Abstract
Objectives
People living with HIV have increased Human Papillomavirus (HPV) related lesions and malignancies. We describe HPV DNA recovered from the cervix and anal canal, explore the effect of vaccination on HPV detection, and examine the durability of vaccine titers in women living with HIV-1 who were vaccinated with the quadrivalent HPV vaccine.
Methods
AIDS Clinical Trials Group A5240 was a prospective study of the quadrivalent HPV (qHPV) vaccine in 315 HIV-1 infected women in three CD4 strata (A: >350, B; 201–350, C: ≤200 cells/mm3). Vaccine was administered at entry, week 8 and week 24. Cervical and anal HPV DNA specimens were collected at baseline, weeks 28 and 52; serum for antibody testing was obtained at baseline, weeks 28 and 72.
Results
Vaccine antibody titers decreased across all four HPV types at week 72 compared to week 28. Lower proportions of sustained seropositivity were observed in women with lower CD4 counts for all four vaccine types, with the lowest titers for HPV 18. Despite the decrease, the geometric mean titer levels were above the seroconversion cut-off levels for all types except HPV 18 in the lowest CD4 stratum. Of the 174 participants who had a negative baseline HPV 16 antibody and developed antibody response at week 28, 95%, 88%, and 86% retained seropositivity at week 72 in strata A, B, and C respectively. Lower antibody retention was observed in women with CD4 < 200 compared to CD4 > 350 (p = 0.016). Anal HPV detection was more prevalent compared to cervical detection at all visits. Among high risk types, type 52, 31, 16, 18 and 51 were the most common in the cervical compartment, while types 16, 35, 18, and 51 were the most prevalent in the anal canal at baseline (listed in the order of prevalence). Later detection of HPV not present at baseline was uncommon in either compartment. Serial recovery of HPV over time was more commonly observed in the anal canal.
Conclusion
The qHPV vaccine elicits durable titer response above the seroconversion cut-off levels in HIV-infected women. However, the titer levels were substantially lower by Week 72, most noticeably in type 18. HPV DNA was detected more frequently in the anal canal. Detection of non-vaccine high risk HPV suggests a role for the nonavalent vaccine.
Abbreviations: ACTG, AIDS Clinical Trials Group; AMC, AIDS Malignancy Consortium; ART, antiretroviral therapy; CI, confidence interval; CIN, cervical intraepithelial neoplasia; DNA, deoxyribonucleic acid; FDA, Food and Drug Administration; GMT, geometric mean titers; HIV, human immunodeficiency virus; HPV, human papillomavirus; mMU, milliMerck units; NA-ACCORD, North American AIDS Cohort Collaboration on Research and Design; nHPV, nonavalent human papillomavirus vaccine; NIAID, National Institute of Allergy and Infectious Diseases; NIDCR, National Institute of Dental and Craniofacial Research; NIH, National Institutes of Health; PCR, polymerase chain reaction; qHPV, quadrivalent human papillomavirus vaccine; VIN, vaginal intraepithelial neoplasia; WIHS, Women's Interagency HIV Study (WIHS)
Keywords: HPV, HIV, Anogenital, Quadrivalent HPV vaccine, Women, Immunogenicity
1. Introduction
People living with HIV are disproportionally infected with human papillomavirus (HPV) and have a higher burden of HPV associated disease in the cervix and anus [1], [2]. Infection with HPV is the primary causative agent in the development of squamous cell cancer of the anogenital tract [3], [4], [5], [6]. The quadrivalent HPV vaccine (qHPV) was designed to prevent infection from four distinct HPV types, HPV16 and 18 (the causative agents of most cervical and anal cancers) and HPV 6 and 11 (which cause most anogenital warts). When given to HIV-uninfected women without prior exposure to HPV16 or 18, qHPV demonstrated 98% efficacy in preventing cervical intraepithelial neoplasia related to the vaccine types and 100% of anogenital warts [7], [8]. In our published primary manuscript, we reported vaccine titer seroconversion proportions 4 weeks after completion of the vaccine series, ranging from 75% to 98% for the 4 HPV vaccine types in women who were seronegative for that type at baseline [9]. The seroconversion proportion was > 90% among HIV infected women with baseline CD4 counts greater than 200 copies. Among women with CD4 counts of 200 or lower, those with HIV RNA viral loads lower than 400 had higher rates of seroconversion.
There is increasing evidence that HPV related disease in the anal canal continues to rise in HIV-infected populations [10], [11], [12], [13], [14], [15], [16], [17], [18]. The U.S. HIV/AIDS Cancer Match Study reported an annual 3.8% increase in anal cancer rates among HIV-infected people from 1996 to 2010 [19]. Data from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) suggests that 3% of HIV infected adults will develop anal cancer by age 60 [20]. The Cancer Risk Group of the French Hospital Database on HIV reported that from 2005 to 2009, relative to the general population, the risk among HIV-infected individuals was 79-fold higher for anal cancer [21]. The risk of anal disease remains even in the setting of immune reconstitution secondary to combination antiretroviral therapy (ART) [2], [10]. The risk for anal cancer in HIV infected women is 35 times that of the non HIV infected population. Recommendations for anal cancer screening of HIV infected women are evolving.
Scant data exists on the efficacy of the qHPV vaccine in anatomical compartments of HIV infected women predisposed to HPV associated cancers. We previously reported titers elicited four weeks after the vaccine series. Here we present study data on HPV DNA detection in the cervix and anal canal, and on the durability of the antibody titer response at study week 72, 48 weeks after the last dose of vaccine.
2. Methods
2.1. Study design
AIDS Clinical Trials Group (ACTG) Protocol 5240 was an international phase II, open-label, single arm study with stratification by CD4+ cell count and HIV-1 RNA viral load designed to assess the immunogenicity and safety of the Quadrivalent Human Papillomavirus (Types 6, 11, 16, 18) Recombinant Vaccine in HIV-1 infected women ages 13–45. Participants were enrolled at ACTG sites in the United States; Fiocruz, Brazil; and Johannesburg, South Africa. CD4 cell count strata were defined as: Stratum A: CD4+cell count > 350 cells/mm³ ; Stratum B: CD4+ cell count > 200 to < 350 cells/mm³ and Stratum C: CD4+ cell count < 200 cells/mm³ . Additional details regarding the study design, enrollment criteria, and schedule of events can be found in the primary paper [9]. Institutional review boards of all of the participating institutions approved the study, and each participant gave written informed consent.
Participants were enrolled regardless of HPV DNA or antibody status. Participants were excluded if they had current or history of high grade cervical intraepithelial neoplasia (CIN II or III), vaginal intraepithelial neoplasia (VIN II or III), or physician diagnosed genital warts within 180 days prior to study entry.
At entry, week 8, and week 24, participants received the quadrivalent HPV recombinant vaccine 0.5 mL intramuscularly. Participants were subsequently seen at weeks 28, 52, and at 72. Serology was obtained 48 weeks later at week 72 for the secondary immunogenicity endpoint to determine the durability of the antibody response to vaccine types in those seronegative for each type at baseline. Samples obtained from the cervix and the anal canal for HPV DNA were obtained at baseline and at week 52 to correspond with annual screening guideline recommendations.
2.2. Role of funding source
The funding source approved the final version of the study, regulated study product control and protocol compliance, and monitored safety and adverse event reporting.
2.3. Serology, typing, and viral detection methods
HPV serological testing was performed using competitive Luminex immunoassay (HPV-4 cLIA; Merck Research Laboratories). Seropositivity for a given vaccine type was measured in milliMerck units (mMU) and defined as ≥ 20 for type 16, ≥ 16 for type 11, ≥ 20 for type 16, ≥ 24 for type 18 (mMU/mL). Anal and cervical HPV DNA polymerase chain reaction (PCR) testing was performed with MY09/MY11 primers and L1 consensus and type-specific probing as described elsewhere [22]. HIV RNA testing was performed locally, using Roche Ultra-Sensitive HIV Real-time (RT) PCR, Roche COBAS AmpliPrep/TaqMan HIV-1, or Roche Amplicor Monitor HIV-1 RT PCR. The HPV types were classified according to their cervical cancer risks as follows. High Risk (HR) types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 66; Low Risk (LR) types: 6/11, 26/69, 32/42, 53, 54, 57/2/27, 61, 62, 71, 72, 81, 83, 84, 86/87, 102/89, 90/106, MIX1 types (7, 13, 40, 43, 44, 55, 74, and 91), and MIX2 types (3, 10, 28, 29, 77, 78, and 94); and Unknown Risk HPV types: 26, 30, 34, 53, 67, 68, 69, 70, 73, 82, 85 and 97.
2.4. Statistical methods
Proportions were estimated with two-sided 95% exact Clopper-Pearson confidence intervals (CI). The CIs around geometric means were calculated assuming t-distribution of log10-transformed antibody titer. Kappa statistics and 95% CIs were assessed for agreement between assays on different specimens. Two-sided Fisher's exact tests were used to compare binary or categorical variables among groups, and a p-value< 0.05 was considered statistically significant. Multiple comparisons were conducted without adjustment for type I error.
3. Results
3.1. Demographics
Of three hundred nineteen participants enrolled in A5240 from March 2008 to July 2011, 315 met study inclusion criteria and initiated study vaccination. Because the study did not restrict enrollment based on HPV serostatus or HPV DNA presence, the analysis sets varied depending on the question being evaluated and sample availability. Participants in all three CD4 strata had similar age and racial distributions, as previously described [6]. Briefly, the median age of participants was 36 years, 56% were Black, non-Hispanic, and 31% were Hispanic. Twenty-one participants were under the age of 25 at baseline. Median baseline CD4 for strata A, B, and C were 519, 287, and 154 cells/mm3 respectively. The proportion of participants who had an undetectable HIV-1 RNA viral load (lower limit of detection of 20 copies) for strata A, B, and C were 38%, 45%, and 38% respectively. Data from the fifty-three participants from Brazil and South Africa were not analyzed separately due to the small number.
3.2. Sustained Seropositivity Rates to Vaccine Types
The geometric mean titers of antibody responses and the proportions of participants who were seronegative for a given HPV type at baseline and seropositive at weeks 28 and 72 are described in Table 1, Table 2, respectively. Across all strata, there was a decrease in the geometric mean titer of type specific antibody responses from week 28 to week 72, most noticeably in type 16. We examined participants who were seronegative at baseline, became seropositive at week 28 for HPV16, and had HPV16 serostatus results at Week 72. Ninety five percent, 88%, and 86% of these participants were seropositive at week 72 (48 weeks post last dose of vaccine) in Strata A, B, and C, respectively. Similar proportions were observed for HPV6 and 11, but lower positive serostatus proportions were observed for HPV18. There was no difference in sustained seropositivity at Week 72 between Strata A and B.
Table 1.
HPV antibody responses over time among participants seronegative for a given type at baseline.
| Participants Seronegative for HPV Type at Baselinea |
||||||
|---|---|---|---|---|---|---|
| Week 28 |
Week 72 |
|||||
| HPV Type | CD4 Stratumb | Nc | GMT mMU/mL (95% CI) | Nc | GMT mMU/mL (95% CI) | |
| HPV 6 | A | 60 | 462 (321–667) | 55 | 113 (82–156) | |
| B | 58 | 349 (242–504) | 53 | 80 (52–123) | ||
| C | 52 | 137 (82–229) | 53 | 43 (29–64) | ||
| HPV 11 | A | 93 | 477 (362–627) | 83 | 122 (90–166) | |
| B | 70 | 417 (287–607) | 66 | 85 (56–129) | ||
| C | 71 | 205 (129–327) | 69 | 53 (35–80) | ||
| HPV 16 | A | 73 | 1200 (871–1654) | 63 | 249 (171–362) | |
| B | 63 | 1117 (746–1672) | 59 | 170 (107–272) | ||
| C | 64 | 571 (328–994) | 64 | 98 (62–156) | ||
| HPV 18 | A | 86 | 175 (126–243) | 75 | 42 (30–60) | |
| B | 80 | 171 (115–255) | 73 | 41 (27–61) | ||
| C | 69 | 94 (59–149) | 69 | 21 (15–30) | ||
Abbreviations: CI, confidence interval; GMT, geometric mean titer of HPV antibodies; HPV, human papillomavirus; mMU/mL, milli-Merck units/mL.
Participants who had HPV antibody results available at both baseline and post-baseline (week 28 or 72, respectively) study visits and who had received either partial or complete vaccination series.
CD4 strata: A: > 350, B; 201–350, C: ≤ 200 cells/mm.
Number of participants in the analysis.
Table 2.
Proportion of participants with sustained seropositivity at week 72.
| HPV type | CD4 Stratum |
|||||
|---|---|---|---|---|---|---|
| A |
B |
C |
||||
| N | % | N | % | N | % | |
| 6 | 52 | 94.2 (84.1 – 98.8) | 52 | 84.6 (71.9 – 93.1) | 40 | 72.5 (56.1 – 85.4) |
| 11 | 80 | 95.0 (87.7 −98.6) | 63 | 82.5 (70.9–90.9) | 58 | 77.6 (64.7–87.5) |
| 16 | 62 | 95.2 (86.5–99.0) | 57 | 87.7 (76.3–94.9) | 55 | 85.5 (73.3–93.5) |
| 18 | 68 | 69.1 (56.7–79.8) | 60 | 71.7 (58.6–82.5) | 47 | 53.2 (38.1–67.9) |
Results reported as proportion (95% CI), N = Number of participants who were seronegative at baseline, became seropositive at week 28 for the given type, and had Week 72 serostatus result available for that type.
Sustained seropositivity was compared pairwise among the CD4 strata in 244 participants with complete data across study visits. These participants were seronegative to at least one of the vaccine HPV types at baseline, hence had opportunity to achieve seropositivity. Participants were considered seropositive at week 28 if they became seropositive at week 28 to any of the types to which they were seronegative at baseline. In Stratum C, the proportion of participants with sustained seropositivity at week 72 was lower than in Stratum A (83.8% vs. 95.6%, p = 0.016). Sustained seropositivity by baseline HIV RNA detection status within each CD4 stratum was assessed. There was no statistically significant difference between the HIV RNA detectable and undetectable participants in Stratum A (97.4% among the undetectable vs. 94.2% among the detectable) and in Stratum B (97.2% vs. 88.4%), despite the trend of lower sustained seropositivity among those with detectable HIV RNA. The difference was statistically significant in Stratum C: 100% (95% CI: 87–100%) sustained seropositivity among those who were HIV RNA undetectable at baseline, compared to 74.5% (95% CI: 60–86%) among those who had detectable HIV RNA (p = 0.003).
3.3. Comparison of HPV DNA in the cervical and anal compartments
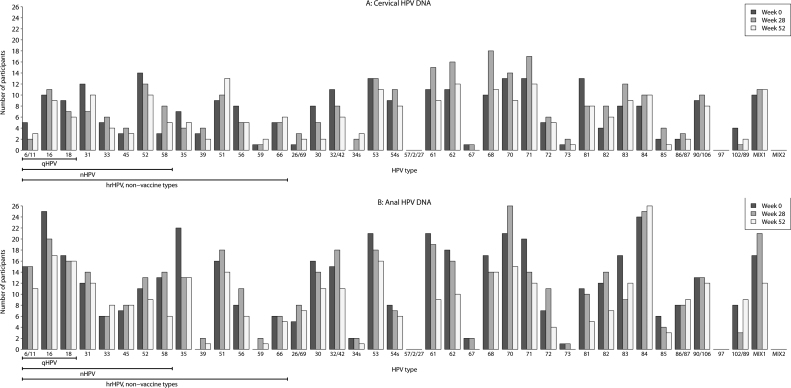
Recovery of HPV DNA in the cervical and anal canals at baseline from both the vaccine and oncogenic high risk HPV types are listed in Table 3. The number of participants with positive HPV DNA in either the cervical or anal compartment was small. Across HPV types, HPV detection in the anal compartment was more prevalent compared to the cervical canal at baseline and at subsequent time points (Fig. 1). Among 298 women with baseline results, 50 women (17%) had any vaccine type HPV DNA detected in anal compartment at baseline, with multiple types detected in 7 women (2.3%). Of the vaccine types, HPV 16 was the most common type detected in the anal canal of 25 participants at study entry, followed by HPV 18, found in 17 participants at baseline. The most prevalent oncogenic, non-vaccine HPV types detected in the anal compartment were types 35, 51, 58, 31, and 52 found in 22, 16, 13, 12, and 11 participants respectively. Recovery of other high-risk types was rare, including HPV 33 and 45 detected in only 6 and 7 participants respectively at baseline.
Table 3.
Baseline detection of vaccine and high risk HPV types from the cervical and anal canal by CD4 stratum.
| HPV DNA type | A |
B |
C |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CERVIX | ANAL CANAL | CERVIX | ANAL CANAL | CERVIX | ANAL CANAL | |||||||||
| (N = 19) | (N = 35) | (N = 17) | (N = 28) | (N = 28) | (N = 41) | |||||||||
| nHPV types | qHPV | 16 | 6 | (31.6) | 11 | (31.4) | 2 | (11.8) | 4 | (14.3) | 2 | (7.1) | 10 | (24.4) |
| 18 | 3 | (15.8) | 4 | (11.4) | 2 | (11.8) | 7 | (25.0) | 4 | (14.3) | 6 | (14.6) | ||
| 31 | 2 | (10.5) | 1 | (2.9) | 5 | (29.4) | 3 | (10.7) | 5 | (17.9) | 8 | (19.5) | ||
| 33 | 4 | (21.1) | 3 | (8.6) | 0 | (0.0) | 0 | (0.0) | 1 | (3.6) | 3 | (7.3) | ||
| 45 | 1 | (5.3) | 2 | (5.7) | 0 | (0.0) | 2 | (7.1) | 2 | (7.1) | 3 | (7.3) | ||
| 52 | 5 | (26.3) | 2 | (5.7) | 5 | (29.4) | 1 | (3.6) | 4 | (14.3) | 8 | (19.5) | ||
| 58 | 0 | (0.0) | 3 | (8.6) | 2 | (11.8) | 3 | (10.7) | 1 | (3.6) | 7 | (17.1) | ||
| hrHPV, non vaccine types | 35 | 1 | (5.3) | 9 | (25.7) | 1 | (5.9) | 6 | (21.4) | 5 | (17.9) | 7 | (17.1) | |
| 39 | 1 | (5.3) | 0 | (0.0) | 1 | (5.9) | 0 | (0.0) | 1 | (3.6) | 0 | (0.0) | ||
| 51 | 2 | (10.5) | 4 | (11.4) | 1 | (5.9) | 2 | (7.1) | 6 | (21.4) | 10 | (24.4) | ||
| 56 | 2 | (10.5) | 0 | (0.0) | 2 | (11.8) | 3 | (10.7) | 4 | (14.3) | 5 | (12.2) | ||
| 59 | 0 | (0.0) | 0 | (0.0) | 1 | (5.9) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| 66 | 2 | (10.5) | 1 | (2.9) | 1 | (5.9) | 3 | (10.7) | 2 | (7.1) | 2 | (4.9) | ||
results reported as n (% of participants with any high risk HPV detected at baseline), CER = cervical, qHPV=quadrivalent HPV vaccine, nHPV=nonavalent HPV vaccine, hrHPV = high risk HPV.
Fig. 1.
HPV DNA detection at baseline and post vaccination by anogenital compartment.
3.4. Relationship between cervical and anal HPV DNA
Combining the baseline high risk HPV types recovered from 285 participants with data from both compartments, 69% of the cervical HPV DNA positives were also positive for anal DNA, and 75% of the cervical HPV DNA negatives were also negative for anal HPV DNA. When high risk HPV types were combined, the presence of HPV DNA in cervical and anal samples were not in agreement for 27% of the study subjects. The kappa statistic on agreement between the two anatomical sites was low at 0.35 (95% CI: 0.24 – 0.47).
3.5. Anogenital HPV DNA detection and persistence
Most participants had no vaccine type HPV DNA detected in either the cervical or anal canal at any time point throughout the study (Table 4). For example, of the 228 women who had cervical HPV DNA data at baseline, week 28, and week 52, 213 were negative for HPV16 at all three time points. Detection of HPV DNA in a compartment that was previously negative for HPV DNA at either entry or week 28 was extremely rare. For HPV 16, only 8 participants who were negative at baseline had HPV 16 DNA detected in the cervical canal at a later visit. Similarly, 9 participants who did not have anal HPV16 DNA detected at baseline had this type detected later in the study. Four participants who had HPV16 DNA detected in the anus at baseline, had no HPV 16 detected at week 28, then subsequently had HPV16 detected at week 52. The detection of HPV18 or 6/11 was similarly infrequent.
Table 4.
HPV DNA detection and persistence over time by anogenital compartment*.
|
CERVIX |
ANUS |
||||||
|---|---|---|---|---|---|---|---|
| week 0, 28, 52 | 6/11 | 16 | 18 | 6/11 | 16 | 18 | |
| N | 291 | 286 | 287 | 283 | 273 | 281 | |
| DNA negative at baseline | -, -, - | 222 | 213 | 219 | 212 | 201 | 213 |
| -, -, + | 2 | 4 | 0 | 0 | 4 | 2 | |
| -, +, + | 0 | 1 | 0 | 3 | 0 | 3 | |
| -, +, - | 0 | 3 | 1 | 2 | 5 | 0 | |
| Missing* | 67 | 65 | 67 | 66 | 63 | 63 | |
| N | 5 | 10 | 9 | 15 | 25 | 17 | |
| DNA positive at baseline | +, +, + | 1 | 3 | 5 | 4 | 7 | 6 |
| +, +, - | 0 | 1 | 1 | 4 | 4 | 2 | |
| +, -, - | 3 | 3 | 1 | 4 | 6 | 4 | |
| +, -, + | 0 | 0 | 1 | 2 | 4 | 1 | |
| Missing* | 1 | 3 | 1 | 1 | 4 | 4 | |
- = DNA not detected, + = DNA detected, * other patterns, including any combination of missed visit(s) after wk 0.
The persistence of HPV detection, defined as the detection of the same type specific HPV for two consecutive time points, was rare but was more frequently observed in the anal canal compared to the cervical compartment.
3.6. Concordance of anogenital HPV and serostatus
Baseline agreement between type specific serostatus and HPV DNA, in either compartment, was poor for all vaccine types (Table 5). Of the 283 participants who had no HPV16 detected in the cervix at baseline, 194 (69%) were seronegative to HPV-16. The remaining 31% were seropositive to HPV-16 indicating a response to previous infection. Similarly, of the 284 women who had no evidence of HPV18 in the cervix at baseline, 227 (80%) were seronegative to HPV18. Among the 199 HPV16 seronegative participants, only 12 (6%) tested positive for anal HPV16 DNA. The proportions of detection of and seropositivity to the same high risk HPV vaccine type in the anal canal were more evenly divided. Of the 25 women with HPV16 DNA and 17 women with HPV18 DNA detected in the anus at baseline, 52% and 47% were seropositive for the same type respectively.
Table 5.
Concordance between HPV DNA and serostatus by compartment at baselinea.
| Cervical DNA |
Anal DNA |
||||
|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | ||
| Type 6/11b | total, N | 287 | 5 | 280 | 14 |
| Seronegative | 154 (53.7) | 1 (20.0) | 157 (56.1) | 1 (7.1) | |
| Seropositive | 133 (46.3) | 4 (80.0) | 123 (43.9) | 13 (92.9) | |
| Type 16 | total, N | 283 | 10 | 270 | 25 |
| Seronegative | 194 (68.6) | 5 (50.0) | 187 (69.3) | 12 (48.0) | |
| Seropositive | 89 (31.4) | 5 (50.0) | 83 (30.7) | 13 (52.0) | |
| Type 18 | total, N | 284 | 9 | 278 | 17 |
| Seronegative | 227 (79.9) | 7 (77.8) | 227 (81.7) | 9 (52.9) | |
| Seropositive | 57 (20.1) | 2 (22.2) | 51 (18.3) | 8 (47.1) | |
Includes participants with baseline data on serostatus and HPV DNA, by DNA status. Data reported as n (% of total either seronegative or seropositive for the given HPV type at entry).
Types 6 and 11 were combined for both DNA and serostatus data for that HPV type.
4. Discussion
This is the first longitudinal study of anogenital HPV DNA detection and immunogenicity over time in women with HIV who were vaccinated with the qHPV vaccine. We previously reported the safety and immunogenicity of the qHPV vaccine one month after the vaccine series in women with HIV regardless of baseline CD4 or HIV viral load [9]. In this follow-up, we demonstrate that the vaccine elicited sustained elevated titer responses one year after vaccination and that the decline observed over time is similar to that detected in women in the general population [23], [24], [25]. Sustained titer response to vaccination was lower in participants who had detectable HIV viral loads at baseline in the lowest CD4 stratum. This finding is consistent with the association of lower seroconversion rates in women with low CD4 counts and HIV viral load that was documented in our previous paper [9].
Despite the observed decline in titer, the vaccine is considered to confer long lasting memory due to the anamnestic immune response, i.e. rapid increase in immune titer upon re-exposure to the antigen. The qHPV vaccine has been shown to invoke an anamnestic response in HIV infected men re-challenged with a 4th dose of qHPV 2 years after completion of the series [26], [27]. The significance of the lower antibody titers over time are unclear as the level of titer required for clinical protection are unknown.
Consistent with previous studies, HPV infection of the anal compartment was more prevalent than infection of the cervical canal across all time points [28], [29]. Anal HPV prevalence in women with HIV was higher in the anal canal compared to the cervical canal in the SUN and WIHS studies, as well as in a French cohort of were anal infection with multiple HPV types was twice as common when compared to the cervical canal [22], [30], [31]. In a meta-analysis of type specific HPV prevalence in the anal canal, HPV 16 was less frequently identified in persons with HIV and high grade lesions or anal cancer compared to people without HIV [32]. These findings suggest that differences in the mucosal microenvironment or the efficacy of the immune response in each compartment promote HPV persistence to varying degrees, especially in the anal canal. Increased detection of other hrHPV is thought to be secondary to the low dependence of HPV-16 on absolute immune deficiency to evade immunologic control. In other words, people living with HIV are more susceptible to HPV types that are less pathogenic in other populations.
It is difficult to determine the significance of the concordance of detected anogenital HPV DNA and serostatus. In our study, we detected HPV at lower rates than previously reported. The detection of HPV DNA can represent either a recently acquired infection or intermittent shedding from an established infection acquired years in the past. Based on serology, at least a third of subjects had evidence of prior HPV 16 exposure, with 40% and 20% with serologic evidence of prior HPV 11 and HPV 18 exposure respectively. For those who are HPV DNA positive yet seronegative for that type, this may signal recent anogenital infection that has not had the opportunity to mount a detectable IgG response.
The currently approved nonavalent HPV vaccine (nHPV) includes five additional hrHPV types (HPV 31, 33, 45, 52, and 58). A meta-analysis of women with HIV worldwide found that in Africa, HPV-45 was more carcinogenic than other nonHPV-16/18 types [33]. Data from the WIHS cohort demonstrated that oncogenic HPV types other than HPV-16 were more prevalent in high grade cervical lesions in women with HIV than compared to women without HIV [34]. The seroprevalence of the seven high risk HPV types in the 2005–2006 US National Health and Nutrition Examination Survey was 30%. Black women, who are also disproportionately infected with HIV, demonstrated higher seroprevalence of 31/33/45/52/58 (36.8% vs 15.9%, p < 0.05) compared to non-Hispanic Whites [35]. High risk types covered by the nonavalent HPV vaccine accounted for 63% and 64% of the individual cervical and anal HPV isolates detected at baseline in our current study. This suggests additional benefit of HPV vaccination despite the older age of this cohort and further, that testing to determine previous exposure to hrHPV by serum titers prior to vaccination would not adequately assess potential benefit.
We recognize the limitations of our observations’ generalizability including the low rates of HPV recovered from either compartment compared to rates observed in previous studies. This observation may be a reflection of the change in the epidemiology of HPV in women over time related to increased uptake of vaccine and herd immunity, as described in other populations [36], [37], [38], [39]. Equally these women may have had more years of undetectable plasma HIV-1 RNA and have been heavily screened with Pap tests and colposcopy due to their increased burden of HPV disease. Much of the data on HPV prevalence in women with HIV has come from large observational cohorts. In a prospective cohort of 333 Brazilian women with HIV observed over 2 years, the prevalence of cervical HPV-16 and HPV-18 were 5.1% and 3.9% respectively, with the hrHPV incidence at 12 months of only 3.7% and a clearance rate of 41.7% [40]. Variables associated with HPV incidence in this cohort were nulliparity, oral contraceptive use, and detectable HIV-1 RNA viral load. The women included in our study who volunteered for an interventional trial may be inherently different in terms of factors associated with HPV acquisition. We did not collect data on socio-economic status, age of coitarche, preferred method of birth control, number of lifetime or new partners, or sexual behavior, including receptive anal sex. The short duration of follow up is another limitation as the natural history and required duration of infection in the anal canal in women with HIV is less certain. HPV in the anal compartment was examined over only one year to coincide with the standard of care annual Pap screening.
A5240 demonstrates the immunogenicity of qHPV vaccination in older women with HIV over time. The inability of antiretroviral therapy alone to impact the control of HPV infection has led to concern that HPV-associated anogenital cancer and dysplasia may continue to increase among individuals with HIV. This highlights the importance of wide adoption of HPV vaccination for adolescent women to reduce the incidence of HPV-related cancer among those who become HIV-infected. Detection of non-vaccine high risk HPV suggests a role for the nonavalent vaccine. Increased detection of HPV in the anal canal suggests that anal disease may prove to be a problem for HIV infected women in the future. Future studies should address which women with HIV may benefit from vaccination in combination with screening to prevent HPV related disease.
Funding sources
This project was supported by Award Number 1U01AI068636 from the National Institute of Allergy and Infectious Diseases and supported by the National Institute of Dental and Craniofacial Research (NIDCR). The work was also supported by the Statistical and Data Management Center of the AIDS Clinical Trials Group, under the National Institute of Allergy and Infectious Diseases grant No. UM1 AI068634. Merck & Co. provided the study product and the assays to measure antibody response.
National Clinical Trial (NCT) Number NCT00604175.
Conflict of interest
MSC and JAA received clinical research support from Gilead Sciences and Glaxo-Smith-Kline/Viiv Healthcare and received scientific advisory board personal fees from Gilead Sciences. JAA received scientific advisory board personal fees from Janssen, Merck, and ViiV Healthcare, all unrelated to the present study. SC reports support for lab testing from, and AS is employed by Merck, the manufacturer of the qHPV vaccine. CG contributed to this paper in her capacity as an NIH employee, but the views expressed in this paper do not necessarily represent those of the NIH. MK, EMK, TU, JWC, RM, CF, and BG have no conflicts to disclose.
References
- 1.Liu G., Sharma M., Tan N., Barnabas R.V. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS. 2018:795–808. doi: 10.1097/QAD.0000000000001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palefsky J.M. Human papillomavirus-associated anal and cervical cancers in HIV-infected individuals: incidence and prevention in the antiretroviral therapy era. Curr. Opin. HIV AIDS. 2017:26–30. doi: 10.1097/COH.0000000000000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piketty C., Darragh T.M., Da Costa M., Bruneval P., Heard I., Kazatchkine M.D., Palefsky J.M. High prevalence of anal human papillomavirus infection and anal cancer precursors among HIV-infected persons in the absence of anal intercourse. Ann. Intern. Med. 2003:453–459. doi: 10.7326/0003-4819-138-6-200303180-00008. [DOI] [PubMed] [Google Scholar]
- 4.Melbye M., Sprogel P. Aetiological parallel between anal cancer and cervical cancer. Lancet. 1991:657–659. doi: 10.1016/0140-6736(91)91233-k. [DOI] [PubMed] [Google Scholar]
- 5.Ogunbiyi O.A., Scholefield J.H., Robertson G., Smith J.H., Sharp F., Rogers K. Anal human papillomavirus infection and squamous neoplasia in patients with invasive vulvar cancer. Obstet. Gynecol. 1994:212–216. [PubMed] [Google Scholar]
- 6.Patel H.S., Silver A.R., Northover J.M. Anal cancer in renal transplant patients. Int. J. Colorectal Dis. 2007:1–5. doi: 10.1007/s00384-005-0023-3. [DOI] [PubMed] [Google Scholar]
- 7.Garland S.M., Hernandez-Avila M., Wheeler C.M., Perez G., Harper D.M., Leodolter S., Tang G.W., Ferris D.G., Steben M., Bryan J., Taddeo F.J., Railkar R., Esser M.T., Sings H.L., Nelson M., Boslego J., Sattler C., Barr E., Koutsky L.A. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 2007:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 8.Future II Study Group Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 2007:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 9.Kojic E.M., Kang M., Cespedes M.S., Umbleja T., Godfrey C., Allen R.T., Firnhaber C., Grinsztejn B., Palefsky J.M., Webster-Cyriaque J.Y., Saah A., Aberg J.A., Cu-Uvin S. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin. Infect. Dis. 2014:127–135. doi: 10.1093/cid/ciu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hidalgo-Tenorio C., de Jesus S.E., Esquivias J., Pasquau J. High prevalence and incidence of HPV-related anal cancer precursor lesions in HIV-positive women in the late HAART era. Enferm. Infecc. Microbiol. Clin. 2017 doi: 10.1016/j.eimc.2017.10.014. (e-published ahead of print) [DOI] [PubMed] [Google Scholar]
- 11.Center for Disease Control and Prevention (CDC) Human papillomavirus-associated cancers - United States, 2004–2008. Morb. Mortal. Wkly. Rep. 2012:258–261. [PubMed] [Google Scholar]
- 12.Clifford G.M., Polesel J., Rickenbach M., Maso L. Dal, Keiser O., Kofler A., Rapiti E., Levi F., Jundt G., Fisch T., Bordoni A., Weck D. De, Franceschi S. Cancer risk in the Swiss HIV cohort study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J. Natl. Cancer Inst. 2005:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 13.Engels E.A., Biggar R.J., Hall H.I., Cross H., Crutchfield A., Finch J.L., Grigg R., Hylton T., Pawlish K.S., McNeel T.S., Goedert J.J. Cancer risk in people infected with human immunodeficiency virus in the United States. Int. J. Cancer. 2008:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 14.Godfrey C., Firnhaber C.S., D'Souza G., Heard I. Anal dysplasia in HIV-infected women: a commentary on the field. Int. J. STD AIDS. 2015:543–549. doi: 10.1177/0956462415615764. [DOI] [PubMed] [Google Scholar]
- 15.Patel P., Hanson D.L., Sullivan P.S., Novak R.M., Moorman A.C., Tong T.C., Holmberg S.D., Brooks J.T. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann. Intern. Med. 2008:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 16.Goeieman B.J., Firnhaber C.S., Jong E., Michelow P., Kegorilwe P., Swarts A., Williamson A.L., Allan B., Smith J.S., Wilkin T.J. Prevalence of anal HPV and anal dysplasia in HIV-infected women from Johannesburg, South Africa. J. Acquir. Immune Defic. Syndr. 2017:e59–e64. doi: 10.1097/QAI.0000000000001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin C., Franceschi S., Clifford G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect. Dis. 2018:198–206. doi: 10.1016/S1473-3099(17)30653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heard I., Etienney I., Potard V., Poizot-Martin I., Moore C., Lesage A.C., Ressiot E., Crenn-Hebert C., Flejou J.F., Cubie H., Costagliola D., Darragh T.M. High prevalence of anal human Papillomavirus-associated cancer precursors in a contemporary cohort of asymptomatic HIV-infected women. Clin. Infect. Dis. 2015:1559–1568. doi: 10.1093/cid/civ049. [DOI] [PubMed] [Google Scholar]
- 19.Robbins H.A., Shiels M.S., Pfeiffer R.M., Engels E.A. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014:881–890. doi: 10.1097/QAD.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverberg M.J., Lau B., Justice A.C., Engels E., Gill M.J., Goedert J.J., Kirk G.D., D'Souza G., Bosch R.J., Brooks J.T., Napravnik S., Hessol N.A., Jacobson L.P., Kitahata M.M., Klein M.B., Moore R.D., Rodriguez B., Rourke S.B., Saag M.S., Sterling T.R., Gebo K.A., Press N., Martin J.N., Dubrow R. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin. Infect. Dis. 2012:1026–1034. doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hleyhel M., Hleyhel M., Bouvier A.M., Belot A., Tattevin P., Pacanowski J., Genet P., De Castro N., Berger J.L., Dupont C., Lavole A., Pradier C., Salmon D., Simon A., Martinez V., Spano J.P., Costagliola D., Grabar S. Risk of non-AIDS-defining cancers among HIV-1-infected individuals in France between 1997 and 2009: results from a French cohort. AIDS. 2014:2109–2118. doi: 10.1097/QAD.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 22.Palefsky J.M., Holly E.A., Ralston M.L., Da Costa M., Greenblatt R.M. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. J. Infect. Dis. 2001:383–391. doi: 10.1086/318071. [DOI] [PubMed] [Google Scholar]
- 23.Nygard M., Saah A., Munk C., Tryggvadottir L., Enerly E., Hortlund M. Evaluation of the Long-term anti-human papillomavirus 6 (HPV6), 11, 16, and 18 immune responses generated by the quadrivalent HPV vaccine. Clin. Vaccin. Immunol. 2015:43–48. doi: 10.1128/CVI.00133-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Einstein M.H., Takacs P., Chatterjee A., Sperling R.S., Chakhtoura N., Blatter M.M. Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: end-of-study analysis of a phase III randomized trial. Hum. Vaccin. Immunother. 2014:3435–3445. doi: 10.4161/hv.36121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naud P.S., Roteli-Martins C.M., De Carvalho N.S., Teixeira J.C., de Borba P.C., Sanchez N. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum. Vaccin. Immunother. 2014:2147–2162. doi: 10.4161/hv.29532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkin T., Lee J.Y., Lensing S.Y., Stier E.A., Goldstone S.E., Berry J.M. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J. Infect. Dis. 2010:1246–1253. doi: 10.1086/656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellsworth G.B., Lensing S.Y., Ogilvie C.B., Lee J.Y., Goldstone S.E. A delayed dose of quadrivalent human papillomavirus vaccine demonstrates immune memory in HIV-1-infected men. Papillomavirus Res. 2018 doi: 10.1016/j.pvr.2018.05.001. (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volpini L.P.B., Boldrini N.A.T., Freitas L.B., Miranda A.E., Spano L.C. The high prevalence of HPV and HPV16 European variants in cervical and anal samples of HIV-seropositive women with normal Pap test results. PLoS One. 2017:e0176422. doi: 10.1371/journal.pone.0176422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menezes L.J., Poongulali S., Tommasino M., Lin H.Y., Kumarasamy N. Prevalence and concordance of human papillomavirus infection at multiple anatomic sites among HIV-infected women from Chennai, India. Int. J. STD AIDS. 2016:543–553. doi: 10.1177/0956462415587226. [DOI] [PubMed] [Google Scholar]
- 30.Kojic E.M., Cu-Uvin S., Conley L., Bush T., Onyekwuluje J., Swan D.C. Human papillomavirus infection and cytologic abnormalities of the anus and cervix among HIV-infected women in the study to understand the natural history of HIV/AIDS in the era of effective therapy (the sun study) Sex. Transm. Dis. 2011:253–259. doi: 10.1097/OLQ.0b013e3181f70253. [DOI] [PubMed] [Google Scholar]
- 31.Heard I., Poizot-Martin I., Potard V., Etienney I., Crenn-Hebert C., Moore C., Touraine P., Cubie H., Costagliola D. Prevalence of and risk factors for anal oncogenic human papillomavirus infection among HIV-infected women in france in the combination antiretroviral therapy era. J. Infect. Dis. 2016:1455–1461. doi: 10.1093/infdis/jiv751. [DOI] [PubMed] [Google Scholar]
- 32.Lin C., Franceschi S., Clifford G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect. Dis. 2018:198–206. doi: 10.1016/S1473-3099(17)30653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clifford G.M., Tully S., Franceschi S. Carcinogenicity of human Papillomavirus(HPV) types in HIV-positive women: a meta-analysis from HPV infection to cervical cancer. Clin. Infect. Dis. 2017:1228–1235. doi: 10.1093/cid/cix135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massad L.S., Xie X., Burk R.D., D'Souza G., Darragh T.M., Minkoff H., Colie C., Burian P., Palefsky J., Atrio J., Strickler H.D. Association of cervical precancer with human papillomavirus types other than 16 among HIV co-infected women. Am. J. Obstet. Gynecol. 2016:e1–e6. doi: 10.1016/j.ajog.2015.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu G., Markowitz L.E., Hariri S., Panicker G., Unger E.R. Seroprevalence of 9 Human Papillomavirus Types in the United States, 2005–2006. J. Infect. Dis. 2016:191–198. doi: 10.1093/infdis/jiv403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kavanagh K., Pollock K.G., Cuschieri K., Palmer T., Cameron R.L., Watt C., Bhatia R., Moore C., Cubie H., Cruickshank M., Robertson C. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect. Dis. 2017:1293–1302. doi: 10.1016/S1473-3099(17)30468-1. [DOI] [PubMed] [Google Scholar]
- 37.Lehtinen M., Luostarinen T., Vänskä S., Söderlund-Strand A., Eriksson T., Natunen K., Apter D., Baussano I., Harjula K., Hokkanen M., Kuortti M., Palmroth J., Petäjä T., Pukkala E., Rekonen S., Siitari-Mattila M., Surcel H.M., Tuomivaara L., Paavonen J., Nieminen P., Dillner J., Dubin G., Garnett G. Gender-neutral vaccination provides improved control of human papillomavirus types 18/31/33/35 through herd immunity: results of a community randomised trial (III) Int. J. Cancer. 2018 doi: 10.1002/ijc.31618. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 38.Brisson M., Bénard E., Drolet M., Bogaards J.A., Baussano I., Vänskä S., Jit M., Boily M.C., Smith M.A., Berkhof J., Canfell K., Chesson H.W., Burger E.A., Choi Y.H., De Blasio B.F., De Vlas S.J., Guzzetta G., Hontelez J.A.C., Horn J., Jepsen M.R., Kim J.J., Lazzarato F., Matthijsse S.M., Mikolajczyk R., Pavelyev A., Pillsbury M., Shafer L.A., Tully S.P., Turner H.C., Usher C., Walsh C. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health. 2016:e8–e17. doi: 10.1016/S2468-2667(16)30001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machalek D.A., Garland S.M., Brotherton J.M.L., Bateson D., McNamee K., Stewart M., Skinner R.S., Liu B., Cornall A.M., Kaldor J.M., Tabrizi S.N. Very low prevalence of vaccine human papillomavirus types among 18- to 35-year old Australian women 9 years following implementation of vaccination. J. Infect. Dis. 2018:1590–1600. doi: 10.1093/infdis/jiy075. [DOI] [PubMed] [Google Scholar]
- 40.Travassos A., Netto E., Xavier-Souza E., Nóbrega I., Adami K. Predictors of HPV incidence and clearance in a cohort of Brazilian HIV-infected women. PLoS One. 2017:e0185423. doi: 10.1371/journal.pone.0185423. [DOI] [PMC free article] [PubMed] [Google Scholar]