1. Introduction
Persistent infection of the anus with high-risk types of human papillomavirus (HPV) causes squamous cell carcinoma, one of the most common non-AIDS-defining cancers [1], [2]. HIV-infected individuals are at much higher risk for developing anal cancer than HIV-uninfected individuals [3], [4], [5], [6]. Approximately 70% of anal cancers are due to HPV types 16 or 18 and most genital warts are associated with HPV types 6 and 11 [7], [8]. The quadrivalent HPV (qHPV) vaccine, which induces neutralizing antibodies titers in recipients against HPV types 6, 11, 16, and 18, prevented 93% of persistent anal infections and 78% of anal squamous intraepithelial lesions caused by these types in HIV-uninfected men who have sex with men (MSM) [9].
While the qHPV vaccine series is highly immunogenic in HIV-infected adults and children, antibody titers are lower than those reported in HIV-uninfected populations [10], [11], [12]. In studies of both HIV infected and HIV-uninfected vaccine recipients, the titers developed in response to the standard three-dose qHPV series peak soon after the third dose then decline. HIV-uninfected women [13] and HIV-infected children [14] demonstrated an anamnestic response to a fourth dose of the qHPV vaccine suggesting that the qHPV vaccine induces immune memory.
Published studies of HIV-infected adults receiving qHPV vaccine have relatively limited follow-up and the characteristics of the long-term humoral response have not yet been described. It is unclear how much immunity wanes in this population and whether the standard 3 dose series generates immune memory which is necessary for long-term protection against new infections. A delayed fourth vaccination can simulate antigen exposure and a robust increase in antibodies suggestive of an anamnestic response indicative of immune memory [13], [14], [15]. When exposed to specific antigens, long-lived memory immune cells generate an immune response to prevent or stop infection [13].
The AIDS Malignancy Consortium Protocol 052 (AMC052) demonstrated the safety and immunogenicity of the standard three dose qHPV vaccine series in HIV-infected adult males [10]. Here we describe the long-term titers after vaccination in this study population. Since there are no accepted criteria for an anamnestic response, we sought to describe the titers at one and four weeks after a fourth dose, similar to what has been shown in HIV-uninfected women [13]. We hypothesized that titers would be higher four weeks after a fourth dose compared with four weeks after the third dose.
2. Materials and methods
2.1. Study population
A summary of study sites, enrollment criteria, and exclusion criteria for AMC052 has been previously reported [10]. Eligible participants for this analysis completed the three-dose series and provided written consent to receive the fourth dose and receive additional follow-up. AMC052 and this add-on study were approved by the Institutional Review Board at each participating site.
2.2. Study treatment
In the AMC052 protocol participants received 0.5 mL of intramuscular qHPV recombinant vaccine (Gardasil, Merck Sharp & Dohme Corporation) at entry and at weeks 8 and 24. Participants received a fourth dose at week 128 (2 years after completion of the original 3 dose series).
2.3. Study monitoring
In addition to previously reported AMC052 data, participants were assessed at weeks 76, 128, 129, and 132. All signs and symptoms were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events [16].
2.4. Laboratory testing
Serum was collected for assessment of antibody responses at entry, and weeks 28, 76, 128, 129, and week 132. Vaccine type epitope-specific neutralizing antibodies to HPV were analyzed with a competitive Luminex-based immunoassay (Merck Research Laboratories) as described previously [17], [18]. These assays were performed under the direction of Merck Research Laboratories at PPD Vaccines and Biologics Laboratory (Wayne, PA).
2.5. Statistical considerations
HPV seropositivity was defined as having titers of ≥ 20, ≥ 16, ≥ 20, and ≥ 24 milli-Merck units per mL (mMU/mL) for types 6, 11, 16, and 18, respectively, for each assay [18]. Participants with undetectable antibody concentrations were nominally assigned a value of 50% of the detection limit for the assay. Participants that received all four doses of vaccine were included in analysis at weeks 128–132 regardless of baseline antibodies to HPV or detection of anal HPV DNA.
Geometric mean titers and corresponding two-sided 95% confidence intervals were calculated for titers. For investigating the impact of the fourth dose, the primary endpoints were the titers of type specific antibodies at weeks 129 and 132 (1 and 4 weeks after the fourth qHPV dose). Median changes in titers for week 129 versus week 128 and week 132 versus week 28 (4 weeks after fourth and third doses respectively) were compared using the paired Wilcoxon signed rank test. Proportions of HPV seropositive participants were computed and paired comparisons were made using McNemar's test. P-values less than 0.05 were considered statistically significant and p-values were not adjusted for multiple comparisons.
3. Results
3.1. Study participants
AMC052 enrolled 112 subjects from 8 sites. 103 patients received the standard three dose vaccine series and were potentially eligible for a fourth dose. Seventy-five (73%) participants consented and received a fourth dose. Of these, 73 had evaluable sera at week 128; 67 and 69 patients had evaluable sera at weeks 129 and 132. Baseline characteristics of the eligible patients are summarized in Table 1.
Table 1.
Demographic summary and selected baseline characteristics of the 103 eligible participants that had received three doses of quadrivalent HPV vaccine in AMC052.
| Demographic and Baseline Characteristics | |
|---|---|
| Ethnicity/Race, N (%) | |
| Hispanic | 17 (17) |
| Non-Hispanic/White | 65 (63) |
| Non-Hispanic/Black | 13 (13) |
| Non-Hispanic/Asian/Pacific Islander | 6 (6) |
| Non-Hispanic/Multiracial | 2 (2) |
| Age in years, Mean (SD) | 43.8 (9.3) |
| Mean CD4, cells/µL (SD) | |
| Current, N = 102 | 563.9 (222.6) |
| Nadir, N = 103 | 103 (249.7) |
| Current use of ART, N (%) | 87 (84%) |
| Plasma HIV− 1 RNA level < 200 copies/mL (%) | 85 (83%) |
3.2. Titers
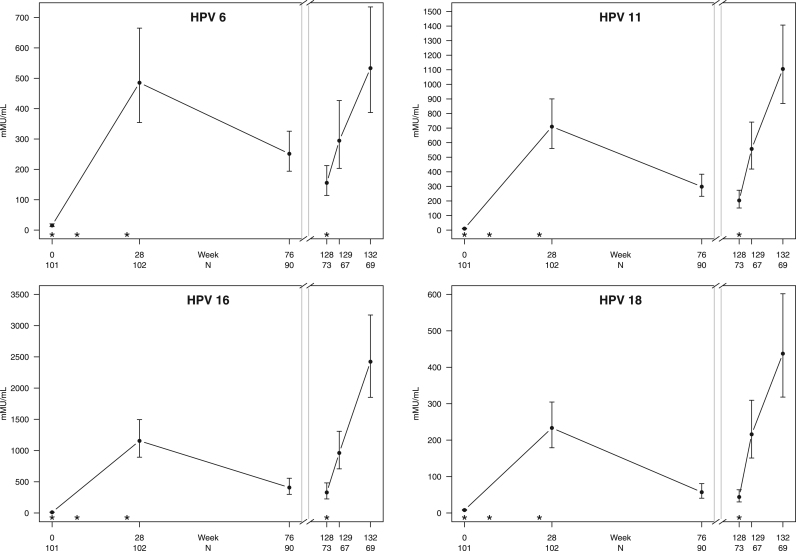
After the third vaccine dose was administered at week 24, titers peaked at week 28. Titers then fell sharply through week 76 (on average 48–76%) followed by a smaller decline (19–38%) through week 128. Fig. 1 graphically summarizes the distribution of geometric mean titers at each time point (see Supplemental Table 1 for detailed data). At week 76, the proportion of participants with HPV seropositivity was greater than 96% for types 6, 11, and 16; type 18 seropositivity was 66%. Despite the further drop in titers through week 128, the proportion with HPV seropositivity was greater than 94% for types 6, 11, and 16%, and 63% for type 18. The fourth dose was given at a median time of 135 weeks (interquartile range 129–141) after vaccine series initiation. Median titers increased significantly one week after vaccination at week 129 for all HPV types including 18, which saw a significant increase to 91% (N = 67 pairwise comparisons, p < 0.001) in the proportion of HPV seropositive participants to antibodies to type 18. Supplemental Table 2 summarizes the proportion of HPV seropositive patients at all visits. Antibody titers to HPV 16 and 18 were significantly higher at week 132 (four weeks after the fourth dose) compared to week 28 (four weeks after the third dose). Antibody titers to HPV 6 and 11 were not significantly different between week 132 and week 28 (Table 2).
Fig. 1.
Trend of geometric mean HPV titers by viral type over time including 95% CI. The asterisks (*) on the x-axis represent vaccine doses administered at 0, 8, 24, and 128 weeks. There were significant increases in peak titers for HPV types 16, 18 at week 132 compared to week 28.
Table 2.
Median increase in titers.
| Median Change (mMU/mL) [Interquartile Range] | p-value | |
|---|---|---|
| Median change in titers one week after fourth dose (week 129 versus week 128) (N = 67): | ||
| HPV 6 | 154 [5, 381] | < 0.001 |
| HPV 11 | 276 [69, 495] | < 0.001 |
| HPV 16 | 497 [154, 1347] | < 0.001 |
| HPV 18 | 115 [54,439] | < 0.001 |
| Median change in titers between week 132 (four weeks after the fourth dose) and week 28 (four weeks after the third dose) (N = 69): | ||
| HPV 6 | 19 [− 526, 685] | 0.901 |
| HPV 11 | 94 [− 508, 836] | 0.245 |
| HPV 16 | 935 [− 375, 3135] | 0.002 |
| HPV 18 | 125 [− 23 629] | 0.001 |
3.3. Safety
There were no grade 3, 4, or 5 events related to the vaccine during the follow up period after 28 weeks.
4. Discussion
In this HIV-infected adult male population, we have shown that titers peak four weeks after a third dose of qHPV vaccine and then decrease through 2.5 years after vaccine series initiation. The titers plateau at lower levels than seen in HIV-uninfected populations or HIV-infected children [12], [14]. We have demonstrated a rapid increase in titers four weeks after a delayed fourth dose of qHPV vaccine consistent with an anamnestic response as has been described in other populations [13], [14]. For types 16 and 18, the titers 4 weeks after the fourth dose are significantly higher than those observed 4 weeks after the third dose. These results are consistent with the induction of immune memory with the standard three dose series and suggest that HPV vaccination generates a memory B-cell population as a mechanism for long-term protection in this HIV-infected adult populations.
Antibodies against HPV 18 are present at lower concentrations than the other three types and are more likely to decline below the assay threshold for HPV seropositivity. This pattern is similar to that seen in HIV-uninfected populations [19]. This is thought to be due to specific characteristics of the neutralizing epitope for the HPV 18 antibody assay rather than lower immunogenicity for the HPV 18 viral-like particle [20]. Breakthrough infection with HPV 18 has not been demonstrated in long-term cohort studies of qHPV vaccine recipients [13], [21], [22]
The majority of participants retained HPV seropositivity for the four vaccine types through 128 weeks after vaccine initiation. After a sharp decline from peak titers, the titers exhibited a smaller decrease from week 76 to week 128 after vaccine initiation. We have shown that a delayed fourth dose of vaccine generates a higher peak titer in antibodies to HPV 16 and 18 but not to HPV 6 and 11. For HPV 16, the titers 4 weeks after the fourth dose in our study was similar to the titers observed in heterosexual HIV-uninfected males aged 16–23 years and 27–45 years after they are vaccinated with three doses (2422 mMU/mL compared to 2622 mMU/mL and 2285 mMU/mL, respectively) [23], [24]. The antibody titer needed for maintenance of protection is not clear. The higher titers elicited by a fourth dose of qHPV vaccine in HIV-infected individuals may or may not lead to greater protection or prolonged immunity against future infection with HPV types contained in the vaccine.
There are limitations to this analysis. Only a subset of AMC052 participants received the fourth dose and the serologic data are incomplete. The serologic assay used in this study only measures a portion of the humoral response induced by HPV infection as it only identifies neutralizing antibodies to limited number of epitopes, specifically those induced by the qHPV vaccine. These assays may underestimate the level of protection provided to vaccine recipients. For a given viral type, it is not possible to discern whether participants were truly immunologically naïve to a given HPV type and serologic responses (undetected by the assay used) may have been modified by prior infection with a specific HPV type. While not a limitation to this study, the generation of an immune response itself does not imply protection in this population given the high rate of current and prior HPV infection at study entry.
In summary, this study demonstrates that HIV-infected adult men receiving the HPV vaccine exhibit a sustained antibody response to the vaccine. They demonstrate a robust humoral immune response to a fourth dose of the vaccine consistent with immune memory resulting from a three dose vaccine series. While a fourth dose of qHPV vaccine induces higher antibody response in HIV-infected adult men than the three dose series, the clinical significance of this finding is unknown.
Acknowledgements
None.
Acknowledgments
Funding
National Institutes of Health [K23 AI 55038 to T.J.W., UL1 RR024996 to Weill Cornell Clinical and Translational Science Center, U01 CA121947–01 to R.T.M., M01-RR00865 to Weill Cornell Clinical and Translational Science Center, T32 AI007613 to Division of Infectious Diseases of Weill Cornell Medical College, and UL1 RR024131 to University of California at San Francisco Clinical and Translational Science Institute]. Merck Laboratories provided funding for the HPV antibody assays and provided the vaccine doses for this study.
Conflict of interest declaration
We wish to draw the attention of the Editor(s) to the following facts which may be considered as potential conflicts of interest and to significant financial contributions to this work.
Mark H. Einstein has advised or participated in educational speaking activities but not received an honorarium from Merck; Montefiore Medical Center had received payment for time spent by M.H.E. for these activities; Montefiore Medical Center has received grant funding from Merck for research-related costs of clinical trials for which M.H.E. was primary investigator. Alfred Saah is an employee of Merck Laboratories and owns stock in Merck Laboratories. Joel M. Palefsky has served as a consultant for Merck; University of California at San Francisco has received grant funding from Merck for advisory board activities of J.M.P. and for research-related costs of clinical trials for which J.M.P. was the primary investigator. Timothy J. Wilkin has served as an ad hoc advisor for GlaxoSmithKline/ViiV Healthcare, and has received grant funding (paid to Weill Cornell Medicine) from GlaxoSmithKline/ViiV Healthcare.
All other authors: None.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pvr.2018.05.001.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.Zaki S.R., Judd R., Coffield L.M., Greer P., Rolston F., Evatt B.L. Human papillomavirus infection and anal carcinoma. Retrospective analysis by in situ hybridization and the polymerase chain reaction. Am. J. Pathol. 1992;140:1345–1355. 〈http://www.ncbi.nlm.nih.gov/pubmed/1318640〉 [PMC free article] [PubMed] [Google Scholar]
- 2.Shiels M.S., Cole S.R., Kirk G.D., Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J. Acquir. Immune Defic. Syndr. 2009;52:611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goedert J.J., Cote T.R., Virgo P., Scoppa S.M., Kingma D.W., Gail M.H. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;351:1833–1839. doi: 10.1016/s0140-6736(97)09028-4. 〈http://www.ncbi.nlm.nih.gov/pubmed/9652666〉 [DOI] [PubMed] [Google Scholar]
- 4.Silverberg M.J., Lau B., Justice A.C., Engels E., Gill M.J., Goedert J.J. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin. Infect. Dis. 2012;54:1026–1034. doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glick S.N., Feng Q., Popov V., Koutsky L.A., Golden M.R. High rates of incident and prevalent anal human papillomavirus infection among young men who have sex with men. J. Infect. Dis. 2014;209:369–376. doi: 10.1093/infdis/jit441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colon-Lopez V., Shiels M.S., Machin M., Ortiz A.P., Strickler H., Castle P.E. Anal cancer risk among people with HIV infection in the United States. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2017 doi: 10.1200/JCO.2017.74.9291. JCO2017749291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garland S.M., Steben M., Sings H.L., James M., Lu S., Railkar R. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J. Infect. Dis. 2009;199:805–814. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 8.Hoots B.E., Palefsky J.M., Pimenta J.M., Smith J.S. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int. J. Cancer. 2009;124:2375–2383. doi: 10.1002/ijc.24215. [DOI] [PubMed] [Google Scholar]
- 9.Giuliano A.R., Palefsky J.M., Goldstone S., Moreira E.D., Penny M.E., Aranda C. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N. Engl. J. Med. 2011;364:401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkin T., Lee J.Y., Lensing S.Y., Stier E.A., Goldstone S.E., Berry J.M. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J. Infect. Dis. 2010;202:1246–1253. doi: 10.1086/656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin M.J., Moscicki A.-B., Song L.-Y., Fenton T., Meyer W.A., Read J.S. Safety and immunogenicity of a quadrivalent human papillomavirus (Types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. JAIDS J. Acquir. Immune Defic. Syndr. 2010;55:197–204. doi: 10.1097/QAI.0b013e3181de8d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kojic E.M., Kang M., Cespedes M.S., Umbleja T., Godfrey C., Allen R.T. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin. Infect. Dis. 2014;59:127–135. doi: 10.1093/cid/ciu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsson S.E., Villa L.L., Costa R.L., Petta C.A., Andrade R.P., Malm C. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007;25:4931–4939. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 14.Levin M.J., Huang S., Moscicki A.B., Song L.Y., Read J.S., Meyer W.A. Four-year persistence of type-specific immunity after quadrivalent human papillomavirus vaccination in HIV-infected children: effect of a fourth dose of vaccine. Vaccine. 2017;35:1712–1720. doi: 10.1016/j.vaccine.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scherer E.M., Smith R.A., Simonich C.A., Niyonzima N., Carter J.J., Galloway D.A. Characteristics of memory B cells elicited by a highly efficacious HPV vaccine in subjects with no pre-existing immunity. PLoS Pathog. 2014;10:e1004461. doi: 10.1371/journal.ppat.1004461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Department of Health and Human Services, National Institutes of Health. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). 〈https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf〉. Date Accessed: 29 January, 2010.
- 17.Opalka D., Lachman C.E., MacMullen S.A., Jansen K.U., Smith J.F., Chirmule N. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin. Diagn. Lab. Immunol. 2003;10:108–115. doi: 10.1128/CDLI.10.1.108-115.2003. 〈http://www.ncbi.nlm.nih.gov/pubmed/12522048〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dias D., Van Doren J., Schlottmann S., Kelly S., Puchalski D., Ruiz W. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin. Diagn. Lab. Immunol. 2005;12:959–969. doi: 10.1128/CDLI.12.8.959-969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Einstein M.H., Takacs P., Chatterjee A., Sperling R.S., Chakhtoura N., Blatter M.M. Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)−16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: end-of-study analysis of a Phase III randomized trial. Human. Vaccin. Immunother. 2014;10:3435–3445. doi: 10.4161/hv.36121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown D.R., Garland S.M., Ferris D.G., Joura E., Steben M., James M. The humoral response to Gardasil over four years as defined by total IgG and competitive Luminex immunoassay. Human. Vaccin. 2011;7:230–238. doi: 10.4161/hv.7.2.13948. 〈http://www.ncbi.nlm.nih.gov/pubmed/21307649〉 [DOI] [PubMed] [Google Scholar]
- 21.Villa L.L., Costa R.L., Petta C.A., Andrade R.P., Paavonen J., Iversen O.E. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br. J. Cancer. 2006;95:1459–1466. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn J.A., Xu J., Kapogiannis B.G., Sleasman J.W. Brief report: antibody responses to quadrivalent HPV vaccination in HIV-infected young women as measured by total IgG and competitive luminex immunoassay. J. Acqui. Immune Defic. Syndr. 2017;75:241–245. doi: 10.1097/QAI.0000000000001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillman R.J., Giuliano A.R., Palefsky J.M., Goldstone S., Moreira E.D., Jr., Vardas E. Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old. Clin. Vaccin. Immunol.: Cvi. 2012;19:261–267. doi: 10.1128/CVI.05208-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giuliano A.R., Isaacs-Soriano K., Torres B.N., Abrahamsen M., Ingles D.J., Sirak B.A. Immunogenicity and safety of Gardasil among mid-adult aged men (27-45 years)--The MAM Study. Vaccine. 2015;33:5640–5646. doi: 10.1016/j.vaccine.2015.08.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material