Abstract
Background
Abdominal obesity, an accumulation of fat in the abdominal region, is a risk factor for several non-communicable diseases. This review aims to identify non-surgical treatment options for abdominal obesity in adults. Interventions with behavioral, dietary, physical activity, or pharmaceutical components were compared to control conditions.
Methods
A systematic literature research for randomized controlled trials was conducted in Medline, Embase, and the Cochrane Central Register of Controlled Trials according to a prespecified and registered protocol (PROSPERO CRD42017057898).
Results
Out of 2954 articles, 15 studies with 2918 participants remained after applying inclusion and exclusion criteria. Altogether the programs achieved a –2.65cm (95% confidence interval (CI) [–3.77, –1.53]) reduction in waist circumference (WC), as a measure of abdominal obesity. Eight behavioral interventions reduced WC by –1.88cm (95% CI [–2.55, –1.22]), and six combined interventions with behavioral plus dietary and/or physical activity components by –4.11cm (95% CI [–6.17, –2.05]). The only pharmaceutical trial did not find any effect on WC.
Conclusion
Overall, the identified interventions showed a moderate effect on WC. One reason may be that in most studies WC was a secondary outcome parameter, while only a small number of interventions primarily targeted abdominal obesity. Further research regarding the treatment of abdominal obesity is urgently needed.
A Meta-Analysis and Systematic Review of Randomized Controlled Trials
The World Health Organization (WHO) defines obesity as abnormal or excessive fat accumulation that may impair health (1). Furthermore, obesity is the visible sign of a metabolic disturbance caused by excessive intake of highly processed foods loaded with sugar of any kind and fat in an unhealthy ratio of omega 6 to omega 3 fatty acids (2). Thus, especially abdominal obesity (AO) is strongly linked to most of the non-communicable diseases (NCD) identified by the WHO to be the main types (3), in particular the following four:
The highest relative mortality risk was detected for the combination of low BMI with high waist circumference (WC) or waist-to-hip ratio (WHR) (8). Although not recognized by many physicians and even less patients (9), AO represents the high risk form of obesity (10). Hence, the identification of effective therapies for AO is of importance to counter the rise in prevalence of both AO and correlated NCDs.
Prevalence and measurement
There exist several measuring methods for assessing AO. The most conventional one is measuring WC and evaluating it in combination with sex-specific cutoffs, most widespread are 80cm for women and 94cm for men (11). A more recent measure is waist-to-height ratio which takes variations in height into account and offers one threshold (0.5) for all (12), yet some researchers propose to apply a threshold of 0.6 for the elderly.
A nationally representative sample of German adults (18–79 years, n=7013) from 1998 revealed abdominal obesity (WC = 94/80 cm) in 58.1% of the male and 59.7% of the female participants (13). More recent data assessed throughout the German federal state of Baden-Württemberg in 2010 show a high prevalence of AO in primary schoolchildren and their parents (24–68 years, n=1659) with 75% of fathers and 47% of mothers being abdominally obese (waist-to-height ratio (WHtR) = 0.5) (14).
The treatment of AO may help to improve public health and save resources. The influence of exercise on abdominal fat has already been systematically reviewed (15, 16), but no systematic review for lifestyle and pharmaceutical interventions could be found in relevant databases. Therefore, the aim of this systematic review is firstly to identify therapeutic options for AO and secondly to establish a basis for further research into specific therapies. The formula depicted in Table 1 was defined according to the “Participants, Interventions, Comparisons and Outcomes (PICO)” framework (17)
Table 1. PICO formula.
| Population | Adults with abdominal obesity, overweight or obesity (BMI ≥ 25) |
| Intervention | Therapeutic interventions for abdominal obesity (excluding surgery) including behavioral, dietary, physical activity, pharmacological and combined approaches |
| Comparison | Usual care, no intervention, intervention with alternative components without influence on the outcome parameter (e.g. general health information), placebo plus the same regimen as the intervention group for pharmaceutical interventions |
| Outcome | Changes in measures of abdominal obesity (WC, WHtR, WHR, visceral adipose tissue [VAT]), treatment harms: any kind of adverse events |
| Study design | Randomized controlled trials (RCTs) |
PICO, „Participants, Interventions, Comparisons and Outcomes“; BMI, Body-mass-Index; WC, “waist circumference“; WHtR, „waist-to-height ratio“; WHR, „waist-to-hip ratio“
Methods
The protocol of this review was registered in advance at the International prospective register of systematic reviews PROSPERO (https://www.crd.york.ac.uk/PROSPERO/, registration number CRD42017057898).
Databases
Medline, Embase and the Cochrane Central Register of Controlled Trials (CENTRAL) were systematically searched for RCTs published between January 1990 and January 2017. The search strategy for Medline is illustrated in the eBox. This strategy was adapted for Embase and the CENTRAL. References were stored in the web-based reference management software package RefWorks (ProQuest LCC, Ann Arbor, MI, USA), and duplicates were eliminated program-controlled.
eBOX. Search strategy in Medline (Ovid).
exp Obesity, Abdominal
Sagittal Abdominal Diameter/ or Waist Circumference/ or Waist-Height Ratio/ or Abdominal Fat/ or Waist-Hip Ratio/ or Subcutaneous Fat, Abdominal/
(waist$ or ((abdominal$ or central$ or visceral$) adj obes$)).mp.
or/1–3
Diet Therapy/ or Exercise Therapy/ or Behavior Therapy/ or Counselling/ or Anti-Obesity Agents/ or Life Style/
Obesity, Abdominal/dt, dh, th
Obesity/dt, dh, th
Metabolic Syndrome X/dt, dh, th
or/5–8
((lifestyle or nutrition or diet$ or weight$ or exercise$ or physical activity) adj3 (program$ or counsel$ or education$ or intervention$ or therapy or trial or study)).ti,ab.
9 or 10
4 and 11
bariatric surgery/ or Obesity, Morbid/ or Pregnancy/ or Child/ or Dietary Supplements/
12 not 13
limit 14 to randomized controlled trial
(random$ adj ((control$ or clinic$) adj trial$)).ti,ab.
14 and 16
15 or 17
limit 18 to (evidence based medicine reviews or „article reviews (acp journal club)“ or „review articles“ or „article reviews (dare)“ or „topic reviews (cochrane)“)
18 not 19
limit 20 to (human and „all adult (19 plus years)“)
Study selection, data extraction and risk of bias
Two researchers (JE, DS) independently screened titles and abstracts of retrieved articles according to the prespecified inclusion and exclusion criteria as depicted in Table 2, and removed further duplicates. Discrepancies were solved by a third researcher (DK). The same procedure was applied when screening the full texts.
Table 2. Inclusion and exclusion criteria for RCTs on therapeutic options for abdominal obesity in adults.
| Inclusion | Exclusion | |
| Population | Adults (18+ years) | Children |
| Abdominal obese, overweight or obese (BMI ≥ 25) | Morbid obesity (BMI ≥ 40) | |
| Pregnancy | ||
| Overweight/obese due to disease (e.g. Hypothyreosis) or medication (e.g. Cortisone) | ||
| Cancer, eating disorder, psychiatric condition or medication, HIV, diseases of the musculoskeletal system (e.g. Parkinson, MS) | ||
| Institutionalized (hospital, retirement home) | ||
| Intervention | Behavioral, dietary, physical activity, pharmacological (e.g. Orlistat, Metformin) and combined interventions | Surgical interventions (bariatric surgery) |
| Dietary supplements | ||
| Control | No intervention, usual care, placebo, interventions without ‧influence on outcome variables (e.g. general health information) | Control condition with any suspected influence on abdominal fat accumulation |
| Outcome | Changes in measures of abdominal obesity: WC, WHtR, WHR, VAT | |
| Study design | Randomized controlled trials (RCT) | |
| Duration of intervention at least 3 months (12 weeks) | ||
| Total time of observation at least 12 months | ||
| Publication | English or German language | Conference abstracts |
BMI, Body mass index; MS, multiple sclerosis; VAT, visceral adipose tissue; WC, waist circumference; WHR, waist-to-hip ratio; WHtR, waist-to-height ratio
The data was extracted and sorted into predefined tables that had been developed as a team effort by all participating researchers. All entries were then checked by a second researcher. Extracted data included basic information on
the study details,
the characteristics of the participants,
the description of the intervention and control condition, and
the numbers of participants in each group as well as
information on drop outs and, finally,
data with regard to outcome measures, results and adverse events.
Missing information was taken from study protocols or other publications that were referenced in the text, whenever possible. The principal outcome measure was the mean difference in longitudinal changes in WC between intervention and control group. Where possible, data from a 12 months observation period, independent of the length of the intervention, was used in the meta-analysis.
Risk of bias of the included RCTs was determined using the Cochrane Collaboration’s tool for assessing risk of bias (18). Again, two researchers independently evaluated the risk of bias and conflicts were solved in discussion with a third researcher. To evaluate a publication bias, a funnel plot was generated, and a rank correlation test for asymmetry conducted.
Analysis
The meta-analysis was conducted applying the statistical software package R Release 3.2.3 for Windows (http://cran.r-project.org). Differences between intervention and control groups regarding changes in WC from baseline to a 12 month follow-up were analyzed. For multi-arm trials the number of controls was equally divided. For those studies reporting data for men and women, sex-specific analyses were conducted. Additionally, the magnitude of the correlation of weight loss in kilogram and reduction in WC was tested utilizing the Pearson correlation coefficient.
Random effect models were applied to account for differences between studies. Group size, length and type of intervention were tested in mixed effects meta-regression as moderator variables. Pooled mean differences were calculated based on raw mean differences in WC in centimeters and are reported as inverse-variance weighted averages with 95% confidence intervals (CI). To quantify heterogeneity the I2 statistic was calculated and Cochran’s Q-test was applied. Sensitivity analysis was performed by leaving out studies with extreme results.
Results
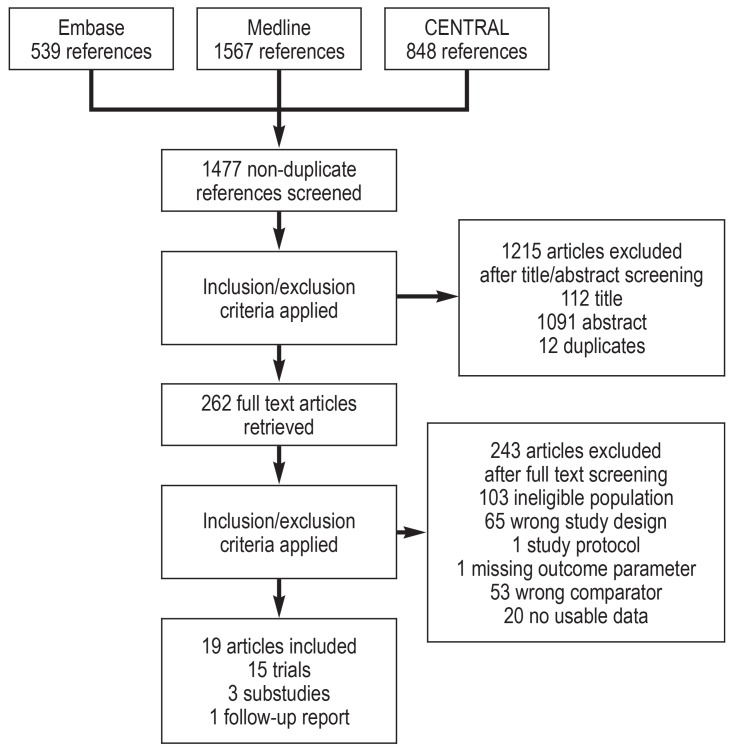
The systematic search returned 2954 records from three medical databases. Within RefWorks 1477 citations were identified as non-duplicates and were subsequently screened applying the predefined inclusion/exclusion criteria. 262 articles were retrieved for the full text screening after which 19 articles remained, reporting 15 trials. Figure 1 shows the PRISMA flow diagram of study selection.
Figure 1.
PRISMA flow diagram of study selection
CENTRAL, Cochrane Central Register of Controlled Trials
Study characteristics
The RCTs were conducted between 2001 and 2011, if specified. There were two trials from the USA and Canada, one from Australia and 12 from Europe. Eight out of 15 trials conducted a behavioral therapy intervention, six an intervention combining behavioral change strategies with a specific diet and/or physical activity program and one a pharmacological intervention. Three studies included more than one intervention group testing different types of intervention. All interventions, except for three, were described as “lifestyle intervention” and incorporated recommendations on diet and physical activity. One of these studies examined the effects of diet and physical activity each separately and combined (e1). The comparator in nine studies was usual care or no intervention, two control groups received a delayed intervention, three investigators provided self-help materials or general recommendations, and the pharmacological intervention was placebo-controlled.
The number of study participants varied between 34 and 439 per study, in sum, 2918 datasets were included in the meta-analysis. The duration of intervention varied between three months and three years with a median duration of 12 months. Measurement data at 12 months were available from 13 studies. For one study, data were available from a 6 months measurement only, and for another study, we had to use data from a 3 year measurement. Seven studies reported baseline and follow-up values, eight studies reported follow-up change scores for intervention and control group. Most studies were conducted in a university hospital or a general practitioner setting. WC in centimeters was measured in all studies and was primary or secondary outcome in eight studies. More details are found in the eTable.
eTable. Main characteristics of the included studies.
|
Author, name of program |
Country, ethnicity, year of recruitment/trial |
Setting | Participants |
Duration intervention, follow-up |
Intervention | Control | Primary outcome |
WC [cm] baseline, m (sd), raw mean difference of change score*, m [se] |
Completion rate* [%] |
| Burke et al., 2005 The Activity, Diet and Blood Pressure Trial (ADAPT) (e5) |
Australia, no year stated |
Research studies unit |
Overweight hypertensive patients aged 40–70 |
4 months, 16 months follow-up |
Lifestyle, nutrition, PA, weight loss (n=123) |
Usual care, information material (n=118) |
Anti-hypertensive drug requirements, ABP, WC |
IG: 96.6 (9.98) CG: 93.7 (9.78) –3.1 (1.35) |
IG: 82.9 CG: 76.3 |
| Clark et al., 2004 (e2) | United Kingdom, no year stated |
UK national Health Service diabetes center |
T2DM patients aged 40–70 |
3 months, 12 months follow-up |
Tailored lifestyle self-management (n=50) |
Usual care (n=50) |
Dietary behavior, PA, BMI, WC |
IG: 104.2 (10.46) CG: 101.3 (11.44) –3.87 (2.14) |
IG: 96 CG: 92 |
| Dekkers et al., 2011 (e11) ALIFE@Work |
Netherlands, 2004–2006 |
Different companies |
Healthy overweight employees |
6 months, 24 months follow-up |
Lifestyle, behavior counseling by phone (n=91) or e-mail (n=93) |
Self-help materials (n=92) |
Body weight, BMI, diet, PA, secondary outcome WC |
Phone: 99.9 (10.2) E-mail: 102.9 (11.1) CG: 101.7 (8.5) Phone: –1.1 (2.04) E-mail: –1.0 (1.94) |
Phone: 48.4 E-mail: 51.6 CG: 53.3 |
| Ferré et al., 2012 (e12) |
Spain, no year stated |
University hospital |
Patients with AO and moderate CV risk, aged 30–75 |
1 year intervention | Therapeutic lifestyle changes, PA program (n=60) |
Conventional medical care (n=82) |
Not explicitly stated, PAT ratio, WC |
IG: 104.8 (8.9) CG: 106.0 (8.7) –1.10 (1.690) |
IG: 88.2 CG: 100 |
| Foster-Schubert et al., 2012 Nutrition and Exercise in Women (NEW) (e1) |
United States of America, non-hispanic whites (85%) 2005–2009 |
Cancer research center |
Overweight-to- obese sedentary post-menopausal women |
1 year intervention |
Lifestyle change intervention, diet (D, n=118), aerobic exercise (E, n=117), combination (DE, n=108) |
Delayed intervention, advised to not change diet or exercise habits (n=87) |
Change in body weight, secondary outcome WC |
D: 94.6 (10.2) E: 95.1 (10.1) DE: 93.7 (9.9) CG: 94.8 (10.2) D: –5.3 (1.48) E: –2.9 (1.41) DE: –7.9 (1.47) |
D: 99.0 E: 90.6 DE: 92.3 CG: 92.0 |
| Franco et al., 2005 (e8) |
Sweden, no year stated |
University hospital |
Abdominal obese post-menopausal women aged 50–65 |
12 months intervention |
Growth hormone treatment (n=20) |
Placebo (n=20) |
Insulin sensitivity |
IG: 104.0 (5.42) CG: 102.0 (6.97) 0.00 (2.44) |
IG: 75 CG: 95 |
| Van Dyck et al., 2013 (e3) |
Belgium, 2007 |
University hospital |
T2DM patients aged 35–75 |
24 weeks, 1 year follow-up |
Behavioral and PA intervention (n=60) |
Usual care (n=32) |
PA and sedentary behavior |
IG: 1.05 (9.0) CG: 1.04 (11.0) –1.0 (2.09) |
IG: 98 CG: 97 |
| Gomez-Huelgas et al., 2015 (e9) |
Spain, no year stated |
Health center in Malaga |
Persons with MetS | 3 years intervention | Lifestyle intervention, recommendations on diet and daily exercise (n=298) |
General health recommendations (n=303) |
Changes in the mean values of components of MetS |
IG: 102.1 (11.4) CG: 103.0 (11.2) –2.40 (0.63) |
IG: 77.2 CG: 58.1 |
| Haapala et al., 2009 (e13) |
Finland, 2001–2002 |
Not stated | Healthy overweight volunteers aged 25–44 |
12 months intervention | Mobile phone operated weight-loss program (n=62) |
No intervention (n=63) |
Changes in body weight and WC |
IG: 98.5 (10.3) CG: 96.6 (10.4) –2.90 (0.88) |
IG: 73 CG: 63 |
| Nanchahal et al., 2017 Camden Weight Loss (CAMWEL) (e4) |
United Kingdom, 2009–2011 |
General practices | Overweight adults | 36 weeks, 12 months follow-up |
Sessions with advisors for behavior change, healthy eating, increased PA (n=191) |
Usual weight management care (n=190) |
Not explicitly stated, weight loss |
IG: 107.6 (12.78) CG: 105.8 (13.01) –1.88 (0.95) |
IG: 53.9 CG: 60.0 |
| Puhkala et al., 2015 (e14) |
Finland, no year stated |
Workplace | Male truck or bus drivers, aged 30–62, WC ≥ 100 cm |
12 months, 24 months follow-up |
Lifestyle counseling on diet, PA and sleep (n=55) |
Delayed shortened intervention (n=58) |
Not explicitly stated, percentage of weight loss |
IG: 113,8 (9.50) CG: 114.9 (10.30) –4.6 (0.99) |
IG: 85 CG: 83 |
| Ross et al., 2012 Prevention and Reduction of Obesity Through Active Living (PROACTIVE) (e6) |
Canada, 2004–2008 |
Family medicine clinics, primary care |
Sedentary, overweight, abdominally obese adults |
2 years intervention | Lifestyle-based behavioral intervention, PA and diet (n=249) |
Usual care (n= 241) |
WC, MetS | IG: 109.1 (11.05) CG: 108.0 (10.87) –1.60 (0.57) |
IG: 83.1 CG: 86.3 |
| ter Bogt et al., 2011 Groningen Overweight and Lifestyle (GOAL) (e7) |
The Netherlands, 2005–2006 |
General practices |
Overweight patients aged 40–70 with hypertension and/or dyslipidemia |
3 years intervention | Lifestyle counseling, PA and diet, pedometer (n=225) |
Usual care (n=232) |
Not explicitly stated, weight loss |
IG: 104 (7.8) CG: 105 (9.5) –1.50 (0.68) |
IG: 80 2 CG: 75.1 |
| Tuomilehto et al., 2009 (e10) |
Finland, 2004–2006 |
University hospital |
Overweight adults with mild obstructive sleep apnea |
12 weeks, 1 year follow-up |
Lifestyle intervention, very low calorie diet (n=40) |
General dietary and exercise counseling (n=41) |
Change of apnea-hypopnea index |
IG: 112.5 (8.7) CG: 105.3 (8.3) –8.6 (1.49) |
IG: 88 CG: 90 |
| Yates et al., 2009 Pre-diabetes Risk Education and PA Recommendation and Encouragement (PREPARE) (e15) |
United Kingdom, 2006–2008 |
Diabetes Research Unit |
Overweight individuals with IGT |
6 months, 12 months follow-up |
Education program promoting walking IG: no pedometer (n=31) IGP: pedometer (n=33) |
Usual care (n=32) |
OGTT 2-hour glucose value |
IG: 103 (11) IGP: 99 (12) CG: 103 (9) IG: 0.2 (2.10) IGP: 1.20 (2.11) |
IG: 94 IGP: 88 CG: 85 |
IIG, intervention group; CG, control group; m (sd), mean (standard deviation); ABP, ambulatory blood pressure; T2DM, type 2 diabetes mellitus; AO, abdominal obesity; CV, cardiovascular; PA, physical activity; PAT, peripheral artery tonometry,
IGT, impaired glucose tolerance; MetS, metabolic syndrome; OGTT, oral glucose tolerance test
* If possible, for reasons of consistency, the values at 12 months were taken
Risk of bias and completion rates
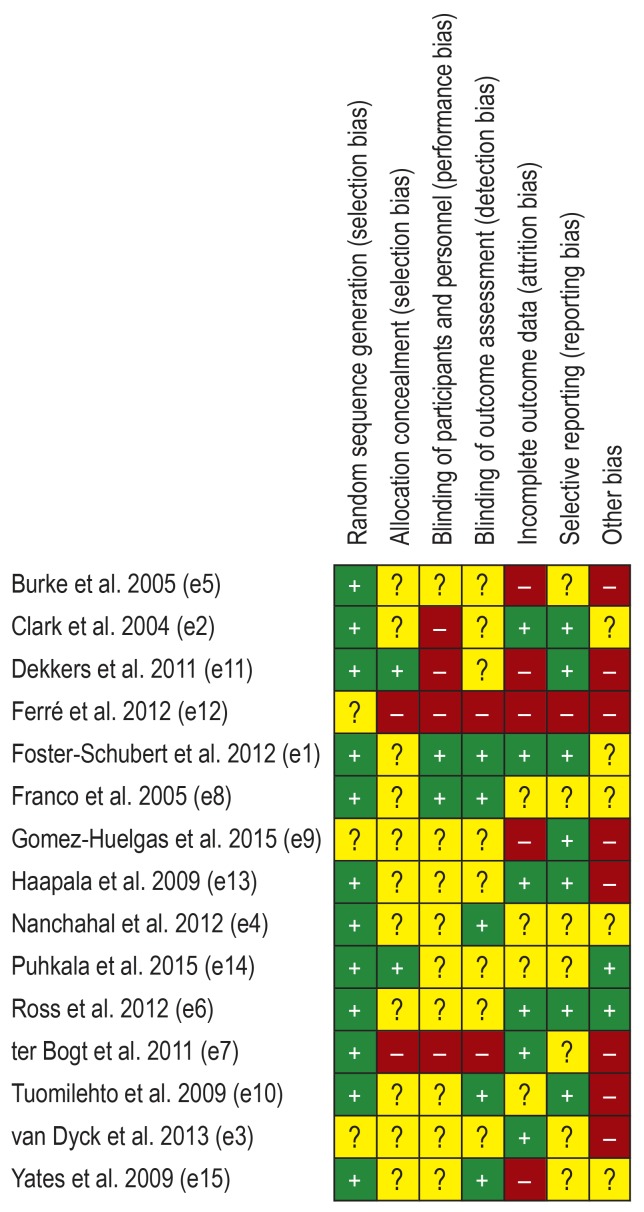
Published study protocols, where available, were taken into account to assess the risk of bias. All studies were randomized but blinding of participants and personnel was only partly possible. Some studies reported significant baseline differences. Only one study reached an overall low risk of bias (e1), all others were at high risk or unclear risk. An overview of the risk of bias in individual studies is depicted in Figure 2. A funnel plot is illustrated in the eFigure. The rank correlation test showed no significant asymmetry (Kendall’s tau=0.123, p=0.489).
Figure 2.
Overview of the risk of bias
“+”, low risk; “-”, high risk; “?”, unclear risk
eFigure.
Funnel plot of the observed outcome in the random effects model
Completion rates varied considerably between 48% and 100%. Most studies performed complete case analyses, four studies followed an intention-to-treat principle (e1– e4).
Meta-analysis
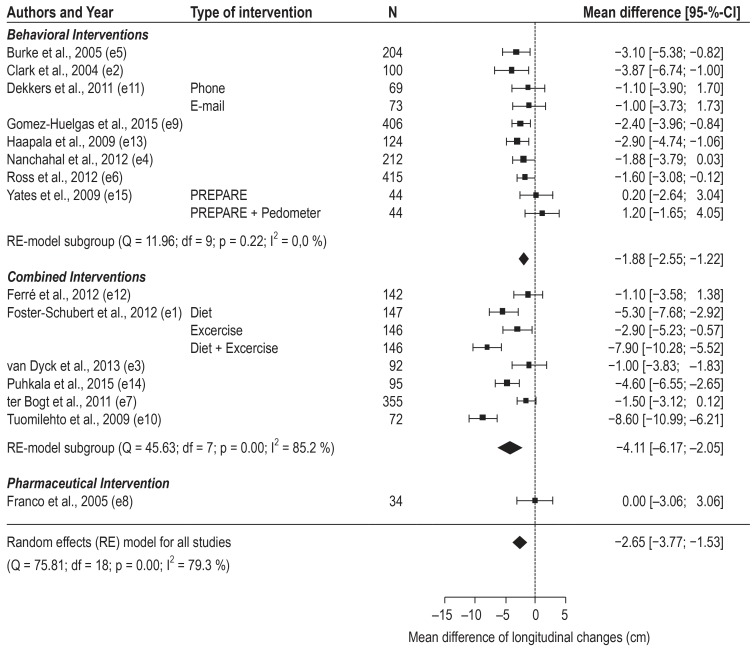
Altogether the programs achieved a –2.65cm (95% CI [–3.77; –1.53]) reduction in waist circumference (WC). Results of the random-effects meta-analysis for all interventions are depicted in Figure 3. Leaving out the sole pharmaceutical intervention leads to a slightly higher overall effect (–2.78 [–3.92; –1.63]). Participants in behavioral change programs reduced their WC on average by –1.88 cm [–2.55; –1.22], those in a combined program lost in mean –4.11cm [–6.17; –2.05]. Meta-regression revealed a significant difference of –2.39cm between both types of programs (p=0.024), favoring a combined approach. When leaving out the study with the greatest effect the difference became smaller (–1.74 [–3.59; 0.12]) and lost its significance. Three studies (e5– e7) reported sub-group analysis by gender (n=1035). Male participants lost a mean of –2.61 cm WC [–4.38; –0.83], female participants reduced their WC on average by –1.63cm [–2.76; –0.49]. Finally, weight loss in kilogram and reduction in WC were strongly correlated (r[16]=0.95, p<0.001).
Figure 3.
Forest plot of raw means of differences in change scores between intervention and control groups of included studies.
95-%-CI, 95-% confidence interval; Q, Cochran’s Q-test; I2, I2 statistic; df, degrees of freedom
Adverse events
Only six studies provided information on adverse events. Clark et al. reported three deaths during the study period, one in the intervention and two in the control group (e2). The growth hormone intervention caused side effects related to fluid retention in mild to moderate severity (e8). Gomez-Huelgas et al. indicate that during three years of study duration 15 participants died and 7 were disabled, but there is no information given regarding their respective group (e9). Ross et al. reported numbers of musculoskeletal events during or after exercise and potential cardiovascular events without differences between intervention and usual care (e6). Ter Bogt et al. lost four participants in the intervention and six in the usual care group because of disease/death (e7). Tuomilehto et al. lost two intervention participants because of intolerance of the meal replacement products used in the weight reduction program (e10).
Discussion
Initially, the conduction of a meta-analysis was not planned in the protocol because of considerable doubt that a consistent summary measure would be found, due to various possibilities of measuring and defining abdominal obesity (19). However, all included studies provided data on WC and therefore a meta-analysis was added. Results showed superiority for approaches combining behavioral counseling with either a diet or exercise program or both. Though, heterogeneity was considerable between combined interventions. The most successful intervention with regard to WC was a lifestyle intervention with a very low calorie diet (e10). The sole pharmaceutical intervention applying a growth hormone treatment did not show any changes in WC, but reported a reduction in visceral adipose tissue, measured with computed tomography scans (e8). This result also indicates a limitation of the use of WC as a proxy for visceral fat. Nonetheless, its use is strongly recommended and of vital importance for the estimation of cardiovascular risk (20).
The overall effect was only moderate with –2.65cm [–3.77; –1.53], representing a 2.5% reduction from a 102.7 cm average baseline value. However, the meta-regression confirmed the superiority of combined interventions over behavioral counseling alone. From six combined interventions four choose a physical activity (PA) component, one a very low calorie diet and one tested diet and PA separately as well as combined, with the combination showing the best result. Basically, continuing exercise training may help to maintain the results and prevent the regain of abdominal fat (21).
The overall effect was also small compared to a loss in WC of 4.4 cm which were associated with a 58% reduction in the incidence of type 2 diabetes mellitus (T2DM) in a prevention study (22). In obese Japanese men at least 3 cm reduction of WC was needed for improving metabolic syndrome (MetS) (23). One possible reason for the restricted effectiveness is that in most studies WC was a secondary outcome parameter, while only a small number of interventions primarily targeted abdominal obesity. The result of this review is also sobering in the light of the urgent need to prevent non-communicable diseases (NCD), the main cause of morbidity and mortality in the world (24).
WC is an obligatory part of the definition of the metabolic syndrome by the International Diabetes Federation (13) and the main determinant of elevated C-reactive protein in patients with metabolic syndrome (25). MetS itself is a cluster of risk factors of cardiovascular disease (CVD) with an estimated prevalence of 20–25% of the world’s adult population, bringing about a new CVD epidemic (13). The close relationship between MetS and T2DM is self-evident as well as the worldwide rise in the incidence and prevalence of both (13). Hence, the reduction of WC or even better, visceral adipose tissue, is a promising preventive measure for CVD, and T2DM, and possibly further NCDs. The American Heart Association criticizes that “WC measurements have not been well adopted in clinical practice” despite WC being “a simple and inexpensive tool for assessing body fat distribution” (26).
Strengths and Limitations
This review was, except for the meta-analysis, conducted according to a registered protocol and is, as far as applicable, reported following the PRISMA statement.
Results have to be treated with caution due to considerable heterogeneity and risk of bias in the included RCTs. Differences in components and contents, duration, intensity, completion rates and adherence to the intervention are among others causes of heterogeneity. The preference of complete-case-analysis over the intention-to-treat approach may also have influenced the results.
Conclusion
Presumably there is no sustainable intervention for AO but a permanent change of lifestyle habits with preventive ability for major NCDs, as proposed by several authors (2, 27, 28). Since losing weight is not obligatory to reduce abdominal fat (16, 29), new strategies that might even be better acceptable for patients need to be developed, easing the pressure of inevitable weight loss. Especially those with greater visceral fat mass might benefit from any intervention because visceral fat seems to be broken down preferentially compared to general body fat (30). More specific research in the treatment of abdominal obesity is urgently needed.
Key Messages.
Abdominal obesity is an underestimated health risk
Therapies for abdominal obesity need not necessarily aim at or lead to weight loss but a reduction in waist circumference
Interventions combining behavioral counseling with diet and/or physical activity are superior to behavioral counseling alone
Post-interventional reductions in waist circumference were more pronounced in males than in females
More research of effective therapies for abdominal obesity is urgently needed
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.World Health Organization (WHO) Obesity and overweight. Fact sheet 2018. www.who.int/mediacentre/factsheets/fs311/en/ (last accessed on 13 February 2018) [Google Scholar]
- 2.Lustig R. Processed food—an experiment that failed. JAMA Pediatr. 2017;171:212–214. doi: 10.1001/jamapediatrics.2016.4136. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organisation (WHO) Noncommunicable diseses. Factsheet 2015. www.who.int/mediacentre/factsheets/fs355/en/ (last accessed on 22 May 2017) [Google Scholar]
- 4.Ritchie SA, Connell JMC. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17:319–326. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Freemantle N, Holmes J, Hockey A, Kumar S. How strong is the association between abdominal obesity and the incidence of type 2 diabetes? Int J Clin Pract. 2008;62:1391–1396. doi: 10.1111/j.1742-1241.2008.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle SL, Donohoe CL, Lysaght J, Reynolds JV. Visceral adiposity, insulin resistance and cancer risk. Proc Nutr Soc. 2012;3 doi: 10.1017/S002966511100320X. [DOI] [PubMed] [Google Scholar]
- 7.Zammit C, Liddicoat H, Moonsie I, Makker H. Obesity and respiratory diseases. Int J Gen Med. 2010;3:335–343. doi: 10.2147/IJGM.S11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 9.Smith SC, Haslam D. Abdominal obesity, waist circumference and cardio-metabolic risk: awareness among primary care physicians, the general population and patients at risk—the Shape of the Nations survey. Curr Med Res Opin. 2007;23:29–47. doi: 10.1185/030079906X159489. [DOI] [PubMed] [Google Scholar]
- 10.Després J, Lemieux I, Prud D. Treatment of obesity: need to focus on high risk abdominally obese patients. Br Med J. 2001;322:716–720. doi: 10.1136/bmj.322.7288.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome 2006. www.idf.org/e-library/consensus-statements.html (last accessed on 24 May 2018) [Google Scholar]
- 12.Ashwell M, Hsieh SD. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. 2005;56:303–307. doi: 10.1080/09637480500195066. [DOI] [PubMed] [Google Scholar]
- 13.Schienkiewitz A, Mensink GBM, Scheidt-Nave C. Comorbidity of overweight and obesity in a nationally representative sample of German adults aged 18-79 years. BMC Public Health. 2012;12 doi: 10.1186/1471-2458-12-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesztyüs D, Lauer R, Kesztyüs T, Kilian R, Steinacker JM. Costs and effects of a state-wide health promotion program in primary schools in Germany—The Baden-Württemberg Study: a cluster-randomized, controlled trial. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172332. e0172332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vissers D, Hens W, Taeymans J, Baeyens J-P, Poortmans J, van Gaal L. The effect of exercise on visceral adipose tissue in overweight adults:a systematic review and meta-analysis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056415. e56415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kay SJ, Fiatarone Singh MA. The influence of physical activity on abdominal fat: a systematic review of the literature. Obes Rev. 2006;7:183–200. doi: 10.1111/j.1467-789X.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- 17.The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions [Internet]. 5.1. Higgings JPT, Green S (eds.): The Cochrane Collaboration; www.handbook.cochrane.org. .(last accessed on 24 May 2018) 2011 Chapter 8 [Google Scholar]
- 18.The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. 5.1. Higgings JPT, Green S (eds.): The Cochrane Collaboration. ww.handbook.cochrane.org (last accessed on 24 May 2018) [Google Scholar]
- 19.Onat A, Avci GS, Barlan MM, Uyarel H, Uzunlar B, Sansoy V. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int J Obes. 2004;28:1018–1025. doi: 10.1038/sj.ijo.0802695. [DOI] [PubMed] [Google Scholar]
- 20.Després JP. Waist circumference as a vital sign in cardiology 20 years after its initial publication in the American Journal of Cardiology. Am J Cardiol. 2014;114:320–323. doi: 10.1016/j.amjcard.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 21.Hunter GR, Brock DW, Byrne NM, Chandler-Laney PC, Del Corral P, Gower BA. Exercise training prevents regain of visceral fat for 1 year following weight loss. Obesity Nature Publishing Group. 2010;18:690–695. doi: 10.1038/oby.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuomilehto J, Lindström J, Eriksson J, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 23.Miyatake N, Matsumoto S, Fujii M, Numata T. Reducing waist circumference by at least 3 cm is recommended for improving metabolic syndrome in obese Japanese men. Diabetes Res Clin Pract. 2008;79:191–195. doi: 10.1016/j.diabres.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Beaglehole R, Bonita R, Horton R, et al. Priority actions for the non-communicable disease crisis. Lancet Elsevier Ltd. 2011;377:1438–1447. doi: 10.1016/S0140-6736(11)60393-0. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura H, Ito H, Egami Y, et al. Waist circumference is the main determinant of elevated C-reactive protein in metabolic syndrome. Diabetes Res Clin Pract. 2008;79:330–336. doi: 10.1016/j.diabres.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Cornier MA, Despres JP, Davis N, et al. Assessing adiposity:a scientific statement from the American Heart Association. Circulation. 2011;124:1996–2019. doi: 10.1161/CIR.0b013e318233bc6a. [DOI] [PubMed] [Google Scholar]
- 27.Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition Elsevier Inc. 2015;31:1–13. doi: 10.1016/j.nut.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Després J, Arsenault BJ, Côté M, Cartier A, Lemieux I. Abdominal obesity: the cholesterol of the 21st century? Can J Cardiol. 2008;24:7D–12D. doi: 10.1016/s0828-282x(08)71043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross R, Dagnone D, Jones PJH, et al. Diet-induced weight loss or exercise-induced weight loss in men. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 30.Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes. 2008;32:619–628. doi: 10.1038/sj.ijo.0803761. [DOI] [PubMed] [Google Scholar]
- E1.Foster-Schubert KE, Alfano CM, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity. 2012;20:1628–1638. doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Clark M, Hampson SE, Avery L, Simpson R. Effects of a tailored lifestyle self-management intervention in patients with type 2 diabetes. Br J Health Psychol. 2004;9:365–379. doi: 10.1348/1359107041557066. [DOI] [PubMed] [Google Scholar]
- E3.van Dyck D, De Greef K, Deforche B, et al. The relationship between changes in steps/day and health outcomes after a pedometer-based physical activity intervention with telephone support in type 2 diabetes patients. Health Educ Res. 2013;28:539–545. doi: 10.1093/her/cyt038. [DOI] [PubMed] [Google Scholar]
- E4.Nanchahal K, Power T, Holdsworth E, et al. A pragmatic randomized controlled trial in primary care of the Camden Weight Loss (CAMWEL) programme. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2011-000793. e000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Burke V, Beilin LJ, Cutt HE, Mansour J, Wilson A, Mori TA. Effects of a lifestyle programme on ambulatory blood pressure and drug dosage in treated hypertensive patients: a randomized controlled trial. J Hypertens. 2005;23:1241–1249. doi: 10.1097/01.hjh.0000170388.61579.4f. [DOI] [PubMed] [Google Scholar]
- E6.Ross R, Lam M, Blair SN, et al. Trial of prevention and reduction of obesity through active living in clinical settings. Arch Intern Med. 2012;172 doi: 10.1001/archinternmed.2011.1972. [DOI] [PubMed] [Google Scholar]
- E7.ter Bogt NCW, Bemelmans WJE, Beltman FW, Broer J, Smit AJ, van der Meer K. Preventing weight gain by lifestyle intervention in a general practice setting. Arch Intern Med. 2011;171:306–313. doi: 10.1001/archinternmed.2011.22. [DOI] [PubMed] [Google Scholar]
- E8.Franco C, Brandberg J, Lönn L, Andersson B, Bengtsson BÅ, Johannsson G. Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J Clin Endocrinol Metab. 2005;90:1466–1474. doi: 10.1210/jc.2004-1657. [DOI] [PubMed] [Google Scholar]
- E9.Gomez-Huelgas R, Jansen-Chaparro S, Baca-Osorio AJ, Mancera-Romero J, Tinahones FJ, Bernal-López MR. Effects of a long-term lifestyle intervention program with Mediterranean diet and exercise for the management of patients with metabolic syndrome in a primary care setting. Eur J Intern Med. 2015;26:317–323. doi: 10.1016/j.ejim.2015.04.007. [DOI] [PubMed] [Google Scholar]
- E10.Tuomilehto HPI, Seppä JM, Partinen MM, et al. Lifestyle intervention with weight reduction: First-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:320–327. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- E11.Dekkers JC, van Wier MF, Ariëns GA, et al. Comparative effectiveness of lifestyle interventions on cardiovascular risk factors among a Dutch overweight working population: a randomized controlled trial. BMC Public Health. 2011;11 doi: 10.1186/1471-2458-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E12.Ferré R, Plana N, Merino J, et al. Effects of therapeutic lifestyle changes on peripheral artery tonometry in patients with abdominal obesity. Nutr Metab Cardiovasc Dis. 2012;22:95–102. doi: 10.1016/j.numecd.2010.04.008. [DOI] [PubMed] [Google Scholar]
- E13.Haapala I, Barengo NC, Biggs S, Surakka L, Manninen P. Weight loss by mobile phone: a 1-year effectiveness study. Public Health Nutr. 2009;12 doi: 10.1017/S1368980009005230. [DOI] [PubMed] [Google Scholar]
- E14.Puhkala J, Kukkonen-Harjula K, Mansikkamäki K, et al. Lifestyle counseling to reduce body weight and cardiometabolic risk factors among truck and bus drivers—a randomized controlled trial. Scand J Work Environ Heal. 2015;41:54–64. doi: 10.5271/sjweh.3463. [DOI] [PubMed] [Google Scholar]
- E15.Yates T, Davies M, Gorely T, Bull F, Khunti K. Effectiveness of a pragmatic education programme aimed at promoting walking activity in individuals with impaired glucose tolerance: a randomized controlled trial. Diabetes Care. 2009;321:404–410. doi: 10.2337/dc09-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]