Abstract
Psychological resilience reflects the capacity to bounce back from stress, which plays an important role in health and well-being. However, less is known about the neural substrate for psychological resilience and the underlying mechanism for how psychological resilience enhances subjective well-being in the healthy brain. To investigate these issues, we employed fractional amplitude of low-frequency fluctuations (fALFF) measured with resting-state fMRI in 100 young healthy adults. The correlation analysis found that higher psychological resilience was related to lower fALFF in the left orbitofrontal cortex (OFC), which is involved in reward-related processing and emotion regulation. Furthermore, the mediation analysis indicated that psychological resilience acted as a full mediator of the association between the fALFF in left OFC and subjective well-being indicators (i.e. life satisfaction and hedonic balance). Importantly, these results remained significant after controlling for the effect of gray matter volume and regional homogeneity in the region. Overall, the present study provides the further evidence for functional neural substrates of psychological resilience and reveals a potential mechanism that psychological resilience mediates the effect of spontaneous brain activity on subjective well-being.
Keywords: resilience, subjective well-being, orbitofrontal cortex, amplitude of low-frequency fluctuations
Introduction
As an important personality trait, psychological resilience is defined as the capacity to bounce back from stress (Smith et al., 2010). It plays a protective role against disorders related to stress such as post-traumatic stress disorder (PTSD) (Agaibi and Wilson, 2005). Furthermore, psychological resilience is considered as a crucial concept in the field of positive psychology, and exhibits a beneficial effect on subjective well-being (Davydov et al., 2010; Hu et al., 2015). Subjective well-being reflects people’s cognitive (life satisfaction) and affective evaluations (hedonic balance) of their lives (Schimmack et al., 2002). A lot of research has found that psychological resilience is positively associated with hedonic balance and life satisfaction (Liu et al., 2012; Di Fabio and Palazzeschi, 2015; Kong et al., 2015d; Bajaj and Pande, 2016; Satici, 2016). In this study, we tried to investigate the neurobiological underpinnings of psychological resilience and the potential mechanism that how psychological resilience influences subjective well-being in the brain with resting-state fMRI (rs-fMRI).
Although psychological resilience is a hot topic in the field of positive psychology, limited work has used fMRI to explore the neurobiological underpinnings of resilience in healthy populations. For example, Waugh et al. (2008) used task-based fMRI (tfMRI) to probe this issue and found that, when facing with a threat, all of participants had prolonged activity in the insula to the aversive stimuli, but only low-resilient participants had prolonged activity in the insula to the neutral stimuli. Furthermore, Reynaud et al. (2013) found that high resilience was associated with increased activity in the amygdala and orbitofrontal cortex when responding to stress-related stimuli. However, these tfMRI results are limited to the regions that are activated by a certain task. Because psychological resilience is a complex construct, it should be related to different brain functions. Consistent with this view, evidence from studies on disorders related to stress has demonstrated that beyond the amygdala, insula and OFC, psychological resilience is also related to other regions within the prefrontal cortex (PFC) including anterior cingulate cortex (ACC) and medial PFC (mPFC) (Liberzon and Sripada, 2008; Milad et al., 2009; Sekiguchi et al., 2015).
Although psychological resilience has been defined as a dynamic process (Lutha and Cicchetti, 2000; Masten, 2001; Waugh and Koster, 2015), it has also often been considered as a stable trait (Jacelon, 1997; Waugh et al., 2011; Waaktaar and Torgersen, 2012), so its neural underpinnings might be related to the overall brain function under task-free conditions, which can be investigated by using the rs-fMRI based on measurements of low-frequency fluctuations (LFFs, 0.01–0.10 Hz) in the BOLD signal (Fox and Raichle, 2007; Biswal, 2012). Two increasingly popular measures of LFFs [e.g. regional homogeneity (ReHo) and fractional amplitude of low-frequency fluctuations (fALFF)] reflect local properties of spontaneous brain activity, but they characterize different aspects of the regional spontaneous activity. ReHo measures the temporal synchronization of the BOLD fluctuations in a given region (i.e. short range connectivity) that may reflect the interaction and integration among local voxels (Zang et al., 2004), whereas fALFF measures the amplitude of the BOLD fluctuations of each voxel (i.e. spontaneous brain activity intensity) (Zou et al., 2008). Importantly, the ReHo/fALFF measures have been used to identify the neural marker of mental disorders (Cheng et al., 2012; Dutta et al., 2014; Liu et al., 2014) and uncover the neural basis of individual differences in behavior in normal populations (Tian et al., 2012; Zou et al., 2013; Kong et al., 2015c, 2016a,b, 2018; Xiang et al., 2016). In addition, previous studies have found that the ReHo/fALFF could successfully predict task-evoked activations and behavioral performance (Mennes et al., 2011; Yuan et al., 2013; Zou et al., 2013). Therefore, these two measures can provide a useful tool to investigate neural correlates of psychological resilience.
Recently, Kong et al. (2015d) used the ReHo measure to explore neural correlates of trait resilience, and found that higher ReHo in the ACC and insula within salience network was associated with lower trait resilience. However, as Kong et al. (2015d) mentioned, this study failed to find an association of resilience with other PFC regions such as OFC, which has been demonstrated in studies on disorders related to stress, as well as studies on healthy populations (Liberzon and Sripada, 2008; Reynaud et al., 2013; Sekiguchi et al., 2015). Because both fALFF and ReHo reflect different aspects of the regional spontaneous activity (An et al., 2013; Tian et al., 2016; Zhao et al., 2016), so we wondered whether the association of psychological resilience with other PFC regions, especially OFC can be seen by using the measures on neuronal activity magnitude (i.e. fALFF). Recently, Wang et al. (2017) found a negative association between higher fALFF in the OFC and trait hope, which is a highly related construct to psychological resilience (Lloyd and Hastings, 2009; Satici, 2016). Thus, we speculated that the fALFF in the OFC could be negatively associated with psychological resilience.
Moreover, as we mentioned previously, behavioral studies have demonstrated that psychological resilience exhibits a beneficial effect on subjective well-being (Bajaj and Pande, 2016; Satici, 2016). Importantly, some regions with the PFC related to psychological resilience (e.g. OFC and ACC) are also found to be implicated in subjective well-being (Van Reekum et al., 2007; Kong et al., 2015a,c,d, 2016a;). Notably, Kong et al. (2015d) revealed that the ReHo in the ACC related to trait resilience could significantly predict the cognitive component of subjective well-being (i.e. life satisfaction), indicating that the ACC is an important site supporting the link between cognitive well-being and psychological resilience. However, there’re still several problems needing further research. On one hand, as mentioned earlier, this study did not find any association of other PFC subregions, especially OFC with psychological resilience and life satisfaction (Liberzon and Sripada, 2008; Reynaud et al., 2013; Sekiguchi et al., 2015; Kong et al, 2015c). On the other hand, this study focused on only the cognitive component of subjective well-being, but it is generally known that subjective well-being includes a cognitive component (i.e. life satisfaction) and an emotional component (i.e. hedonic balance).
To explore these issues, we used a standard instrument to measure psychological resilience in a sample of young healthy adults (N = 100). Then, we conducted a correlation analysis to investigate the relationship of psychological resilience with the fALFF across the brain. Based on previous neuroimaging studies on resilience, we speculated that the fALFF in the OFC would be negatively associated with psychological resilience. Finally, we conducted a mediation analysis to examine how the fALFF in these regions related to resilience, especially the OFC, affects two components of subjective well-being (e.g. hedonic balance and life satisfaction) through resilience. Based on previous behavioral and neuroimaging studies on resilience and well-being, we speculated that psychological resilience would mediate the relationship of the fALFF in the OFC with two components of subjective well-being.
Materials and methods
Participants
One hundred Chinese healthy individuals (42 men; mean age =20.86 years; s.d. =2.01) from South China Normal University took part in the study. The current participants were completely non-overlapping with those in the study by Kong et al. (2015d). All participants were right-handed and none of participants reported history of neurological or psychiatric disorders. Two participants were excluded due to lack of behavioral data and three participants were excluded because they had incomplete rs-fMRI scans (i.e. lack of several EPI images). The Edinburgh Handedness Inventory (Oldfield, 1971) was used to assess handedness. The study was approved by the Institutional Review Board of South China Normal University, and all participants gave written informed consent.
Behavioral tests
We used the Chinese version of Connor–Davidson Resilience Scale (CD-RISC) to measure participants’ levels of psychological resilience (Wang et al., 2010; Kong et al., 2015d). The scale has ten items (e.g. ‘I can achieve goals despite obstacles’) with a one-factor structure (Campbell-Sills and Stein, 2007). Respondents rate items on a scale from 1 (strongly disagree) to 6 (strongly agree). The Chinese version of the CD-RISC has good reliability and validity (Wang et al., 2010; Kong et al., 2015d). In our dataset, the CD-RISC showed satisfactory reliability (α = 0.91).
We used the five-item Satisfaction with Life Scale (SWLS) to measure participants’ levels of life satisfaction, which is the cognitive component of subjective well-being (Diener et al., 1985). Respondents rate items on a scale from 1 (strongly disagree) to 6 (strongly agree). The Chinese version of the SWLS has good reliability and validity (Kong and You, 2013; Sun and Kong, 2013; Kong et al., 2015b,c, 2017). In our dataset, the SWLS showed satisfactory reliability (α = 0.89).
We used the Chinese version of Positive and Negative Affect Schedule (PANAS) to assess the affective component of subjective well-being (Qiu et al., 2008). The Chinese PANAS was revised based on the original PANAS (Watson et al., 1988). The scale consists of 18 affect words (9 positive and 9 negative), for example, ‘Enthusiastic’ and ‘Distressed’. Respondents indicate to what extent they generally feel each affect states using a five-point scale. The Chinese version of the PANAS has good reliability and validity (Qiu et al., 2008; Liang and Zhu, 2015). In our dataset, the PA scale (α = 0.93) and NA scale (α = 0.86) showed satisfactory reliability. We used hedonic balance (the relative amount of positive affect to negative affect) as an index of the affective component of subjective well-being (Bradburn, 1969; Diener et al., 1995; Schimmack et al., 2002, 2008). Hedonic balance scores were computed by subtracting the NA scores from the PA scores, with higher scores indicating a relatively greater tendency to experience positive affect.
MRI data acquisition and preprocessing
Data acquisitions were performed on a 3T scanner (Siemens Magnetom Trio, A Tim System) that was located at South China Normal University. The rs-fMRI scans included 240 contiguous EPI images (TR/TE = 2000 ms/30 ms; flip angle =90°; number of slices =33; matrix =64 × 64; FOV =22 ×22 cm2; acquisition voxel size =3.2 × 3.2 × 4.2 mm3). During the resting-state acquisitions, participants were instructed to lie still and relax in the scanner with their eyes closed. After the rs-fMRI scans, high-resolution T1-weighted images were acquired with MPRAGE sequence (TR/TE/TI =1900/2.52/900 ms; flip angle =7°; matrix =256 × 256; acquisition voxel size =1 × 1 × 1 mm3).
Data preprocessing was conducted using the DPARSF software (Yan et al., 2016), which employs the functions of Statistical Parametric Mapping program (SPM8, Wellcome Department of Cognitive Neurology, London, UK). The first four volumes of the data were discarded due to instability of MRI signal. Slice timing and head motion correction were performed for the remaining images. One participant was excluded because the angular and displacement motion exceeded ±2.5° or ±2.5 mm. No participant was excluded due to excessive mean framewise displacement (FD >0.25 mm). Then, these images were co-registered to each participant’s own T1 image and normalized to the SPM8 EPI template with 3 × 3 × 3 mm3 resolution. Next, the normalized data were smoothed using a 6-mm full-width at half-maximum. Finally, the images were linearly detrended to remove drifts and a Friston 24-parameter model was used to regress out head motion effects from the smoothed data.
fALFF-behavior correlation analysis
Following the methods proposed by Zou et al. (2008), the time courses for each voxel were first converted to the frequency domain, and then the square root of the power spectrum was computed and averaged across the specified frequency range (0.01–0.1 Hz) at each voxel. The fALFF was then computed as the fractional sum of the amplitudes across a low-frequency range (0.01–0.1 Hz) divided by the sum of the amplitudes across entire frequency range (0–0.25 Hz). Then, the normalized fALFF (mfALFF) was obtained by dividing the fALFF of each voxel by the global mean fALFF value. Calculations were conducted using the DPARSF software (Yan et al., 2016).
To examine the association between psychological resilience and spontaneous brain activity, a whole-brain correlation analysis was conducted. The resilience scores were treated as the covariates of interest, and age and gender were treated as the confounding covariates. To correct for multiple comparisons, a threshold of family-wise error (FWE) corrected threshold of P < 0.05 at the cluster level was set, combined with an uncorrected threshold of P < 0.001 at the voxel level.
Mediation analysis
We conducted a mediation analysis to check if the fALFF in regions related to resilience affects two components of subjective well-being through resilience using the PROCESS macro in SPSS (Hayes, 2013). In the mediation model, the mediator variable (MV) is psychological resilience, the independent variable (IV) is the fALFF in brain regions and the dependent variable (DV) is subjective well-being. Psychological resilience can be considered a mediator if the effect of the fALFF in brain regions on subjective well-being reduces significantly when the MV and the IV are included in the model. We assess the significance of the MV using a bootstrapping approach (5000 iterations). The mediating effect is significant, if a 95% confidence interval (CI) do not include zero.
Results
The neural correlates of psychological resilience
Table 1 presents means, s.d., skewness and kurtosis for all measures. The kurtosis and skewness were acceptable for the normality assumption, with the range between −2 and 2 (Lawson et al., 2004; Chalbot et al., 2010). In addition, the resilience scores had no significant relations with age (r =−0.07, P > 0.05) or gender (r =−0.02, P > 0.05). Next, we explored the neural substrates of psychological resilience.
Table 1.
Descriptive statistics for all the measures
| Minimum | Maximum | Mean | SD | Skewness | Kurtosis | |
|---|---|---|---|---|---|---|
| Age | 18 | 26 | 20.86 | 2.01 | 0.63 | −0.37 |
| Resilience | 27 | 66 | 52.07 | 8.51 | −1.03 | 0.59 |
| Life satisfaction | 8 | 35 | 19.66 | 6.60 | 0.29 | −0.93 |
| Hedonic balance | −16 | 29 | 10.38 | 9.26 | −0.75 | 0.64 |
| Positive affect | 11 | 44 | 27.47 | 7.13 | −0.19 | −0.44 |
| Negative affect | 9 | 36 | 17.09 | 6.04 | 1.03 | 0.92 |
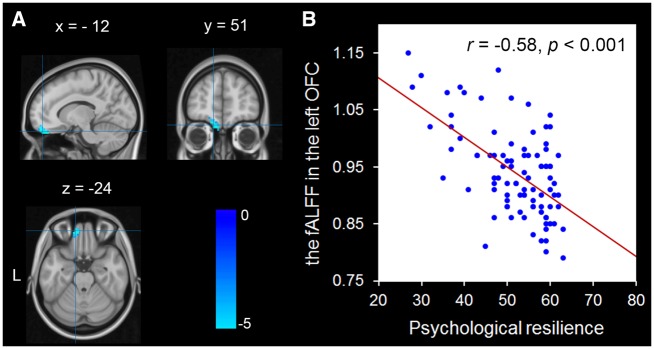
To detect the relationships of psychological resilience with spontaneous brain activity, we correlated the resilience scores with the fALFF of each voxel across the brain. After controlling for age and gender, the resilience scores has a negative association with the fALFF in the left OFC (MNI: –12, 51, –24; r =−0.58; T =−6.11; Cluster size =1215 mm3; P < 0.05) (Figure 1; Table 2). No other significant correlations were observed. Given that affect resting-state brain activity can be influenced by head motion (Power et al., 2012), so we also tested whether our results are specific to head motion. To assess possible effects of motion, mean FD was calculated for each participant. After controlling for age, gender and FD, the resilience scores was negatively associated with the fALFF in the left OFC (MNI: –12, 51, –24; r =−0.58; T =−5.84; Cluster size =999 mm3; P < 0.05) (Table 2). The region was identical to that obtained in the above analysis.
Fig. 1.
Brain regions that are correlated with psychological resilience. (A) The fALFF in the left orbitofrontal cortex (OFC) was negatively associated with psychological resilience. The coordinate is shown in the MNI stereotactic space. (B) Scatter plots depicting correlations between the fALFF in the left OFC and psychological resilience (r=−0.58, P < 0.001).
Table 2.
Brain regions that are correlated with psychological resilience
| Region | Side | BA | MNI coordinate |
T | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| After controlling for age and gender | |||||||
| Orbitofrontal cortex | L | 11 | –12 | 51 | –24 | –6.11 | 1215* |
| After controlling for age, gender and FD | |||||||
| Orbitofrontal cortex | L | 11 | –12 | 51 | –24 | –5.84 | 999* |
P < 0.05 corrected at the cluster level.
MNI, Montreal Neurological Institute; L, left; BA, Brodmann area.
Brain regions link resilience and subjective well-being
After obtaining the neural basis of psychological resilience, we further examined whether the fALFF in the left OFC can influence the two components of subjective well-being through psychological resilience. First, we replicated the significant positive association of resilience with hedonic balance (r = 0.59, P < 0.001) and life satisfaction (r = 0.56, P < 0.001) in our dataset. Importantly, the regression analysis found that the variable accounted for additional variance in hedonic balance (ΔR2 =35.9%) and life satisfaction (ΔR2 =29.4%) after controlling for age, gender and FD.
Second, we checked if the fALFF in the left OFC was related to the two components of subjective well-being. The results showed that the fALFF in the left OFC was negatively associated with hedonic balance (r =−0.31, P = 0.012, Bonferroni corrected) and life satisfaction (r =−0.31, P = 0.008, Bonferroni corrected). Notably, we did not find any significant association with positive affect (r =−0.20, P > 0.05, Bonferroni corrected) and negative affect (r = 0.25, P > 0.05, Bonferroni corrected), consistent with the view that reports of general happiness are based on the affect balance between positive and negative affect (Bradburn, 1969). Furthermore, the regression analysis found that the region accounted for additional variance in life satisfaction (ΔR2 =8.2%) and hedonic balance (ΔR2 =11.9%) after controlling for age, gender and FD.
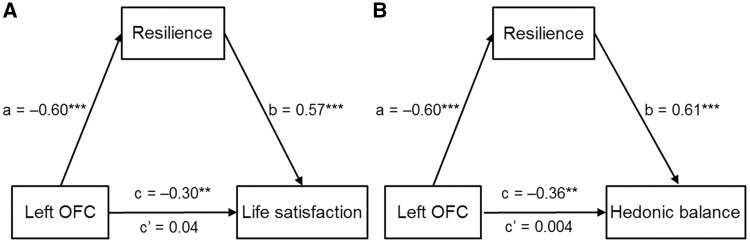
To examine whether psychological resilience could mediate the influence of the fALFF in the left OFC on the two components of subjective well-being, we carried out a mediation analysis. Interestingly, psychological resilience played a fully mediating role in the association of the fALFF in the left OFC with affect balance [indirect effect =−0.36; 95% CI =(–0.54, –0.21), P < 0.05] and life satisfaction [indirect effect =−0.34; 95% CI =(–0.49, –0.21), P < 0.05]. Even when age, gender and FD were adjusted for, psychological resilience still mediated the association of the OFC with affect balance [indirect effect =−0.35; 95% CI =(–0.53, –0.20), P > 0.05; Figure 2] and life satisfaction [indirect effect =−0.33; 95% CI =(–0.50, –0.20), P < 0.05; Figure 2].
Fig. 2.
Psychological resilience mediates the influence of the fALFF in the orbitofrontal cortex on life satisfaction (A) and hedonic balance (B). Standard regression coefficients are presented in path diagram. *P < 0.05; **P < 0.01; ***P < 0.001.
In addition, we carried out another mediation analysis to examine the directionality of the variables in our mediation model. In this model, psychological resilience was the IV, the two components of subjective well-being were the DV, and the fALFF in the OFC was the MV. The results found that the fALFF in the OFC could not mediate the effect of psychological resilience on life satisfaction [95% CI =(–0.15, 0.07), P > 0.05] and hedonic balance [95% CI =(–0.12, 0.08), P > 0.05]. Thus, these results suggested that the OFC influenced subjective well-being through psychological resilience.
Supplementary analyses
To check if our results were caused by structural brain differences, we applied voxel-based morphometry to explore the relationship of psychological resilience with regional gray matter volume (rGMV) in our dataset. We first preprocessed MRI data using standard procedures in SPM8 (Xiang et al., 2017) and then performed a correlation analysis between psychological resilience and rGMV across the brain, with gender, age and total brain volume (TBV) as confounding variables. To correct for multiple comparisons, a threshold of FWE corrected threshold of P < 0.05 at the cluster level was set, combined with an uncorrected threshold of P < 0.001 at the voxel level. Even when no multiple comparisons correction was performed, no clusters were significantly associated with psychological resilience.
Next, we checked whether psychological resilience was related to the rGMV in the OFC at the ROI level. We extracted the mean rGMV values from the OFC identified in the previous fALFF analysis. First, we found that the rGMV in the OFC was significantly associated with hedonic balance (r =−0.29, P = 0.005), and had a non-significant correlation with life satisfaction (r =−0.18, P > 0.05) and psychological resilience (r =−0.16, P > 0.05) after controlling for age, gender, FD and TBV. Second, psychological resilience was still reliably correlated with the fALFF in the OFC (r =−0.58, P < 0.001) after controlling for age, gender, TBV, FD and the rGMV in the OFC. Notably, even when age, gender, TBV, FD and the rGMV in the OFC were adjusted for, psychological resilience still mediated the influence of the OFC on affect balance [indirect effect =−0.35; 95% CI =(–0.55, –0.20), P > 0.05] and life satisfaction [indirect effect =−0.33; 95% CI =(–0.50, –0.19), P < 0.05]. Taken together, our findings were not caused by structural brain differences.
In addition, although Kong et al. (2015d) found that the ReHo in the OFC was not related to psychological resilience, we still checked if our results were influenced by the ReHo in the region. Following the methods proposed by Zang et al. (2004), we calculated the ReHo of each voxel and then extracted the mean ReHo values from the region identified in the previous fALFF analysis. We found that the ReHo in the OFC was significantly associated with psychological resilience (r =−0.30, P = 0.005), but not life satisfaction (r =−0.08, P > 0.05) and hedonic balance (r = –0.15, P > 0.05). However, after controlling for age, gender, FD, and the ReHo in the OFC, psychological resilience was still reliably correlated with the fALFF in the OFC (r =−0.53, P < 0.001). Furthermore, psychological resilience mediated the influence of the OFC on affect balance [indirect effect =−0.31; 95% CI =(–0.47, –0.18), P > 0.05] and life satisfaction [indirect effect =−0.29; 95% CI =(–0.43, –0.17), P < 0.05], after controlling for age, gender, FD and the ReHo in the OFC. These results suggest that our findings were not caused by local synchronization of the OFC, and the fALFF and ReHo measures indeed capture different aspects of the regional spontaneous activity.
Finally, we also tested whether psychological resilience separately mediates the influence of OFC activity on positive and negative affect although the correlation between OFC activity and positive and negative affect was not significant. Interestingly, psychological resilience mediated the influence of the OFC on positive affect [indirect effect =−0.26; 95% CI =(–0.44, –0.12), P > 0.05] and negative affect [indirect effect =0.25; 95% CI =(0.11, 0.44), P > 0.05]. Furthermore, after controlling for age, gender, FD and the ReHo/rGMV in the OFC, psychological resilience still mediated the influence of the OFC on positive affect [indirect effect =−0.22; 95% CI =(–0.39, –0.09), P > 0.05] and negative affect [indirect effect =0.19; 95% CI =(0.07, 0.35), P > 0.05]. Taken together, these results suggest that psychological resilience could mediate the influence of OFC activity on positive and negative affect.
Discussion
The present study sought to explore the neurobiological substrates of psychological resilience and their association with subjective well-being in healthy adults using the fALFF measured with rs-fMRI. Two main results were obtained. First, the whole brain correlation analyses showed that higher psychological resilience was associated with lower fALFF in the left OFC. Second, psychological resilience fully mediated the effect of the fALFF in the left OFC on the two components of subjective well-being (i.e. life satisfaction and hedonic balance). These findings remained even when controlling for structural brain differences (e.g. GMV) or local synchronization (i.e. ReHo). In short, our results provide the further evidence that the brain function of OFC is linked to psychological resilience, and offer a potential mechanism that psychological resilience plays a mediating role in the association between spontaneous brain activity and subjective well-being.
Confirming our first hypothesis, psychological resilience was negatively correlated with the fALFF in the left OFC. The negative correlation of resilience with spontaneous brain activity is consistent with a body of studies reporting enhanced fALFF in the OFC in stress-related psychopathologies such as major depressive disorder (Liu et al., 2014), PTSD (Bing et al., 2013), bipolar disorder (Xu et al., 2014) and anxiety disorder (Qiu et al., 2015). Alternatively, increased fALFF in the OFC during resting state may develop as a compensatory (but not sufficiently efficient) mechanism for those difficulties or outcomes induced by a reduction in resilience or lack of resilience among low-resilient participants. In fact, the compensatory mechanism to offset structural or functional defects has been demonstrated in many other studies (Yang et al., 2011; Bing et al., 2013; Yang et al., 2014; Gallea et al., 2015; Li et al., 2015; Sun et al., 2016). Moreover, our results also fit well with prior findings in healthy populations showing an association of higher fALFF in the left OFC with lower trait hope (Wang et al., 2017) and that of smaller gray matter volume in the left OFC with trait optimism (Dolcos et al., 2016). Previous studies have demonstrated that trait optimism and trait hope are two highly related constructs to psychological resilience (Lloyd and Hastings, 2009; Wang et al., 2010). In addition, consistent with the notion that left hemisphere is more involved in processing positive information (Hecht, 2013; Dolcos et al., 2016), our results were identified only in the left OFC.
The OFC has been known to play a crucial role in encoding the reward value of pain or pleasure (Gottfried et al., 2003; Kringelbach, 2005; Leknes and Tracey, 2008; Kahnt et al., 2010; Sescousse et al., 2010; Grabenhorst and Rolls, 2011; Berridge and Kringelbach, 2013), which is in accordance with behavioral studies reporting a close positive correlation of psychological resilience with reward dependence (Simeon et al., 2007; Kim et al., 2013) and reward experience (Geschwind et al., 2010). Furthermore, this region is also considered to be a critical node of brain networks underlying emotion regulation (Banks et al., 2007; Welborn et al., 2009; Golkar et al., 2012; Petrovic et al., 2016; Shiba et al., 2016), which corresponds to the view that ‘psychologically resilient individuals are emotionally intelligent’ (Salovey et al., 1999; Tugade and Fredrickson, 2004). Thus, increased fALFF in the OFC among low-resilient individuals may reflect the compensatory mechanism to offset a poor capacity to process the reward value of different stimuli and regulate daily emotions that then results in fewer resilient behaviors such as attentional bias toward positive stimuli and flexible adaptation to negative stressors.
Importantly, our study revealed that psychological resilience fully mediated the influence of the fALFF in the left OFC on subjective well-being. Previous research has consistently shown that psychological resilience is strongly correlated with the cognitive and affective components of subjective well-being (Liu et al., 2012; Di Fabio and Palazzeschi, 2015; Bajaj and Pande, 2016; Satici, 2016). Furthermore, we found that the variable explained additional variance in hedonic balance (ΔR2 =33.3%) and life satisfaction (ΔR2 =28.6%), even when controlling for age, gender and FD. Thus, psychological resilience is a crucial factor for achieving a happy life. On the other hand, our finding concurs with those previously reporting the association of the OFC with life satisfaction (Kong et al., 2015a,c). Specifically, lower life satisfaction was related to smaller rGMV in the OFC/vmPFC (Kong et al., 2015a) and higher fALFF in the OFC (Kong et al., 2015c). In addition, although the OFC has been found to be implicated in hedonic experience (Kringelbach, 2005; Berridge and Kringelbach, 2013; Kong et al., 2016a), our results suggest that the OFC is an important neural site where positive and negative affect are balanced. Given the role of the OFC in reward processing and emotion regulation, the involvement of the OFC may help acquire more resilient behaviors such as attentional bias toward positive stimuli and flexible adaptation to negative stressors, which further increase feelings of well-being. Briefly, these results substantiate that psychological resilience can serve as an underlying mechanism that accounted for the influence of the fALFF in the left OFC on subjective well-being.
In summary, our study provides the further evidence for a neural marker for psychological resilience by demonstrating spontaneous brain activity in the OFC was negatively related to psychological resilience. Furthermore, our study offers a potential mechanism that psychological resilience plays a mediating role in the association between OFC spontaneous brain activity and subjective well-being. Despite these advantages, several limitations and future directions deserve consideration. First, all instruments depended on self-report, although they had adequate reliability and validity. Further studies should use other methods to lower the impact of response bias. Second, our results depended on the local measure of spontaneous brain activity, so further studies should also test the neural basis of psychological resilience at the functional connectivity and network level. Finally, our study invites further work to investigate how to develop the neurofeedback training of psychological resilience to promote well-being.
Funding
This work was supported by the Research Foundation for Advanced Talents of Shaanxi Normal University, the Fundamental Research Funds for the Central Universities (GK201703090), and the Young Talent fund of University Association for Science and Technology in Shaanxi, China (20180206).
Conflict of interest. None declared.
References
- Agaibi C.E., Wilson J.P. (2005). Trauma, PTSD, and resilience: a review of the literature. Trauma, Violence, & Abuse, 6(3), 195–216. [DOI] [PubMed] [Google Scholar]
- An L., Cao Q.-J., Sui M.-Q., et al. (2013). Local synchronization and amplitude of the fluctuation of spontaneous brain activity in attention-deficit/hyperactivity disorder: a resting-state fMRI study. Neuroscience Bulletin, 29(5), 603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj B., Pande N. (2016). Mediating role of resilience in the impact of mindfulness on life satisfaction and affect as indices of subjective well-being. Personality and Individual Differences, 93, 63–7. [Google Scholar]
- Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Phan K.L. (2007). Amygdala—frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2(4), 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Kringelbach M.L. (2013). Neuroscience of affect: brain mechanisms of pleasure and displeasure. Current Opinion in Neurobiology, 23(3), 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing X., Ming-guo Q., Ye Z., et al. (2013). Alterations in the cortical thickness and the amplitude of low-frequency fluctuation in patients with post-traumatic stress disorder. Brain Research, 1490, 225–32. [DOI] [PubMed] [Google Scholar]
- Biswal B.B. (2012). Resting state fMRI: a personal history. Neuroimage, 62(2), 938–44. [DOI] [PubMed] [Google Scholar]
- Bradburn N.M. (1969) The Structure of Psychological Well-Being. Chicago: Alpine. [Google Scholar]
- Campbell-Sills L., Stein M.B. (2007). Psychometric analysis and refinement of the Connor–Davidson Resilience Scale (CD–RISC): validation of a 10-item measure of resilience. Journal of Traumatic Stress, 20(6), 1019–28. [DOI] [PubMed] [Google Scholar]
- Chalbot S., Zetterberg H., Blennow K., Fladby T., Grundke-Iqbal I., Iqbal K. (2010). Cerebrospinal fluid secretory Ca 2+-dependent phospholipase A2 activity: a biomarker of blood–cerebrospinal fluid barrier permeability. Neuroscience Letters, 478(3), 179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W., Ji X., Zhang J., Feng J. (2012). Individual classification of ADHD patients by integrating multiscale neuroimaging markers and advanced pattern recognition techniques. Frontiers in Systems Neuroscience, 6, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydov D.M., Stewart R., Ritchie K., Chaudieu I. (2010). Resilience and mental health. Clinical Psychology Review, 30(5), 479–95. [DOI] [PubMed] [Google Scholar]
- Di Fabio A., Palazzeschi L. (2015). Hedonic and eudaimonic well-being: the role of resilience beyond fluid intelligence and personality traits. Frontiers in Psychology, 6, 1367.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E., Emmons R.A., Larsen R.J., Griffin S. (1985). The satisfaction with life scale. Journal of Personality Assessment, 49(1), 71–5. [DOI] [PubMed] [Google Scholar]
- Diener E., Wolsic B., Fujita F. (1995). Physical attractiveness and subjective well-being. Journal of Personality and Social Psychology, 69(1), 120–9. [Google Scholar]
- Dolcos S., Hu Y., Iordan A.D., Moore M., Dolcos F. (2016). Optimism and the brain: trait optimism mediates the protective role of the orbitofrontal cortex gray matter volume against anxiety. Social Cognitive and Affective Neuroscience, 11(2), 263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A., McKie S., Deakin J.W. (2014). Resting state networks in major depressive disorder. Psychiatry Research: Neuroimaging, 224, 139–51. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews. Neuroscience, 8(9), 700–11. [DOI] [PubMed] [Google Scholar]
- Gallea C., Popa T., García-Lorenzo D., et al. (2015). Intrinsic signature of essential tremor in the cerebello-frontal network. Brain, 138(10), 2920–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N., Peeters F., Jacobs N., et al. (2010). Meeting risk with resilience: high daily life reward experience preserves mental health. Acta Psychiatrica Scandinavica, 122(2), 129–38. [DOI] [PubMed] [Google Scholar]
- Golkar A., Lonsdorf T.B., Olsson A., et al. (2012). Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One, 7(11), e48107.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried J.A., O'Doherty J., Dolan R.J. (2003). Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science, 301(5636), 1104–7. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F., Rolls E.T. (2011). Value, pleasure and choice in the ventral prefrontal cortex. Trends in Cognitive Sciences, 15(2), 56–67. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. (2013) Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York: Guilford Press. [Google Scholar]
- Hecht D. (2013). The neural basis of optimism and pessimism. Experimental Neurobiology, 22(3), 173–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T., Zhang D., Wang J. (2015). A meta-analysis of the trait resilience and mental health. Personality and Individual Differences, 76, 18–27. [Google Scholar]
- Jacelon C.S. (1997). The trait and process of resilience. Journal of Advanced Nursing, 25(1), 123–9. [DOI] [PubMed] [Google Scholar]
- Kahnt T., Heinzle J., Park S.Q., Haynes J.-D. (2010). The neural code of reward anticipation in human orbitofrontal cortex. Proceedings of the National Academy of Sciences, 107(13), 6010–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.W., Lee H.-K., Lee K. (2013). Influence of temperament and character on resilience. Comprehensive Psychiatry, 54(7), 1105–10. [DOI] [PubMed] [Google Scholar]
- Kong F., You X. (2013). Loneliness and self-esteem as mediators between social support and life satisfaction in late adolescence. Social Indicators Research, 110(1), 271–9. [Google Scholar]
- Kong F., Ding K., Yang Z., et al. (2015a). Examining gray matter structures associated with individual differences in global life satisfaction in a large sample of young adults. Social Cognitive and Affective Neuroscience, 10(7), 952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F., Ding K., Zhao J. (2015b). The relationships among gratitude, self-esteem, social support and life satisfaction among undergraduate students. Journal of Happiness Studies, 16(2), 477–89. [Google Scholar]
- Kong F., He Q., Liu X., Chen X., Wang X., Zhao J. (2018). Amplitude of low-frequency fluctuations during resting state differentially predicts authentic and Hubristic Pride. Journal of Personality, 86(2), 213–9. [DOI] [PubMed] [Google Scholar]
- Kong F., Hu S., Wang X., Song Y., Liu J. (2015c). Neural correlates of the happy life: the amplitude of spontaneous low frequency fluctuations predicts subjective well-being. Neuroimage, 107, 136–45. [DOI] [PubMed] [Google Scholar]
- Kong F., Wang X., Hu S., Liu J. (2015d). Neural correlates of psychological resilience and their relation to life satisfaction in a sample of healthy young adults. Neuroimage, 123, 165–72. [DOI] [PubMed] [Google Scholar]
- Kong F., Wang X., Song Y., Liu J. (2016a). Brain regions involved in dispositional mindfulness during resting state and their relation with well-being. Social Neuroscience, 11, 331–43. [DOI] [PubMed] [Google Scholar]
- Kong F., Xue S., Wang X. (2016b). Amplitude of low frequency fluctuations during resting state predicts social well-being. Biological Psychology, 118, 161–8. [DOI] [PubMed] [Google Scholar]
- Kong F., You X., Zhao J. (2017). Evaluation of the gratitude questionnaire in a Chinese sample of adults: factorial validity, criterion-related validity, and measurement invariance across sex. Frontiers in Psychology, 8, 1498.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M.L. (2005). The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience, 6(9), 691–702. [DOI] [PubMed] [Google Scholar]
- Lawson J., Baron-Cohen S., Wheelwright S. (2004). Empathising and systemising in adults with and without Asperger Syndrome. Journal of Autism and Developmental Disorders, 34(3), 301–10. [DOI] [PubMed] [Google Scholar]
- Leknes S., Tracey I. (2008). A common neurobiology for pain and pleasure. Nature Reviews Neuroscience, 9(4), 314.. [DOI] [PubMed] [Google Scholar]
- Li C., Yang J., Yin X., et al. (2015). Abnormal intrinsic brain activity patterns in leukoaraiosis with and without cognitive impairment. Behavioural Brain Research, 292, 409–13. [DOI] [PubMed] [Google Scholar]
- Liang Y., Zhu D. (2015). Subjective well-being of Chinese landless peasants in relatively developed regions: measurement using PANAS and SWLS. Social Indicators Research, 123(3), 817–35. [Google Scholar]
- Liberzon I., Sripada C.S. (2008). The functional neuroanatomy of PTSD: a critical review. Progress in Brain Research, 167, 151–69. [DOI] [PubMed] [Google Scholar]
- Liu J., Ren L., Womer F.Y., et al. (2014). Alterations in amplitude of low frequency fluctuation in treatment-naïve major depressive disorder measured with resting‐state fMRI. Human Brain Mapping, 35(10), 4979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang Z.-H., Li Z.-G. (2012). Affective mediators of the influence of neuroticism and resilience on life satisfaction. Personality and Individual Differences, 52(7), 833–8. [Google Scholar]
- Lloyd T.J., Hastings R. (2009). Hope as a psychological resilience factor in mothers and fathers of children with intellectual disabilities. Journal of Intellectual Disability Research, 53(12), 957–68. [DOI] [PubMed] [Google Scholar]
- Lutha S.S., Cicchetti D. (2000). The construct of resilience: implications for interventions and social policies. Development and Psychopathology, 12(4), 857–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten A. (2001). Ordinary magic. Resilience processes in development. The American Psychologist, 56(3), 227–38. [DOI] [PubMed] [Google Scholar]
- Mennes M., Zuo X.-N., Kelly C., et al. (2011). Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage, 54(4), 2950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R., Pitman R.K., Ellis C.B., et al. (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry, 66(12), 1075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Petrovic P., Ekman C.J., Klahr J., et al. (2016). Significant grey matter changes in a region of the orbitofrontal cortex in healthy participants predicts emotional dysregulation. Social Cognitive and Affective Neuroscience, 11(7), 1041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59(3), 2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C., Feng Y., Meng Y., et al. (2015). Analysis of altered baseline brain activity in drug-naive adult patients with social anxiety disorder using resting-state functional MRI. Psychiatry Investigation, 12(3), 372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L., Zheng X., Wang Y. (2008). Revision of the positive affect and negative affect scale. Chinese Journal of Applied Psychology, 14, 249–54. [Google Scholar]
- Reynaud E., Guedj E., Souville M., et al. (2013). Relationship between emotional experience and resilience: an fMRI study in fire-fighters. Neuropsychologia, 51(5), 845–9. [DOI] [PubMed] [Google Scholar]
- Salovey P., Bedell B.T., Detweiler J.B., Mayer J.D. (1999) Coping intelligently: emotional intelligence and the coping process In: Snyder C.R., editor. Coping: The Psychology of What Works. New York: Oxford University Press, pp. 141–64. [Google Scholar]
- Satici S.A. (2016). Psychological vulnerability, resilience, and subjective well-being: the mediating role of hope. Personality and Individual Differences, 102, 68–73. [Google Scholar]
- Schimmack U., Radhakrishnan P., Oishi S., Dzokoto V., Ahadi S. (2002). Culture, personality, and subjective well-being: integrating process models of life satisfaction. Journal of Personality and Social Psychology, 82(4), 582–93. [PubMed] [Google Scholar]
- Schimmack U., Schupp J., Wagner G.G. (2008). The influence of environment and personality on the affective and cognitive component of subjective well-being. Social Indicators Research, 89(1), 41–60. [Google Scholar]
- Sekiguchi A., Kotozaki Y., Sugiura M., et al. (2015). Resilience after 3/11: structural brain changes 1 year after the Japanese earthquake. Molecular Psychiatry, 20(5), 553–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G., Redouté J., Dreher J.-C. (2010). The architecture of reward value coding in the human orbitofrontal cortex. Journal of Neuroscience, 30(39), 13095–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y., Santangelo A., Roberts A. (2016). Beyond the medial regions of prefrontal cortex in the regulation of fear and anxiety. Frontiers in Systems Neuroscience, 10, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeon D., Yehuda R., Cunill R., Knutelska M., Putnam F.W., Smith L.M. (2007). Factors associated with resilience in healthy adults. Psychoneuroendocrinology, 32(8–10), 1149–52. [DOI] [PubMed] [Google Scholar]
- Smith B., Tooley E., Christopher P., Kay V. (2010). Resilience as the ability to bounce back from stress: a neglected personal resource? Journal of Positive Psychology, 5(3), 166–76. [Google Scholar]
- Sun P., Kong F. (2013). Affective mediators of the influence of gratitude on life satisfaction in late adolescence. Social Indicators Research, 114(3), 1361–9. [Google Scholar]
- Sun Y., Dai Z., Li Y., et al. (2016). Subjective cognitive decline: mapping functional and structural brain changes—a combined resting-state functional and structural MR imaging study. Radiology, 281(1), 185–92. [DOI] [PubMed] [Google Scholar]
- Tian L., Ren J., Zang Y. (2012). Regional homogeneity of resting state fMRI signals predicts Stop signal task performance. Neuroimage, 60(1), 539–44. [DOI] [PubMed] [Google Scholar]
- Tian X., Wei D., Du X., et al. (2016). Assessment of trait anxiety and prediction of changes in state anxiety using functional brain imaging: a test–retest study. Neuroimage, 133, 408–16. [DOI] [PubMed] [Google Scholar]
- Tugade M.M., Fredrickson B.L. (2004). Resilient individuals use positive emotions to bounce back from negative emotional experiences. Journal of Personality and Social Psychology, 86(2), 320.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reekum C.M., Urry H.L., Johnstone T., et al. (2007). Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. Journal of Cognitive Neuroscience, 19(2), 237–48. [DOI] [PubMed] [Google Scholar]
- Waaktaar T., Torgersen S. (2012). Genetic and environmental causes of variation in trait resilience in young people. Behavior Genetics, 42(3), 366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Shi Z., Zhang Y., Zhang Z. (2010). Psychometric properties of the 10-item Connor–Davidson Resilience Scale in Chinese earthquake victims. Psychiatry and Clinical Neurosciences, 64(5), 499–504. [DOI] [PubMed] [Google Scholar]
- Wang S., Xu X., Zhou M., et al. (2017). Hope and the brain: trait hope mediates the protective role of medial orbitofrontal cortex spontaneous activity against anxiety. Neuroimage, 157, 439–47. [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. (1988). Development and Validation of Brief Measures of Positive and Negative Affect. Journal of Personality and Social Psychology, 54(6), 1063–70. [DOI] [PubMed] [Google Scholar]
- Waugh C.E., Koster E.H. (2015). A resilience framework for promoting stable remission from depression. Clinical Psychology Review, 41, 49–60. [DOI] [PubMed] [Google Scholar]
- Waugh C.E., Thompson R.J., Gotlib I.H. (2011). Flexible emotional responsiveness in trait resilience. Emotion (Washington, DC), 11, 1059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh C.E., Wager T.D., Fredrickson B.L., Noll D.C., Taylor S.F. (2008). The neural correlates of trait resilience when anticipating and recovering from threat. Social Cognitive and Affective Neuroscience, 3(4), 322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welborn B.L., Papademetris X., Reis D.L., Rajeevan N., Bloise S.M., Gray J.R. (2009). Variation in orbitofrontal cortex volume: relation to sex, emotion regulation and affect. Social Cognitive and Affective Neuroscience, 4(4), 328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Kong F., Wen X., Wu Q., Mo L. (2016). Neural correlates of envy: regional homogeneity of resting-state brain activity predicts dispositional envy. Neuroimage, 142, 225–30. [DOI] [PubMed] [Google Scholar]
- Xiang Y., Zhao S., Wang H., Wu Q., Kong F., Mo L. (2017). Examining brain structures associated with dispositional envy and the mediation role of emotional intelligence. Scientific Reports, 7, 39947.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Liu H., Li H., et al. (2014). Amplitude of low-frequency fluctuations in bipolar disorder: a resting state fMRI study. Journal of Affective Disorders, 152–4, 237–42. [DOI] [PubMed] [Google Scholar]
- Yan C.-G., Wang X.-D., Zuo X.-N., Zang Y.-F. (2016). DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics, 14(3), 339–51. [DOI] [PubMed] [Google Scholar]
- Yang H., Wu Q.-Z., Guo L.-T., et al. (2011). Abnormal spontaneous brain activity in medication-naive ADHD children: a resting state fMRI study. Neuroscience Letters, 502(2), 89–93. [DOI] [PubMed] [Google Scholar]
- Yang M., Chen H.-J., Liu B., et al. (2014). Brain structural and functional alterations in patients with unilateral hearing loss. Hearing Research, 316, 37–43. [DOI] [PubMed] [Google Scholar]
- Yuan R., Di X., Kim E.H., Barik S., Rypma B., Biswal B.B. (2013). Regional homogeneity of resting-state fMRI contributes to both neurovascular and task activation variations. Magnetic Resonance Imaging, 31(9), 1492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y., Jiang T., Lu Y., He Y., Tian L. (2004). Regional homogeneity approach to fMRI data analysis. Neuroimage, 22(1), 394–400. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Li J., Liu X., et al. (2016). Altered spontaneous neural activity in the occipital face area reflects behavioral deficits in developmental prosopagnosia. Neuropsychologia, 89, 344–55. [DOI] [PubMed] [Google Scholar]
- Zou Q., Ross T.J., Gu H., et al. (2013). Intrinsic resting—state activity predicts working memory brain activation and behavioral performance. Human Brain Mapping, 34(12), 3204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q.-H., Zhu C.-Z., Yang Y., et al. (2008). An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. Journal of Neuroscience Methods, 172(1), 137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]