 20.9; range 25–96).
In 749 samples (85.4% of samples with positive urine culture), only one organism was identified, whereas 128 (14.6%) had more than one organism. E. coli was the most commonly isolated pathogen in 653 samples (74.5%) (table 1). The frequency of occurrence of additional information about patients with E.coli detected can be seen in eTable 1. In 342 (52.4%) of the E. coli-positive samples, patients were diagnosed with uncomplicated UTIs, in 311 (47.6%) with complicated UTIs (figure 1).
Table 1. Isolated pathogens in patients with positive urine culture.
| Pathogen | Number | Percent*1 |
| Escherichia coli | 653 | 74.5 |
| Enterococcus spp. *2 | 73 | 8.3 |
| Klebsiella pneumoniae | 48 | 5.5 |
| Streptococcus group B/S.agalactiae | 35 | 4.0 |
| Proteus mirabilis | 34 | 3.9 |
| Citrobacter spp. *3 | 19 | 2.2 |
| Staphylococcus saprophyticus | 17 | 1.9 |
| Staphylococcus aureus | 11 | 1.3 |
| other | 132 | 15.1 |
| Total | 1022 |
Multiple answers were permitted
*1 The total is more than 100% as it is based on the number of patients with a positive urine culture and, in 14.6% of cases, more than one infecting organism was isolated from the urine sample.
*2 Enterococcus spp. comprise Enterococcus spp., E. faecalis, E. faecium, and E. gallinarium
*3 Citrobacter spp. comprise Citrobacter amalonaticus,
C. freundii, C. braakii, and C. koseri
eTable 1. Additional information related to patients with detected Escherichia coli.
| Number | in % | |
| At least one UTI within the last 6 months | 217 | 33.2 |
| Diabetes mellitus | 93 | 14.2 |
| Antibiotic treatment within the last 2 weeks | 72 | 11.0 |
| Urologic/renal disease | 48 | 7.4 |
| Functional/anatomic abnormalities | 42 | 6.4 |
| Immunosuppression | 18 | 2.8 |
| Indwelling urinary catheter | 11 | 1.7 |
| Pregnancy | 3 | 0.5 * |
Multiple answers were permitted;
* Percentage refers to the number of female patients with E. coli isolation
(n= 611); UTI, urinary tract infection
Figure 1.
Composition of the study population
UTI, urinary tract infection
Results of susceptibility testing
Antimicrobial susceptibility testing did not always include all antibiotics. For amoxicillin/clavulanic acid and ampicillin, the proportion of tested isolates was below 50%, and for all other antibiotics above 90%. The following proportions refer to the total number of isolates tested for each antibiotic.
For uncomplicated UTIs, the proportions of resistance of E. coli to TMP and TMP/SMX were 15.2% and 13.0%, respectively, and for complicated UTIs 26.1% and 23.3%, respectively. The corresponding proportions of resistance in the ARS routine data from urine samples collected in 2015 were 25.3% and 24.4%, respectively (table 2).
Table 2. Resistance rates of E. coli to trimethoprim and co-trimoxazole.
| Trimethoprim | Co-trimoxazole | ||||||
| Comparison group | Number of practices (n) | R in % | 95% CI | n tested | R in % | 95% CI | n tested |
| Study uncomplicated UTIs | 58 | 15.2 | [11.3; 19.2] | 315 | 13.0 | [9.4; 16.7] | 330 |
| Study complicated UTIs | 58 | 26.1 | [21.0; 31.3] | 283 | 23.3 | [18.5; 28.0] | 301 |
| Study practices May 2014 to February 2015 *1 | 51*2 | 23.4 | [18.4; 28.5] | 273 | 22.6 | [19.0; 26.2] | 526 |
| ARS 2014 | 6909 | 24.6 | [23.9; 25.4] | 13 020 | 25.4 | [25.0; 25.8] | 47 149 |
| ARS 2015 | 11 235 | 25.3 | [24.6; 25.9] | 18 347 | 24.4 | [24.0; 24.7] | 59 653 |
| ARS 2016 | 13 962 | 25.0 | [24.6; 25.5] | 32 456 | 23.2 | [22.9; 23.5] | 75 035 |
*1 Period is one year before the study period. ARS routine data without distinguishing between uncomplicated/complicated UTIs
*2 For this period, data of 51 of the total of 58 study practices are available in ARS.
ARS, Antimicrobial Resistance Surveillance; UTI, urinary tract infection; CI, confidence interval; R, proportion of resistance
The highest proportions of resistance with regard to the additional information were found among patients with previous antibiotic treatment, including 30.9% resistance to TMP (95% confidence interval: [20.2; 43.3]) and 27.1% to TMP/SMX (95% CI: [17.2; 39.1]). Similarly among patients who experienced at least two UTIs within the last 6 months, 28.9% resistance to TMP (95% CI: [22.7; 35.6]) and 25.0% to TMP/SMX (95% CI: [19.3; 31.4]), respectively, were found.
In the univariable analysis, the proportion of resistance of E. coli to TMP and TMP/SMX was significantly associated with the occurrence of = 2 UTIs within the last 6 months (TMP: Odds Ratio [OR] = 2.09; 95% CI: [1.39; 3.13], pcorr= 0,0035, TMP/SMX: OR = 1.97, 95% CI: [1.3; 2.98], pcorr= 0.013). No statistically significant associations were found for the other factors included in the analysis (etable 2). The results of the multivariable analysis are listed in eTable 3.
eTable 3. Multivariable logistic regression.
| Trimethoprim | Co-trimoxazole | ||||
| Variable | Category |
Multivariable logistic regression OR (95% CI) |
p-value |
Multivariable logistic regression OR ((95% CI) |
p-value |
| Frequency of UTI within the last 6 months (including current episode) |
1 UTI / not specified | (Reference) | (Reference) | ||
| ≥ 2 UTIs | 1.86 [1.21; 2.86] | 0.005 | 1.97 [1.29; 3.00] | 0.002 | |
| Antibiotic therapy within the last 2 weeks | no | (Reference) | (Reference) | ||
| yes | 1.46 [0.79; 2.62] | 0.213 | 1.46 [0.80; 2.59] | 0.203 | |
UTI, urinary tract infection; OR, odds ratio; 95% CI, 95% confidence interval
The proportion of resistance of E. coli to TMP stratified by laboratories/regions showed only minor variations for uncomplicated UTIs, while the regional differences were more distinct for complicated UTIs (table 3). However, none of these differences were statistically significant.
Table 3. Stratified proportions of resistance of Escherichia coli to trimethoprim in the participating laboratories.
| Uncomplicated UTIs | Complicated UTIs | |||||
| Laboratory/region | R in % | 95% CI | n tested | R in % | 95% CI | n tested |
| North | 13.4 | [6.9; 22.7] | 82 | 21.7 | [12.7; 33.3] | 69 |
| East | 16.3 | [9.4; 25.5] | 92 | 32.6 | [22.8; 43.5] | 86 |
| West | 13.8 | [6.1; 25.4] | 58 | 30.4 | [18.8; 44.1] | 56 |
| Southwest | 16.9 | [9.5; 26.7] | 83 | 19.4 | [11.1; 30.5] | 72 |
UTI, urinary tract infection; CI, confidence interval; R, proportion of resistance
Susceptibility testing of other antibiotics in patients with uncomplicated UTIs revealed lower proportions of resistance for nitrofurantoin (0.6%), fosfomycin (0.6%) and ciprofloxacin (4.5%), as well as high proportions of resistance for amoxicillin/clavulanic acid (27.3%) and ampicillin (29.3%) (etable 4). From 2013 to 2016, the proportions of resistance to TMP and fosfomycin remained constant in the ARS routine data, while a decreasing trend was noted for the resistance to TMP/SMX and nitrofurantoin (etable 5).
eTable 4. Proportions of resistance of E. coli to various antibiotics in uncomplicated/complicated UTIs.
| Uncomplicated UTIs | Complicated UTIs | |||||
| Antibiotic | R in % | 95% CI | n tested | R in % | 95% CI | n tested |
| Trimethoprim | 15.2 | [11.3; 19.2] | 315 | 26.1 | [21.0; 31.3] | 283 |
| Co-trimoxazole | 13.0 | [9.4; 16.7] | 330 | 23.3 | [18.5; 28.0] | 301 |
| Nitrofurantoin | 0.6 | [0.0; 1.5] | 327 | 1.0 | [0.0; 2.1] | 297 |
| Fosfomycin | 0.6 | [0.0; 1.5] | 325 | 2.0 | [0.4; 3.6] | 298 |
| Ciprofloxacin | 4.5 | [2.3; 6.8] | 330 | 15.6 | [11.5; 19.7] | 301 |
| Amoxicillin/clavulanic acid | 27.3 | [20.9; 33.8] | 183 | 41.0 | [33.4; 48.6] | 161 |
| Ampicillin | 29.3 | [21.9; 36.6] | 147 | 48.9 | [40.5; 57,3] | 137 |
E. coli. Escherichia coli; UTI, urinary tract infection; R, proportion of resistance; 95% CI, 95% confidence interval
Requesting of urine cultures
According to the guideline recommendations, a urine culture would have been indicated in 649 (52.1%) of the patients. According to the physicians, a routine urine culture would actually have been ordered for 409 (63.0%) patients. In 251 (42.1%) of the 596 patients in whom a urine culture would not have been necessary according to the guideline recommendations, a urine culture was requested nonetheless (etable 6). The physicians’ decision for or against requesting a urine culture did not always correlate with the guideline recommendations (phi coefficient f = 0.21).
eTable 5. Proportions of resistance of Escherichia coli in ARS routine data 2013–2016.
| 2013 | 2014 | 2015 | 2016 | ||
| Trimethoprim | R in % | 26.0 | 25.5 | 24.2 | 25.2 |
| 95% CI | [25.0; 27.0] | [24.5; 26.5] | [23.2; 25.1] | [24.2; 26.2] | |
| n tested | 7521 | 7281 | 8244 | 7318 | |
| Co-trimoxazole | R in % | 26.6 | 25.6 | 24.3 | 23.6 |
| 95% CI | [26.1; 27.2] | [25.1; 26.1] | [23.9; 24.8] | [23.1; 24.1] | |
| n tested | 25 043 | 30 960 | 32 868 | 31 247 | |
| Fosfomycin | R in % | 1.2 | 1.1 | 1.3 | 1.1 |
| 95% CI | [1.0; 1.3] | [0.9; 1.2] | [1.1; 1.4] | [1.0; 1.3] | |
| n tested | 20 457 | 24 230 | 27 952 | 26 473 | |
| Nitrofurantoin | R in % | 1.9 | 2.0 | 1.6 | 1.1 |
| 95% CI | [1.7; 2.0] | [1.8; 2.2] | [1.5; 1.8] | [1.0; 1.3] | |
| n tested | 20 314 | 24 065 | 27 810 | 26 402 | |
| Amoxicillin/clavulanic acid | R in % | 30.3 | 32.9 | 33.4 | 34.1 |
| 95% CI | [29.6; 31.0] | [32.2; 33.6] | [32.7; 34.1] | 33.5; 34.8] | |
| n tested | 14 765 | 18 083 | 18 380 | 21 352 | |
| Ciprofloxacin | R in % | 18.2 | 18.0 | 17.1 | 17.1 |
| 95% CI | [17.7; 18.7] | [17.5; 18.4] | [16.7; 17.5] | 16.7; 17.6] | |
| n tested | 23 956 | 26 467 | 28 109 | 26 609 | |
Routine data without differentiation between uncomplicated and complicated UTIs of general medicine/internal medicine practices regularly participating in the ARS from 2013–2016; ARS, Antimicrobial Resistance Surveillance system; UTI, urinary tract infection; R, proportion of resistance; 95% CI, 95% confidence interval
Discussion
In our study, we determined the proportions of resistance of E. coli in patients with community-acquired uncomplicated UTIs in 58 medical practices of community-based internists and general practitioners in various regions of Germany and compared the results with the corresponding data from the ARS system.
To the best of our knowledge, our study is the first to include all patients with the clinical diagnosis of UTI and in which the classification of uncomplicated and complicated UTIs was made at the time of the analysis, based on the additional patient information. This approach ensured that the UTIs were classified accurately according to the predefined criteria. Furthermore, the costs for all microbiological tests ordered as part of the study were covered. In this way, the risk of inclusion bias was largely minimized.
The proportions of resistance of E. coli to TMP and TMP/SMX in uncomplicated UTIs were significantly lower in our study compared to the corresponding values of the Antimicrobial Resistance Surveillance system during the same period. It can therefore be suggested that the selection of an antibiotic for the empiric therapy of an uncomplicated UTI based on ARS routine data is only permissible under certain conditions. One option to make the best use of ARS is to select sentinel practices, regularly providing urine samples of all patients with suspected UTI for microbiological testing, and to classify these patients into the groups “uncomplicated“ and “complicated“ UTIs in order to obtain data of higher quality and with lower risk of bias (25). Alternatively, validation studies could be performed on a regular basis.
The low proportions of resistance of fosfomycin, nitrofurantoin and TMP support the current recommendation in the German clinical practice guideline to use these antibiotics as first-line agents for the empiric therapy of uncomplicated UTIs (6, 20). Even though our study also found low proportions of resistance of TMP/SMX and ciprofloxacin, these drugs should not be used as first-line agents due to their unfavorable adverse effect profile. Because of its broad activity spectrum, ciprofloxacin should be reserved for the treatment of severe infections.
In recent studies from Germany, similar proportions of resistance of 17.5% (TMP) and 15.0% (TMP/SMX), respectively, were found for uncomplicated UTIs. Studies from neighboring countries arrived at similar results: Austria 15.8% (TMP)/14.4% (TMP/SMX), France 17.5% (TMP) (7, 26– 28). Since the use of TMP is associated with an increase in TMP resistance, the development of the resistance situation should be monitored, considering that TMP is now again recommended as a first-line agent (29).
The study practices’ proportions of resistance from the previous year (May 2014 until February 2015) did not differ significantly from those in all practices of general practitioners and internists (ARS 2014 and 2015) (table 2). Thus, selection bias with regard to the participating physicians appears unlikely.
The only factor in the univariable analyses that had a statistically significant association after multiple testing corrections with the proportions of resistance of E. coli to TMP and TMP/SMX was “= 2 UTIs in the previous 6 months“. Although the factor “antibiotic treatment in the past 2 weeks” was not found to be significant after multiple testing corrections, the p-value was marginally significant without this correction, thus, we believe it is also an important factor to consider. For the other studied factors even uncorrected p-values were considerably higher than 0.05. In part, this lack of significant associations may be attributed to the small sample sizes in the various categories (for example only 3 pregnant women). In patients with UTI and previous antibiotic treatment or with recurrent UTIs, switching to another antibiotic should primarily be considered in the light of the high proportions of resistance, and microbiological testing may need to be performed. In individual cases, it can be an option to treat the UTI after microbiological testing.
Physicians following the guideline recommendations send urine samples of patients with complicated UTIs for microbiological testing on a routine basis.
However, our study found only a weak association between the guideline recommendations and the decision of physicians to request a urine culture. No urine cultures were requested despite the recommendation to do so, while, on the other hand, microbiological testing was performed without recommendation. Finding differences in the approach to community-based diagnosis and treatment of UTIs compared to the guidelines is not surprising as these have already been demonstrated in various studies (25, 30, 31). This situation is not so much caused by unawareness of the existing recommendations, but it is rather related to attitudes, behavior and external barriers (32, 33).
Limitations
No information about previous hospital stays was obtained. Thus, the possibility cannot be ruled out that a small proportion of the UTIs were not community-acquired UTIs.
There is potential for selection bias because the group of patients who presented with signs and symptoms of UTI in a practice but who were not enrolled in the study is unknown.
Conclusion
The currently available evidence supports the clinical practice guideline’s recommendation of TMP, fosfomycin and nitrofurantoin for the empiric therapy in community-acquired uncomplicated UTI. Because of high proportions of resistance among patients with recurrent UTIs or after antibiotic treatment within the previous 2 weeks, it is recommended to select the appropriate antibiotic agent in this patient population based on microbiological testing.
Routine UTI resistance data obtained from surveillance systems which mainly include data on complicated UTIs with high proportions of resistance, are of limited use for treatment planning in patients with uncomplicated UTIs without additional clinical information. Validation studies or intensified surveillance by means of sentinel practices are possibilities to improve the data basis.
The decision to request microbiological testing relies only in part on the relevant guideline recommendations.
Supplementary Material
eMethods
Antimicrobial Resistance Surveillance System
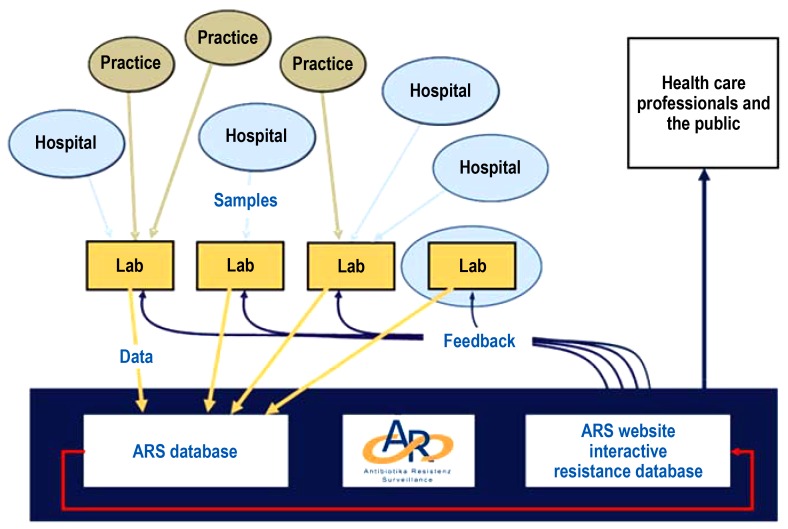
In 2008, the Antimicrobial Resistance Surveillance System (ARS) was launched at the Robert Koch Institute in accordance with the provisions of the German Antimicrobial Resistance Strategy (Deutsche Antibiotika-Resistenzstrategie, DART) (e1). The aim of the continuous monitoring and evaluation of the resistance situation and resistance development in medical facilities is to identify local areas of concern at an early stage so that appropriate interventions can be initiated (12). The voluntarily participating microbiology laboratories transfer their pathogen identification and resistance testing results from routine microbiological diagnostic assessments to ARS in a timely manner (e2). The surveillance covers all clinically relevant bacterial pathogens from all sample materials obtained in community-based practice care and hospital inpatient care. The data are transferred in a standardized form via an electronic interface to the Robert Koch Institute where the incoming data are checked for plausibility and then stored in a central database (efigure2). These data are continuously analyzed and published once a year. For most common pathogens, the resistance data of a specific period (broken down by region and level of care of the facility) are made publicly available on the website in aggregated form. The laboratories participating in ARS receive a structured feedback with sender-specific resistance statistics which they can make available to their clients (www.ars.rki.de). As a national surveillance system, ARS is a cooperation partner of the European Antimicrobial Resistance Surveillance Network (EARS-Net) (e3). For the year 2016, the ARS database contains validated data from more than 400 hospitals, 45 rehabilitation facilities and almost 14 000 medical practices.
Classification into uncomplicated and complicated urinary tract infections
Using the classification system of the clinical practice guideline, patients were assigned to either the uncomplicated urinary tract infection (UTI) or the complicated UTI group (6).
The criterion for classification as a “complicated urinary tract infections“ was the presence of at least 1 of the following factors: male sex, indwelling catheter, antibiotic treatment within the previous 2 weeks, immunosuppression, occurrence of at least 1 previous UTI within the last 6 months (recurrent UTI) as well as functional/anatomic signs and symptoms or urologic/renal disease. In all other patients and in pregnant women, patients with diabetes mellitus and elderly patients, the urinary tract infection was classified as uncomplicated, if no criterion for complicated UTI had been mentioned in the additional information (6).
Men aged younger than 30 years may also have uncomplicated UTIs; however, most cases of UTI in men are complicated UTIs. Consequently, all UTIs affecting men were classified as complicated UTIs in this study.
Recurrent UTIs can take an uncomplicated or complicated course, depending on the presence/absence of complicating factors. In our study, recurrent UTIs were classed as complicated UTIs because of the high resistance rates and the associated increased risk of treatment failure.
Microbiological testing
The clinical practice guideline recommends performing a urine culture in patients with complicated UTIs, pregnant women and patients with recurrent UTIs. For postmenopausal women, no clear recommendation is available due to the lack of high-quality studies. Provided no complicating factors were present, postmenopausal women were included in the group without recommendation for urine culture.
ARS comparative data
The data obtained in our study were compared with the resistance data of the study practices from the year before the study period (May 2014–February 2015). From altogether 51 of the 58 practices that participated in the study, urine samples had been sent to an ARS laboratory for microbiological testing during that period (table 2).
In addition, the study data were compared with ARS routine data of urine samples from community-based practices of general practitioners and internists from the years 2013 to 2016. In the years 2013, 2014, 2015, and 2016, the numbers of practices amounted to 7049, 6909, 11235, and 13 962, respectively.
Statistical methods
As a test family for the Bonferroni-Holm correction for multiple comparisons, the covariables for trimethoprim (TMP) and co-trimoxazole (TMP/SMX), respectively, were used (e4). Because the resistances to these agents are very similar, the implicit assumption of independence of a correction when looking at the regressions over both agents would be too conservative. P-values corrected in this manner are referred to as pcorr. The results of the univariable analysis were regarded significant if pcorr<0.05. Fisher’s exact test (level of significance: pcorr<0.05) was used to test for independence in 2 × 2 contingency tables.
For the multivariable analysis, all variables with p<0.05 from the univariable analysis were selected. Likelihood-ratio tests were used to check for other variables capable of improving the model (p<0.05).
Based on the information from the questionnaires, the patients were grouped according to the criterion whether or not a urine culture would have been recommended according to the clinical practice guideline. This classification was compared with the information provided by the physicians as to whether the urine samples would have been sent for microbiological testing as part of the practice routine too or only as part of this study. As a measure of the strength of this association, the Phi coefficient (f) was used.
Key messages.
In the SARHA study, the proportions of resistance of Escherichia coli to trimethoprim (TMP) or co-trimoxazole(TMP/SMX) was 15.2% and 13.0%, respectively, for uncomplicated urinary tract infections (UTIs) and thus considerably below the ARS (Antimicrobial Resistance Surveillance) urine culture routine data collected in 2015.
A statistically significant association between the proportions of resistance of E. coli to TMP or TMP/SMX and recurrent UTIs (= 2/last 6 months) was found.
In patients with recurrent UTIs and previous antibiotic treatment, microbiological testing before initiating antibiotic treatment for UTI according to the guideline is recommended.
Whether or not the participating physicians requested a urine culture correlated only in part with the recommendation in the guideline.
Routine data-based surveillance systems like for instance ARS can be used to reflect trends, but the data may overestimate resistance in uncomplicated UTIs.
eFigure 2.
Structure of the ARS network
ARS, Antimicrobial Resistance Surveillance System; RKI, Robert Koch Institute
(From: Noll I, Schweickert B, Abu Sin M, Feig M, Claus H, Eckmanns T: [Antimicrobial resistance in Germany. Four years of antimicrobial resistance surveillance (ARS)]. Bundesgesundheitsblatt – Gesundheitsforschung – Gesundheitsschutz 2012; 55: 1370–6 [12]; by courtesy of Springer Nature).
eTable 2. Prediction of resistance of Escherichia coli to trimethoprim or co-trimoxazole using univariable logistic regression.
| Trimethoprim | Co-trimoxazole | ||||
| Variable | Category |
Univariable logistic regression OR (95% CI) |
p-value (p corr) |
Univariable logistic regression OR (95% CI) |
p-value (p corr) |
|
Frequency of UTI within the last 6 months (including current episode) |
1 UTI / not known |
(reference) | (reference) | ||
| ≥ 2 HWI | 2.09 [1.39; 3.13] |
0.0004 (0.0035) |
1.97 [1.3; 2.98] |
0.001 (0.013) |
|
|
Antibiotic therapy within the last 2 weeks |
no | (reference) | (reference) | ||
| yes | 1.85 [1.04; 3.19] |
0.032 (0.254) |
1.83 [1.01; 3.19] |
0.039 (0.316) |
|
| Sex | Female | (reference) | (reference) | ||
| Male | 0.94 [0.37; 2.08] |
0.883 (1.0) |
1.16 [0.48; 2.47] |
0.722 (1.0) |
|
|
Indwelling urinary catheter |
no | (reference) | (reference) | ||
| yes | 0.99 [0.15; 4.01] |
0.989 (1.0) |
2.73 [0.71; 9.21] |
0.114 (0.798) |
|
| Immunosuppression | no | (reference) | (reference) | ||
| yes | 1.33 [0.37; 3.89] |
0.630 (1.0) |
1.32 [0.37; 3.77] |
0.630 (1.0) |
|
|
Urologic/renal disease |
no | (reference) | (reference) | ||
| yes | 0.92 [0.38; 1.95] |
0.830 (1.0) |
1.26 [0.58; 2.54] |
0.535 (1.0) |
|
|
Functional/anatomic abnormalities |
no | (reference) | (reference) | ||
| yes | 0.79 [0.29; 1.82] |
0.607 (1.0) |
0.87 (0.32; 2.01] |
0.768 (1.0) |
|
| Pregnancy | no | (reference) | (reference) | ||
| yes | 4.12 [0.16; 104.68] |
0.318 (1.0) |
4.89 [0.19; 124.48] |
0.263 (1.0) |
|
| Diabetes mellitus | no | (reference) | (reference) | ||
| yes | 0.93 [0.50; 1.63] |
0.804 (1.0) |
1.05 [0.58; 1.83] |
0.857 (1.0) |
|
UTI, urinary tract infection; 95% CI, 95% confidence interval; OR, odds ratio
eTable 6. Comparison between the physician’s decision to request a urine culture and the clinical practice guideline recommendation*.
| Physician-related categorization | Urine culture recommended | Urine culture not recommended | Total |
| a) Urine culture would have been part of practice routine | 409 | 251 | 660 |
| b) Urine culture was performed only because of the study | 239 | 344 | 583 |
| Not specified | 1 | 1 | 2 |
| Total | 649 | 596 | 1245 |
* Guideline recommendation (according to UTI clinical practice (S3) guideline [6])
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest statement The authors declare no conflict of interest.
References
- 1.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 2.Naber KG. [New aspects on diagnostics and therapy of uncomplicated cystitis] Der Urologe. 2014, Ausgabe A;53:1489–1494. doi: 10.1007/s00120-014-3564-7. [DOI] [PubMed] [Google Scholar]
- 3.Schulz M, Kern W, Hering R, Schulz M, Bätzing-Feigenbaum J. Antibiotikaverordnungen in der ambulanten Versorgung in Deutschland bei bestimmten Infektionserkrankungen in 2009 - Teil 1 und 2 Zentralinstitut für die kassenärztliche Versorgung in Deutschland (Zi). Versorgungsatlas-Bericht Nr. 14/04. Berlin 2014. www.versorgungsatlas.de/themen/versorgungsprozesse/?tab=6&uid=46 (last accessed on 22 January 2018) [Google Scholar]
- 4.Dicheva S. Glaeske G, Schicktanz C, editors. Harnwegsinfekte bei Frauen. BARMER GEK Arzneimittelreport. 2015:107–137. [Google Scholar]
- 5.Stamm WE. An epidemic of urinary tract infections? N Engl J Med. 2001;345:1055–1057. doi: 10.1056/NEJM200110043451409. [DOI] [PubMed] [Google Scholar]
- 6.Leitlinienprogramm DGU Interdisziplinäre S3 Leitlinie. Epidemiologie, Diagnostik, Therapie, Prävention und Management unkomplizierter, bakterieller, ambulant erworbener Harnwegsinfektionen bei erwachsenen Patienten Langversion 1.1-2, 2017 AWMF Registernummer: 043/044. www.awmf.org/uploads/tx_szleitlinien/043-044l_S3_Harnwegsinfektionen (last accessed on 20 June 2017) [Google Scholar]
- 7.Schmiemann G, Gagyor I, Hummers-Pradier E, Bleidorn J. Resistance profiles of urinary tract infections in general practice—an observational study. BMC Urol. 2012;12 doi: 10.1186/1471-2490-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagenlehner FME, Wagenlehner C, Savov O, Gualco L, Schito G, Naber KG. Klinik und Epidemiologie der unkomplizierten Zystitis bei Frauen. Urologe. 2010;49:253–261. doi: 10.1007/s00120-009-2145-7. [DOI] [PubMed] [Google Scholar]
- 9.Stamm WE, Hooton TM. Management of urinary tract infections in adults. N Engl J Med. 1993;329:1328–1334. doi: 10.1056/NEJM199310283291808. [DOI] [PubMed] [Google Scholar]
- 10.Rothberg MB, Wong JB. All dysuria is local A cost-effectiveness model for designing site-specific management algorithms. J Gen Intern Med. 2004;19:433–443. doi: 10.1111/j.1525-1497.2004.10440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RKI. Antibiotika-Resistenz-Surveillance. https://ars.rki.de/ (last accessed on 11 June 2018) [Google Scholar]
- 12.Noll I, Schweickert B, Abu Sin M, Feig M, Claus H, Eckmanns T. [Antimicrobial resistance in Germany. Four years of antimicrobial resistance surveillance (ARS)] Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2012;55:1370–1376. doi: 10.1007/s00103-012-1559-3. [DOI] [PubMed] [Google Scholar]
- 13.Wagenlehner FME, Hoyme U, Kaase M, Fünfstück R, Naber KG, Schmiemann G. Clinical practice guideline: uncomplicated urinary tract infections. Dtsch Arztebl int. 2011;108:415–423. doi: 10.3238/arztebl.2011.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNulty CA, Richards J, Livermore DM, et al. Clinical relevance of laboratory-reported antibiotic resistance in acute uncomplicated urinary tract infection in primary care. J Antimicrob Chemother. 2006;58:1000–1008. doi: 10.1093/jac/dkl368. [DOI] [PubMed] [Google Scholar]
- 15.Kronenberg A, Koenig S, Droz S, Muhlemann K. Active surveillance of antibiotic resistance prevalence in urinary tract and skin infections in the outpatient setting. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2011;17:1845–1851. doi: 10.1111/j.1469-0691.2011.03519.x. [DOI] [PubMed] [Google Scholar]
- 16.Nicolle LE. Complicated urinary tract infection in adults. Can J Infect Dis. 2005;16:349–360. doi: 10.1155/2005/385768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamm WE, Norrby SR. Urinary tract infections: disease panorama and challenges. J Infect Dis. 2001;183(1):1–4. doi: 10.1086/318850. [DOI] [PubMed] [Google Scholar]
- 18.Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women Infectious Diseases Society of America (IDSA) Clin Infect Dis. 1999;29:745–758. doi: 10.1086/520427. [DOI] [PubMed] [Google Scholar]
- 19.Kahlmeter G. An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO.SENS Project. J Antimicrob Chemother. 2003;51:69–76. doi: 10.1093/jac/dkg028. [DOI] [PubMed] [Google Scholar]
- 20.Kranz J, Schmidt S, Lebert C, Schneidewind L, Schmiemann G, Wagenlehner F. Uncomplicated bacterial community-acquired urinary tract infection in adults. Dtsch Arztebl Int. 2017;114:866–873. doi: 10.3238/arztebl.2017.0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmiemann G, Kniehl E, Gebhardt K, Matejczyk MM, Hummers-Pradier E. The diagnosis of urinary tract infection: a systematic review. Dtsch Arztebl Int. 2010;107:361–367. doi: 10.3238/arztebl.2010.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982;307:463–468. doi: 10.1056/NEJM198208193070802. [DOI] [PubMed] [Google Scholar]
- 23.R Core Team. www.R-project.org (last accessed on 20 January 2018) Austria; A language and environment for statistical computing. R Foundation for Statistical Computing V, [Google Scholar]
- 24.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 25.Chin TL, McNulty C, Beck C, MacGowan A. Antimicrobial resistance surveillance in urinary tract infections in primary care. J Antimicrob Chemother. 2016;71:2723–2728. doi: 10.1093/jac/dkw223. [DOI] [PubMed] [Google Scholar]
- 26.Kahlmeter G, Ahman J, Matuschek E. Antimicrobial resistance of escherichia coli causing uncomplicated urinary tract infections: a European update for 2014 and comparison with 2000 and 2008. Infect Dis Ther. 2015;4:417–423. doi: 10.1007/s40121-015-0095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamenski G, Wagner G, Zehetmayer S, Fink W, Spiegel W, Hoffmann K. Antibacterial resistances in uncomplicated urinary tract infections in women: ECOSENS II data from primary health care in Austria. BMC Infect Dis. 2012;12 doi: 10.1186/1471-2334-12-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zwirner M, Bialek R, Roth T, et al. Local resistance profile of bacterial isolates in uncomplicated urinary tract infections (LORE study) Kongressabstract DGHM. 2016 [Google Scholar]
- 29.Pouwels KB, Freeman R, Muller-Pebody B, et al. Association between use of different antibiotics and trimethoprim resistance: going beyond the obvious crude association. J Antimicrob Chemother. 2018;731:700–707. doi: 10.1093/jac/dky031. [DOI] [PubMed] [Google Scholar]
- 30.Christoffersen T, Bjerrum L, Nielsen AB. General practitioners do not systematically adhere to regional recommendations on treatment of uncomplicated urinary tract infections. Dan Med J. 2014;61 [PubMed] [Google Scholar]
- 31.Lindback H, Lindback J, Melhus A. Inadequate adherence to Swedish guidelines for uncomplicated lower urinary tract infections among adults in general practice. APMIS. 2017;125:816–821. doi: 10.1111/apm.12718. [DOI] [PubMed] [Google Scholar]
- 32.Lugtenberg M, Burgers JS, Zegers-van Schaick JM, Westert GP. Guidelines on uncomplicated urinary tract infections are difficult to follow: perceived barriers and suggested interventions. BMC Fam Pract. 2010;11 doi: 10.1186/1471-2296-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuehlein T, Goetz K, Laux G, Gutscher A, Szecsenyi J, Joos S. Antibiotics in urinary-tract infections. Sustained change in prescribing habits by practice test and self-reflection: a mixed methods before-after study. BMJ Qual Saf. 2011;20:522–526. doi: 10.1136/bmjqs.2010.047357. [DOI] [PubMed] [Google Scholar]
- E1.Bundesministerium für Gesundheit. DART- Deutsche Antibiotika-Resistenzstrategie. www.bundesgesundheitsministerium.de/themen/praevention/antibiotika-resistenzen/antibiotika-resistenzstrategie/ (last accessed on 15 February 2018) [Google Scholar]
- E2.Noll I, Eckmanns T, Abu Sin M. Antibiotikaresistenz: Vergleich mit europäischen Daten. Dtsch Arztebl. 2017;114:A2209 A2210. [Google Scholar]
- E3.Noll I, Eckmanns T. ARS - Antibiotika-Resistenz-Surveillance in Deutschland. Krankenhhyg up2date. 2013;08:125–138. [Google Scholar]
- E4.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Antimicrobial Resistance Surveillance System
In 2008, the Antimicrobial Resistance Surveillance System (ARS) was launched at the Robert Koch Institute in accordance with the provisions of the German Antimicrobial Resistance Strategy (Deutsche Antibiotika-Resistenzstrategie, DART) (e1). The aim of the continuous monitoring and evaluation of the resistance situation and resistance development in medical facilities is to identify local areas of concern at an early stage so that appropriate interventions can be initiated (12). The voluntarily participating microbiology laboratories transfer their pathogen identification and resistance testing results from routine microbiological diagnostic assessments to ARS in a timely manner (e2). The surveillance covers all clinically relevant bacterial pathogens from all sample materials obtained in community-based practice care and hospital inpatient care. The data are transferred in a standardized form via an electronic interface to the Robert Koch Institute where the incoming data are checked for plausibility and then stored in a central database (efigure2). These data are continuously analyzed and published once a year. For most common pathogens, the resistance data of a specific period (broken down by region and level of care of the facility) are made publicly available on the website in aggregated form. The laboratories participating in ARS receive a structured feedback with sender-specific resistance statistics which they can make available to their clients (www.ars.rki.de). As a national surveillance system, ARS is a cooperation partner of the European Antimicrobial Resistance Surveillance Network (EARS-Net) (e3). For the year 2016, the ARS database contains validated data from more than 400 hospitals, 45 rehabilitation facilities and almost 14 000 medical practices.
Classification into uncomplicated and complicated urinary tract infections
Using the classification system of the clinical practice guideline, patients were assigned to either the uncomplicated urinary tract infection (UTI) or the complicated UTI group (6).
The criterion for classification as a “complicated urinary tract infections“ was the presence of at least 1 of the following factors: male sex, indwelling catheter, antibiotic treatment within the previous 2 weeks, immunosuppression, occurrence of at least 1 previous UTI within the last 6 months (recurrent UTI) as well as functional/anatomic signs and symptoms or urologic/renal disease. In all other patients and in pregnant women, patients with diabetes mellitus and elderly patients, the urinary tract infection was classified as uncomplicated, if no criterion for complicated UTI had been mentioned in the additional information (6).
Men aged younger than 30 years may also have uncomplicated UTIs; however, most cases of UTI in men are complicated UTIs. Consequently, all UTIs affecting men were classified as complicated UTIs in this study.
Recurrent UTIs can take an uncomplicated or complicated course, depending on the presence/absence of complicating factors. In our study, recurrent UTIs were classed as complicated UTIs because of the high resistance rates and the associated increased risk of treatment failure.
Microbiological testing
The clinical practice guideline recommends performing a urine culture in patients with complicated UTIs, pregnant women and patients with recurrent UTIs. For postmenopausal women, no clear recommendation is available due to the lack of high-quality studies. Provided no complicating factors were present, postmenopausal women were included in the group without recommendation for urine culture.
ARS comparative data
The data obtained in our study were compared with the resistance data of the study practices from the year before the study period (May 2014–February 2015). From altogether 51 of the 58 practices that participated in the study, urine samples had been sent to an ARS laboratory for microbiological testing during that period (table 2).
In addition, the study data were compared with ARS routine data of urine samples from community-based practices of general practitioners and internists from the years 2013 to 2016. In the years 2013, 2014, 2015, and 2016, the numbers of practices amounted to 7049, 6909, 11235, and 13 962, respectively.
Statistical methods
As a test family for the Bonferroni-Holm correction for multiple comparisons, the covariables for trimethoprim (TMP) and co-trimoxazole (TMP/SMX), respectively, were used (e4). Because the resistances to these agents are very similar, the implicit assumption of independence of a correction when looking at the regressions over both agents would be too conservative. P-values corrected in this manner are referred to as pcorr. The results of the univariable analysis were regarded significant if pcorr<0.05. Fisher’s exact test (level of significance: pcorr<0.05) was used to test for independence in 2 × 2 contingency tables.
For the multivariable analysis, all variables with p<0.05 from the univariable analysis were selected. Likelihood-ratio tests were used to check for other variables capable of improving the model (p<0.05).
Based on the information from the questionnaires, the patients were grouped according to the criterion whether or not a urine culture would have been recommended according to the clinical practice guideline. This classification was compared with the information provided by the physicians as to whether the urine samples would have been sent for microbiological testing as part of the practice routine too or only as part of this study. As a measure of the strength of this association, the Phi coefficient (f) was used.