Abstract
Purpose
Postcholecystectomy syndrome (PCS) is characterized by abdominal symptoms following gallbladder removal. However, there is no consensus for the definition or treatment for PCS. The purpose of this study was to define PCS among various symptoms after laparoscopic cholecystectomy, and to identify risk factors affecting PCS.
Methods
This study was conducted at Dongguk University Ilsan Hospital and Chung-Ang University Hospital (2012–2013). Outcomes were assessed using European Organization for Research and Treatment of Cancer QLQ–C30 questionnaire. Symptom cluster for determining PCS was made by factor analysis. Cluster analysis evaluating risk factors of PCS was made by Ward methods and Dentogram.
Results
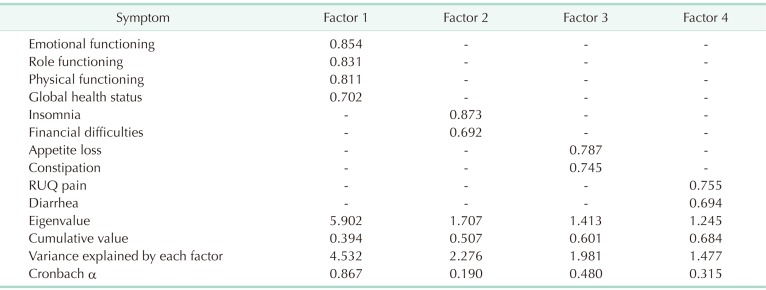
Factor analysis revealed three distinct symptom clusters, those are ‘insomnia and financial difficulties (eigenvalue, 1.707; Cronbach α, 0.190),’ ‘appetite loss and constipation (eigenvalue, 1.413; Cronbach α, 0.480),’ and ‘right upper quadrant (RUQ) pain and diarrhea (eigenvalue, 1.245; Cronbach α, 0.315).’ Among these symptom clusters, the cluster of ‘RUQ pain and diarrhea’ was determined as PCS. However, we could not find any risk factors between high symptomatic group and low symptomatic group.
Conclusion
PCS could consist of RUQ pain and diarrhea. Well-designed prospective trials are needed to determine risk factors of PCS.
Keywords: Cholecystectomy, Postcholecystectomy syndrome, Quality of life
INTRODUCTION
Since its introduction in 1986, laparoscopic cholecystectomy (LC) has been more widely used and it is now considered the treatment of choice for various gallbladder (GB) diseases [1,2,3,4,5,6]. However, many patients remain symptomatic after cholecystectomy. As a result, when LC is recommended, many patients wonder about the relief of their symptoms and the occurrence of new symptoms after removing the GB [6,7].
Symptom clusters are defined as 2 or more concurrent symptoms that are related with or without a common cause [8,9,10,11,12]. Previous studies have shown that postoperative patients experiencing multiple concurrent symptoms may be characterized as symptom clusters [9,13]. Therefore, studying about symptom clusters is important for its implications regarding patient management. A consensus on appropriate research methodology is vital [10]. However, up to date, no studies have been conducted to explore whether concurrent symptoms experienced by patients after cholecystectomy could be grouped as symptom clusters also known as postcholecystectomy syndrome (PCS).
This exploratory analysis assessed symptom clusters as PCS among patients undergoing LC to identify distinct subgroups of patients who experienced differential burden for symptom cluster and assessed whether patient subgroups were associated with deleterious quality of life (QoL) outcomes. As a result, this study was designed to cluster the physical, emotional, and psychosomatic symptoms after LC, and define PCS among various symptoms after LC using a validated questionnaire. We also explored possible risk factors of PCS in this study.
METHODS
Patient
The study was conducted as part of a former Institutional Review Board (IRB) (approval number: DUIH-2014-109) approved randomized controlled trial to investigate the effects of Rowachol on prevention of postcholecystectomy pain after LC (NCT01765465) [6]. For the current study, a total of 138 patients, with various GB diseases after LC, were enrolled.
Study design
Between May of 2013 and January of 2014, 160 patients were assessed for eligibility at Dongguk University Ilsan Hospital and Chung-Ang University Hospital as described in a previous study [6]. The IRB at each hospital approved the study protocol. Written informed consent was obtained from all patients before enrollment. All operations were conducted by experienced laparoscopic surgeons using single or multiport methods. Technical difficulties were assessed as present (score of 1) or absent (score of 0) for each of the following 5 operative steps: (1) access into the peritoneal cavity, (2) dissection of adhesions from the GB, (3) dissection of the triangle formed by the common bile duct, cystic duct, and liver (Calot triangle), (4) dissection of the GB bed, and (5) extraction of the GB from the abdominal cavity. All GB specimens were sent for histopathology analysis [6,14].
Symptomatic evaluation
Since there are no validated or translated questionnaires in Korean suitable for evaluating symptoms, symptomatic evaluation were made using the validated Korean version of European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire in Korean in outpatient clinics three months postoperatively as aforementioned study [6,15]. These questionnaires were self-reported by patients. A trained nurse was available for patients who required help in completing the surveys. Raw data underwent linear transformation to standardize the raw scores, ranging from 0 to 100, as recommended by the EORTC QLQ-C30 scoring manual.
Demographic information such as age, sex, body mass index, and the American Society of Anesthesiologists (ASA) physical status classification and pertinent surgical information such as the presence of gallstone, type of surgery, conversion rate, operation time, and difficulty score were all predefined. Outcomes were assessed by dedicated study nurses who deposited these data into a dedicated computerized database (MDB, Seoul, Korea).
Symptom cluster for determining PCS
Factor analysis by Varimax rotation and K-mean methods was used to identify symptom clusters based on the severity of patients' symptom experiences. Applying factor analysis to symptom cluster research is an effective statistical approach to identify common factors that explain the correlation between symptoms and find the communality that “binds” 2 or more symptoms together into a common concept. Accordingly, exploratory factor analysis with principal axis factoring was used to identify symptom clusters [16]. Varimax rotation was used to maximize the variance of loadings within each component while assuming the independence of component structure [11,16].
Determining patient subgroups
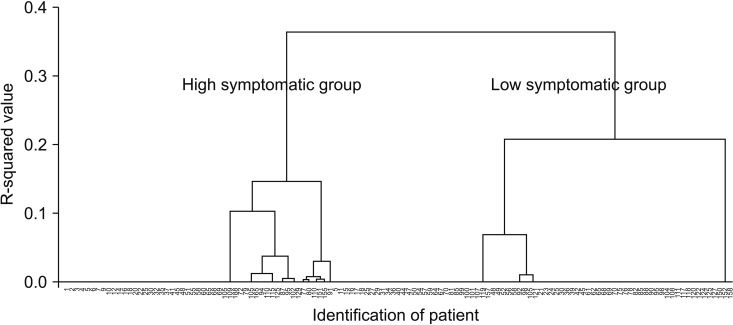
To determine whether distinct subgroups of patients experiencing differing levels of symptom burden might be having PCS, we modeled our cluster analysis on Ward methods and Dentogram. After cluster analysis, 2 patient subgroups, the high and low symptomatic group for PCS, were identified (Fig. 1).
Fig. 1. To determine whether distinct subgroups of patients experiencing differing levels of symptoms burden might be having PCS, we modeled our cluster analysis on Ward methods and Dentogram. After cluster analysis, two patient subgroups, the high and low symptomatic group for PCS, were identified. PCS, postcholecystectomy syndrome.
Statistical analysis
All statistical analyses were analyzed by using SAS ver. 9.3 (SAS Institute, Cary, NC, USA). All statistical tests were 2-sided, and the level of significance was set at 0.05. Continuous variables were expressed as the mean ± standard deviation and categorical variables were described by number and percentage in parentheses.
Several factor analyses procedures were conducted to identify domains of PCS measures response. Specifically, principal components analysis was performed with varimax rotations. Factors with eigenvalues values greater than one were retained for interpretation. Eigenvalue is a measure of how much each factor describes the variance of the overall symptom. And Cronbach α coefficient is an internal consistency coefficient that assesses the reliability of each symptom to measure the factor. The standardized Z-scores within each factor were averaged to produce the PCS. All Z-scores were determined such that positive scores on the PCS indicate higher symptomatic group for PCS and negative scores indicate lower PCS.
Cluster analysis using the PCS scores, was performed to identify homogeneous subgroups or clusters representing specific patterns of PCS sensitivity. Hierarchical cluster analysis was chosen employing Ward's clustering method with squared Euclidean distances as the similarity measure in order to be sensitive to differences in elevation as well as profile shape.
RESULTS
Patients
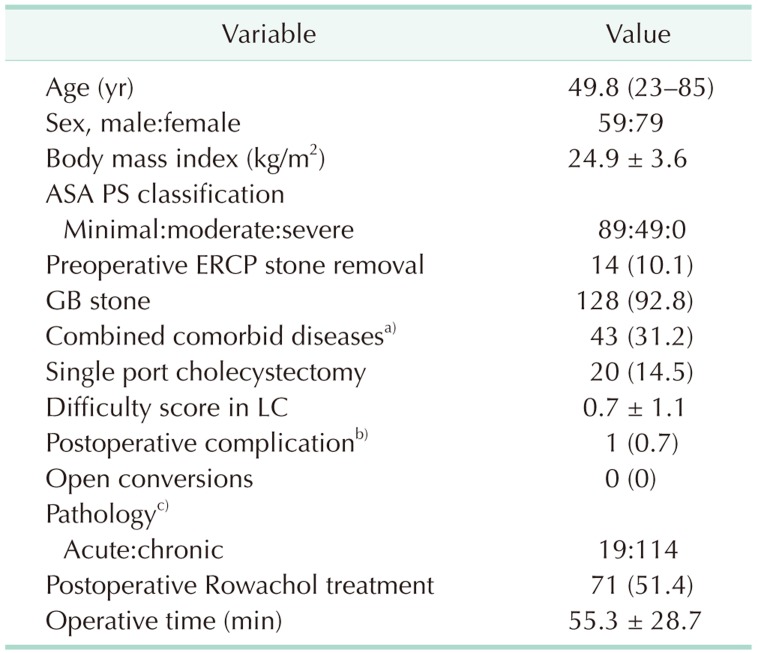
Of the 160 patients who were screened, 22 were excluded, including 8 who gave informed refusal as described in the previous study [6]. As a result, 138 patients were enrolled in this study, including 59 male and 79 female patients. The mean age was 49.8 years old and the most common ASA PS classification was I. Total complication case was 1 case (0.7%), postoperative minor bile leak which decreased spontaneously without any intervention. Open conversion rate was 0.7%. And there was no open conversion case. Postoperative Rowachol treatment was performed in 71 (51.4%) patients (Table 1).
Table 1. Patient characteristics in the intent-to-treat population (n =138).
Values are presented as median (range), mean ± standard deviation, or number of patients (%).
ASA PS, American Society of Anesthesiologists physical status; ERCP, endoscopic retrograde cholangiopancreatography; GB, gallbladder; LC, laparoscopic cholecystectomy.
a)Cardiovascular, cerebrovascular, diabetes mellitus, chronic obstructive lung disease, chronic renal failure, etc. b)One case of minor bile leak only, no other complications occurred. c)Three cases of xanthogranulomatous cholecystitis, 2 cases of T1 GB cancer.
Symptom cluster and risk factors
Among 138 patients, 127 patients completed the questionnaire at 3 months postoperatively. After excluding symptoms such as nausea, vomiting, fatigue, cognitive functioning, and social functioning by factor analysis, 1 functional cluster (factor 1, emotional functioning, role functioning, physical functioning, global health status) and 3 symptom clusters (factor 2, insomnia, and financial difficulty; factor 3, appetite loss and constipation; factor 4, right upper quadrant (RUQ) pain and diarrhea) were identified. Among these, factor 4 of RUQ pain and diarrhea (eigenvalue, 1.245; Cronbach α, 0.315) was decided as PCS (Table 2). Twelve patients (8.6%) had RUQ pain which was an EORTC QLQ C-30 score for abdominal pain exceeding 30 points. The patients who had diarrhea were 79 (57.2%). Among these patients, 5 patients (3.6%) complained of RUQ pain and diarrhea.
Table 2. Symptom clusters after factor analysis at 3 months after laparoscopic cholecystectomy.
RUQ, right upper quadrant.
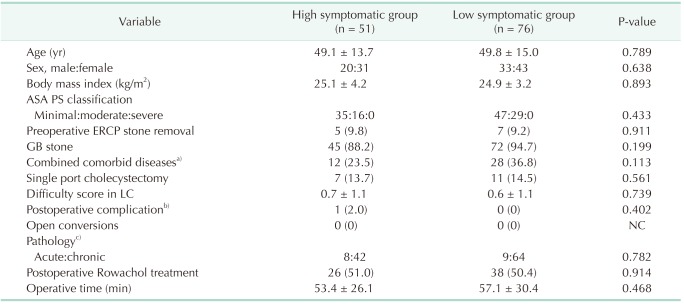
After cluster analysis using Ward methods and Dentogram, all patients were divided into 2 groups, high symptomatic group (n = 51, 40.2%) and low symptomatic group (n = 76, 59.8%) of PCS according to factor 4, RUQ pain and diarrhea (Fig. 1). To find any risk factors of PCS, various clinical parameters were compared between the 2 groups. However, there was no statistically significant different clinical parameter between high symptomatic group and low symptomatic group (Table 3).
Table 3. Risk factor analysis affecting postcholecystectomy syndrome.
Values are presented as mean ± standard deviation or number of patients (%).
ASA PS, American Society of Anesthesiologists physical status; ERCP, endoscopic retrograde cholangiopancreatography; GB, gallbladder; LC, laparoscopic cholecystectomy; NC, not calculated.
a)Cardiovascular, cerebrovascular, diabetes mellitus, chronic obstructive lung disease, chronic renal failure, etc. b)One case of minor bile leak only, no other complications occurred. c)Three cases of xanthogranulomatous cholecystitis, 2 cases of T1 GB cancer.
DISCUSSION
Postsurgical patients often experience multiple symptoms which can independently predict changes in prognosis, QoL, and functional status. And patients are seldom present with a single symptom which may explain why treating one symptom may not necessarily improve their QoL [10]. Understanding the synergistic effect of concurrent symptoms holds promise to develop effective strategies capable of ameliorating specific groups of treatment- and disease-related symptoms and improve patients' QoL [17]. In this study, factor 4 (RUQ pain and diarrhea) was defined as PCS because both RUQ pain and diarrhea were common and representative postoperative presentations [1,6,7,18,19,20,21,22]. This study identified distinct patient subgroup who could be called PCS based on symptoms after cholecystectomy obtained from self-reported questionnaire.
Previously, we have demonstrated that Rowachol might be beneficial for preventing pain after LC. A limitation of our previous report was that we did not include a relationship analysis for pain with other symptoms [6]. However, this present study clearly suggested that postoperative pain was usually accompanied by diarrhea in a single symptom cluster (Table 2). As a result, it is important for surgeons to assess not only the pain but also diarrhea in this patient population. Follow-up treatment should include questions on both pain and diarrhea in order to facilitate their access to adequate treatment or psychosocial support.
Chronic diarrhea developed after cholecystectomy is another well-known clinical problem. Bile acid malabsorption has been incriminated as an important cause of postcholecystectomy diarrhea. Diarrhea could be a consequence of altered bile flow in the bowel induced by cholecystectomy [13]. Removal of the GB can result in a more continuous enterohepatic cycling of bile acids, which can increase the spill-over of bile acids from the terminal ileum into the colon [13,21,23]. When aqueous concentrations of fecal bile acids exceed a critical secretory level, chologenic diarrhea will occurs [20,24]. These might be the reasons why diarrhea is one of the components of PCS in this study.
At present, however, no obvious reasons can be drawn from this study to explain why pain and diarrhea are synchronous after cholecystectomy. The questionnaire using this study was the Korean version of EORTC QLQ C-30, which was designed for cancer patients. As a result, we could not precisely evaluate the relationship between postoperative pain and diarrhea because of the shortage of question items. An accurate and abundant report of patient-reported outcomes may be important to surgeons as feedback on the care they have provided. This can decrease variations in practice. Further study is needed to evaluate postoperative symptoms using specialized questionnaire for gastrointestinal symptoms, such as, Gastrointestinal Quality-of-Life Index.
From a clinical point of view, cluster analysis can be used to identify subgroups of patients who are more likely to benefit from an additional intervention [23]. Subgroups of patients identified using symptom data could benefit from interventions that could reduce symptom burden if identified early or if symptom burden is correlated with factors amenable to intervention [17]. However, regarding our exploratory aim, we found no differences between high and low symptomatic group of PCS based on demographic, disease, or treatment characteristics (Table 3). This could be due to the relatively small sample size used in the study. As a result, future research with an expanded sample size should be performed.
This study has some potential limitations. First, the types and proportions of persistent symptoms were reported to be different from those that occur de novo, suggesting that these two entities may have different causes [22]. In this study, symptomatic evaluation using the EORTC QLQ C-30 questionnaire was done once at 3 months postoperatively. Therefore, we could not differentiate persistent symptoms from de novo postoperative symptoms. To reduce the number of patients with persistent or new symptoms, more evidence of the exact relationship between cholecystectomies and symptoms is needed. In addition, there might be a selection bias because our samples were drawn from tertiary level academic centers, and might differ from the typical patient treated in a community-based clinical practice.
In conclusion, factor analysis revealed that PCS might consist of RUQ pain and diarrhea based on symptoms present in postcholecystectomy patients using a self-reported questionnaire. However, there was no statistically significant different clinical parameter between high symptomatic group and low symptomatic group. We should be able to develop effective strategies capable of ameliorating specific groups of treatment- and disease-related symptoms to improve patients' QoL after cholecystectomy.
Footnotes
This study was presented at 12th IHPBA Sao Paulo 2016, taking place on April 20–23, 2016.
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Jaunoo SS, Mohandas S, Almond LM. Postcholecystectomy syndrome (PCS) Int J Surg. 2010;8:15–17. doi: 10.1016/j.ijsu.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 2.McPherson K, Wennberg JE, Hovind OB, Clifford P. Small-area variations in the use of common surgical procedures: an international comparison of New England, England, and Norway. N Engl J Med. 1982;307:1310–1314. doi: 10.1056/NEJM198211183072104. [DOI] [PubMed] [Google Scholar]
- 3.Lirici MM, Califano AD, Angelini P, Corcione F. Laparo-endoscopic single site cholecystectomy versus standard laparoscopic cholecystectomy: results of a pilot randomized trial. Am J Surg. 2011;202:45–52. doi: 10.1016/j.amjsurg.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds W., Jr The first laparoscopic cholecystectomy. JSLS. 2001;5:89–94. [PMC free article] [PubMed] [Google Scholar]
- 5.Tiong L, Oh J. Safety and efficacy of a laparoscopic cholecystectomy in the morbid and super obese patients. HPB (Oxford) 2015;17:600–604. doi: 10.1111/hpb.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han IW, Kwon OC, Oh MG, Choi YS, Lee SE. Effects of Rowachol on prevention of postcholecystectomy pain after laparoHongbeom scopic cholecystectomy: prospective multicenter randomized controlled trial. HPB (Oxford) 2016;18:664–670. doi: 10.1016/j.hpb.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim GH, Lee HD, Kim M, Kim K, Jeong Y, Hong YJ, et al. Fate of dyspeptic or colonic symptoms after laparoscopic cholecystectomy. J Neurogastroenterol Motil. 2014;20:253–260. doi: 10.5056/jnm.2014.20.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28:465–470. [PubMed] [Google Scholar]
- 9.Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN. Symptom cluster research: conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006;31:85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Fan G, Filipczak L, Chow E. Symptom clusters in cancer patients: a review of the literature. Curr Oncol. 2007;14:173–179. doi: 10.3747/co.2007.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryu E, Kim K, Cho MS, Kwon IG, Kim HS, Fu MR. Symptom clusters and quality of life in Korean patients with hepatocellular carcinoma. Cancer Nurs. 2010;33:3–10. doi: 10.1097/NCC.0b013e3181b4367e. [DOI] [PubMed] [Google Scholar]
- 12.Steel JL, Kim KH, Dew MA, Unruh ML, Antoni MH, Olek MC, et al. Cancer-related symptom clusters, eosinophils, and survival in hepatobiliary cancer: an exploratory study. J Pain Symptom Manage. 2010;39:859–871. doi: 10.1016/j.jpainsymman.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wanjura V, Sandblom G. How do quality-of-life and gastrointestinal symptoms differ between post-cholecystectomy patients and the background population? World J Surg. 2016;40:81–88. doi: 10.1007/s00268-015-3240-0. [DOI] [PubMed] [Google Scholar]
- 14.Cho KS, Baek SY, Kang BC, Choi HY, Han HS. Evaluation of preoperative sonography in acute cholecystitis to predict technical difficulties during laparoscopic cholecystectomy. J Clin Ultrasound. 2004;32:115–122. doi: 10.1002/jcu.20001. [DOI] [PubMed] [Google Scholar]
- 15.Yun YH, Park YS, Lee ES, Bang SM, Heo DS, Park SY, et al. Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res. 2004;13:863–868. doi: 10.1023/B:QURE.0000021692.81214.70. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Abraham IL. Statistical approaches to modeling symptom clusters in cancer patients. Cancer Nurs. 2008;31:E1–E10. doi: 10.1097/01.NCC.0000305757.58615.c8. [DOI] [PubMed] [Google Scholar]
- 17.Gwede CK, Small BJ, Munster PN, Andrykowski MA, Jacobsen PB. Exploring the differential experience of breast cancer treatment-related symptoms: a cluster analytic approach. Support Care Cancer. 2008;16:925–933. doi: 10.1007/s00520-007-0364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson RG, Macintyre IM. Symptomatic outcome after laparoscopic cholecystectomy. Br J Surg. 1993;80:439–441. doi: 10.1002/bjs.1800800410. [DOI] [PubMed] [Google Scholar]
- 19.O'Donnell LJ. Post-cholecystectomy diarrhoea: a running commentary. Gut. 1999;45:796–797. doi: 10.1136/gut.45.6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauter GH, Moussavian AC, Meyer G, Steitz HO, Parhofer KG, Jüngst D. Bowel habits and bile acid malabsorption in the months after cholecystectomy. Am J Gastroenterol. 2002;97:1732–1735. doi: 10.1111/j.1572-0241.2002.05779.x. [DOI] [PubMed] [Google Scholar]
- 21.Berger MY, Olde Hartman TC, Bohnen AM. Abdominal symptoms: do they disappear after cholecystectomy? Surg Endosc. 2003;17:1723–1728. doi: 10.1007/s00464-002-9154-6. [DOI] [PubMed] [Google Scholar]
- 22.Lamberts MP, Lugtenberg M, Rovers MM, Roukema AJ, Drenth JP, Westert GP, et al. Persistent and de novo symptoms after cholecystectomy: a systematic review of cholecystectomy effectiveness. Surg Endosc. 2013;27:709–718. doi: 10.1007/s00464-012-2516-9. [DOI] [PubMed] [Google Scholar]
- 23.Nagel GC, Schmidt S, Strauss BM, Katenkamp D. Quality of life in breast cancer patients: a cluster analytic approach. Empirically derived subgroups of the EORTC-QLQ BR 23--a clinically oriented assessment. Breast Cancer Res Treat. 2001;68:75–87. doi: 10.1023/a:1017975609835. [DOI] [PubMed] [Google Scholar]
- 24.McJunkin B, Fromm H, Sarva RP, Amin P. Factors in the mechanism of diarrhea in bile acid malabsorption: fecal pH--a key determinant. Gastroenterology. 1981;80:1454–1464. [PubMed] [Google Scholar]