Abstract
Facial expressions offer an ecologically valid model for examining individual differences in affective decision-making. They convey an emotional signal from a social agent and provide important predictive information about one’s environment (presence of potential rewards or threats). Although some expressions provide clear predictive information (angry, happy), others (surprised) are ambiguous in that they predict both positive and negative outcomes. Thus, surprised faces can delineate an individual’s valence bias, or the tendency to interpret ambiguity as positive or negative. Our initial negativity hypothesis suggests that the initial response to ambiguity is negative, and that positivity relies on emotion regulation. We tested this hypothesis by comparing brain activity during explicit emotion regulation (reappraisal) and while freely viewing facial expressions, and measuring the relationship between brain activity and valence bias. Brain regions recruited during reappraisal showed greater activity for surprise in individuals with an increasingly positive valence bias. Additionally, we linked amygdala activity with an initial negativity, revealing a pattern similarity in individuals with negative bias between viewing surprised faces and maintaining negativity. Finally, these individuals failed to show normal habituation to clear negativity. These results support the initial negativity hypothesis, and are consistent with emotion research in both children and adult populations.
Keywords: ambiguity, emotion regulation, amygdala, pattern similarity, habituation
Introduction
Among the extensive and varied catalog of social interactions, humans are often faced with the task of interpreting another person’s social signals. Facial expressions are nonverbal signals of emotion which can be predictive of motivationally relevant variables in the environment (Ekman and Friesen, 1971). Although some expressions (happy, angry) provide clear predictive information, other expressions are less clear. For example, surprised facial expressions may be associated with both pleasant (surprise party) and unpleasant (witnessing a car accident) outcomes. Without a clarifying context, individuals must rely on personal experiences and biases in order to make decisions about the valence of these faces. Along these lines, ratings of surprised relative to angry or happy expressions comprise longer reaction times and larger variability across individuals (Neta et al., 2009; 2013; Neta and Tong, 2016). This variability in ratings of surprised faces provides insight into a stable, trait-like individual difference in valence bias (the tendency to interpret surprised faces as positive or negative).
Despite individual differences in ratings of emotional ambiguity, initial responses toward ambiguous stimuli tend to be more negative compared with delayed responses (Kim et al., 2003; Kaffenberger et al., 2010; Neta and Whalen, 2010; Neta et al., 2011; Neta and Tong, 2016). For example, reaction times are longer when rating ambiguous cues as positive compared with negative (Neta and Tong, 2016), and surprised faces are detected more quickly as an oddball among positive (happy) compared to among negative (angry) faces (Neta et al., 2011). Other work has shown that faster visual processing of surprised faces results in more negative interpretations (Neta and Whalen, 2010), and that positive ratings are associated with a strong attraction toward the competing (negative) response option (Neta et al., in preparation).
Taken together, our working model suggests that the initial response to the emotional ambiguity of surprised faces is negative, and that positive interpretations may require some regulatory mechanism that overrides this initial negativity. Indeed, domain-general cognitive control regions are recruited when participants make decisions in an effort to resolve ambiguity (Neta et al., 2013, 2014), suggesting that some form of top-down control or regulatory process is important for processing ambiguity. However, there is no evidence to suggest that this mechanism allows for positivity bias (overriding negativity), or that it is related to emotion regulation per se. Other work has shown that a more negative interpretation of surprise is associated with activity in the amygdala, whereas a more positive interpretation is associated with activity in medial prefrontal cortex (Kim et al., 2003), a region that is structurally connected with the amygdala (Price, 2005) and regulates the amygdala in some instances (Ochsner and Gross, 2005; Urry et al., 2006; Winecoff et al., 2011; Silvers et al., 2016). However, little has been done to directly link valence bias with emotion regulation, which is the focus of the present work.
As described above, most of the previous work testing the initial negativity hypothesis has focused on demonstrating that negativity is faster and likely first, and has relied on behavioral measures. A goal of the present study, therefore, was to provide evidence for both the initial negativity and the regulation needed for positivity using converging neuroimaging methods. First, we identified regions that are recruited during an explicit emotion regulation task, and examined brain activity in these regions when viewing surprised faces as a function of valence bias. We hypothesized that if the positive valence bias relies on emotion regulation, then there would be greater activity in these regions in individuals with an increasingly positive bias.
Second, we employ a multivariate neuroimaging approach that has been increasingly used to explore patterns of brain activity associated with a particular stimulus or behavior (Kriegeskorte et al., 2008; Kriegeskorte and Kievit, 2013). As opposed to more traditional univariate analyses, which are often used to identify brain regions recruited under specific conditions, multivariate approaches such as pattern similarity examine activity patterns associated with those conditions (Hsieh et al., 2014; Kragel and Labar, 2016). This approach has been recently used to demonstrate that patterns of amygdala activity reflect emotional valence (Jin et al., 2015) and learning (Visser et al., 2015). However, this research is limited with respect to individual differences in processing emotional ambiguity. One study related amygdala patterns to anxiety-related biases in processing morphed expressions (Bishop et al., 2015), but did not probe responses to the more ecologically valid intact surprised facial expressions. Given that we propose the initial response is negative, we hypothesized that individuals with a more negative valence bias would show similar patterns of amygdala activity for free viewing expressions of surprise as for maintaining negative affect (as opposed to downregulating that negativity).
Finally, one important feature of amygdala activity is that it tends to decrease with repeated exposures to clear negativity (i.e . habituation; Breiter et al., 1996; Whalen et al., 1998, 2001; Phelps et al., 2001; Phillips et al., 2001; Wright et al., 2001; Somerville et al., 2004). Interestingly, individual differences in amygdala activity are often lost when examining activation magnitudes (Schuyler et al., 2014), but the change in activity over time (habituation) has been shown to relate to stable individual differences. For example, slower habituation to negative stimuli is associated with decreased well-being (Davidson, 2004), more inhibited temperament (Blackford et al., 2013), and greater trait anxiety (Hare et al., 2008) and PTSD (van den Bulk et al., 2016). Here, we will build on these findings by examining habituation to clear negativity as a function of individual differences in valence bias. We hypothesized that individuals with a more negative bias, like those high in trait anxiety, will show weaker amygdala habituation.
Materials and methods
Participants
We tested 57 participants (28 female; ages 17–30 years, mean age = 20.8, s.d. = 2.93) who were right-handed, had no history of psychological or neurological disorders, and were not taking any psychotropic medication. Additionally, all participants were Caucasian to control for any cross-race effects when making judgments about emotional expressions of Caucasian faces. Three participants were excluded because they failed to provide accurate ratings of clearly valenced faces (angry, happy) on at least 60% of trials, as in previous work (Neta et al., 2009, 2013, 2018; Neta and Tong, 2016; Brown et al., 2017). Three additional participants were removed because they did not complete the neuroimaging portion of the task, resulting in a final sample of 51 participants (26 female; ages 17–30 years, mean age = 20.7, s.d. = 2.93). The local Institutional Review Board approved all research protocols, and participants gave written informed consent prior to testing in accordance with the Declaration of Helsinki.
Procedure
Session 1: assessing valence bias
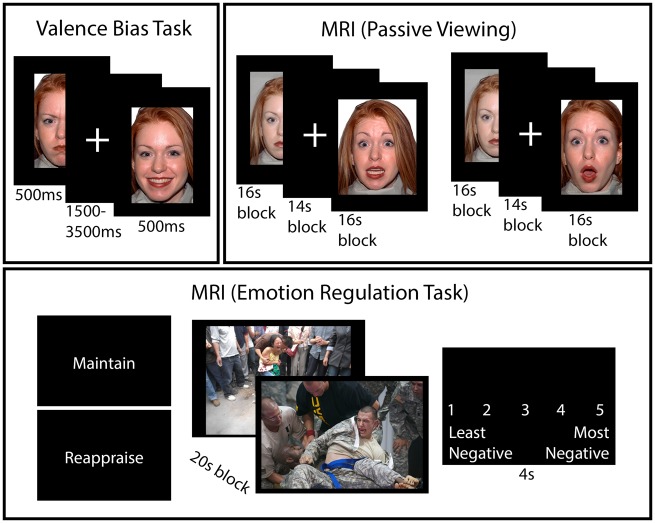
We used E-Prime software (Psychology Software Tools, Pittsburgh, PA, USA) in all behavioral testing. In Session 1, participants performed a task to assess their baseline valence bias in which they viewed images of happy, angry, and surprised faces and rated (via keyboard press) each image as positive or negative. Images included 34 discrete identities, with 14 of them (7 females, ages 21–30 years) taken from the NimStim Set of Facial Expressions (Tottenham et al., 2009) and 20 of them (10 females, age 20–30 years) taken from the Karolinska Directed Emotional Faces database (Goeleven et al., 2008). Stimuli were presented for 500 ms with an interstimulus interval of 1500 ms. Each block of stimuli included 24 images (eight of each expression) presented in a pseudorandom order, and blocks were counterbalanced between participants (see Figure 1 for a depiction of tasks). We calculated the valence bias for each participant using percent negative ratings of surprised faces (i.e. the percent of trials a face was rated as negative out of the total number of surprised faces presented, excluding omissions).
Fig. 1.
Depiction of behavioral tasks. The valence bias task was completed a week prior to scanning. Participants viewed happy, angry and surprised faces, and rated each face as positive or negative. In the MRI, participants passively viewed a new set of faces (i.e. not overlapping with the valence bias task, despite the overlap shown here due to copyright issues). There were two runs with blocks of surprised and neutral faces, and two runs with blocks of fearful and neutral faces. The emotion regulation task, also in the MRI, included blocks with instructions to “maintain”, and others to “reappraise” the response to negatively valenced scenes (IAPS). After each block, participants rated their negative affect on a scale of 1–5. Images shown here were not the actual stimuli, but rather they were pulled from the public domain.
Session 2: magnetic resonance imaging
One week later, participants returned for a follow-up session in the magnetic resonance imaging (MRI) scanner. Participants freely viewed blocks of faces in four runs: two runs with blocks of surprise and blocks of neutral faces, then two runs of fear and neutral blocks. Because of the study’s primary focus on surprised faces, and to prevent possible priming effects of the fearful faces, blocks containing only surprise expressions always preceded the blocks containing fear expressions. It is also worth noting that, although we examined neural responses to fear, we did not collect subjective ratings of fear faces at any point. However, our other work that has used surprised and fearful faces interleaved (e.g. Neta and Whalen, 2010; Neta and Dodd, 2018; Neta et al., in preparation) has shown that participants responded to fear consistently negatively, whereas there was a range of individual differences in response to surprise.
We used a new set of faces from the Umeå University Database of Facial Expressions (Samuelsson et al., 2012), and included four male and four female identities. The same neutral expressions were presented in surprise and fear runs. Each block contained 32 faces (in a pseudorandom order) with each face shown for 200 ms, followed by a fixation cross for 300 ms, as in previous work (Kim and Whalen, 2009). There were 14 s of fixation between blocks. Each of the four runs included six blocks of faces with three blocks of emotion (surprised or fearful) and three blocks of neutral expressions, and the order of blocks was counterbalanced between participants.
Following this free viewing task, participants performed an explicit emotion regulation task that focused on the reappraisal strategy. Given that surprised faces have a dual valence ambiguity (both positive and negative interpretations are valid), participants are thought to be overriding the initial negativity by following a more positive (re)interpretation of the expression. In other words, a positive bias is not likely the result of distancing or suppressing the negative interpretation, but rather by interpreting the expression as having a positive meaning. We adopted an fMRI paradigm (Phan et al., 2005) in which we asked participants to regulate their emotions as they viewed images of negatively valenced scenes from the International Affective Picture System (IAPS) (Lang et al., 1997). In half of the blocks, participants were asked to maintain their initial response to the images (Maintain), whereas in the other half of the blocks, they were asked to regulate their natural response so that they experienced less negative, or potentially positive, emotion (Reappraise). Importantly, before beginning the emotion regulation task, we trained participants on how to complete the Maintain and Reappraise task, and participants completed one of each block as practice. The images used in these practice blocks differed from the 80 images used in the task. For the emotion regulation task, Maintain and Reappraise blocks occurred in a pseudorandom order, counterbalanced between participants. Stimuli were presented in eight blocks per run (four per condition), where each block contained 4000 ms presentations of five consecutive images. At the end of each block, participants had 4000 ms to rate via button press their level of negative affect on a scale from 1 (least negative) to 5 (most negative). To calculate a reappraisal success score for each participant that accounted for the intensity of the negative affect in the ‘natural’ response reported during Maintain blocks, we computed the difference between the average Maintain and Reappraise score for each participant, and then multiplied this value by their Maintain score.
MRI acquisition and processing
Scan parameters
All MRI scans were performed on a Siemens 3 T Skyra scanner using a 32-channel head coil at the University of Nebraska-Lincoln, Center for Brain, Biology & Behavior. We acquired structural images using a T1-weighted MPRAGE sequence with the following parameters: TR = 2.2 s, TE =3.37 ms, slices = 192 interleaved, voxel size = 1.0 × 1.0 × 1.0 mm, matrix =256 × 256 mm, FOV = 256 mm, flip angle = 7°, total acquisition time = 5:07. While participants freely viewed faces, we tracked blood oxygen level-dependent (BOLD) activity using an EPI sequence with the following parameters: TR = 2.5 s, TE =30 ms, slices = 42 interleaved, voxel size = 2.5 × 2.5 × 3.0 mm, matrix = 88 × 88 mm, FOV = 220 mm, flip angle = 80°, total acquisition time = 3:24. Slices were acquired parallel with the intercommissural plane, and the volume positioned to cover the entire brain. We used identical parameters for the emotion regulation task, except the total acquisition time was increased to 6:49.
MRI preprocessing
We analyzed imaging data using MATLAB (The MathWorks) and the Analysis of Functional Neuroimages (AFNI) suite of programs (Cox, 1996). The first four volumes acquired were discarded to allow for equilibration. We corrected for incidental head motion by registering all BOLD volumes to the minimum outlying anatomical volume and blurring the images with a 6.0 mm (full width at half maximum) Gaussian filter. To perform group-level analyses, we warped each participant’s scans to a Talairach template atlas (Talairach and Tournoux, 1988) using linear transformation and re-sliced the images to 3.0 mm isotropic voxels. We normalized the functional data by dividing the signal of each voxel by the mean intensity of the same voxel in a time series and multiplying by 100, thereby using voxel-wise percent of the mean intensities in regression analyses. We used a general linear model with a boxcar block design consisting of six motion regressors (three rotational and three translational vectors) and task-related regressors. For the task of free viewing faces, one model included Fear, Neutral and Surprise regressors, while another included Early Fear, Late Fear, Early Neutral, Late Neutral, Early Surprise and Late Surprise regressors. We defined Early stimuli as items presented during the first of two runs for that expression (surprise or fear), and Late as those that were presented in the second of two runs. For the emotion regulation task, the model included two regressors—Maintain and Reappraise—in addition to the six motion regressors. Nuisance regressors were also included to model slow temporal drifts. Given the different lengths of the runs, the blocks of freely viewed faces modeled a linear and quadratic trend, whereas the longer emotion regulation runs contained an additional cubic trend, the application of which was roughly equal to 0.0026 Hz highpass filtering. Volumes in which there was a significant motion event (>0.3° rotation) were excluded from the GLM. All regressors were convolved with a canonical hemodynamic response function.
To isolate neural responses to emotional expressions and to compare activity for Fear and Surprise, we used the Neutral trials associated with each particular run as a baseline in all of our analyses involving facial expressions, as in previous work (Kim et al., 2003; Kim and Whalen, 2009). In the emotion regulation task, fixation served as a baseline, and we calculated activity for Reappraise and Maintain blocks compared with baseline.
Defining regions of interest
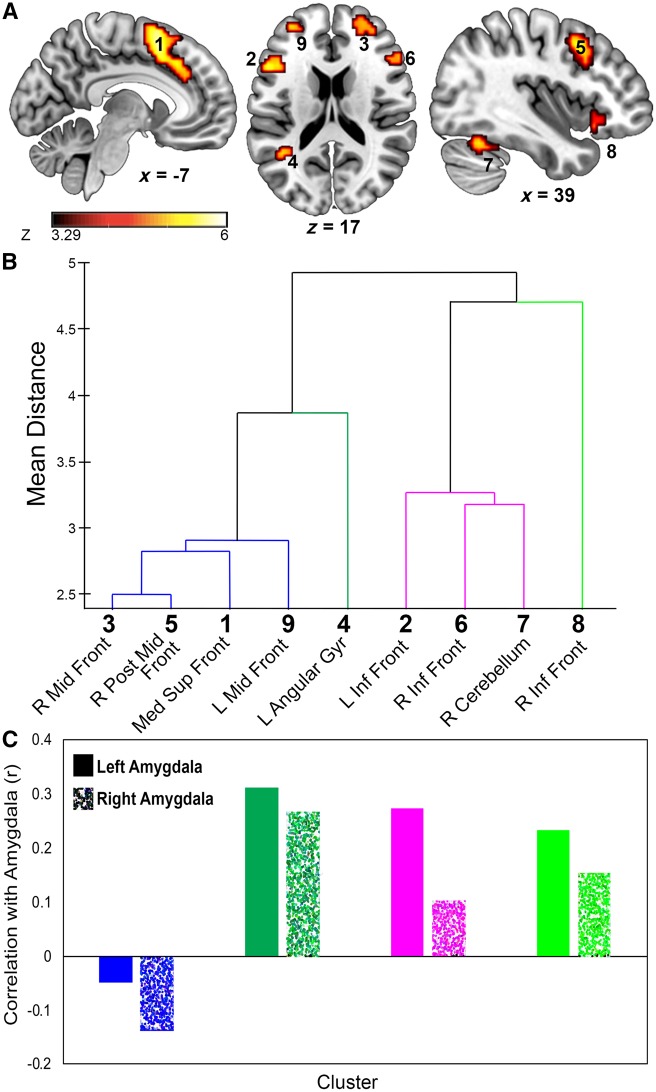
Regions of interest (ROIs) were defined based on effects observed during the explicit emotion regulation task. First, we isolated functional amygdala clusters by calculating voxels across subjects that had a significant task (Maintain plus Reappraise) vs baseline effect during the emotion regulation task. To identify voxels in the amygdala most active during the emotion regulation task, a voxel-wise threshold P-value <1.0 × 10−11 (uncorrected) was used. This strict threshold was used to isolate only the most sensitive regions of the amygdala to enhance the interpretability of the results (Woo et al. 2014). This resulted in clusters in left (Talairach: −20, −2, −13) and right (Talairach: 32, 2, −16) amygdala consisting of 17 and 62 voxels, respectively.
We also isolated brain regions that were active for explicit emotion regulation, specifically relying on a Reappraise >Maintain contrast. Clusters were corrected for multiple comparisons by implementing a series Monte Carlo simulations on the functional data using AFNI’s 3dClustSim as implemented in the “3dttest++” command to estimate noise. This method is a non-parametric approach which, across 10 000 iterations, simulates noise by randomizing the sign of residual data and calculating cluster sizes. These estimates are used to generate cluster forming probabilities. Using this method, clusters were considered significant at the P <0.05 level if exceeding a P <0.001 threshold across 29 contiguous (face-touching) voxels. Anatomical labeling of clusters was conducted using the Eickoff-Zilles (Eickoff et al., 2007) macro level atlas as implemented in AFNI. This analysis resulted in nine significant ROIs (Table 1; Figure 2A). To characterize the relationship between these nine ROIs, we conducted an unweighted pair group method with arithmetic mean (UPGMA) hierarchical clustering analysis (Sneath and Sokal, 1973). We included β weights for Reappraise and Maintain trials contrasted with baseline as well as Fear and Surprise trials contrasted with Neutral trials in the clustering analysis. This divided the ROIs into groups according to their response in both tasks (Figure 2B). Because the prevailing literature suggests a negative correlation between amygdala and prefrontal regions (Hariri et al., 2003; Lieberman et al., 2007; Etkin et al., 2010), particularly during reappraisal (Ochsner et al., 2002; Goldin et al., 2008; Drabant et al., 2009; Kim et al., 2011), we tested regions that (i) were closely related in their response across tasks and (ii) had a negative correlation with amygdala during emotion regulation. The average β weights for the ROIs in each of these four clusters were correlated with the β weights from the left and right amygdala. The first cluster of ROIs, which included the right middle frontal, right posterior middle frontal region, medial superior frontal region and left middle frontal, correlated negatively with the amygdala, whereas the three other clusters correlated positively with the amygdala (Figure 2C). Having identified the first cluster as our primary ROIs, we tested correlations of activity in these regions with behavioral measures (i.e. reappraisal success and valence bias). Given that the distribution of valence bias scores was not normally distributed (according to the Shapiro–Wilk test), and the distribution of reappraisal scores contained outlying data (>3 s.d. above the median) all correlations used Spearman’s rank correlation.
Table 1.
ROIs with significant effects for Reappraise>Maintain
| ROI | Voxels | Peak x | Peak y | Peak z | Peak Voxel z stat |
|---|---|---|---|---|---|
| 1. Medial Superior Frontala | 975 | −8 | 11 | 54 | 5.63 |
| 2. L Inferior Frontal (p. Triangularis) | 167 | −52 | 20 | 14 | 5.16 |
| 3. R Middle Frontal | 116 | 20 | 50 | 30 | 4.29 |
| 4. L Angular Gyrus | 109 | −44 | −62 | 24 | 4.45 |
| 5. R Posterior Middle Frontal | 84 | 38 | 10 | 44 | 5.18 |
| 6. R Inferior Frontal (p. Triangularis) | 70 | 52 | 26 | 14 | 4.03 |
| 7. R Cerebellum (Crus I) | 67 | 34 | −50 | −28 | 4.81 |
| 8. R Inferior Frontal (p. Orbitalis) | 42 | 40 | 26 | −10 | 4.27 |
| 9. L Middle Frontal | 33 | −32 | 50 | 14 | 4.82 |
All voxels contain Ps<0.05 corrected. Coordinates are in Talairach space. Clustered are anatomically labeled using the Eickoff-Zilles macro label atlas provided as implemented in AFNI.
Peak-z value coordinates of the third local maxima, representative of this extensive, bilateral cluster.
Fig. 2.
Regions sensitive to the explicit emotion regulation task. (A) Activation map showing increased activity for Reappraise relative to Maintain trials. A total of nine regions survived threshold (k = 29, P < 0.001). (B) UPGMA hierarchical clustering of the 9 regions, using their averaged βs for Maintain and Reappraise relative to baseline, as well as Surprise and Fear trials relative to Neutral. Based on this hierarchical clustering analysis, these nine regions were divided into four cluster groups. (C) Regions from each cluster were correlated with amygdala activity for the Reappraise>Maintain contrast. Only the first cluster (blue) showed a negative correlation with amygdala.
Pattern similarity analysis
To probe the relationship between emotion regulation and valence bias in the amygdala, we performed a pattern similarity analysis on amygdala activity comparing individuals with a positive vs negative valence bias. Amygdala activity patterns were defined as the β weights from Surprise, Maintain and Reappraise trials across each individual voxel within amygdala masks across trials for each participant. Pearson correlations were calculated for patterns of amygdala activity between Maintain and Surprise, and between Reappraise and Surprise trials for each subject. Then, we tested the relationship between pattern similarity for each individual subject and both measures of reappraisal success and valence bias.
Psychological–physiological interaction
The previously described analyses aimed to investigate individual differences in reactivity to facial expressions in regions negatively associated with amygdala activity across individuals. One possibility is that individual differences in valence bias may be related to differences in functional connectivity in the amygdala. Indeed, it has been demonstrated that one single area may not be explicative of the cognitive functions underlying the processing of a stimulus, instead the strength of the connections may vary and be more informative (Pessoa, 2014; Diano et al., 2017). A psychological–physiological interaction (PPI) analysis was conducted, aimed at identifying regions functionally connected to the amygdala for Surprise relative to Neutral trials. Here, BOLD activity from the bilateral amygdala was multiplied with a boxcar regressor modeling Surprise (+1) greater than Neutral (−1), convolved with the HRF, and treated as a regressor in a GLM, which otherwise included regressors modeling trial onsets and nuisance regressors. The β values associated with this PPI repressor thus represented the difference in functional connectivity between Surprise relative to Neutral trials. To identify clusters of BOLD activity showing this condition-specific connectivity with the amygdala, β values were submitted to a one-sample t-test, and cluster thresholds determined using Monte Carlo simulations (as described above). As an additional exploratory analysis, these β weights were correlated with valence bias separately at each voxel using a Spearman rank correlation across all participants. Multiple comparison corrections for these correlation coefficients was accomplished using a cluster-extent (k = 13) and cluster-wise (P = 0.001) threshold, calculated based on random field theory (Friston et al., 1994; Hayasaka and Nichols, 2003) according to the voxels sizes and spatial smoothing parameters used in this study.
Results
Behavioral
Valence bias task
Valence ratings—characterizing valence bias
The dependent measure we used was percent negative ratings. Participants rated angry faces as negative (Mean=95.5, s.d.=6.6; range=75–100), and happy faces as positive (Mean=6.2, s.d.=8.9; range=0–38). In contrast, there were individual differences in ratings of surprised expressions (Mean=59.1, s.d.=24.4; range=0–100), which represented the baseline valence bias for each individual.
Reaction time
Given that the focus of this study was to examine responses to surprised faces, the clearly valenced (happy and angry) expressions were included only to serve as anchors for participants’ ratings of ambiguity. As such, we focus our behavioral analyses on the surprised faces, as in previous work (Neta et al., 2013). We correlated valence bias and reaction times, which revealed a significant negative correlation [r(49)=−0.454; P=0.001], such that individuals with a more positive valence bias took longer to rate surprised expressions.
Valence ratings over time
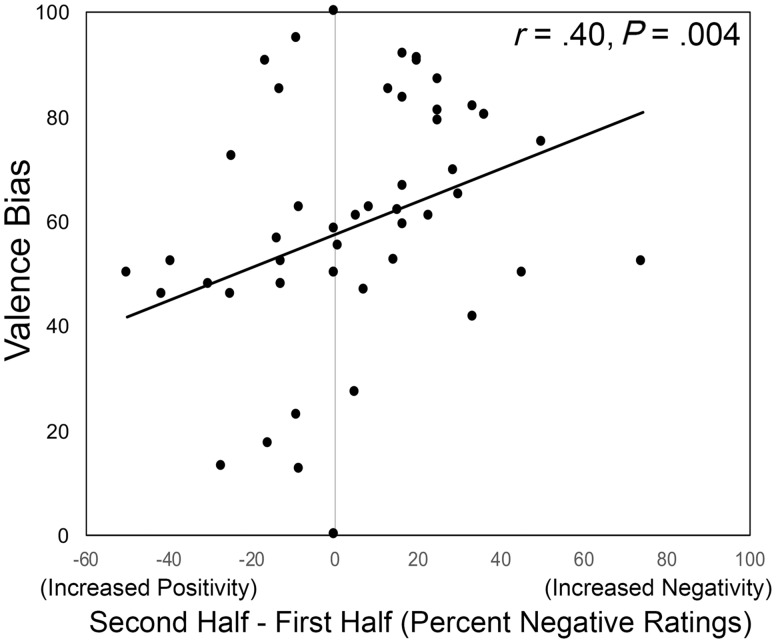
Because we were interested in the relationship between habituation and valence bias, we divided this task into two halves and compared ratings of Surprise for each half across groups. Specifically, we calculated a difference score for percent negative ratings in the second half of trials minus the first half of trials, representing increased negativity over time. We correlated this difference score with valence bias, which revealed a significant positive correlation [r(49)=0.397; P=0.004; Figure 3], such that individuals with a more negative bias showed an increase in negativity over time.
Fig. 3.
Change in valence bias over time. Individuals with a more negative valence bias showed increased negativity in their ratings over time.
Emotion regulation task
Reappraisal success was calculated as the Maintain – Reappraise ratings multiplied by the Maintain rating, where high values represented high reappraisal success (i.e. greatest decrease in negativity from Maintain to Reappraise, accounting for the level of negativity when asked to simply Maintain). The average reappraisal success was 5.9620 (s.d.=3.4776; range=0.2812–16.8750).
Imaging
Neuroimaging evidence of the initial negativity hypothesis
Reappraise > Maintain activity within the first cluster of regions correlated negatively with activity in the amygdala (Figure 2C). This inverse relationship with the amygdala is considered a marker of emotion regulation. Averaging across the four ROIs in this cluster, we compared Reappraise β values to individual differences in reappraisal success and valence bias in order to further support a link between activity in these regions and these behavioral measures. There was a trend for a positive correlation between reappraisal success and Reappraise BOLD activity across participants [r(49)=0.2645; P=0.0607], indicating that those with more activity in regions sensitive to the explicit emotion regulation task were more successful at explicit reappraisal of negatively valenced images. However, Reappraise BOLD activity was not related to valence bias [r(49)=0.2024, P=0.1544].
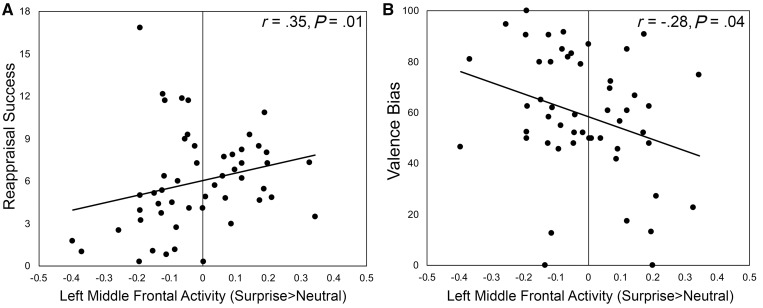
Next, we tested the relationship between activity in each of the individual ROIs during free viewing of surprised relative to neutral faces and both reappraisal success and valence bias. Activity to freely viewed surprised faces in the right [r(49)=0.3380, P=0.0153] and left middle frontal region [r(49)=0.3539, P=0.0108; Figure 4A] as well as the right posterior middle frontal region [r(49)=0.3112, P=0.0262] was positively related to reappraisal success, indicating that individuals that were better able to explicitly regulate their emotions showed more activity in these bilateral frontal cortical regions in response to surprised faces. The medial superior frontal region was not related to reappraisal success [r(49)=0.1946, P =0.1712].
Fig. 4.
Activity for surprised relative to neutral faces in a region recruited during explicit emotion regulation. (A) Activity in the left middle frontal cortex correlated with reappraisal success, such that greater activity was associated with greater success, and (B) activity in the same region also correlated with valence bias, such that greater activity was associated with a more positive bias.
Activity in the left [r(49)=−0.2818, P=0.0451; Figure 4B], but not right [r(49)=−0.1395, P=0.3289], middle frontal region was negatively correlated with valence bias, such that those who rated surprised faces as more negative had less activation of this emotion regulation region when freely viewing surprised faces. Activity in the right posterior middle frontal [r(49)=−0.1078, P=0.4513] and the medial superior frontal [r(49)=−0.0904, P=0.5283] regions were not correlated with valence bias.
Pattern similarity analysis
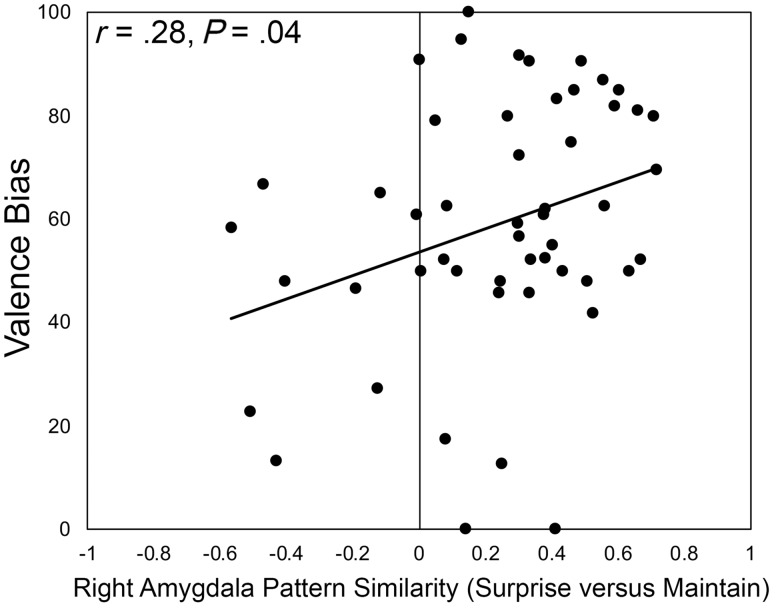
Next, patterns of amygdala activity were compared between Surprise β values and the β values for Maintain and Reappraise by correlating the β weights across all amygdala voxels for each participant separately. These two indices of pattern similarity from each participant were compared with valence bias. Similarity scores between Surprise and Maintain were positively correlated with valence bias in the right [r(49)=0.2838, P=0.0436; Figure 5] but not left [r(49)=0.1399, P=0.3277] amygdala, such that those who rated surprise more negatively showed more similar right amygdala activity when freely viewing surprised faces and when maintaining their natural or initial affective response during the explicit emotion regulation task. Similarity scores between Surprise and Reappraise β values were not correlated with valence bias in either the left [r(49)=0.1172, P=0.4127] or right [r(49)=0.1670, P =0.2414] amygdala.
Fig. 5.
Amygdala pattern similarity between Surprise and Maintain trials correlated with valence bias; β values from each voxel in the amygdala were correlated between Surprise and Maintain trials for each participant separately, producing values of pattern similarity. These similarity scores were positively correlated with valence bias, such that individuals with a more negative bias showed more similar patterns of activation between Surprise and Maintain. This provides some evidence linking the negative bias with the initial (i.e. not regulated) response to surprised faces.
Individual differences in valence bias and amygdala habituation
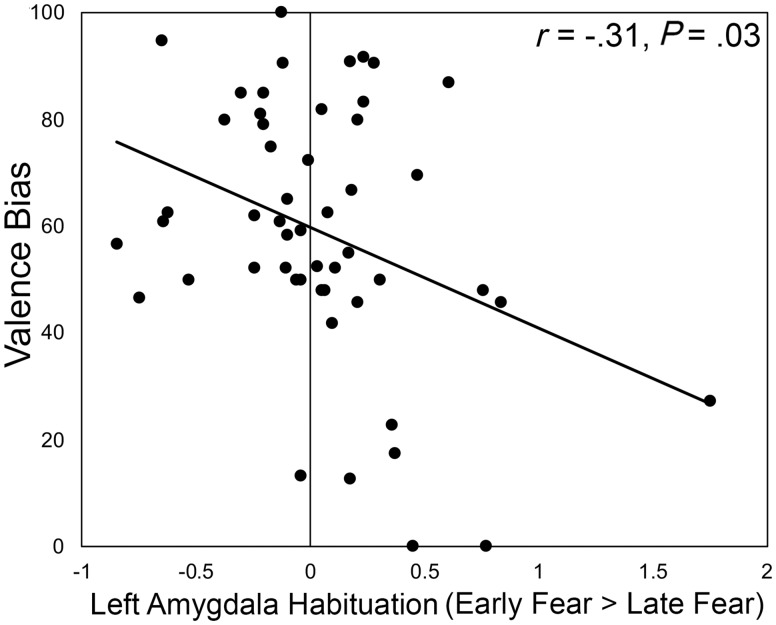
For analyses of habituation, we focused on activity associated with viewing fearful faces given that fear is accompanied by a marked habituation response, whereas surprise is not (Whalen and Phelps, 2009). To test whether differences in amygdala activity across the course of the experiment differed based on differences in valence bias, amygdala habituation to clearly valenced (fearful) faces (i.e. β values for early > late trials) was correlated with valence bias across participants. Habituation in the left amygdala was negatively correlated with valence bias [r(49)=−0.3122, P =0.0257; Figure 6], indicating that individuals who show less habituation in the amygdala are more likely to rate surprised faces negatively. This relationship was not observed in the right amygdala [r(49)=−0.0992, P =0.4884].
Fig. 6.
Amygdala habituation correlated with valence bias. Individuals with a more negative valence bias showed weaker amygdala habituation, as represented by greater activity for fearful faces in early relative to late trials.
Psychological–physiological interaction
The PPI analysis aimed to first identify regions functionally connected to the amygdala in Surprise relative to Neutral trials. This analysis revealed two regions that showed inverse connectivity with the amygdala. One region was located in the right middle cingulate cortex (peak-z=−4.50, k = 89; x=−7.5, y = 35, z=−10), and the second was located in the left rectal gyrus (peak-z=−4.72, k = 94; x = 1.5, y=−23, z = 33), consistent with previous work (Gee et al., 2013). Next, we identified regions showing different degrees of Surprise > Neutral amygdala connectivity as a function of valence bias. No clusters survived thresholds in either positive or negative directions. As a follow-up analysis, we implemented a more lenient cluster-wise threshold of P=0.05 and limited our search to voxels in overlapping with the Reappraise > Maintain regions. Overlapping voxels were observed between seven of the nine Reappraise > Maintain regions and voxel clusters showing a positive relationship between amygdala connectivity and valence bias (i.e. voxels showing greater amygdala connectivity in individuals with a more positive valence bias). In the medial superior frontal region, a total of 84 voxels across 5 voxel clusters correlated with valence bias. In the left angular gyrus, a total of 5 voxels between 2 voxel clusters shared this correlation. In addition, this correlation was observed in 2 voxels within the left inferior frontal region, 6 voxels within the right middle frontal region, 6 voxels in the right posterior middle frontal, 2 voxels in the right inferior frontal region and 14 voxels in the right cerebellum. The results from this exploratory analysis indicate that these regions sensitive to explicit emotion regulation show more negative connectivity with the amygdala in individuals with a more positive valence bias.
Discussion
The findings in the present study suggest that one mechanism underlying individual differences in valence bias is the differential activity in brain regions sensitive to explicit emotion regulation. First, the left middle frontal gyrus showed increased activity during explicit emotion regulation while viewing IAPS scenes. This same region also showed greater activity for freely viewed surprised faces in individuals with an increasingly positive valence bias, and also in individuals with greater emotion regulation success. Also, a pattern similarity analysis revealed that individuals with a negative valence bias showed amygdala responses to surprised faces that were similar to maintaining negative affect towards negatively valence IAPS scenes during an emotion regulation task. Taken together, this study supports the prevailing hypothesis that positive interpretations of ambiguous stimuli involve mechanisms common to reappraisal during explicit emotion regulation (Neta and Tong, 2016), and is consistent with the notion that negative interpretations tend to be the initial response (Kim et al., 2003; Kaffenberger et al., 2010; Neta and Whalen, 2010; Neta et al., 2011).
Here, we unpack our findings within the framework of our primary objectives: (i) To demonstrate that a positive valence bias is associated with a mechanism common to emotion regulation, (ii) to examine patterns of amygdala activity for evidence that the initial response is negative and (iii) to examine change in amygdala activity (habituation) as a function of individual differences in valence bias.
Positive valence bias is associated with emotion regulation
Lateral prefrontal regions are important for monitoring and altering behavior to conform with one’s goals (Miller and Cohen, 2001), and specifically for emotion regulation ( Ochsner et al., 2002; Phelps and LeDoux, 2005; see Buhle et al., 2014 for a meta-analysis of cognitive reappraisal). Much of the research on emotion regulation varies considerably as to the exact location of lateral prefrontal regions that are recruited (Delgado et al., 2008; see also Ochsner and Gross, 2005); however, the regions we reported overlap with those previously linked with emotion regulation (Phan et al., 2005; Harenski and Hamann, 2006; Ohira et al., 2006; Kim and Hamann, 2007). Additionally, left middle frontal region activity during surprise trials was correlated with both reappraisal success and valence bias. In the context of explicit emotion regulation, this region is consistent with previous studies in which participants are instructed to judge the valence of ambiguous, non-face stimuli (Grimm et al., 2006; Jung et al., 2008). But, to our knowledge, this is the first study to examine how regions that are defined during emotion regulation are also related to individual differences in valence bias.
The present results highlight the psychological benefits of successful reappraisal of negative stimuli and the role of prefrontal activity during emotion regulation. Successful reappraisal is often thought to be related to one’s ability to find a positive outlook in negative situations, a hallmark of what is known as resiliency (Tugade and Fredrickson, 2004). As with resiliency, results of studies on individual differences in emotion regulation indicate that better reappraisal success is associated with increased well-being (Masten et al., 1999; Gross and John, 2003; Phelps and LeDoux, 2005). Neuroimaging results indicate that mindfulness training can lead to an increase in lateral prefrontal cortex activity and a concomitant decrease in anxiety (Hölzel et al., 2013). Therefore, it appears that perhaps with training, individuals could improve reappraisal success, and consequentially well-being. While some have found that mindfulness or compassion training improve emotion regulation (Goldin and Gross, 2010; Jazaieri et al., 2012, 2014) and that mindfulness training is associated with decreased amygdala reactivity to negative stimuli (Goldin and Gross, 2010), future studies might consider specifically testing the effects of mindfulness or other training on prefrontal cortex activity and whether such training may influence valence bias.
Taken together, these findings suggest that one characteristic of individuals with a positive valence bias is the increased recruitment of mechanisms involved in explicit reappraisal. This is consistent with our hypothesis that one strategy associated with positive valence bias is the reappraisal of the initial negative interpretation of an ambiguous emotional expression.
While other emotion regulation strategies involve distancing or suppressing the negative alternative, a positive valence bias is most likely related to (re)interpreting an ambiguous expression as having a positive meaning. Whether such regulation of ambiguous expressions is implicit or explicit, however, should be subject to future research. Indeed, implicit and explicit emotion regulation have been associated with dissociable neural networks (Gyurak et al., 2011; Etkin et al., 2015). However, activity in regions demonstrably involved in explicit regulation was also correlated with what we assume to be a task mostly involving implicit regulation (freely viewing facial expressions).
One potential consideration is that, even though the behavioral session took place a week before the MRI session, the first session could have primed participants to evaluate the valence of the faces that they were instructed to freely view during the MRI session. Future studies might include a thorough debriefing in order to determine whether or not participants are evaluating the valence of the faces and if they are of using an explicit emotion regulation strategy. Regardless, these results suggest that participants with a positive valence bias recruited emotion regulation regions when freely viewing surprised faces more than participants with a negative valence bias.
Patterns of amygdala activity support an initial negativity
Our pattern similarity results provide additional evidence that the initial response to surprised faces is negative. Specifically, the similarity of activation patterns in these amygdala clusters suggests that while freely viewing surprised faces, individuals with a more negative valence bias tended to show amygdala activity similar to when instructed to maintain their negative rating of negative IAPS scenes. These findings are consistent with work that linked changes in amygdala activity patterns to interpretations of morphed facial expressions (Bishop et al., 2015), and even a valence continuum in olfaction (Jin et al., 2015). We build on these findings by demonstrating that patterns of amygdala activity were associated with stable individual differences in valence bias. These findings also provide further evidence that the negative interpretation of emotional ambiguity (as represented by the amygdala response in individuals with a negative bias) may be more represented in the initial response. Interestingly, there was not a significant correlation in patterns of amygdala activity for reappraisal and surprise faces in individual with a positive valence bias. This suggests that, although explicit regulation may overlap with our putative implicit regulation process in some areas of the brain (lateral prefrontal), the mechanism by which the amygdala is recruited while freely viewing surprised faces and reappraising negative emotions may be more distinct.
Weaker amygdala habituation is associated with a more negative valence bias
Some individual differences in amygdala activity are lost when examining activation magnitudes (Schuyler et al., 2014), whereas changes in activity (habituation) offer unique information associated with stable individual differences. Indeed, we found that a more negative valence bias was associated with weaker habituation to clearly negative facial expressions and a concomitant increase in negative ratings of surprise as the task progressed. This is consistent with previous work showing that both negativity bias and weaker habituation are associated with trait anxiety (Hare et al., 2008). These findings are also consistent with evidence that weaker habituation is correlated with weaker amygdala-ventral prefrontal connectivity (Hare et al., 2008), given that children show weaker habituation and weaker amygdala-prefrontal connectivity than adults (Guyer et al., 2008), and they also show a more negative valence bias than adults (Tottenham et al., 2013).
Importantly, while the amygdala habituates towards stimuli with clear negative valence, no habituation occurs if stimuli hold ambiguous valence (see also Whalen and Phelps, 2009), unless the surprised faces are presented in a temporal context that suggests a more clearly negative interpretation (Davis et al., 2016). Taken together, examining the amygdala response to stimuli with clear negativity over time offers new insight into understanding the stable individual differences in valence bias.
Amygdala connectivity may be related to valence bias
The previous analyses demonstrated that valence bias is related to a set of regions sensitive to explicit reappraisal as well as activity in the amygdala. Given that the amygdala is highly connected with multiple cortical regions (Pessoa and Adolphs, 2010), its activity may functionally influence the activity of these cortical reappraisal regions. In the context of emotional regulation, inhibition of the amygdala via these frontal functional connections is thought to underlie successful reappraisal of emotional valence during regulation (Ochsner and Gross, 2005; Urry et al., 2006; Winecoff et al., 2011). The question arises whether these functional inhibitory connections may be implicated in positive valence bias. This question was explored using a PPI analysis to assess amygdala connectivity differences between surprise and neutral trials, and then submitting these connectivity indices to a correlation with valence bias. Correlations across all voxels did not survive multiple comparison corrections, but were significant at more lenient thresholds in voxels overlapping with the explicit emotion regulation regions. These results suggest that individuals with a more positive valence bias tend to have more negative functional connections between reappraisal regions and the amygdala while freely viewing surprised faces. However, future work is needed to more rigorously test these findings.
Limitations
The commonality in brain activity between explicit emotion regulation while viewing IAPS scenes and individual differences in valence bias (in response to surprised facial expressions) does not necessarily indicate identical mental processes across the tasks. Instead, the current findings indicate only that similar regions predict variability in explicit emotion regulation activity and valence bias. Furthermore, additional brain regions beyond those studied here (i.e. not sensitive to the emotion regulation task) may also relate to valence bias differences. Future work will be useful in providing a more comprehensive description of the relationship between valence bias and emotion regulation including the involvement of more widespread brain regions.
In a similar vein, an involvement of the amygdala in valence bias does not reflect the participants’ negativity prima vista. Indeed, previous work has shown that the amygdala responds to both negative and positive information, and could represent arousal or vigilance (Whalen et al., 1998; Lindquist et al., 2016). However, in the context of surprised faces, activity in the amygdala is associated with a more negative interpretation (Kim et al., 2003, 2004; note also that the amygdala is more active towards negative compared to positive emotional scenes; Lane et al., 1997; Sabatinelli et al., 2005; Straube et al., 2008). Notably, the coordinates for our amygdala ROIs (left amygdala: −20, −2, −13; right amygdala: 32, 2, −16) are located in the ventral amygdala (z-plane < −10), which tends to activate uniquely toward negative stimuli (Whalen et al., 2001). Indeed, previous work has demonstrated that the ventral amygdala primarily comprises basolateral nuclei and the cortical nucleus, which is related to the detection and discrimination of presented stimuli, for example, in terms of their valence (primarily negativity). The basolateral nuclei (putatively ventral amygdala) send projections to central nucleus (putatively dorsal amygdala), which projects to hypothalamic and brainstem target areas (Schwaber et al., 1982; Amaral et al., 1992), and to all major neuromodulatory centers (e.g. cholinergic, dopaminergic, serotonergic and noradrenergic source neurons; see Kapp et al., 1992). In other words, dorsal amygdala activation is thought to increase when the predictive nature of presented stimuli is unclear. Taken together, there is some evidence that suggests that the ventral amygdala (our ROIs) is more important for valence/negativity signals, whereas the dorsal amygdala is more important for vigilance/arousal (see Whalen et al., 2001; Kim et al., 2003; Neta and Whalen, 2010). Although future research will be important for explicitly testing the role of the ventral amygdala in differentiating positive from negative valence that is equally salient/arousing, there is some evidence that supports the notion that the amygdala activity reported here are suggestive of a negative affective response rather than a general increase in arousal.
Furthermore, and perhaps more importantly, if our reported responses in the amygdala were representing arousal, then we would predict that both individuals with a positive and a negative valence bias would show similar responses in amygdala (e.g. pattern similarity between viewing surprised faces and negative pictures). Indeed, previous work has shown that there are no surprise-related skin conductance differences between positive and negative valence bias groups (Neta et al., 2009). As such, we do not believe that the differences as a function of valence bias are attributed to differences in arousal.
Conclusions
The present study elucidates the neural mechanisms underlying individual differences in valence bias and provides support for the initial negativity hypothesis, which proposes that the initial response to emotional ambiguity is more negative, and that positivity is associated with an emotion regulation mechanism that allows for overcoming the initial negativity. Individuals who display more positivity to ambiguity are more likely to recruit brain regions that are involved with explicit emotion regulation (reappraisal). Thus, it appears that a positivity bias might be the result of some emotion regulatory mechanism that could represent greater resilience when confronted with uncertain negativity. Furthermore, individuals with a negativity bias respond to surprised faces in a similar manner as when they are instructed to maintain their natural response to clearly negative images, further supporting the notion that negativity represents the initial response to ambiguity. Finally, the negativity bias corresponds with increased negativity over time and a concomitant weaker amygdala habituation. Thus, individuals who interpret emotional ambiguity in a positive light may need to overcome the initial response (i.e. interpreting ambiguity as a potential threat) using neural mechanisms that are associated with greater resilience and overall well-being. Future research might set out to determine if this emotion regulation strategy is a useful intervention for increasing positivity bias.
Funding
This work was supported in part by NIMH111640 (PI: Neta), and by Nebraska Tobacco Settlement Biomedical Research Enhancement Funds.
Conflict of interest. None declared.
References
- Amaral D.G., Price J.L., Pitkänen A., Carmichael S.T. (1992). Anatomical organization of the primate amygdaloid complex In: Aggleton J.P., editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. New York: Wiley-Liss, pp. 1–66. [Google Scholar]
- Bishop S.J., Aguirre G.K., Nunez-Elizalde A.O., Toker D. (2015). Seeing the world through non rose-colored glasses: anxiety and the amygdala response to blended expressions. Frontiers in Human Neuroscience, 9, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford J.U., Allen A.H., Cowan R.L., Avery S.N. (2013). Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Social Cognitive and Affective Neuroscience, 8(2), 143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter H.C., Etcoff N.L., Whalen P.J., et al. (1996). Response and habituation of the human amygdala during visual processing of facial expression. Neuron, 17(5), 875–87. [DOI] [PubMed] [Google Scholar]
- Brown C.C., Raio C.M., Neta M. (2017). Cortisol responses enhance negative valence perception for ambiguous facial expressions. Scientific Reports, 7(1), 15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. [DOI] [PubMed] [Google Scholar]
- Davidson R.J. (2004). Well-being and affective style: neural substrates and biobehavioural correlates. Philosophical Transactions-Royal Society of London Series B Biological Sciences, 359(1449), 1395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis F.C., Neta M., Kim M.J., Moran J.M., Whalen P.J. (2016). Interpreting ambiguous social cues in unpredictable contexts. Social, Cognitive, and Affective Neuroscience, 11(5), 775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R., Nearing K.I., LeDoux J.E., Phelps E.A. (2008). Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron, 59(5), 829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano M., Tamietto M., Celeghin A., et al. (2017). Dynamic changes in amygdala psychophysiological connectivity reveal distinct neural networks for facial expressions of basic emotions. Scientific Reports, 7, 45260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant E.M., McRae K., Manuck S.B., Hariri A.R., Gross J.J. (2009). Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biological Psychiatry, 65(5), 367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Paus T., Caspers S., Grosbras M.H., Evans A.C., Zilles K., Amunts K. (2007). Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage, 36(3), 511–21. [DOI] [PubMed] [Google Scholar]
- Ekman P., Friesen W.V. (1971). Constants across cultures in face and emotion. Journal of Personality and Social Psychology, 17(2), 124. [DOI] [PubMed] [Google Scholar]
- Etkin A., Buchel C., Gross J.J. (2015). The neural bases of emotion regulation. Nature Reviews Neuroscience, 16(11), 693. [DOI] [PubMed] [Google Scholar]
- Etkin A., Prater K.E., Hoeft F., Menon V., Schatzberg A.F. (2010). Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. American Journal of Psychiatry, 167(5), 545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Worsley K.J., Frackowiak R.S., Mazziotta J.C., Evans A.C. (1994). Assessing the significance of focal activations using their spatial extent. Human Brain Mapping, 1(3), 210–20. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., et al. (2013). Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences, 110(39), 15638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeleven E., De Raedt R., Leyman L., Verschuere B. (2008). The Karolinska directed emotional faces: a validation study. Cognition & Emotion, 22(6), 1094–118. [Google Scholar]
- Goldin P.R., Gross J.J. (2010). Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion, 10(1), 83.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P.R., McRae K., Ramel W., Gross J.J. (2008). The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry, 63(6), 577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S., Schmidt C.F., Bermpohl F., et al. (2006). Segregated neural representation of distinct emotion dimensions in the prefrontal cortex—an fMRI study. Neuroimage, 30(1), 325–40. [DOI] [PubMed] [Google Scholar]
- Gross J.J., John O.P. (2003). Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85(2), 348–62. [DOI] [PubMed] [Google Scholar]
- Guyer A.E., Monk C.S., McClure-Tone E.B., et al. (2008). A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience, 20(9), 1565–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A., Gross J.J., Etkin A. (2011). Explicit and implicit emotion regulation: a dual-process framework. Cognition & Emotion, 25(3), 400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry, 63(10), 927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski C.L., Hamann S. (2006). Neural correlates of regulating negative emotions related to moral violations. Neuroimage, 30(1), 313–24. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., Fera F., Weinberger D.R. (2003). Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry, 53(6), 494–501. [DOI] [PubMed] [Google Scholar]
- Hayasaka S., Nichols T.E. (2003). Validating cluster size inference: random field and permutation methods. Neuroimage, 20(4), 2343–56. [DOI] [PubMed] [Google Scholar]
- Hölzel B.K., Hoge E.A., Greve D.N., et al. (2013). Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. Neuroimage-Clinical, 2, 448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh L.T., Gruber M.J., Jenkins L.J., Ranganath C. (2014). Hippocampal activity patterns carry information about objects in temporal context. Neuron, 81(5), 1165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazaieri H., Goldin P.R., Werner K., Ziv M., Gross J.J. (2012). A randomized trial of MBSR versus aerobic exercise for social anxiety disorder. Journal of Clinical Psychology, 68(7), 715–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazaieri H., McGonigal K., Jinpa T., Doty J.R., Gross J.J., Goldin P.R. (2014). A randomized controlled trial of compassion cultivation training: effects on mindfulness, affect, and emotion regulation. Motivation and Emotion, 38(1), 23–35. [Google Scholar]
- Jin J.W., Zelano C., Gottfried J.A., Mohanty A. (2015). Human amygdala represents the complete spectrum of subjective valence. Journal of Neuroscience, 35(45), 15145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y.-C., Park H.-J., Kim J.-J., et al. (2008). Reciprocal activation of the orbitofrontal cortex and the ventrolateral prefrontal cortex in processing ambivalent stimuli. Brain Research, 1246, 136–43. [DOI] [PubMed] [Google Scholar]
- Kaffenberger T., Bruhl A.B., Baumgartner T., Jancke L., Herwig U. (2010). Negative bias of processing ambiguously cued emotional stimuli. Neuroreport, 21(9), 601–5. [DOI] [PubMed] [Google Scholar]
- Kapp B.S., Whalen P.J., Supple W.F., Pascoe J.P. (1992). Amygdaloid contributions to conditioned arousal and sensory information processing In: Aggleton J.P., editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction .New York: Wiley-Liss, pp. 229–54. [Google Scholar]
- Kim H., Somerville L.H., Johnstone T., Alexander A.L., Whalen P.J. (2003). Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport, 14(18), 2317–22. [DOI] [PubMed] [Google Scholar]
- Kim H., Somerville L.H., Johnstone T., et al. (2004). Contextual modulation of amygdala responsivity to surprised faces. Journal of Cognitive Neuroscience, 16(10), 1730–45. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Whalen P.J. (2009). The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. Journal of Neuroscience, 29(37), 11614–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Loucks R.A., Palmer A.L., et al. (2011). The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural Brain Research, 223(2), 403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Hamann S. (2007). Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience, 19(5), 776–98. [DOI] [PubMed] [Google Scholar]
- Kragel P.A., Labar K.S. (2016). Decoding the nature of emotion in the brain. Trends in Cognitive Sciences, 20(6), 444–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Kievit R.A. (2013). Representational geometry: integrating cognition, computation, and the brain. Trends in Cognitive Sciences, 17(8), 401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Mur M., Ruff D.A., et al. (2008). Matching categorical object representations in inferior temporal cortex of man and monkey. Neuron, 60(6), 1126–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R.D., Reiman E.M., Bradley M.M., et al. (1997). Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia, 35(11), 1437–44. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (1997). International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Lieberman M.D., Eisenberger N.I., Crockett M.J., Tom S.M., Pfeifer J.H., Way B.M. (2007). Putting feelings into words—affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science, 18(5), 421–8. [DOI] [PubMed] [Google Scholar]
- Lindquist K.A., Satpute A.B., Wager T.D., Weber J., Barrett L.F. (2016). The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex, 26(5), 1910–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten A.S., Hubbard J.J., Gest S.D., Tellegen A., Garmezy N., Ramirez M. (1999). Competence in the context of adversity: pathways to resilience and maladaptation from childhood to late adolescence. Development and Psychopathology, 11(1), 143–69. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202. [DOI] [PubMed] [Google Scholar]
- Neta M., Berkebile M., Freeman J.B. (in preparation). The dynamic process of ambiguous emotion perception. [DOI] [PMC free article] [PubMed]
- Neta M., Dodd M.D. (2018). Through the eyes of the beholder: simulated Eye-movement Experience (“SEE”) modulates valence bias in response to emotional ambiguity. Emotion; doi:10.1037/emo0000421 (Advanced online publication). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M., Tong T.T. (2016). Don't like what you see? Give it time: longer reaction times associated with increased positive affect. Emotion, 16(5), 730–9. [DOI] [PubMed] [Google Scholar]
- Neta M., Whalen P.J. (2010). The primacy of negative interpretations when resolving the valence of ambiguous facial expressions. Psychological Science, 21(7), 901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M., Davis F.C., Whalen P.J. (2011). Valence resolution of ambiguous facial expressions using an emotional oddball task. Emotion, 11(6), 1425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M., Kelley W.M., Whalen P.J. (2013). Neural responses to ambiguity involve domain-general and domain-specific emotion processing systems. Journal of Cognitive Neuroscience, 25(4), 547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M., Norris C.J., Whalen P.J. (2009). Corrugator muscle responses are associated with individual differences in positivity-negativity bias. Emotion, 9(5), 640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M., Schlaggar B.L., Petersen S.E. (2014). Separable responses to error, ambiguity, and reaction time in cingulo-opercular task control regions. Neuroimage, 99, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M., Tong T.T., Henley D.J. (2018). It’s a matter of time (perspectives): shifting valence responses to emotional ambiguity. Motivation and Emotion, 42(2), 258–66. [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–9. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D.E. (2002). Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14(8), 1215–29. [DOI] [PubMed] [Google Scholar]
- Ohira H., Nomura M., Ichikawa N., et al. (2006). Association of neural and physiological responses during voluntary emotion suppression. Neuroimage, 29(3), 721–33. [DOI] [PubMed] [Google Scholar]
- Pessoa L. (2014). Understanding brain networks and brain organization. Physics of Life Reviews, 11(3), 400–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., Adolphs R. (2010). Emotion processing and the amygdala: from a ′low road′ to ′many roads′ of evaluating biological significance. Nature Reviews Neuroscience, 11(11), 773.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Moore G.J., Uhde T.W., Tancer M.E. (2005). Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry, 57(3), 210–9. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron, 48(2), 175–87. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., O'Connor K.J., Gatenby J.C., Gore J.C., Grillon C., Davis M. (2001). Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience, 4(4), 437–41. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Medford N., Young A.W., et al. (2001). Time courses of left and right amygdalar responses to fearful facial expressions. Human Brain Mapping, 12(4), 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L. (2005). Free will versus survival: brain systems that underlie intrinsic constraints on behavior. Journal of Comparative Neurology, 493(1), 132–9. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D., Bradley M.M., Fitzsimmons J.R., Lang P.J. (2005). Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage, 24(4), 1265–70. [DOI] [PubMed] [Google Scholar]
- Samuelsson H., Jarnvik K., Henningsson H., Andersson J., Carlbring P. (2012). The Umea University database of facial expressions: a validation study. Journal of Medical Internet Research, 14(5), e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler B.S., Kral T.R.A., Jacquart J., et al. (2014). Temporal dynamics of emotional responding: amygdala recovery predicts emotional traits. Social Cognitive and Affective Neuroscience, 9(2), 176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaber J.S., Kapp B.S., Higgins G.A., Rapp P.R. (1982). Amygdaloid basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. Journal of Neuroscience, 2(10), 1424–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Insel C., Powers A., et al. (2016). vlPFC–vmPFC–amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cerebral Cortex, 27(7), 3502–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneath P.H.A., Sokal R.R. (1973). Numerical Taxonomy: The Principles and Practice of Numerical Classification. San Francisco, CA: W. H. Freeman and Co. [Google Scholar]
- Somerville L.H., Kim H., Johnstone T., Alexander A.L., Whalen P.J. (2004). Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biological Psychiatry, 55(9), 897–903. [DOI] [PubMed] [Google Scholar]
- Straube T., Pohlack S., Mentzel H.J., Miltner W.H. (2008). Differential amygdala activation to negative and positive emotional pictures during an indirect task. Behavioural Brain Research, 191(2), 285–8. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. (1988). Co-Planar Stereotaxic Atlas of the Human Brain. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Stuttgart: Thieme. [Google Scholar]
- Tottenham N., Phuong J., Flannery J., Gabard-Durnam L., Goff B. (2013). A negativity bias for ambiguous facial-expression valence during childhood: converging evidence from behavior and facial corrugator muscle responses. Emotion, 13(1), 92.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., et al. (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research, 168(3), 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugade M.M., Fredrickson B.L. (2004). Resilient individuals use positive emotions to bounce back from negative emotional experiences. Journal of Personality and Social Psychology, 86(2), 320.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry H.L., van Reekum C.M., Johnstone T., et al. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience, 26(16), 4415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bulk B.G., Somerville L.H., van Hoofa M.J., et al. (2016). Amygdala habituation to emotional faces in adolescents with internalizing disorders, adolescents with childhood sexual abuse related PTSD and healthy adolescents. Developmental Cognitive Neuroscience, 21, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser R.M., Kunze A.E., Westhoff B., Scholte H.S., Kindt M. (2015). Representational similarity analysis offers a preview of the noradrenergic modulation of long-term fear memory at the time of encoding. Psychoneuroendocrinology, 55, 8–20. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Phelps E.A. (2009). The Human Amygdala. New York: Guilford Press. [Google Scholar]
- Whalen P.J., Rauch S.L., Etcoff N.L., McInerney S.C., Lee M.B., Jenike M.A. (1998). Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience, 18(1), 411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen P.J., Shin L.M., McInerney S.C., Fischer H., Wright C.I., Rauch S.L. (2001). A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion, 1(1), 70–83. [DOI] [PubMed] [Google Scholar]
- Winecoff A., LaBar K.S., Madden D.J., Cabeza R., Huettel S.A. (2011). Cognitive and neural contributors to emotion regulation in aging. Social Cognitive and Affective Neuroscience, 6(2), 165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C.W., Krishnan A., Wager T.D. (2014). Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage, 91, 412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C.I., Fischer H., Whalen P.J., McInerney S., Shin L.M., Rauch S.L. (2001). Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport, 12(2), 379–83. [DOI] [PubMed] [Google Scholar]