Abstract
Major depressive disorder (MDD) has been associated with abnormalities in cortical thickness and autonomic function. Adolescence is a time notable for brain development and MDD onset. In healthy adolescents, greater resting state vagal activity (RVA) is associated with lower cortical thickness. The relationship between brain structural thickness and RVA in adolescents with MDD has not previously been studied. This secondary analysis drew on a sample of 37 non-depressed controls and 53 adolescents with MDD. Resting state heart rate and two indices of RVA (HF-HRV and RMSSD) were recorded during a neuroimaging session. Cortical thickness within fronto-limbic regions of interest was measured using Freesurfer analysis of T1-weighted high-resolution structural images. Self-reports of depression severity showed a significant interaction with cortical thickness of the right insula in predicting RMSSD [t = 2.22, P=0.030, β = 5.44; model fit of the interaction term as indicated by the ‘Bayes Factor’ (BF): 7.58] and HF-HRV (t = 2.09, P=0.041, β = 4.72; BF: 7.94). Clinician ratings of depression severity showed further interactions. Findings underscore the important relationships between RVA and cortical development, suggesting two possible explanations: (i) in adolescent MDD, greater fronto-limbic thickness is compensatory for deficits in autonomic regulation or (ii) increased autonomic arousal results in delayed fronto-limbic maturation. Longitudinal research is necessary to further clarify the nature of the relationship between autonomic functioning and cortical development.
Keywords: major depressive disorder, autonomic function, cortical thickness, adolescents, vagal activity
Introduction
Major depressive disorder (MDD) is one of the leading causes of disability worldwide (Vos et al., 2016), affecting individuals of all ages. Among adolescents, the worldwide prevalence of any depressive disorder is estimated at 2.6% (Polanczyk et al., 2015). MDD is characterized by differences in brain structure, including some evidence of abnormal cortical thickness (Kempton et al., 2011; Sacher et al., 2012). Independent of age, MDD is associated with autonomic nervous system (ANS) dysfunction, indexed by decreased resting state vagal activity (RVA) (Kemp et al., 2010; Koenig et al., 2016). In healthy adults, greater RVA is associated with greater cortical thickness of the prefrontal cortex (PFC) and anterior cingulate cortex (ACC) (Winkelmann et al., 2016; Yoo et al., 2018). Previously no study addressed the brain structural concomitants of altered RVA in adults or adolescent with MDD. Identifying the brain structural concomitants of altered RVA in patients with MDD may provide insights into neurobiological mechanism of MDD pathology, thereby informing novel treatment targets and markers to monitor treatment outcome.
Meta-analyses have consistently shown lower cortical thickness of the medial PFC and ACC in adult MDD patients (Koolschijn et al., 2009; Kempton et al., 2011; Arnone et al., 2012; Bora et al., 2012; Lai, 2013; Zhao et al., 2014). Recently, the largest meta-analysis to date demonstrated bilateral cortical thinning compared with controls in adult MDD in regions including the medial PFC, rostral anterior and posterior cingulate cortex, insula and fusiform gyrus, as well as the left middle temporal gyrus, right inferior temporal and right caudal ACC (Schmaal et al., 2017a). In contrast to the adult literature, findings from studies on cortical thickness in adolescent MDD have been mixed. While the recent meta-analysis found no differences in cortical thickness (Schmaal et al., 2017a), it should be noted that the majority of included adolescents were 18–21 years of age. Other studies with younger adolescent samples (aged 12–18 years) have found volumetric changes such as decreased hippocampal growth and a lack of putamen volume reduction among adolescents with MDD compared with controls (Whittle et al., 2014) and lower cortical thickness in the pericalcarine gyrus and postcentral gyrus in pediatric patients with MDD compared with controls (Fallucca et al., 2011). Further, another study found greater cortical thickness in adolescents with MDD compared with controls in the bilateral rostral middle frontal gyrus (MFG) and the left caudal ACC (Reynolds et al., 2014). Longitudinal evidence is limited. Recently, a 8-year follow-up study (12–19 years) assessed brain structural changes comparing different trajectories in the development of depressive symptoms across adolescence (Schmaal et al., 2017b). Adolescents who reported early onset of MDD symptoms that decreased over time showed alterations of surface area of the ACC and orbitofrontal cortex (OFC), moderated by sex. No effects on structural thickness were found (Schmaal et al., 2017b). Another longitudinal study using a large sample suggested that depressive symptoms are negatively associated with cortical thickness at younger ages (<9 years), but positively associated with cortical thickness at older ages (18–22 years) (Ducharme et al., 2014). MDD might be associated with a delay in cortical maturation that is related to the age of MDD onset (Truong et al., 2013). Prospective machine learning analysis suggests that cortical thickness of the OFC (in particular the right medial OFC), precentral gyrus, ACC and insula may predict the first onset of MDD in adolescents, as reductions of the thickness in these regions is associated with greater risk for the development of MDD, particularly in girls between 10 and 15 years of age (Foland-Ross et al., 2015b).
MDD-related changes in cortical thickness, particularly in regions of the fronto-limbic network, may explain differences in the top-down control of cardiac function resulting in decreased resting state RVA and increased autonomic arousal observed in depressed children, adolescents (Koenig et al., 2016) and adults (Kemp et al., 2010). The ANS is separated into two branches: the parasympathetic nervous system (PNS) and the sympathetic nervous system (SNS). PNS and SNS dually innervate many organs of the body (including the heart) and their adaptive interplay is one of the most prominent factors linking psychological processes to somatic health and disease (Thayer and Sternberg, 2006). The vagus nerve is the primary nerve of the PNS and a major inhibitory pathway adaptively regulating inflammatory, immune, endocrine, and cardiovascular function (Thayer and Sternberg, 2006; Thayer and Lane, 2009; Jarczok et al., 2014). RVA, indexed by time- or frequency-domain measures of heart rate variability (HRV) reflecting fast parasympathetic modulation of autonomic control of the heart, is inversely related to affective instability in daily life (Koval et al., 2013), self-reports on difficulties in emotion regulation (Berna et al., 2014; Beauchaine, 2015; Williams et al., 2015) and depression severity in adults (Kemp et al., 2010). Functional neuroimaging studies and human lesion studies have shown several fronto-limbic brain regions and networks involved in the neural control of RVA (Thayer et al., 2012).
Recently, Koenig et al (2018) showed that in healthy adolescents, RVA is inversely associated with cortical thickness, particularly of the rostral ACC. We found that the developmentally normative processes of (i) cortical thinning and (ii) increased RVA, go hand-in-hand; disruptions in one process may be related to disruption in the other (Silvetti et al., 2001). However, these relationships have not been examined in adolescent MDD. Investigating the interplay between brain structure and altered physiological functioning in adolescent MDD is important to understand processes of neurovisceral development and guide new treatment approaches for this age group [i.e. vagus nerve stimulation (VNS)].
This study is the first to examine cortical thickness, RVA, and depression symptoms in adolescents with and without a diagnosis of MDD, with the goal of understanding the interplay of these indices. Based on previous findings in healthy adolescents (Koenig et al. 2018), it was hypothesized that adolescents with MDD would show greater cortical thickness and lower RVA compared with non-depressed controls (CTRL), and that greater cortical thickness would be related to lower RVA. Furthermore, we considered the possibility that due to neuroadaptive processes that take place in the course of illness of depression in adolescents, the relationships between cortical thickness and RVA may differ between adolescents with vs without depression. Therefore, we examined the interaction between cortical thickness and depression [using both a categorical (MDD vs CTRL) approach and a continuous variable (depression severity based on self-reports and clinician ratings) approach] in predicting RVA, indexed by time- and frequency-domain measures of HRV, and mean heart rate (HR).
Materials and methods
General procedures
Data for this secondary analysis were taken from previous imaging studies in adolescents with MDD (Cullen et al., 2014, 2016; Hall et al., 2014; Klimes-Dougan et al., 2014; Musgrove et al., 2015; Sommerfeldt et al., 2016). The original study protocol was approved by the University of Minnesota Institutional Review Board. All participants or their legal guardians (<18 years) provided written informed consent or assent when appropriate. Adolescents with MDD and CTRL (12–19 years) were recruited to participate in the study through community postings and referrals from local mental health services. MDD participants were eligible to participate if they had a primary diagnosis of MDD. While patients were not excluded based on their medication status, most of the patients had not received any treatment with psychotropic medication in the past 2 months. Participants in the CTRL group were matched to the MDD group on age and sex and were eligible to participate if they had no current or past Axis I DSM-IV-TR psychiatric diagnoses as assessed by the ‘Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (K-SADS-PL)’ (Kaufman et al., 1997). Independent of group, participants were excluded if they endorsed the presence of a neurological or chronic medical condition, mental retardation, pervasive developmental disorder, substance use disorder, bipolar disorder, schizophrenia, or if they had an intelligence quotient (IQ) of <80 as determined by the ‘Wechsler Abbreviated Scale of Intelligence (WASI)’ (Wechsler, 1999). Additionally, participants were also excluded if they had any MRI contraindications such as braces, claustrophobia, or non-MRI safe implants.
Clinical assessments
All participants meeting the inclusion criteria and not meeting any exclusion criteria completed a comprehensive diagnostic assessment after the informed consent process. All participants completed clinical interviews that were conducted separately with adolescents and parents, and included the K-SADS-PL (Kaufman et al., 1997). MDD participants also completed the clinician-administered ‘Children’s Depression Rating Scale—Revised (CDRS-R)’ (Poznanski et al., 1985). All participants provided self-reports on depression severity in the past 2 weeks using the ‘Beck Depression Inventory II (BDI-II)’ (Beck et al., 1996; Osman et al., 2004). Parent socioeconomic status (SES) was calculated using the ‘Hollingshead Four Factor Index’ (Hollingshead, 1975), and handedness was assessed using the ‘Edinburgh Handedness Inventory’ (Oldfield, 1971).
Structural neuroimaging
Data were acquired at the Center for Magnetic Resonance Research at UMN using a Siemens 3T TIM Trio scanner. A five-minute structural scan was acquired using a T1-weighted high-resolution magnetization prepared gradient echo sequence: TR = 2530 ms; TE = 3.65 ms; TI = 1100 ms; flip angle = 7°; 1 mm slices, FOV = 256, voxel size 1 × 1 × 1 mm; GRAPPA = 2. FreeSurfer Version 5.3 (surfer.nmr.mgh.harvard.edu) was used to process T1 data including brain extraction and parcellation of data into a standard set of anatomically based regions of white and grey matter. Cortical thickness was calculated using automated cortical parcellation from FreeSurfer version 5.3 using the Desikan–Killiany atlas (Desikan et al., 2006). This method uses both intensity and continuity information from the entire 3D MR volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white matter boundary to the gray matter/CSF boundary at each vertex on the tessellated surface (Fischl and Dale, 2000). The technical details of these procedures are described in prior publications (Fischl et al., 2004; Han et al., 2006; Jovicich et al., 2006). Analyses of structural thickness included nine ROIs each for the left and the right hemisphere, defined prior to the analysis, covering brain areas implicated in depression and autonomic control: ‘Caudal ACC’, ‘Caudal MFG’, ‘Lateral OFC’, ‘Medial OFC’, ‘Rostral ACC’, ‘Rostral MFG’, ‘Superior Frontal’, ‘Frontal Pole’ and the ‘Insula’. The selection of ROIs was based on previous studies addressing the association of structural thickness and RVA (Woodward et al., 2008; Winkelmann et al., 2016; Koenig et al. 2018; Yoo et al., 2018).
Analysis of HR and its variability
Reporting of HRV processing and analyses adheres to the ‘Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH)’ (Quintana et al., 2016). Pulse-to-pulse intervals were derived using the built-in Siemens MRI-compatible ‘photoplethysmograph (PPG)’ placed on the right index finger, recorded at a sample rate of 50 Hz for 6 min during an initial resting state scan. During the resting state scan, subjects were instructed to rest awake with their eyes closed, and to not think about anything in particular. A segment of 5 min was selected for analysis of HR and HRV, discarding 30 s each at the beginning and end of the recoding. Pulse-to-pulse intervals are a sufficient proxy for cardiac inter-beat-intervals (IBIs) and suitable for analysis of HR and HRV (Selvaraj et al., 2008; Lu et al., 2009; Gil et al., 2010). Readout of pulse oximetry data was automated in MATLAB to determine IBIs based on the built-in Siemens peak detection algorithm, as described in Gaebler et al. (2013). IBIs were written in a text file and later analyzed using Kubios HRV analysis package 2.2 (Tarvainen et al., 2014), allowing for the calculation of time- and frequency-domain indices of RVA. Data processing was done by the first author, who was blinded to group membership of the participants at the time of processing the data. The level of artifact removal was selected based on visual inspection of the IBI signal in Kubios. In total, brain imaging data from n = 110 subjects were available (n = 70 with MDD). Simultaneous PPG data from n = 20 subjects (n = 17 with MDD) were either not available or had too many noise artifacts. PPG recordings of n = 7 subjects were lost due to a technical error. PPG data from n = 13 participants were not properly recorded or too noisy as determined by visual inspection and removed from further analysis during processing. Group differences on subjects with PPG data and those with missing PPG data are provided in Supplementary Table S1. In brief, the majority of subjects with missing data were female MDD patients. Smoothing priors were selected as detrending method (λ500) for IBI data. The frequency band for the assessment of power in the HF band was adjusted to the adolescent population as recently suggested (0.2–1 Hz) (Zisner and Beauchaine, 2017). The square root of the mean squared difference of successive IBIs (RMSSD) measured in milliseconds (ms) and HF-HRV were derived as time- and frequency-domain measure of RVA (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). HF-HRV was determined as absolute power (ms2) from autoregressive models. HF-HRV data were not normally distributed (χ2 = 12.83, P=0.002), and successfully log-transformed before analysis (χ2=0.94, P=0.624). RMSSD (χ2=3.44, P=0.179), HR (χ2=1.76, P=0.418) showed no skewness or kurtosis.
Statistical analysis
Subjects with missing HR/HRV data were excluded case-wise from analyses. Subjects were not excluded from analyses if they had any other missing data. Differences between groups on sociodemographic, clinical variables, indices of cardiac function and structural thickness were first tested using two-sided independent-samples t tests for continuous variables and χ2 tests for categorical variables. Cohen’s D and its 95% confidence interval (CI) were derived as measure of effect size. Group differences on HR, HF-HRV and RMSSD, as well as cortical thickness were first analyzed using two-sided independent-samples t tests, followed by regression analysis adjusting for sex and medication status. To measure the association between indices of cardiac function, cortical thickness and continuous measures of depression severity (CDRS, BDI), regression analyses adjusting for sex and medication status were calculated. To assess differences in the brain structural concomitants of cardiac function between groups and based on depression severity, regression analyses were calculated. Main and interaction effects of depression (categorical: MDD; continuous: BDI, CDRS) with structural thickness (continuous) in selected ROIs in predicting indices of cardiac function (HR, HF-HRV or RMSSD) were tested. Standardized beta-coefficients (β) were derived from regression models. Models revealing a significant F-test and interaction were subjected to further analyses to determine the model fit, and additional variance explained by the interaction. Models including the interaction were compared with models including main effects only using the ‘Bayes Factor (BF)’. The interpretation of the BF by Raftery (1995) was used to interpret the level of evidence (i.e. a BF ≥ 3 was considered as positive evidence). This approach was chosen to account for multiple testing in a sequential frequentist approach. Only models with superior model fit of the interaction term (indicated by a BF ≥ 3) are reported. Full reporting of all results from regression analyses is provided in the Supplementary data. All analyses were performed using Stata (Version 14; StataCorp LP, College Station, TX, USA), at an α level of 0.05. All Graphs were prepared using GraphPad Prism version 7.0 (GraphPad Software Inc.), with the exception of contour plots to illustrate continuous by continuous interactions that were prepared in Stata.
Results
Sample characteristics
A sample of 90 adolescents was available for analyses (53 MDD patients; 12–19 years, mean age: 16.04; SD = 1.93; 67.8% female). Adolescents with MDD most frequently endorsed comorbid diagnoses of generalized anxiety disorder or attention deficit-hyperactivity disorder. Patients with MDD and CTRL did not differ on sex [χ2(1) = 0.24, P=0.621], race [χ2(1) = 0.907, P=0.341], age [t(88) = 1.15, P=0.254], weight [t(88) =−0.29, P=0.772] or height [t(88)=−0.32, P=0.749]. There were no significant group differences on the number of right handed participants [χ2(1)=0.79, P=0.375), laterality [t(80)=−0.053, P=0.957], and right hand index [t(80)=0.076, P=0.940] or IQ [t(77)=1.25, P=0.216]. Groups significantly differed on self-reports of depressive symptoms [t(67)=−9.74, P < 0.0001]. Sociodemographic and clinical chracteristics by group are provided in Table 1.
Table 1.
Sociodemographic and clinical characteristics by group
| CTRL | MDD | Cohen’s D [95% CI] | |
|---|---|---|---|
| N (female %) | 37 (64.86) | 53 (69.81) | −0.10 [−0.52; 0.32] |
| Caucasian, n (%) | 23 (62.16) | 38 (71.70) | .20 [−0.22; 0.62] |
| Age, mean years (SD) | 16.32 (2.02) | 15.84 (1.85) | .25 [−0.18; 0.67] |
| Weight, mean pounds (SD) | 145.61 (35.15) | 147.99 (40.29) | −0.06 [−0.48; 0.36] |
| Height, mean inch (SD) | 65.36 (4.04) | 65.62 (3.61) | −0.07 [−0.49; 0.35] |
| Medication, yes n (%) | 0 (0.00) | 11 (20.75) | −0.66 [−1.09; −0.23] |
| Parents SESa | 50.54 (11.28) | 46.09 (11.22) | 0.34 [−0.04; 0.83] |
| Right handedness, n (%) | 34 (94.44) | 49 (98.00) | 0.19[−0.24; 0.62] |
| Laterality | 62.61 (37.14) | 63.01 (29.05) | −0.01 [−0.45; 0.43] |
| Right hand ratio | 107.88 (45.26) | 107.14 (41.23) | 0.02 [−0.42; 0.46] |
| IQ | 110.03 (12.94) | 105.53 (17.39) | 0.29 [−0.17; 0.74] |
| HR, mean bpm (SD) | 67.32 (8.00) | 70.98 (9.40) | −0.41 [−0.84; 0.01] |
| RMSSD, mean ms (SD) | 61.76 (18.20) | 57.77 (18.18) | 0.22 [−0.20; 0.64] |
| HF, mean log (SD) | 6.96 (0.61) | 6.80 (0.63) | 0.25 [−0.17; 0.67] |
| CDRS, mean (SD) | n.a. | 76.66 (6.96) | n.a. |
| BDIa, mean (SD) | 2.43 (2.99) | 26.34 (12.73) | −2.39 [−3.01; −1.75] |
| Current comorbid diagnoses, n (%) | |||
| Attention deficit-hyperactivity disorder | n.a. | 8 (15.09) | n.a. |
| Anorexia nervosa | n.a. | 1 (1.89) | n.a. |
| Generalized anxiety disorder | n.a. | 19 (35.85) | n.a. |
| Obsessive-compulsive disorder | n.a. | 2 (3.77) | n.a. |
| Oppositional defiant disorder | n.a. | 2 (3.77) | n.a. |
| Post-traumatic stress disorder | n.a. | 2 (3.77) | n.a. |
| Separation anxiety disorder | n.a. | 1 (1.89) | n.a. |
| Social anxiety disorder | n.a. | 6 (11.32) | n.a. |
| Dysthymia | n.a. | 4 (7.55) | n.a. |
| Panic disorder | n.a. | 1 (1.89) | n.a. |
| Specific phobia | n.a. | 1 (1.89) | n.a. |
| Seasonal affective disorder | n.a. | 1 (1.89) | n.a. |
Incomplete data: handedness: complete data by group CTRL, n = 34; MDD, n = 50; laterality/right hand ratio: complete data by group CTRL, n = 33; MDD, n = 49; IQ: complete data by group CTRL, n = 32; MDD, n = 47; BDI: complete data by group CTRL, n = 28; MDD, n = 41; parents SES: complete data by group CTRL, n = 37; MDD, n = 46; CDRS: complete data by group MDD, n = 37.
CTRL, non-depressed control group; MDD, major depressive disorder group; SD, standard deviation; SES, socio economic status; IQ, intelligence quotient; HR, heart rate; RMSSD, root mean square of successive differences; HF, high-frequency heart rate variability; CDRS, children’s depression rating scale total summary score; BDI, beck depression inventory; CI, confidence interval.
Resting autonomic function
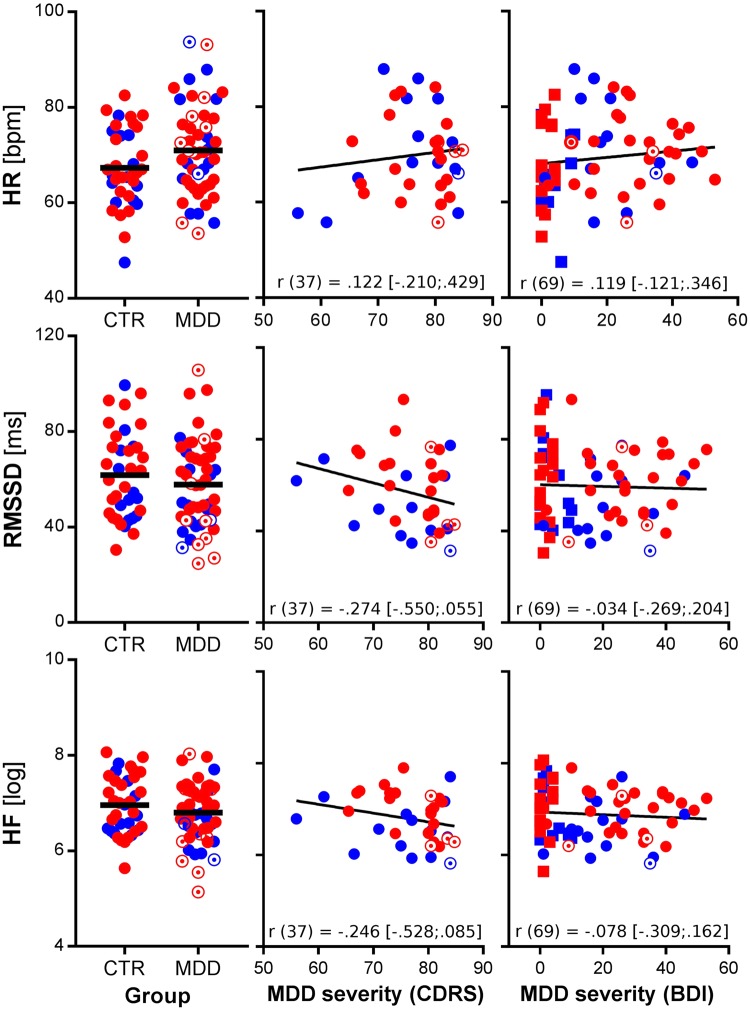
There were no significant group differences between MDD and CTRL on mean HR [t(88)=−1.93, P=0.057], HF-HRV [t(88)=1.16, P=0.249] or RMSSD [t(88)=1.02, P=0.309], as illustrated in Figure 1. Regression analysis on HF-HRV and RMSSD showed significant effects of sex and medication status independent of group. Analysis indicated greater HF-HRV and RMSSD in female subjects compared with their male counterparts and lower HF-HRV and RMSSD in medicated compared with un-medicated patients. No such effects were found on mean HR and there was no main effect of MDD, self-reports of depression severity (BDI) or clinician ratings (CDRS) on any index of cardiac function (see Supplementary Table S2). Models including clinician ratings (CDRS) showed significant effects of depression severity when adjusting for sex on RMSSD [F(2, 34) = 3.76, P=0.033; CDRS: t =−2.44, P=0.020, β=−0.32] but not HF-HRV [F(2, 34) = 2.86, P=0.071; CDRS: t=−1.97, P=0.057, β=−0.30). The effect was no longer significant when further adjusting for medication status (see Supplementary Table S2).
Fig. 1.
Measures of resting cardiac function by group and depression severity. Notes: HR, heart rate; RMSSD, root mean square of successive differences between adjacent R–R intervals in milliseconds; HF, high-frequency HR variability; CTR, non-depressed controls; MDD, major depressive disorder; CDRS, children’s depression rating scale; BDI, Beck Depression Inventory; red symbols, female subjects; blue symbols, male subjects; open circles, MDD patients with current medication; squares, CTRL participants (in the illustration of BDI scores); refer to Table 1 for missing data; r values represent correlation coefficients and their 95% CIs from zero-order correlations.
Cortical thickness
Groups showed significant differences on cortical thickness of the caudal ACC [t(88) =−3.43, P <0.0001], ‘rostral MFG’ [t(88) =−2.53, P=0.013] and ‘frontal pole’ [t(88) =−2.22, P=0.029] in the left hemisphere. Complete reporting of descriptive statistics and effect sizes for group differences are provided in Table 2. Regression analysis adjusted for sex and medication status supported significant main effects of MDD on thickness of the left caudal ACC, and rostral MFG, indicating greater thickness in adolescents with MDD. Independent of group, structural thickness of the bilateral frontal pole significantly differed by sex indicating greater thickness in females. Self-reports of depression severity (BDI) were associated with thickness of the bilateral caudal ACC, right medial OFC and bilateral frontal pole. Clinicians’ ratings of depression severity (CDRS) were associated with thickness of the left frontal pole only (see Supplementary Table S3).
Table 2.
Group differences on cortical thickness in selected regions of interest by hemisphere
| CTRL |
MDD |
Cohen’s D [95%CI] |
||||
|---|---|---|---|---|---|---|
| LH | RH | LH | RH | LH | RH | |
| Caudal ACC | 2.63 (0.18) | 2.57 (0.24) | 2.77 (0.18) | 2.66 (0.18) | −0.73 [−1.17; −0.30] | −0.42 [−0.84; 0.01] |
| Caudal MFG | 2.75 (0.13) | 2.66 (0.11) | 2.78 (0.12) | 2.68 (0.11) | −0.32 [−0.74; 0.11] | −0.20 [−0.62; 0.22] |
| Lateral OFC | 2.67 (0.13) | 2.62 (0.14) | 2.69 (0.13) | 2.64 (0.14) | −0.15 [−0.57; 0.27] | −0.11 [−0.53; 0.31] |
| Medial OFC | 2.48 (0.12) | 2.46 (0.12) | 2.48 (0.13) | 2.49 (0.16) | −0.01 [−0.43; 0.41] | −0.20 [−0.62; 0.23] |
| Rostral ACCa | 2.98 (0.19) | 2.98 (0.22) | 3.03 (0.17) | 2.98 (0.19) | −0.27 [−0.70; 0.15] | −0.02 [−0.44; 0.40] |
| Rostral MFG | 2.53 (0.12) | 2.44 (0.11) | 2.60 (0.12) | 2.49 (0.13) | −0.54 [−0.97; −0.11] | −0.37 [−0.80; 0.05] |
| Superior frontal | 2.91 (0.12) | 2.86 (0.10) | 2.97 (0.13 | 2.91 (0.13) | −0.40 [−0.82; 0.03] | −0.40 [−0.82; 0.03] |
| Frontal pole | 2.73 (0.36) | 2.72 (0.34) | 2.90 (0.33) | 2.86 (0.31) | −0.48 [−0.90; −0.05] | −0.41 [−0.83; 0.02] |
| Insulaa | 3.14 (0.09) | 3.04 (0.12) | 3.14 (0.11) | 3.05 (0.13 | −0.03 [−0.45; 0.39] | −0.13 [−0.55; 0.29] |
Incomplete data: rostral ACC-LH: complete data by group CTRL, n = 36; MDD, n = 53; Insula-LH: complete data by group CTRL, n = 37; MDD, n = 52; all data given as mean and standard deviation.
CTRL, non-depressed control group; MDD, major depressive disorder group; LH, left hemisphere; RH, right hemisphere; ACC, anterior cingulate cortex; MFG, middle frontal gyrus; OFC, orbitofrontal cortex.
Brain s tructure and a utonomic f unction 1
Interaction effects: group level and self-reports on depression severity
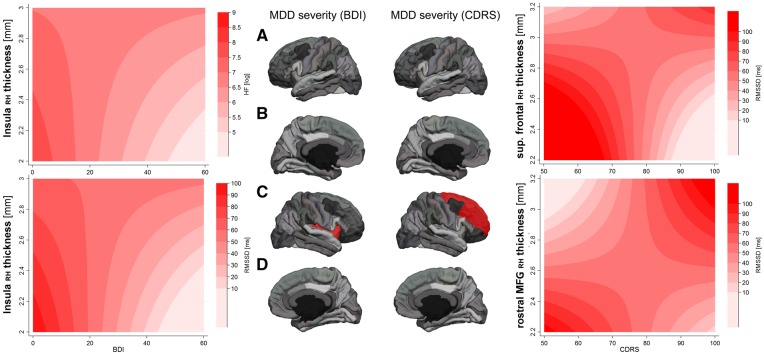
Results from all regression analyses predicting cardiac function are provided in Supplementary Table S4. Regression analyses including group as predictor showed no significant effects in predicting HR, RMSSD or HF-HRV. Regression analyses predicting RMSSD [F(5,63) = 2.89, P=0.021) and HF-HRV [F(5,63) = 2.96, P=0.018], showed a significant cross-over interaction between right insula thickness and BDI scores in predicting RMSSD (interaction: t = 2.22, P=0.030, β = 5.44], and HF-HRV (interaction: t = 2.09, P=0.041, β = 4.72), in the absence of significant main effects for cortical thickness but significant main effects for BDI (see Supplementary data). The model-fit for the interaction predicting RMSSD (BIC: 4.05; BF: 7.58) and HF-HRV (BIC: 4.14; BF: 7.94) was superior compared with the respective models including main effects only. These findings, suggesting a cross-over interaction of BDI and cortical thickness of the right insula in predicting vagal activity are illustrated in Figure 2. None of the significant interactions between cortical thickness and BDI in predicting HR showed a superior model fit (left medial OFC: BIC: .55; BF: 1.32; rostral MFG: BIC: −1.86; BF: 0.39).
Fig. 2.
Interaction of Cortical Thickness with Self-Reports (BDI) and Clinician Ratings (CDRS) of Depression Severity in Predicting RVA. Notes: RMSSD, root mean square of successive differences between adjacent R–R intervals in milliseconds; HF, high-frequency HR variability; RH, right hemisphere; BDI, Beck depression inventory; CDRS, children's depression rating scale; contour plots based on margins from regression analysis at selected levels of HF-HRV/RMSSD and cortical thickness; darker colors illustrate greater RVA; (A) left hemisphere lateral view; (B): left hemisphere medial view; (C): right hemisphere lateral view; (D): right hemisphere medial view; ROIs highlighted in red show a significant interaction with depression severity in predicting RVA. Note: For clarity the superior frontal gyrus is only highlighted in the lateral view; illustrated are predicted levels of the dependent variable (here RMSSD or HF) by different color intensities. Darker shades of red illustrate greater RMSSD or HF, respectively. Changes in RMSSD or HF are plotted as a function of the interaction between cortical thickness in ROIs (y-axis) and depression severity (x-axis) indexed by self-reports (BDI) or clinicians rating (CDRS) on a 2D space. For example: considering only the effect of depression severity, the graph on the bottom left shows that RMSSD is greater at lower depression severity and decreases with increasing levels of depression severity, independent of cortical thickness. On the other hand, the effect of cortical thickness of the right Insula (y-axis) seems to account for variance in RMSSD, only at moderate to high levels of depression severity, as the effect of cortical thickness on RMSSD in those with no or very low depression severity is very small. The graphs on the right illustrate more complex interactions between cortical thickness of frontal brain regions and clinicians ratings of depression severity in predicting RMSSD.
Interaction effects: clinician ratings of depression severity
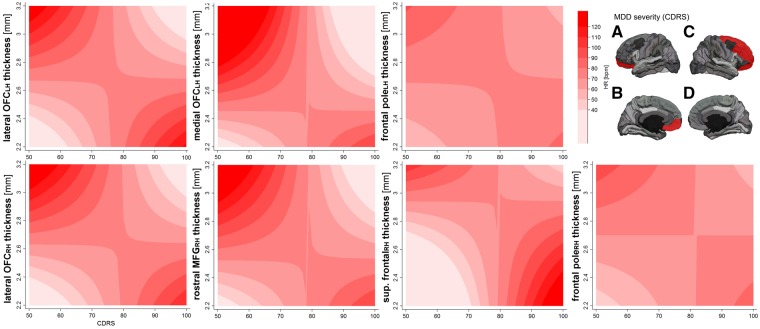
Models in depressed patients only, including the clinicians rating of depression severity in predicting mean HR, showed three significant interaction effects with superior model fit of cortical thickness of the left lateral OFC (BIC: 3.38; BF: 5.41), the left medial OFC (BIC: 3.30; BF: 5.20), and the left frontal pole (BIC: 2.97; BF: 4.41).2 Regarding the right hemisphere, there were significant interaction effects in predicting HR of cortical thickness and CDRS scores for the right lateral OFC (BIC: 3.37; BF: 5.39), the right rostral MFG (BIC: 2.67; BF: 3.80), the right superior frontal (BIC: 3.42; BF: 5.53) and the right frontal pole (BIC: 2.55; BF: 3.59). Findings for HR are illustrated in Figure 3. Models predicting HF-HRV showed no superior model-fit (superior frontal: BIC: 1.89; BF: 2.58). Analysis of RMSSD showed a significant interaction effect of thickness and CDRS scores for the right rostral MFG (BIC: 3.34; BF: 5.30), and the right superior frontal (BIC: 2.75; BF: 3.95). Findings are illustrated in Figure 2 (RMSSD) or Figure 3 (HR) respectively.
Fig. 3.
Interaction of cortical thickness and CDRS in predicting resting state HR. Notes: HR, heart rate in beats per minute; RH, right hemisphere; LH, left hemisphere; CDRS, children’s depression rating scale; contour plot based on margins from regression analysis at selected levels of HR and cortical thickness; darker colors illustrate greater mean HR; (A) left hemisphere lateral view; (B) left hemisphere medial view; (C) right hemisphere lateral view; (D) right hemisphere medial view; ROIs highlighted in red show a significant interaction with depression severity in predicting HR. Note: For clarity the superior frontal gyrus is only highlighted in the lateral view.
Discussion
The present study aimed to elucidate the relationships among cortical thickness, autonomic functioning, and depression symptoms in adolescents with MDD and non-depressed controls. On a group level, we found no significant effect of cortical thickness on autonomic functioning measures, suggesting that the diagnostic category per se is not related to altered brain structural concomitants of RVA or HR. However, dimensional analyses revealed some important findings with regard to how symptom severity impacts the relationship between cortical thickness and autonomic functioning. Analyses in the entire sample emphasized a unique interaction between insula thickness and self-reported depression severity in predicting RVA (but not HR). Specifically, for those with lower levels of depression (including controls), greater insula thickness was associated with lower RVA, but in those with higher depression severity, greater insula thickness was associated with relatively higher RVA. Both RVA and HR were significantly predicted by interactions between depression severity (indexed by clinician interviews) and cortical thickness in predominantly prefrontal areas in patients with MDD. In these analyses, for those patients with lower levels of depression severity, greater frontal thickness was associated with lower RMSSD, while in those with more severe depression, greater frontal thickness was associated with relatively greater RMSSD. The opposite pattern was observed for analyses examining the severity-thickness interactions in predicting HR. These findings suggest that the relationship between cortical thickness and autonomic functioning varies as a function of depression severity, and that patterns vary across specific fronto-limbic brain regions, and even across specific measures of autonomic functioning.
Regarding alterations of resting autonomic activity as a function of psychopathology, our categorical (MDD vs. CTRL) analyses did not replicate previous findings on group differences comparing adolescents with MDD and non-depressed controls on RVA. However, the effect was in the same direction and the magnitude was within the 95% CI of the exiting results from meta-analyses (Kemp et al. 2010; Koenig et al., 2016). The present study included a diverse (heterogeneity in sex, medication status and comorbidity) sample of adolescents with MDD, which might partially explain why group-level comparisons did not reach the set level of significance. However, dimensional analyses revealed that depression severity, as assessed by clinical interviews, negatively correlated with RVA (indexed by RMSSD) when adjusting for sex differences. In line with results from the previous meta-analysis, we found no statistical significant correlation between self-reported depression severity and RVA in the entire sample including non-depressed controls. Again, the direction and magnitude of the effect (see Supplementary Material and Figure 1) was similar to the one reported in the meta-analysis (Koenig et al., 2016).
Albeit previous findings on group difference in structural thickness comparing depressed adolescents and non-depressed controls are mixed (Fallucca et al., 2011; Reynolds et al., 2014; Whittle et al., 2014; Schmaal et al., 2017a), we found that adolescents with MDD show significantly greater cortical thickness in ROIs of the left hemisphere including the caudal ACC, rostral MFG, and frontal pole. Differences for the caudal ACC and rostral MFG remained after adjusting for sex and medication status. In line with these findings, self-reports (BDI) and clinician ratings (CDRS) of depression severity showed positive correlations with selected ROIs even after adjusting for sex and medication status. Greater cortical thickness in adolescent MDD might reflect a deviation from the neurotypical pattern of cortical thinning during adolescence, specifically in these regions. Given that the cortical thinning is attributed to pruning and increased efficiency of brain functioning, adolescents with MDD may thus exhibit a delay in neuro-cortical development associated with deficits in executive control.
Our analyses using depression as a continuous variable predictor were more successful in revealing significant findings than analyses using diagnosis as a categorical variable. Specifically, we found no effects of group on the association between cortical thickness and RVA; analyses including self-reports of depression severity (BDI) illustrated important effects specific to RVA (no robust findings for HR), highlighted the importance of the insula. Regression models (see Supplementary Table S5) showed that greater depressive symptoms were associated with lower RVA when adjusting for cortical thickness of the right insula. The significant cross-over interaction suggests that in subjects with lower depressive symptoms, lower right insula cortical thickness was associated with greater RVA. This finding is in line with prior research in non-depressed adolescents, illustrating that greater RVA is associated with reduced cortical thickness in subjects of younger age (Koenig et al. 2018), contrary to a positive association reported in adults (Woodward et al., 2008; Winkelmann et al., 2016; Yoo et al., 2018). The prior study in adolescents did not support an association between insula thickness and RVA, but highlighted the rostral ACC in models not controlling for depressive symptoms. However, a positive correlation between RVA and insula thickness has previously been demonstrated in adults (Wood et al., 2017). Interestingly, in contrast to our hypothesis that greater cortical thickness is associated with reduced RVA in adolescents with MDD, the present findings suggest that in adolescents reporting severe depressive symptoms, greater thickness of the insula aids to maintain relatively normal (in contrast to adolescents with likewise depressive symptoms but lower cortical thickness) levels of RVA (indexed by HF-HRV and RMSSD). Analyses suggest that while reduced cortical thickness is beneficial for RVA (or vice versa) in adolescents with low levels of depressive symptoms, this association changes with increasing depression severity, such that adolescents with MDD show an ‘adult-like pattern’ of a positive association between cortical thickness of the insula and RVA (Wood et al., 2017).
Previous studies have widely documented the importance of the insular cortex in cardiac regulation and MDD. Classic lesion studies have shown that the insula (in particular the left insula) is responsible for parasympathetic control (Oppenheimer et al., 1996), such that patients with insula stroke exhibit sympathetic hyper-activation (Walter et al., 2013). Our analyses highlight a potential role of the right insula in MDD-related sympathetic hyperactivity in depressed adolescents. Animal studies in rats have previously shown that lesions to the right posterior insula can increase blood pressure and HR (Zhang et al., 1998). Human development studies have also highlighted the importance of the insula, illustrating that greater cortical thickness of the right insula is positively associated with age in girls with high risk for the development of MDD and negatively associated in girls with low risk for depression (Foland-Ross et al., 2015a). fMRI studies on (transcutaneous) VNS (tVNS)—a potential treatment option for children and adolescents with treatment resistant depression (Yu et al., 2008; Koenig et al., 2016)—have shown that acute tVNS compared with sham in depressed adults leads to greater activity in the left anterior insula and that greater insula activation during tVNS predicts better clinical outcomes (Hamilton Depression Rating Scale) after 4 weeks of tVNS treatment (Fang et al., 2017). The latter findings provide preliminary evidence for a link between depressive symptoms, vagal activity and the insula.
Beyond the insula, addressing the association of depression severity and RVA in patients only, we found a host of regions associated with HR and RVA. Most interestingly, we found that greater depression severity—indexed by clinician ratings of depression severity (CDRS)—and reduced cortical thickness in frontal brain regions, including the bilateral lateral OFC, and bilateral frontal pole, as well as the left medial OFC, right caudal and rostral MFG, and right superior frontal were associated with increased HR. In line with these findings, two of these regions, the right rostral MFG and the right superior frontal gyrus, were supported by analysis of RVA (indexed by RMSSD). Unlike findings on self-reports of depression severity (BDI) and insula thickness, these analyses showed robust main effects for both depression severity and thickness, indicating that greater cortical thickness and greater depression severity are both associated with lower RVA and greater HR. Again, significant interactions suggest that this relationship changes at greater levels of depression severity and greater cortical thickness. Findings suggest that reduced cortical thickness of frontal regions at greater levels of depression severity is associated with reduced RVA and increased HR. Taken together, while analyses with the entire sample (allowing for a broader continuum of depression symptoms) highlighted the importance of the insula, findings from these MDD-only analyses suggest prefrontal regions are highly relevant to autonomic function in adolescents with MDD.
While the causality in the link between cortical thickness and RVA is unclear, we have previously suggested that greater RVA might be beneficial for cortical thinning during adolescence (Koenig et al. 2018). Considering environmental influences, lower RVA may be a consequence of increased stress and in turn is associated with greater stress vulnerability in children and adolescents (Porges, 1992; Michels et al., 2013). Stress-induced ANS hyper-arousal may have bottom-up consequences on cortical-development via afferent vagal pathways or may be the consequence of poor inhibitory cortical control of peripheral function via efferent vagal fibers. With respect to findings on the insula, we hypothesize that in adolescents with MDD, environmental stressors alter normative cortical maturation of the insula via increased autonomic arousal that subsequently leads to increased depressive symptoms. In general support of this notion are animal studies illustrating that chronic stress in adolescent rats leads to significant remodeling of neurons in the PFC and amygdala associated with depressive-like behavior (Eiland et al., 2012). In humans, chronic reductions of RVA have been shown to be associated with a disturbance of the positive valence system (Gruber et al., 2015; Duarte and Pinto-Gouveia, 2017), difficulties in inhibiting conditioned fear (i.e. decreased capacity of the PFC to inhibit subcortical fear responses in the presence of safety) (Pappens et al., 2014; Wendt et al., 2015), and greater difficulties in emotion regulation (Williams et al., 2015; Visted et al., 2017)—importantly, decreased RVA has been shown to precede (Jandackova et al., 2016) the development of depressive symptoms.
However, the present findings also illustrate that at greater levels of depression severity, greater cortical thickness is associated with greater levels of RVA, suggesting that greater cortical thickness—particularly of the PFC—may be compensatory for this developmental-trajectory in adolescents with MDD. While we can only speculate on the exact nature of this compensatory mechanism, we suggest that greater PFC thickness in severe cases of adolescent MDD may be ‘beneficial’ to maintain autonomic balance. Previous studies in women with borderline personality disorder were able to show increased cortical thickness in the rostral MFG that was positively associated with emotion regulation and cortical thickness of the insula (Bruehl et al., 2013). Based on our findings we speculate on similar effects. As discussed above, we suggest that vagal projections from the nucleus tractus solitarius to the amygdala may inhibit normative cortical thinning of the insula as a consequence of increased and prolonged autonomic arousal (afferent vagal loop). Following this line of reasoning, we suggest that ‘efferent vagal loops’ try to compensate for this hyper-arousal: greater PFC thickness is necessary to inhibit greater insula activity, in line with research demonstrating that greater ventromedial PFC thickness is associated with greater reduction of activation in the left amygdala during suppression of negative emotional states (Foland-Ross et al., 2010). This compensatory mechanism may lead to long term alterations of cortical development, characterized by deficient neural pruning, inversing the relationship between RVA and cortical thickness in adolescents with MDD that show an ‘adult-like’ pattern. Longitudinal studies are necessary to clarify if this development is the consequence of MDD or subsequently leads to MDD, as suggested. Furthermore, potential genetic variants of this development and environmental influences such as maternal support for regulating emotions are subject to further research. Recently, genetic variants associated with heritable variation in vagal control of autonomic function have been identified (Nolte et al., 2017), and previous studies suggest that, e.g. maternal supportive emotion socialization is linked to vagal activity during active emotion regulation in youth (Hastings et al., 2014).
The present study has several limitations that need to be addressed. First, this is a secondary analysis that used pulse oximetry data. Although pulse oximetry is acceptable for the analyses of HRV, future studies would do well to implement recordings of RVA using ECG at a higher sample-rate (Ellis et al., 2015). In a first step, the analyses focused on difference in structural thickness in selected ROIs, given that the majority of previous studies in healthy subjects addressed measures of structural thickness in association with HRV (Woodward et al., 2008; Winkelmann et al., 2016; Koenig et al. 2018; Yoo et al., 2018). However, research suggests that alterations in the volumetric development of brain structure are implicated in MDD onset in adolescents (Whittle et al., 2014). Furthermore, previous analyses from the present sample have shown altered resting state functional connectivity (RSFC) in adolescents with MDD (Cullen et al., 2014). Analysis of RSFC may help to better understand the interplay between the PFC and insula in association with RVA as discussed above, as previous studies have shown that stronger amygdala medial PFC RSFC is associated with greater RVA (Sakaki et al., 2016) and that states of higher vagal activity are associated with stronger coupling of PFC-amygdala activity when recording temporal changes in whole-brain functional connectivity (Chang et al., 2013). Therefore, following up on the present structural findings we will subsequently analyze the respective volumetric and RSFC data in relation to RVA to extend on the present findings. Beyond an extension to volumetric measures and measures of RSFC, studies in clinical samples should cover important sites of the brainstem (Farmer et al., 2016), including the locus coeruleus that has recently been shown to be involved in the regulation of vagal activity, indexed by HRV (Mather et al., 2017). Finally, although participants were instructed to lie awake with their eyes closed, data were not collected on whether or not participants were able to remain awake throughout the scan. Thus, it is unclear whether some of our HRV data were collected during sleep, thus impacting cardiac metrics due to possibly slower HR and greater RVA.
The sample size of the present study prohibited a more in-depth analysis of potential differences in the reported associations as a function of sex and medication. We found significant effects for sex and medication status on RVA, where females showed greater RVA (in line with previous studies in adults; Koenig and Thayer, 2016) and where patients on antidepressant medication showed lower RVA (also as previously seen in adults; Kemp et al., 2010, 2016). While our number of patients on medication was relatively small (n = 11), to the best of our knowledge, this is the first study to replicate this finding in adolescents with MDD. Addressing sex differences in the neural concomitants of RVA in depressed patients seems promising, as sex differences in the association between HRV and regional cerebral blood flow have previously been reported (Allen et al., 2015). Furthermore, there is some evidence suggesting that depressive symptoms are associated with reduced RVA in men but greater RVA in women (Thayer et al., 1998; Chambers and Allen, 2007), highlighting predominant sympathetic modulation in males (Voss et al., 2011). Large-scale neuroimaging studies are necessary to address potential sex differences in thickness and RVA in depressed and non-depressed adolescents. The cross-sectional study design limits any inferences with regard to the timeline of events of the course of development. For example, it has previously been shown that reduced RVA (Jandackova et al., 2016) and increased HR (Latvala et al., 2016) precede the development of depressive symptoms. Longitudinal studies are needed to elucidate how abnormalities of HRV, cortical thickness and their relationship unfold across adolescent development. Similarly, our findings with relation to medication status are limited by the cross-sectional design. Prior treatment studies have shown that some measures of HRV predict treatment outcome in MDD (Jain et al., 2014) and that improvements in MDD symptoms are associated with subsequent increase of RVA (Chambers and Allen, 2002). Previously, one study addressed fMRI changes in the medial visceromotor network in association with RVA in patients with MDD over 12 weeks of sertraline treatment, illustrating that treatment significantly increased brain-RVA covariation (Schafer et al., 2015). To better understand how successful treatment impacts cortical thickness, HRV and their relationship in adolescents, multi-modal neurobiological assessment studies, including measurements of sympathetic modulation (Schumann et al., 2017), nested within the context of clinical trials are needed.
Conclusion
The present study illustrates that at greater levels of depression severity, lower cortical thickness of the right insula is associated with decreased parasympathetic control over resting cardiac function in adolescents. In addition, adolescents endorsing diagnostic criteria for MDD show a pattern of prefrontal brain regions associated with reduced parasympathetic cardiac control (i.e. rostral MFG, superior frontal) and increased resting state HR (i.e. lateral, medial, and rostral MFG, frontal pole and superior frontal). Findings in adolescents with MDD point towards a potential compensatory mechanism, illustrating that greater cortical thickness in frontal brain regions at greater levels of depression severity are associated with relative increased levels of RVA. We suggest that greater cortical thickness of frontal brain regions in adolescents with MDD may serve a compensatory mechanism, beneficial to maintain autonomic balance. Longitudinal studies are needed to address the nature of the association between cortical thickness and RVA in MDD.
Supplementary Material
Acknowledgements
The authors would like to first and foremost thank the adolescents and families that contributed to this study. We thank Dr Michael Gaebler for his helpful support regarding the Siemens PPG data format and readout.
Funding
The study was funded by grants to Dr Cullen including the National Institute of Mental Health (K23MH090421), the National Alliance for Research on Schizophrenia and Depression, the University of Minnesota Graduate School and the Minnesota Medical Foundation, as well as a grant to Dr Klimes-Dougan from the Deborah E. Powell Center for Women’s Health at the University of Minnesota. Dr Koenig is supported by a Physician-Scientist-Fellowship provided by the Medical School, University of Heidelberg, Germany, and acknowledges the financial support through a Post-Doctoral Scholarship provided by the Daimler and Benz Foundation (Ladenburg, Germany) and the Thrasher Research Fund Early Career Award provided by the Thrasher Research Fund (Salt Lake City, UT, USA). These resources supported the roles of design and conduct of the study; collection, management and analysis of the data; and interpretation of results and preparation of the publication. We acknowledge financial support by the Deutsche Forschungsgemeinschaft within the funding programme Open Access Publishing, by the Baden-Württemberg Ministry of Science, Research and the Arts and by the Ruprecht-Karls-Universität Heidelberg.
Conflict of interest. None declared.
Footnotes
Only models with a significant F-test and interaction effect are reported that showed a superior model fit indicated by a BF ≥ 3. Full reporting is provided in the Supplementary data.
Note: Based on a reviewer comment, we tested the influence of age on the reported findings. Age was unrelated to RMSSD (r =0.058, P =0.585) and HF (r =0.050, P=0.640), but showed a moderate association with HR (r=−0.307, P =0.004). Subsequently, we conducted sensitivity analysis, further adjusting all models, significantly predicting HR for age. None of the regression results changed.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Allen B., Jennings J.R., Gianaros P.J., Thayer J.F., Manuck S.B. (2015). Resting high-frequency heart rate variability is related to resting brain perfusion. Psychophysiology, 52(2), 277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D., McIntosh A.M., Ebmeier K.P., Munafò M.R., Anderson I.M. (2012). Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. European Neuropsychopharmacology, 22(1), 1–16. [DOI] [PubMed] [Google Scholar]
- Beauchaine T.P. (2015). Future directions in emotion dysregulation and youth psychopathology. Journal of Clinical Child & Adolescent Psychology, 44(5), 875–96. [DOI] [PubMed] [Google Scholar]
- Beck A., Steer R., Brown K. (1996). Beck Depression Inventory - Revised. San Antonio: Harcourt Brace. [Google Scholar]
- Berna G., Ott L., Nandrino J.-L. (2014). Effects of emotion regulation difficulties on the tonic and phasic cardiac autonomic response. PLoS One, 9(7), e102971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E., Fornito A., Pantelis C., Yücel M. (2012). Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. Journal of Affective Disorders, 138(1–2), 9–18. [DOI] [PubMed] [Google Scholar]
- Bruehl H., Preißler S., Heuser I., Heekeren H.R., Roepke S., Dziobek I. (2013). Increased prefrontal cortical thickness is associated with enhanced abilities to regulate emotions in PTSD-free women with borderline personality disorder. PLoS One, 8(6), e65584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers A.S., Allen J.J.B. (2002). Vagal tone as an indicator of treatment response in major depression. Psychophysiology, 39(6), 861–4. [DOI] [PubMed] [Google Scholar]
- Chambers A.S., Allen J.J.B. (2007). Sex differences in cardiac vagal control in a depressed sample: implications for differential cardiovascular mortality. Biological Psychology, 75(1), 32–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Metzger C.D., Glover G.H., Duyn J.H., Heinze H.-J., Walter M. (2013). Association between heart rate variability and fluctuations in resting-state functional connectivity. NeuroImage, 68, 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K.R., Klimes-Dougan B., Vu D.P., et al. (2016). Neural correlates of antidepressant treatment response in adolescents with major depressive disorder. Journal of Child and Adolescent Psychopharmacology, 26(8), 705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K.R., Westlund M., Klimes-Dougan B., et al. (2014). Abnormal Amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry, 71(10), 1138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–80. [DOI] [PubMed] [Google Scholar]
- Duarte J, Pinto-Gouveia J. (2017). Positive affect and parasympathetic activity: evidence for a quadratic relationship between feeling safe and content and heart rate variability. Psychiatry Research, 257, 284–9. [DOI] [PubMed] [Google Scholar]
- Ducharme S., Albaugh M.D., Hudziak J.J., et al. (2014). Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cerebral Cortex, 24(11), 2941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L., Ramroop J., Hill M.N., Manley J., McEwen B.S. (2012). Chronic Juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology, 37(1), 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R.J., Zhu B., Koenig J., Thayer J.F., Wang Y. (2015). A careful look at ECG sampling frequency and R-peak interpolation on short-term measures of heart rate variability. Physiological Measurement, 36(9), 1827–52. [DOI] [PubMed] [Google Scholar]
- Fallucca E., MacMaster F.P., Haddad J., et al. (2011). Distinguishing between major depressive disorder and obsessive-compulsive disorder in children by measuring regional cortical thickness. Archives of General Psychiatry, 68(5), 527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J., Egorova N., Rong P., et al. (2017). Early cortical biomarkers of longitudinal transcutaneous vagus nerve stimulation treatment success in depression. NeuroImage: Clinical, 14, 105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer D.G.S., Dutschmann M., Paton J.F.R., Pickering A.E., McAllen R.M. (2016). Brainstem sources of cardiac vagal tone and respiratory sinus arrhythmia. Journal of Physiology, 594(24), 7249–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., et al. (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14, 11–22. [DOI] [PubMed] [Google Scholar]
- Foland-Ross L.C., Altshuler L.L., Bookheimer S.Y., et al. (2010). Amygdala reactivity in healthy adults is correlated with prefrontal cortical thickness. Journal of Neuroscience, 30(49), 16673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross L.C., Gilbert B.L., Joormann J., Gotlib I.H. (2015a). Neural markers of familial risk for depression: an investigation of cortical thickness abnormalities in healthy adolescent daughters of mothers with recurrent depression. Journal of Abnormal Psychology, 124(3), 476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross L.C., Sacchet M.D., Prasad G., Gilbert B., Thompson P.M., Gotlib I.H. (2015b). Cortical thickness predicts the first onset of major depression in adolescence. International Journal of Developmental Neuroscience, 46, 125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaebler M., Daniels J.K., Lamke J.-P., Fydrich T., Walter H. (2013). Heart rate variability and its neural correlates during emotional face processing in social anxiety disorder. Biological Psychology, 94(2), 319–30. [DOI] [PubMed] [Google Scholar]
- Gil E., Orini M., Bailón R., Vergara J.M., Mainardi L., Laguna P. (2010). Photoplethysmography pulse rate variability as a surrogate measurement of heart rate variability during non-stationary conditions. Physiological Measurement, 31(9), 1271–90. [DOI] [PubMed] [Google Scholar]
- Gruber J., Mennin D.S., Fields A., Purcell A., Murray G. (2015). Heart rate variability as a potential indicator of positive valence system disturbance: a proof of concept investigation. International Journal of Psychophysiology, 98(2), 240–8. [DOI] [PubMed] [Google Scholar]
- Hall L.M.J., Klimes-Dougan B., Hunt R.H., et al. (2014). An fMRI study of emotional face processing in adolescent major depression. Journal of Affective Disorders, 168, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Jovicich J., Salat D., et al. (2006). Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage, 32(1), 180–94. [DOI] [PubMed] [Google Scholar]
- Hastings P.D., Klimes-Dougan B., Kendziora K.T., Brand A., Zahn-Waxler C. (2014). Regulating sadness and fear from outside and within: mothers’ emotion socialization and adolescents’ parasympathetic regulation predict the development of internalizing difficulties. Development and psychopathology, 26(4pt2), 1369–84. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. (1975). Four-Factor Index of Social Status [Unpublished Manuscript]. New Haven, CT: Yale University. [Google Scholar]
- Jain F.A., Cook I.A., Leuchter A.F., et al. (2014). Heart rate variability and treatment outcome in major depression: a pilot study. International Journal of Psychophysiology, 93(2), 204–10. [DOI] [PubMed] [Google Scholar]
- Jandackova V.K., Britton A., Malik M., Steptoe A. (2016). Heart rate variability and depressive symptoms: a cross-lagged analysis over a 10-year period in the Whitehall II study. Psychological Medicine, 46(10), 2121–31. [DOI] [PubMed] [Google Scholar]
- Jarczok M.N., Koenig J., Mauss D., Fischer J.E., Thayer J.F. (2014). Lower heart rate variability predicts increased level of C-reactive protein 4 years later in healthy, nonsmoking adults. Journal of Internal Medicine, 276(6), 667–71. [DOI] [PubMed] [Google Scholar]
- Jovicich J., Czanner S., Greve D., et al. (2006). Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. NeuroImage, 30(2), 436–43. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., et al. (1997). Schedule for affective disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–8. [DOI] [PubMed] [Google Scholar]
- Kemp A.H., Fráguas R., Brunoni A.R., et al. (2016). Differential associations of specific selective serotonin reuptake inhibitors with resting-state heart rate and heart rate variability: implications for health and well-being. Psychosomatic Medicine, 78(7), 810–8. [DOI] [PubMed] [Google Scholar]
- Kemp A.H., Quintana D.S., Gray M.A., Felmingham K.L., Brown K., Gatt J.M. (2010). Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biological Psychiatry, 67(11), 1067–74. [DOI] [PubMed] [Google Scholar]
- Kempton M.J., Salvador Z., Munafò M.R., et al. (2011). Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Archives of General Psychiatry, 68(7), 675–90. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B., Eberly L.E., Schreiner M.W., et al. (2014). Multilevel assessment of the neurobiological threat system in depressed adolescents: interplay between the limbic system and hypothalamic–pituitary–adrenal axis. Development and psychopathology, 26(4pt2), 1321–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J., Thayer J.F. (2016). Sex differences in healthy human heart rate variability: a meta-analysis. Neuroscience & Biobehavioral Reviews, 64, 288–310. [DOI] [PubMed] [Google Scholar]
- Koenig J., Kemp A.H., Beauchaine T.P., Thayer J.F., Kaess M. (2016). Depression and resting state heart rate variability in children and adolescents—a systematic review and meta-analysis. Clinical Psychology Review, 46, 136–50. [DOI] [PubMed] [Google Scholar]
- Koenig J., Parzer P., Reichl C., et al. (2018). Cortical thickness, resting state heart rate, and heart rate variability in female adolescents. Psychophysiology, 55(5), e13043. [DOI] [PubMed] [Google Scholar]
- Koolschijn P.C.M.P., van Haren N.E.M., Lensvelt-Mulders G.J.L.M., Hulshoff Pol H.E., Kahn R.S. (2009). Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Human Brain Mapping, 30(11), 3719–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval P., Ogrinz B., Kuppens P., Van den Bergh O., Tuerlinckx F., Sütterlin S. (2013). Affective instability in daily life is predicted by resting heart rate variability. PLoS One, 8(11), e81536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-H. (2013). Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatry Research, 211(1), 37–46. [DOI] [PubMed] [Google Scholar]
- Latvala A., Kuja-Halkola R., Rück C., et al. (2016). Association of resting heart rate and blood pressure in late adolescence with subsequent mental disorders: a longitudinal population study of more than 1 million men in Sweden. JAMA Psychiatry, 73(12), 1268–75. [DOI] [PubMed] [Google Scholar]
- Lu G., Yang F., Taylor J.A., Stein J.F. (2009). A comparison of photoplethysmography and ECG recording to analyse heart rate variability in healthy subjects. Journal of Medical Engineering & Technology, 33(8), 634–41. [DOI] [PubMed] [Google Scholar]
- Mather M., Joo Yoo H., Clewett D.V., et al. (2017). Higher locus coeruleus MRI contrast is associated with lower parasympathetic influence over heart rate variability. NeuroImage, 150, 329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels N., Sioen I., Clays E., et al. (2013). Children’s heart rate variability as stress indicator: association with reported stress and cortisol. Biological Psychology, 94(2), 433–40. [DOI] [PubMed] [Google Scholar]
- Musgrove D.R., Eberly L.E., Klimes-Dougan B., et al. (2015). Impaired bottom-up effective connectivity between Amygdala and subgenual anterior cingulate cortex in unmedicated adolescents with major depression: results from a dynamic causal modeling analysis. Brain Connect, 5(10), 608–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte I.M., Munoz M.L., Tragante V., et al. (2017). Genetic loci associated with heart rate variability and their effects on cardiac disease risk. Nature Communications, 8, 15805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Oppenheimer S.M., Kedem G., Martin W.M. (1996). Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clinical Autonomic Research, 6, 131–40. [DOI] [PubMed] [Google Scholar]
- Osman A., Kopper B.A., Barrios F., Gutierrez P.M., Bagge C.L. (2004). Reliability and validity of the Beck depression inventory–II with adolescent psychiatric inpatients. Psychological Assessment, 16(2), 120–32. [DOI] [PubMed] [Google Scholar]
- Pappens M., Schroijen M., Sütterlin S., et al. (2014). Resting heart rate variability predicts safety learning and fear extinction in an interoceptive fear conditioning paradigm. PLoS One, 9(9), e105054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G.V., Salum G.A., Sugaya L.S., Caye A., Rohde L.A. (2015). Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. Journal of Child Psychology and Psychiatry, 56(3), 345–65. [DOI] [PubMed] [Google Scholar]
- Porges S.W. (1992). Vagal tone: a physiologic marker of stress vulnerability. Pediatrics, 90(3 Pt 2), 498–504. [PubMed] [Google Scholar]
- Poznanski E., Freman L., Mokros H. (1985). Children’s depression rating scale-revised. Psychopharmacology Bulletin, 979, 989. [Google Scholar]
- Quintana D.S., Alvares G.A., Heathers J. a J. (2016). Guidelines for reporting articles on psychiatry and heart rate variability (GRAPH): recommendations to advance research communication. Translational Psychiatry, 6(5), e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery A.E. (1995). Bayesian model selection in social research. Sociological Methodology, 25, 111–63. [Google Scholar]
- Reynolds S., Carrey N., Jaworska N., Langevin L.M., Yang X.-R., MacMaster F.P. (2014). Cortical thickness in youth with major depressive disorder. BMC Psychiatry, 14(1), 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J., Neumann J., Fünfstück T., Soliman A., Villringer A., Schroeter M.L. (2012). Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. Journal of Affective Disorders, 140(2), 142–8. [DOI] [PubMed] [Google Scholar]
- Sakaki M., Yoo H.J., Nga L., Lee T.-H., Thayer J.F., Mather M. (2016). Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. NeuroImage, 139, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer S.M., Wager T.D., Mercado R.A., Thayer J.F., Allen J.J.B., Lane R.D. (2015). Partial amelioration of medial visceromotor network dysfunction in major depression by sertraline. Psychosomatic Medicine, 77(7), 752–61. [DOI] [PubMed] [Google Scholar]
- Schmaal L., Hibar D.P., Sämann P.G., et al. (2017a). Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Molecular Psychiatry, 22(6), 900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L., Yücel M., Ellis R., et al. (2017b). Brain structural signatures of adolescent depressive symptom trajectories: a longitudinal magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry, 56(7), 593–601.e9. [DOI] [PubMed] [Google Scholar]
- Schumann A., Andrack C., Bär K.-J. (2017). Differences of sympathetic and parasympathetic modulation in major depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 79, 324–31. [DOI] [PubMed] [Google Scholar]
- Selvaraj N., Jaryal A., Santhosh J., Deepak K.K., Anand S. (2008). Assessment of heart rate variability derived from finger-tip photoplethysmography as compared to electrocardiography. Journal of Medical Engineering & Technology, 32(6), 479–84. [DOI] [PubMed] [Google Scholar]
- Silvetti M.S., Drago F., Ragonese P. (2001). Heart rate variability in healthy children and adolescents is partially related to age and gender. International Journal of Cardiology, 81(2–3), 169–74. [DOI] [PubMed] [Google Scholar]
- Sommerfeldt S.L., Cullen K.R., Han G., Fryza B.J., Houri A.K., Klimes-Dougan B. (2016). Executive attention impairment in adolescents with major depressive disorder. Journal of Clinical Child and Adolescent Psychology, 45(1), 69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarvainen M.P., Niskanen J.-P., Lipponen J.A., Ranta-Aho P.O., Karjalainen P.A. (2014). Kubios HRV—heart rate variability analysis software. Computer Methods and Programs in Biomedicine, 113(1), 210–20. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. (1996). Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation, 93, 1043–65. [PubMed] [Google Scholar]
- Thayer J.F., Sternberg E. (2006). Beyond heart rate variability: vagal regulation of allostatic systems. Annals of the New York Academy of Sciences, 1088(1), 361–72. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Lane R.D. (2009). Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews, 33(2), 81–8. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Ahs F., Fredrikson M., Sollers J.J., Wager T.D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health.Neuroscience & Biobehavioral Reviews, 36(2), 747–56. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Smith M., Rossy L.A., Sollers J.J., Friedman B.H. (1998). Heart period variability and depressive symptoms: gender differences. Biological Psychiatry, 44(4), 304–6. [DOI] [PubMed] [Google Scholar]
- Truong W., Minuzzi L., Soares C.N., et al. (2013). Changes in cortical thickness across the lifespan in major depressive disorder. Psychiatry Research, 214(3), 204–11. [DOI] [PubMed] [Google Scholar]
- Visted E., Sørensen L., Osnes B., Svendsen J.L., Binder P.-E., Schanche E. (2017). The association between self-reported difficulties in emotion regulation and heart rate variability: the salient role of not accepting negative emotions. Frontiers in Psychology, 8, doi:10.3389/fpsyg.2017.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T., Allen C., Arora M., et al. (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet, 388(10053), 1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss A., Boettger M.K., Schulz S., Gross K., Bär K.-J. (2011). Gender-dependent impact of major depression on autonomic cardiovascular modulation. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 35(4), 1131–8. [DOI] [PubMed] [Google Scholar]
- Walter U., Kolbaske S., Patejdl R., et al. (2013). Insular stroke is associated with acute sympathetic hyperactivation and immunodepression.European Journal of Neurology, 20(1), 153–9. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1999. Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation: Harcourt Brace & Company. [Google Scholar]
- Wendt J., Neubert J., Koenig J., Thayer J.F., Hamm A.O. (2015). Resting heart rate variability is associated with inhibition of conditioned fear. Psychophysiology, 52(9), 1161–6. [DOI] [PubMed] [Google Scholar]
- Whittle S., Lichter R., Dennison M., et al. (2014). Structural brain development and depression onset during adolescence: a prospective longitudinal study. American Journal of Psychiatry, 171(5), 564–71. [DOI] [PubMed] [Google Scholar]
- Williams D.P., Cash C., Rankin C., Bernardi A., Koenig J., Thayer J.F. (2015). Resting heart rate variability predicts self-reported difficulties in emotion regulation: a focus on different facets of emotion regulation. Frontiers in Psychology, 6(261), doi:10.3389/fpsyg.2015.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann T., Thayer J.F., Pohlack S., Nees F., Grimm O., Flor H. (2016). Structural brain correlates of heart rate variability in a healthy young adult population. Brain Structure & Function, 222(2), 1061–8. [DOI] [PubMed] [Google Scholar]
- Wood K.N., Badrov M.B., Speechley M.R., Shoemaker J.K. (2017). Regional cerebral cortical thickness correlates with autonomic outflow. Autonomic Nervous System, 207, 28–36. [DOI] [PubMed] [Google Scholar]
- Woodward S.H., Kaloupek D.G., Schaer M., Martinez C., Eliez S. (2008). Right anterior cingulate cortical volume covaries with respiratory sinus arrhythmia magnitude in combat veterans. Journal of Rehabilitation Research and Development, 45(3), 451–63. [DOI] [PubMed] [Google Scholar]
- Yoo H.J., Thayer J., Greening S., et al. (2018). Brain structural concomitants of resting state heart rate variability in the young and old—evidence from two independent samples. Brain Structure & Function, 223(2), 727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.J., Weller R.A., Sandidge K., Weller E.B. (2008). Vagus nerve stimulation: can it be used in adolescents or children with treatment-resistant depression? Current Psychiatry Reports, 10(2), 116–22. [DOI] [PubMed] [Google Scholar]
- Zhang Z.-H., Rashba S., Oppenheimer S.M. (1998). Insular cortex lesions alter baroreceptor sensitivity in the urethane-anesthetized rat. Brain Research, 813(1), 73–81. [DOI] [PubMed] [Google Scholar]
- Zhao Y.-J., Du M.-Y., Huang X.-Q., et al. (2014). Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychological Medicine, 44(14), 2927–37. [DOI] [PubMed] [Google Scholar]
- Zisner A., Beauchaine T. (2017). Psychophysiological methods and developmental psychopathology In: Cicchetti D., editor. Developmental Psychopathology, 3rd edn Hoboken: Wiley. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.