Abstract
Since prior diffusion tensor imaging (DTI) studies reported no significant differences in white matter organizations between individuals with autism spectrum disorder (ASD) and their unaffected siblings, the neural correlates for developing a clinical diagnosis among people with endophenotypes remain undetermined. We obtained DTI data from a total of 60 participants consisting of 30 people with endophenotypes and 30 people without. We first followed a conventional approach by comparing individuals with ASD and their unaffected siblings. Using region-of-interest approach, we then performed bootstrapping to examine whether the differences in white matter organizations between individuals with ASD and their unaffected siblings were substantially large, considering the distribution of differences between typically developing (TD) siblings. Conventional approaches revealed no significant differences in white matter organizations between individuals with ASD and their unaffected siblings. Bootstrapping revealed a significantly large difference in axial diffusivity in the left stria terminalis between individuals with ASD and their unaffected siblings after accounting for the distribution of differences in axial diffusivity among TD siblings (99.998 percentile). The results remained significant after controlling for multiple comparisons with Bonferroni method. We assumed that one aspect of this tract was associated with the development of a clinical diagnosis.
Keywords: ASD, bootstrapping, diffusion tensor imaging, endophenotypes, siblings
Introduction
Autism spectrum disorder and white matter
Autism spectrum disorder (ASD) is a developmental disorder characterized by social interaction deficits and restricted and repetitive behaviors (American Psychiatric Association, 2013). Neuroimaging studies have shown a miswiring connectome among individuals with ASD (Di Martino et al., 2014b; Ecker and Murphy, 2014; Ameis and Catani, 2015). Diffusion tensor imaging (DTI) is a modality for examining connectivity in the brain, and DTI studies have identified atypical structural connectivity in individuals with ASD (reviewed in Travers et al., 2012; Aoki et al., 2013). Although some results varied, the studies consistently showed atypical DTI parameters, including fractional anisotropy (FA), radial diffusivity (RD), mean diffusivity (MD) and axial diffusivity (AD), among individuals with ASD (Itahashi et al., 2015; Catani et al., 2016; Aoki et al., 2017).
Endophenotype
Endophenotypes are heritable and measurable components that are between diseases and genes (McGuffin et al., 1987). Given that ASD shows heritability (Hallmayer et al., 2011), endophenotypes emerge in the siblings of individuals with ASD (Lisiecka et al., 2015; Chien et al., 2017), regardless of their diagnostic status. Structural connectivity in the brain is one example of an ASD endophenotype (Gottesman and Gould, 2003). Indeed, prior DTI studies have revealed that individuals with ASD share alterations with their unaffected siblings (Barnea-Goraly et al., 2010; Jou et al., 2016; Chien et al., 2017), supporting the existence of a white matter ASD endophenotype. Intriguingly, no differences in any DTI parameters were observed between individuals with ASD and their unaffected siblings (Barnea-Goraly et al., 2010; Jou et al., 2016; Chien et al., 2017), leaving the neural correlates for the development of ASD among individuals with endophenotypes undetermined. However, identifying the neural basis for the development of a clinical diagnosis of ASD according to endophenotypes is informative because it may provide a clue to understanding the mechanism of the development of ASD among people with endophenotypes.
Typical variance of brain volume
The brain shows substantial growth after birth. Although the brain is under a strong genetic influence, interactions among genes and environmental factors impact growth (Peper et al., 2007). The influence of these interactions is particularly evident in white matter (Peper et al., 2007) and varies regionally within the brain (Kochunov et al., 2015). Thus, the variance of DTI parameters differs both within the brain and across individuals.
Aim of this study
The aim of this study is to examine the neural correlates for an ASD clinical diagnosis among people with ASD endophenotype. To achieve this goal, we enrolled not only individuals with ASD and their unaffected siblings but pairs of typically developing (TD) siblings. The enrollment of pairs of TD siblings is motivated by the following two concerns. First, difference between individuals with ASD and their unaffected siblings may be influenced by both similarity between siblings and ASD endophenotype. Second, we assume that the DTI parameters of individuals with ASD and their unaffected siblings may not be normally distributed because of ASD endophenotype and influence of clinical diagnosis. In other words, differences between individuals with ASD and their unaffected siblings may encompass neural correlates for the development of a clinical diagnosis. However, ASD endophenotype may impact the differences in DTI parameters between individuals with ASD and their unaffected siblings, compared with that of TD siblings without ASD endophenotype. Prior neuroimaging studies involving individuals with ASD and their unaffected siblings included only one group of TD individuals as a control. Thus, they did not account for the distribution of white matter differences between TD siblings. This might have resulted in an underestimation of the differences in white matter between siblings with and those without a clinical diagnosis of ASD. To overcome this potential drawback, the overarching goal of this study was to dissociate white matter correlates of diagnosis from ASD endophenotypes. To achieve this goal, this study examined the differences in DTI parameters between siblings discordant for ASD diagnosis while accounting for the variance of differences in parameters by enrolling pairs of TD siblings. To compare the results from our novel approach with those from prior studies, we first followed the conventional approaches to explore the differences in white matter between individuals with ASD and their unaffected siblings. Practically, we performed Tract-Based Spatial Statistics (TBSS) analyses contrasting individuals with ASD and their unaffected siblings. Second, using region-of-interest (ROI) analyses, we used paired t-tests to compare the DTI values between individuals with ASD and their unaffected siblings. Then, the similarity of white matter organizations between individuals with ASD and their unaffected siblings and the similarity between TD siblings were separately assessed using the intraclass correlation. Finally, as a novel approach, we implemented bootstrapping to examine the difference in mean DTI values between individuals with ASD and their unaffected siblings were substantially large when overlaid on the distribution of DTI parameter differences between TD siblings. To increase the homogeneity among participants, we recruited only adult males without intellectual disabilities. Furthermore, given that motion influences the DTI results, we ensured that the participants were only permitted a small amount of head motion and that head motion was accounted for in all the analyses.
Materials and methods
Participants
We analyzed data from 60 male individuals consisting of 30 pairs of biological siblings. In total 15 pairs of participants were discordant for ASD. The other set of data consisted of 15 pairs of TD siblings. The diagnosis of ASD was based on the DSM-IV-TR (American Psychiatric Association, 2000). Furthermore, the parents of the participants with ASD and their siblings were interviewed using the Autism Diagnostic Interview-Revised (ADI-R) (Tsuchiya et al., 2013) to confirm the absence of a diagnosis of ASD in the unaffected sibling. Handedness was evaluated using the Edinburgh Handedness Inventory (Oldfield, 1971). The intelligence quotient (IQ) was assessed using either the Wechsler Adult Intelligence Scale-Third Edition or the WAIS-Revised (Wechsler, 1981, 1997). All the participants completed the Japanese version of the Autism-Spectrum Quotient (Wakabayashi et al., 2006). Two of the participants had psychiatric comorbidities: one had attention-deficit/hyperactivity disorder, and the one had learning disabilities. Five of the participants were taking medication at the time of scanning: three were taking benzodiazepine, three were taking anti-depressants, and one was taking a stimulant. The absence of an Axis I diagnosis per the DSM-IV-TR was confirmed with the Mini-International Neuropsychiatric Interview (Hergueta et al., 1998). Further, the absence of a history of psychotropic medication use was required for inclusion in the TD group. The exclusion criteria for all the participants were known genetic diseases or an estimated full IQ of below 80. After a complete explanation of the study to the participants, written informed consent was obtained from all the participants. The study protocol was approved by the Ethics Committee of Showa University and was prepared in accordance with the ethical standards of the Declaration of Helsinki.
Comparison of demographic characteristics
Demographic characteristics, such as age, full IQ, verbal IQ, performance IQ, and the Edinburgh Handedness Inventory, were compared between individuals with ASD and their unaffected siblings using paired t-tests. Furthermore, paired t-tests were performed to compare the AQ scores between individuals with ASD and their unaffected siblings. The differences in abovementioned demographic characteristics and the AQ scores were also examined between TD siblings (younger brothers vs. older brothers). Finally, we used analysis of variance (ANOVA) to examine differences in the AQ scores between individuals with ASD, their unaffected siblings, and all the TD (younger and older siblings combined groups). Statistical threshold for significance was set at P < .05 for all the comparisons.
Data acquisition
All MRI data were acquired using a 3.0-T MRI scanner (MAGNETON Verio; Siemens Medical Systems, Erlangen, Germany) with a 12-channel head coil. Diffusion-weighted images were collected using an echo-planar imaging spin echo sequence with 10 b0-images and 65 directions of diffusion gradients (in-plane resolution, 2.0 × 2.0 mm; 2-mm slice thickness with no gap; repetition time, 13.7 s; echo time, 79 ms; b-value, 1000 s/mm2; matrix size, 100 × 100; phase encoding, anterior-to-posterior; 74 transversal slices; number of excitations, 1). The b0 images were acquired in an intermittent manner, and the directions of the gradients were optimized according to a previously described method (Caruyer et al., 2013).
Pre-processing and head motion
Pre-processing and the analyses were performed using FMRIB Software Library, Version 5 (FSL; http://www.fmrib.ox.ac.uk). Pre-processing included eddy current and motion corrections. Our head motion index was the mean absolute inter-volume displacement with respect to the first image of each run; the root-mean-square deviation was calculated using the FSL rmsdiff function. Since motion introduces artifacts in the DTI findings (Yendiki et al., 2014), we set the inclusion criterion for motion as a mean head motion <2.5 mm.
TBSS pre-processing
First, the data were skull-stripped using the FSL Brain Extraction Tool and registered to the Montreal Neurological Institute 152 standard space using non-linear registration. The FA images were estimated using the function ‘dtifit’ implemented in the FMRIB’s Diffusion Toolbox (FDT) software, which is a part of the FSL. Then, all the FA images were averaged to create the mean FA image and skeleton, which represents the centers of the major tracts common to the group (Smith et al., 2006). The FA threshold was set at 0.3 (Barnea-Goraly et al., 2010). Other DTI parameters, including MD, RD and AD, were projected onto the mean FA skeleton. We conducted TBSS pre-processing for two groups: the first group included only individuals with ASD and their unaffected siblings, while the second group included all the participants.
Statistical analysis
TBSS analyses
ASD vs unaffected siblings
To examine the difference in DTI parameters between individuals with ASD and their unaffected siblings, we conducted paired t-tests using the function ‘randomise’ implemented in the FSL. Age and motion were included as nuisance covariates. Statistical significance was set at a family-wise error rate (α = 0.05)-corrected Threshold-Free Cluster Enhancement P < 0.05, with a minimum cluster size of 10 voxels (Bloemen et al., 2010; Aoki et al., 2018).
ROI analyses
ROI setting
We overlaid the International Consortium for Brain Mapping DTI-81 atlas on the TBSS skeleton and created 48 ROIs focusing on the centers of the major tracts (Mori et al., 2008). The DTI values were controlled for age and motion in the following ROI analyses.
Paired t-tests
For each ROI, we conducted a paired t-test to further examine the difference in DTI metrics between adult males with ASD and their brothers. The statistical threshold for significance was set at a two-tailed P < 0.001 (=0.05/48), correcting for multiple comparisons.
Intraclass correlation
Similarity between TD siblings
We performed intraclass correlation analyses between adult male typical siblings. To divide TD sibling pairs into two groups, we focused on their birth order. This is because the advanced paternal age is associated with ASD risk. In addition, the birth order may also matter although the statistically significant difference in ASD risk was shown in comparison between the first and the third or later born offspring (Reichenberg et al., 2006; Durkin et al., 2008). To examine similarity between males with ASD and their unaffected siblings, we also performed intraclass correlation analyses for each DTI metric for each ROI. We utilized the single fixed rater intraclass correlation coefficient (ICC) to compute the genetic contribution on the DTI (Budisavljevic et al., 2016). Statistical significance was set at P < 0.001 (=0.05/48).
Bootstrapping
To evaluate the difference in DTI metrics between adult males with ASD and their unaffected brothers while accounting for the typical distribution of differences in DTI metrics between siblings, we performed bootstrapping. First, we randomly assigned each of the siblings into two TD groups and calculated the mean difference between the two groups, which provided the distribution of the differences in DTI parameters for a given ROI between TD siblings. Because of the stringent threshold for statistical significance (see below), we repeated this procedure with 100 000 iterations. Then, we overlaid the mean difference in DTI parameters between adult males with ASD and their unaffected brothers on the distribution of DTI parameters between TD siblings. The statistical threshold for significance was set at either above the 99.998 or below the 0.002 percentile, which was equivalent to a two-tailed P < 0.001 (=0.05/48).
Results
Participants’ characteristics
ASD vs unaffected siblings
There were no significant differences in age, full IQ, verbal IQ, performance IQ or handedness between individuals with ASD and their unaffected siblings. Individuals with ASD had significantly higher total and AQ subscale scores, compared with their unaffected siblings (Table 1). Unaffected siblings scored below the cutoff threshold of the ADI-R (Table 1).
Table 1.
Characteristics of the participants
| ASD (n = 15) |
Unaffected ASD siblings (n = 15) |
Statisticsa |
TDb (n = 15) |
TDc (n = 15) |
Statisticsd |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | t-value | P-value | df | t-value | P-value | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||||
| Age (years) | 28.3 | 6.1 | 28.0 | 7.3 | 14 | 0.45 | 0.657 | 28.4 | 6.5 | 25.1 | 5.3 | 14 | 5.62 | <0.001 |
| Full IQ | 111.8 | 16.0 | 107.4 | 13.2 | 14 | 0.77 | 0.452 | 115.9 | 15.7 | 114.9 | 12.1 | 14 | 0.24 | 0.815 |
| Verbal IQ | 106.3 | 31.2 | 107.1 | 16.8 | 14 | 0.08 | 0.940 | 117.1 | 15.9 | 116.7 | 12.5 | 14 | 0.18 | 0.859 |
| Performance IQ | 108.1 | 16.4 | 105.3 | 9.8 | 14 | 0.62 | 0.547 | 109.4 | 13.6 | 107.7 | 7.9 | 14 | 0.42 | 0.678 |
| Handedness | 61.8 | 68.4 | 99.3 | 2.9 | 14 | 2.11 | 0.054 | 89.2 | 26.4 | 80.7 | 51.4 | 14 | 0.55 | 0.594 |
| ADI-Re | ||||||||||||||
| Social interaction | 20.7 | 6.3 | 0.8 | 1.4 | 13 | 11.56 | <0.001 | |||||||
| Communication (verbal) | 12.6 | 5.0 | 0.4 | 1.1 | 13 | 8.83 | <0.001 | |||||||
| RRB | 3.9 | 2.0 | 0.0 | 0.0 | 13 | 7.02 | <0.001 | |||||||
| AQ | ||||||||||||||
| Total | 34.2 | 6.2 | 19.4 | 6.9 | 12 | 6.27 | <0.001 | 17.6 | 5.5 | 14.9 | 6.0 | 14 | 1.12 | 0.280 |
| AS | 7.4 | 1.6 | 3.9 | 1.8 | 12 | 6.30 | <0.001 | 4.0 | 2.1 | 3.7 | 1.4 | 14 | 0.54 | 0.596 |
| ATD | 5.4 | 1.9 | 3.2 | 1.9 | 12 | 2.67 | 0.020 | 3.4 | 2.1 | 3.9 | 1.6 | 14 | 0.88 | 0.396 |
| COM | 7.6 | 1.6 | 3.6 | 2.6 | 12 | 4.16 | 0.001 | 3.1 | 2.5 | 2.6 | 2.3 | 14 | 0.50 | 0.625 |
| IMG | 6.0 | 2.0 | 4.1 | 1.4 | 12 | 2.31 | 0.040 | 3.7 | 1.7 | 2.8 | 1.7 | 14 | 1.26 | 0.229 |
| SS | 7.9 | 2.0 | 4.5 | 2.7 | 12 | 4.26 | 0.001 | 3.4 | 2.7 | 2.0 | 2.1 | 14 | 1.38 | 0.191 |
| SESf | 5.5 | 1.1 | 5.5 | 1.1 | 14 | 0.20 | 0.843 | 5.9 | 1.2 | 5.7 | 1.1 | 13 | 0.46 | 0.655 |
| Mean head motion | 1.5 | 0.2 | 1.5 | 0.3 | 14 | 0.12 | 0.906 | 1.6 | 0.5 | 1.5 | 0.1 | 14 | 0.95 | 0.356 |
| Max head motion | 2.3 | 0.3 | 2.5 | 1.0 | 14 | 1.05 | 0.313 | 2.3 | 0.8 | 2.2 | 0.2 | 14 | 0.85 | 0.407 |
Statistics show the results of comparisons between adult males with ASD and their unaffected brothers.
Older siblings.
Younger siblings.
Statistics show the results of comparisons between typical older and younger siblings.
The ADI-R score was missing for one person.
A higher score indicates a lower socioeconomic status (Okada et al., 2014).
Abbreviations: AQ, autism-spectrum quotient; AS, attention switching/tolerance of change; ASD, autism spectrum disorder; ATD, attention to detail; COM, communication skills; IMG, imagination; IQ, intelligence quotient; RRB, restricted repetitive behaviors; SD, standard deviation; SES, socioeconomic status; SS, social skills; TD, typical development.
TD vs their TD siblings
There were no significant differences in full IQ, verbal IQ, performance IQ, handedness or AQ scores between the older and younger siblings (Table 1).
ASD vs unaffected siblings vs all TD
An ANOVA showed significant effects of group on the total and AQ subscores. Post-hoc analyses revealed that these effects were driven by the group of individuals with ASD. In terms of social skills subscale, the unaffected siblings of the individuals with ASD had significantly higher scores than the all-TD group combining both younger and older siblings (Table 2).
Table 2.
Comparison of individuals with ASD, their unaffected siblings and TD participants
| Statisticsa |
||||
|---|---|---|---|---|
| df | f-value | P-value | Post-hoc | |
| Age (years) | 2, 57 | 0.37 | 0.693 | |
| Full IQ | 2, 57 | 1.61 | 0.209 | |
| Verbal IQ | 2, 57 | 1.92 | 0.157 | |
| Performance IQ | 2, 57 | 0.38 | 0.688 | |
| Handedness | 2, 57 | 2.73 | 0.073 | |
| AQ | ||||
| Total | 2, 55 | 41.55 | <0.001 | ASD>sib=TD |
| AS | 2, 55 | 22.30 | <0.001 | ASD>sib=TD |
| ATD | 2, 55 | 5.42 | 0.007 | ASD>sib=TD |
| COM | 2, 55 | 20.93 | <0.001 | ASD>sib=TD |
| IMG | 2, 55 | 12.17 | <0.001 | ASD>sib=TD |
| SS | 2, 55 | 20.96 | <0.001 | ASD>sib>TD |
| SESb | 2, 56 | 0.31 | 0.732 | |
| Mean head motion | 2, 57 | 0.08 | 0.920 | |
| Max head motion | 2, 57 | 0.93 | 0.399 | |
Results of a comparison of three groups, individuals with ASD, their unaffected siblings, and all TD participants.
A higher score indicates a lower socioeconomic status.
Abbreviations: AQ, autism-spectrum quotient; AS, attention switching/tolerance of change; ASD, autism spectrum disorder; ATD, attention to detail; COM, communication skills; IMG, imagination; IQ, intelligence quotient; SD, standard deviation; SES, socioeconomic status; SS, social skills; TD, typical development.
TBSS analysis
No significant differences in any of the DTI parameters were seen between the individuals with ASD and their unaffected siblings.
ROI analyses
Paired t-tests
ASD vs unaffected ASD siblings
Paired t-tests showed no significant differences in any of the DTI parameters between individuals with ASD and their unaffected siblings. These results did not change when age and motion were not regressed out.
Intraclass correlation
TD and TD siblings
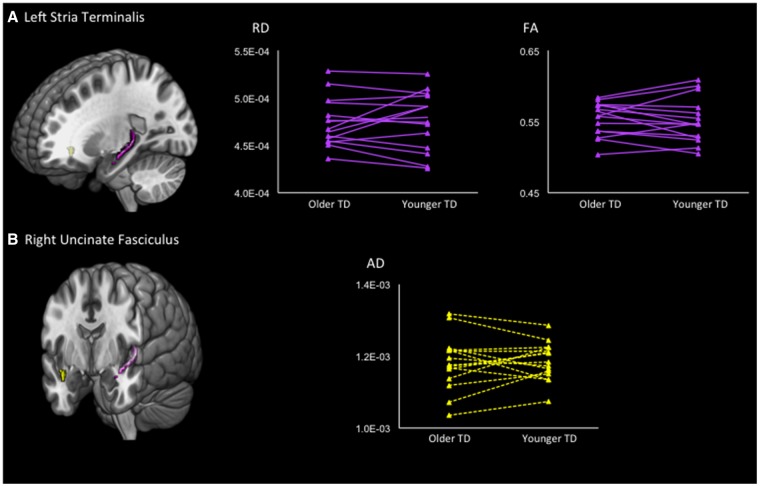
The intraclass correlation analyses showed a significant similarity in the left stria terminalis for RD (ICC = 0.772, P < 0.001) and a marginally significant correlation for FA (ICC = 0.704, P = 0.001) among typical siblings. The AD analysis showed a marginally significant intraclass correlation in the right uncinate fasciculus (ICC = 0.682, P = 0.002) (Figure 1).
Fig. 1.
Results of intraclass correlation analyses between siblings with TD. The ROIs are shown in the left column. (A) Upper panel shows the left stria terminalis. In the right column, the line graphs show the RD and FA values of pairs of siblings with TD. The left dots of each line show the DTI values of the older siblings. Intraclass correlation analyses showed a statistically significant similarity in the mean RD (ICC = 0.772, P < 0.001) and FA (ICC = 0.704, P = 0.001). (B) Axial diffusivity in the right uncinate fasciculus exhibited a statistically significant intraclass correlation (ICC = 0.682, P = 0.002).
ASD and unaffected siblings
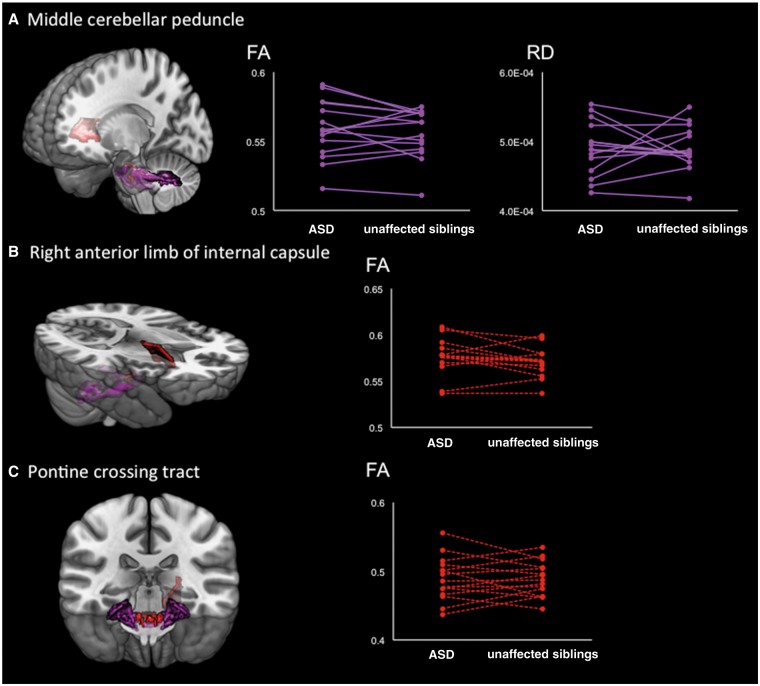
Significant intraclass correlations between individuals with ASD and their unaffected siblings were observed for FA (ICC = 0.756, P < 0.001) and RD (ICC = 0.748, P < 0.001) in the middle cerebellar peduncle. Furthermore, the FA analyses showed a marginally significant intraclass correlation in the right anterior limb of the internal capsule (ICC = 0.683, P = 0.002) and the pontine crossing tract (ICC = 0.678, P = 0.002) (Figure 2).
Fig. 2.
Results of intraclass correlation analyses between individuals with ASD and their unaffected brothers. (A) Upper panel demonstrates the results of intraclass correlation coefficients in the middle cerebellar peduncle. The mean FA (ICC = 0.756, P < 0.001) and RD (ICC = 0.748, P < 0.001) showed statistically significant similarities between individuals with ASD and their unaffected siblings. (B and C) Mean FA values in the right anterior limb of the internal capsule (ICC = 0.683, P = 0.002) and pontine crossing tract (ICC = 0.678, P = 0.002) show significant similarities at the trend level. The dots on the left tip of the lines represent individuals with ASD, while the dots on the right tip of the lines indicate their unaffected siblings.
Bootstrapping
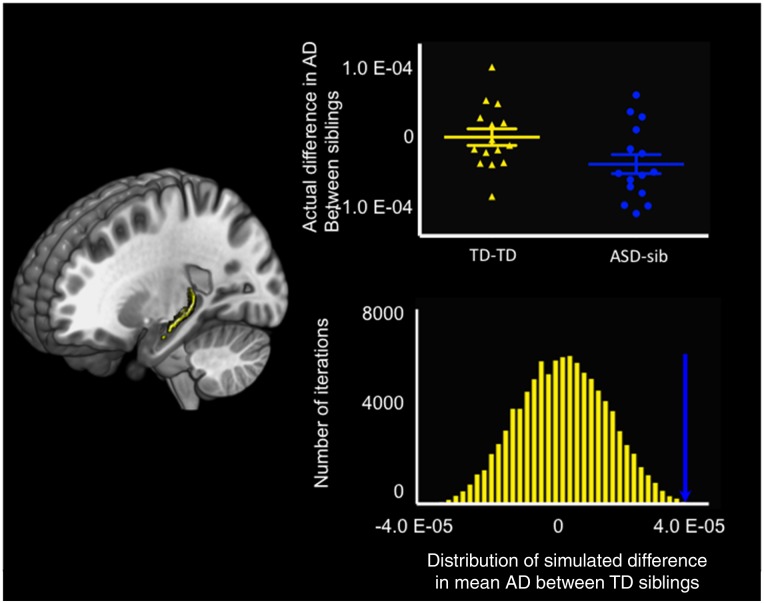
The AD analysis revealed a significantly large difference in the mean AD value between individuals with ASD and their unaffected siblings in the left stria terminalis (99.998 percentile). No other DTI metrics showed any significantly large differences in mean values between adult males with ASD and their unaffected brothers after accounting for the typical distribution of the mean differences in DTI parameters for each ROI between siblings (Figure 3). These results remained substantially unchanged when age and motion were not regressed out.
Fig. 3.
Results of bootstrapping. The left column shows the extension of the left stria terminalis. The upper subpanel in the left column demonstrates the actual difference in the mean AD values between TD siblings (shown in yellow) and the difference between individuals with ASD and their unaffected brothers (shown in blue). In the lower subpanel in the left column, the distribution of the differences in mean AD values between TD siblings as computed using bootstrapping with 100 000 iterations is shown in yellow. The blue arrow demonstrates the actual difference in mean AD values in the left stria terminalis between individuals with ASD and their unaffected brothers.
Discussion
Utilizing DTI data from 30 pairs of siblings, we dissociated white matter neural correlates for the development of a clinical ASD diagnosis from the white matter ASD endophenotype, accounting for the distribution of differences in white matter organization between TD siblings. Our novel approach revealed that the left stria terminalis was related to the development of an ASD diagnosis. On the other hand, the results of conventional approaches in this study were consistent with the results of prior studies (Barnea-Goraly et al., 2010; Jou et al., 2016; Chien et al., 2017). Specifically, comparisons between adult males with ASD and their unaffected brothers did not show any significant differences in white matter organization, regardless of whether a voxel- or cluster-level approach was used. Our novel framework suggested that such conventional approaches may underestimate differences in white matter organization between siblings discordant for ASD by not accounting for the typical distribution of responses to environmental factors of a given structure.
The stria terminalis is a bilateral structure connecting the bed nucleus of the stria terminalis (BNST) and the amygdala (Dumont, 2009). Although the function of the white matter part of the stria terminalis has not been fully elucidated, a variety of social behaviors, including social recognition and attachment, are related to both the BNST and amygdala (Insel and Young, 2001; Lebow and Chen, 2016). Research has also showed that these areas are largely influenced by genes and exhibit sexual dimorphism (Zhou et al., 1995; Goldstein et al., 2001). Despite the strong influences of genes, sex dimorphism in the BNST becomes substantial only during adulthood as a result of long-term interactions between genes and sex hormones (Chung et al., 2002). Since the current study enrolled only adult males, the present finding that typically developing siblings exhibit a high degree of similarity in RD for this region may reflect a high contribution of genes and the similar accumulated response to sex hormones.
On the other hand, adult males with ASD and their unaffected brothers showed a substantially large difference in AD in the stria terminalis. Assuming that AD reflects axonal damage (Budde et al., 2009), one potential interpretation of this result is that the difference in axonal damage may represent a consequence of the development of ASD. Another potential explanation is that the damage in this structure is related to a disorder-specific interaction between gene and sex hormones that may eventually lead to a clinical diagnosis of ASD. Intriguingly, longitudinal neuroimaging studies involving individuals with hormone replacement therapy have revealed that the white matter is vulnerable to the administration of sex hormones (reviewed in Brinton, 2016). We speculated that although the influence of sex hormones in this structure is consistent among people without ASD endophenotypes, reactions to hormones in this structure can vary among people with ASD endophenotypes. Sexual dimorphism of the BNST and amygdala, the vulnerability of white matter to sex hormones, and sex differences in ASD may support our speculation (Baron-Cohen et al., 2005; Ruta et al., 2011; Floris et al., 2013; Nordahl et al., 2015; Loomes et al., 2017).
The middle cerebellar peduncle showed high similarity between individuals with ASD and their unaffected brothers but not between TD siblings. Since we enrolled pairs of TD siblings and did not have any reasonable means of selecting one sibling from each sibling pair, this study did not compare individuals with ASD and TD. However, the ASD-specific similarities of the DTI parameters in this tract may be consistent with the results of a previous report on DTI showing an abnormality in this tract among individuals with ASD (Catani et al., 2008; Shukla et al., 2010). Therefore, our findings for the middle cerebellar peduncle may represent white matter endophenotypes of ASD.
Limitations
This study had several limitations. Because enrollment of TD siblings is the strength of the current study, we did not examine difference between individuals with ASD and TD like other studies with a larger number of participants. In addition, practical difficulties prevented us from enrolling monozygotic twins. Monozygotic twins have identical genes and are more suitable to the aim of this project. In addition, for the same reason, it was not possible to recruit siblings concordant for ASD, which may have enabled us to further confirm our findings of neural correlates for a clinical ASD diagnosis. Future studies involving multiple centers of investigation might be able to recruit a large enough sample of monozygotic twins who are either discordant or concordant for ASD. Second, although we adopted stringent thresholds for significance in novel and sound analyses, we cannot assume a causal relationship between the neuroimaging findings and the development of ASD. Furthermore, typical age for the onset of ASD is the first three years of life. Although ASD is believed to be a lifetime diagnosis, neuroimaging studies have shown atypical aging effect (Aoki et al., 2012a; Kleinhans et al., 2012; Di Martino et al., 2014a), especially during childhood (Redcay and Courchesne, 2005; Aoki et al., 2012b). Because we recruited only adults when the ASD brain does not change dramatically, the results of this study can be homogeneous. However, it cannot be generalized to children or adolescents, and the results from young children can directly address the development of the diagnosis (Hazlett et al., 2017). Future research with a longitudinal design may overcome these limitations. Third, to increase the between-subject homogeneity, we included only males. This could explain why we successfully detected a difference in the stria terminalis, which is a sexually dimorphic structure. However, the results of this study cannot be generalized to females. Given the male-skewed prevalence of ASD, a larger number of collaborators is necessary to recruit female siblings discordant for ASD, compared with the recruitment of male siblings. Open data sharing is one potential solution (Milham, 2012).
Conclusions
We utilized a novel framework to dissociate neural correlates for the development of a clinical diagnosis of ASD from neuroendophenotypes, recruiting not only siblings discordant for ASD but also pairs of typically developing siblings. The RD similarity in the left stria terminalis between TD siblings may represent genetic contribution; the AD in the structure may be related to the development of a clinical diagnosis of ASD among those who have ASD endophenotype.
Funding
This work is partially supported by JSPS KAKENHI [25861030] and the Joint Usage/Research Program of Medical Institute of Developmental Disabilities Research, Showa University (to B.Y.). This work is also partially supported by the Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from the Japan Agency for Medical Research and Development (AMED) (to R.H.).
Conflict of interest. None declared.
References
- Ameis S.H., Catani M. (2015). Altered white matter connectivity as a neural substrate for social impairment in autism spectrum disorder. Cortex, 62, 158–81. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and Statistical Manual, 4th Edn, Text Revision (DSM-IV-TR). Washington: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Arlington: American Psychiatric Pub. [Google Scholar]
- Aoki Y., Abe O., Nippashi Y., Yamasue H. (2013). Comparison of white matter integrity between autism spectrum disorder subjects and typically developing individuals: a meta-analysis of diffusion tensor imaging tractography studies. Molecular Autism, 4(1), 25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y., Abe O., Yahata N., et al. (2012a). Absence of age-related prefrontal NAA change in adults with autism spectrum disorders. Translational Psychiatry, 2(10), e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y., Kasai K., Yamasue H. (2012b). Age-related change in brain metabolite abnormalities in autism: a meta-analysis of proton magnetic resonance spectroscopy studies. Translational Psychiatry, 2(1), e69.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y., Yoncheva Y.N., Chen B., et al. (2017). Association of white matter structure with autism spectrum disorder and attention-deficit/hyperactivity disorder. JAMA Psychiatry, 74(11), 1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y., Cortese S., Castellanos F.X. (2018). Diffusion tensor imaging studies of attention-deficit/hyperactivity disorder: meta-analyses and reflections on head motion. Journal of Child Psychology and Psychiatry, 59(3), 193–202. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N., Lotspeich L.J., Reiss A.L. (2010). Similar white matter aberrations in children with autism and their unaffected siblings: a diffusion tensor imaging study using tract-based spatial statistics. Archives of General Psychiatry, 67(10), 1052–60. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Knickmeyer R.C., Belmonte M.K. (2005). Sex differences in the brain: implications for explaining autism. Science, 310(5749), 819–23. [DOI] [PubMed] [Google Scholar]
- Bloemen O.J.N., Deeley Q., Sundram F., et al. (2010). White matter integrity in Asperger syndrome: a preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Research, 3(5), 203–13. [DOI] [PubMed] [Google Scholar]
- Brinton R.D. (2016). Neuroendocrinology: oestrogen therapy affects brain structure but not function. Nature Reviews Neurology, 12(10), 561–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde M.D., Xie M., Cross A.H., Song S.K. (2009). Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. Journal of Neuroscience, 29(9), 2805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budisavljevic S., Kawadler J.M., Dell'Acqua F., et al. (2016). Heritability of the limbic networks. Social Cognitive and Affective Neuroscience, 11(5), 746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruyer E., Lenglet C., Sapiro G., Deriche R. (2013). Design of multishell sampling schemes with uniform coverage in diffusion MRI. Magnetic Resonance in Medicine, 69(6), 1534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Dell’Acqua F., Budisavljevic S., et al. (2016). Frontal networks in adults with autism spectrum disorder. Brain, 139(2), 616–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Jones D.K., Daly E., et al. (2008). Altered cerebellar feedback projections in Asperger syndrome. Neuroimage, 41(4), 1184–91. [DOI] [PubMed] [Google Scholar]
- Chien Y.L., Chen Y.J., Hsu Y.C., Tseng W.I., Gau S.S. (2017). Altered white-matter integrity in unaffected siblings of probands with autism spectrum disorders. Human Brain Mapping, 38(12), 6053–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W., De Vries G., Swaab D. (2002). Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. Journal of Neuroscience, 22(3), 1027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Fair D.A., Kelly C., et al. (2014a). Unraveling the miswired connectome: a developmental perspective. Neuron, 83(6), 1335–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Yan C.-G., Li Q., et al. (2014b). The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Molecular Psychiatry, 19(6), 659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont E.C. (2009). What is the bed nucleus of the stria terminalis? Progress in Neuropsychopharmacology and Biological Psychiatry, 33(8), 1289–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin M.S., Maenner M.J., Newschaffer C.J., et al. (2008). Advanced parental age and the risk of autism spectrum disorder. American Journal of Epidemiology, 168(11), 1268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C., Murphy D. (2014). Neuroimaging in autism–from basic science to translational research. Nature Reviews Neurology, 10(2), 82–91. [DOI] [PubMed] [Google Scholar]
- Floris D.L., Chura L.R., Holt R.J., et al. (2013). Psychological correlates of handedness and corpus callosum asymmetry in autism: the left hemisphere dysfunction theory revisited. Journal of Autism and Developmental Disorders, 43(8), 1758–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.M., Seidman L.J., Horton N.J., et al. (2001). Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex, 11(6), 490–7. [DOI] [PubMed] [Google Scholar]
- Gottesman I.I., Gould T.D. (2003). The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry, 160(4), 636–45. [DOI] [PubMed] [Google Scholar]
- Hallmayer J., Cleveland S., Torres A., et al. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry, 68(11), 1095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett H.C., Gu H., Munsell B.C., et al. (2017). IBIS Network; Clinical Sites; Data Coordinating Center; Image Processing Core; Statistical Analysis. Early brain development in infants at high risk for autism spectrum disorder. Nature, 542(7641), 348–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergueta T., Baker R., Dunbar G.C. (1998). The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IVand ICD-10. Journal of Clinical Psychiatry, 59, 2233. [PubMed] [Google Scholar]
- Insel T.R., Young L.J. (2001). The neurobiology of attachment. Nature Reviews Neuroscience, 2(2), 129–36. [DOI] [PubMed] [Google Scholar]
- Itahashi T., Yamada T., Nakamura M., et al. (2015). Linked alterations in gray and white matter morphology in adults with high-functioning autism spectrum disorder: a multimodal brain imaging study. Neuroimage: Clinical, 7, 155–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou R.J., Reed H.E., Kaiser M.D., Voos A.C., Volkmar F.R., Pelphrey K.A. (2016). White matter abnormalities in autism and unaffected siblings. The Journal of Neuropsychiatry and Clinical Neurosciences, 28(1), 49–55. [DOI] [PubMed] [Google Scholar]
- Kleinhans N.M., Pauley G., Richards T., et al. (2012). Age-related abnormalities in white matter microstructure in autism spectrum disorders. Brain Research, 1479, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P., Jahanshad N., Marcus D., et al. (2015). Heritability of fractional anisotropy in human white matter: a comparison of Human Connectome Project and ENIGMA-DTI data. Neuroimage, 111, 300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow M.A., Chen A. (2016). Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry, 21(4), 450–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisiecka D.M., Holt R., Tait R., et al. (2015). Developmental white matter microstructure in autism phenotype and corresponding endophenotype during adolescence. Translational Psychiatry, 5(3), e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes R., Hull L., Mandy W.P.L. (2017). What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 56(6), 466–74. [DOI] [PubMed] [Google Scholar]
- McGuffin P., Farmer A., Gottesman I. (1987). Is there really a split in schizophrenia? The genetic evidence. British Journal of Psychiatry, 150, 581–92. [DOI] [PubMed] [Google Scholar]
- Milham M.P. (2012). Open neuroscience solutions for the connectome-wide association era. Neuron, 73(2), 214–8. [DOI] [PubMed] [Google Scholar]
- Mori S., Oishi K., Jiang H., et al. (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage, 40(2), 570–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl C.W., Iosif A.M., Young G.S., et al. (2015). Sex differences in the corpus callosum in preschool-aged children with autism spectrum disorder. Molecular Autism, 6(1), 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N., Kasai K., Takahashi T., Suzuki M., Hashimoto R., Kawakami N. (2014). Brief rating scale of socioeconomic status for biological psychiatry research among Japanese people: a scaling based on an educational history. Japanese Journal of Biological Psychiatry, 25, 115–7. [Google Scholar]
- Oldfield R. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97.. [DOI] [PubMed] [Google Scholar]
- Peper J.S., Brouwer R.M., Boomsma D.I., Kahn R.S., Pol H., Hilleke E. (2007). Genetic influences on human brain structure: a review of brain imaging studies in twins. Human Brain Mapping, 28(6), 464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E., Courchesne E. (2005). When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biological Psychiatry, 58(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Reichenberg A., Gross R., Weiser M., et al. (2006). Advancing paternal age and autism. Archives of General Psychiatry, 63(9), 1026–32. [DOI] [PubMed] [Google Scholar]
- Ruta L., Ingudomnukul E., Taylor K., Chakrabarti B., Baron-Cohen S. (2011). Increased serum androstenedione in adults with autism spectrum conditions. Psychoneuroendocrinology, 36(8), 1154–63. [DOI] [PubMed] [Google Scholar]
- Shukla D.K., Keehn B., Lincoln A.J., Muller R.A. (2010). White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. Journal of the American Academy of Child and Adolescent Psychiatry, 49(12), 1269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Jenkinson M., Johansen-Berg H., et al. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage, 31(4), 1487. [DOI] [PubMed] [Google Scholar]
- Travers B.G., Adluru N., Ennis C., et al. (2012). Diffusion tensor imaging in autism spectrum disorder: a review. Autism Research, 5(5), 289–313., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K.J., Matsumoto K., Yagi A., et al. (2013). Reliability and validity of autism diagnostic interview-revised, Japanese version. Journal of Autism and Developmental Disorders, 43(3), 643–62. [DOI] [PubMed] [Google Scholar]
- Wakabayashi A., Baron-Cohen S., Wheelwright S., Tojo Y. (2006). The Autism-Spectrum Quotient (AQ) in Japan: a cross-cultural comparison. Journal of Autism and Developmental Disorders, 36(2), 263–70. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1981). Wechsler Adult Intelligence Scale—Revised. New York: Harcourt Brace Jovanovich. [Google Scholar]
- Wechsler D. (1997). WAIS-III: Wechsler Adult Intelligence Scale. Psychological Corporation. [Google Scholar]
- Yendiki A., Koldewyn K., Kakunoori S., Kanwisher N., Fischl B. (2014). Spurious group differences due to head motion in a diffusion MRI study. Neuroimage, 88, 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.N., Hofman M.A., Gooren L.J., Swaab D.F. (1995). A sex difference in the human brain and its relation to transsexuality. Nature, 378(6552), 68–70. [DOI] [PubMed] [Google Scholar]