Abstract
SEC23B is a component of coat protein complex II (COPII) vesicles that transport secretory proteins from the endoplasmic reticulum (ER) to the Golgi apparatus. Loss-of-function SEC23B mutations cause a rare form of anemia, resulting from decreased SEC23B levels. We recently identified germline heterozygous SEC23B variants as potentially cancer-predisposing. Mutant SEC23B associated with ER stress-mediated tumorigenesis, without decreased SEC23B expression. However, our understanding of the processes behind these observations remain limited. Here, we show mutant SEC23B exists within nucleoli, in addition to classical distribution at the ER/Golgi. This occurs independent of other COPII proteins and does not compromise secretory function. Mutant cells have increased ribosomal protein and translation-related gene expression, and enhanced translational capacity, in the presence of ER stress. We show that mutant SEC23B binds to UBF transcription factor, with increased UBF transcription factor binding at the ribosomal DNA promoter. Our data indicate SEC23B has potential non-canonical COPII-independent function, particularly within the ribosome biogenesis pathway, and that may contribute to the pathogenesis of cancer-predisposition.
Introduction
High-throughput and agnostic gene mutation screening, such as whole-exome and whole-genome sequencing, has been predicted to accelerate the discovery of previously unidentified genotype-phenotype associations (1). Relatedly, hereditary cancer syndromes serve as powerful models to uncover cancer-relevant genes. We utilized a combined exome sequencing cum family studies approach and identified SEC23B (MIM 610512) as a candidate cancer predisposition gene, specifically in the context of germline heterozygous variants in Cowden syndrome-associated and apparently sporadic thyroid cancer (2). Cowden syndrome (CS [MIM 158350]) is an underdiagnosed difficult-to-recognize autosomal dominant disorder characterized by multiple hamartomas and an increased lifetime risk of cancer, with epithelial thyroid carcinoma being a major clinical component (3).
SEC23B encodes Sec23 homolog B, a component of coat protein complex II (COPII). COPII vesicles function in the anterograde transport of proteins from the endoplasmic reticulum (ER) to the Golgi apparatus (4,5). Interestingly, germline loss-of-function homozygous or compound heterozygous SEC23B mutations cause an unrelated rare disorder, Congenital Dyserythropoietic Anemia Type II (CDA II [MIM 224100]) (6,7). Interestingly, in vivo studies show that SEC23B-deficient mice do not have CDAII but have secretory organ degeneration due to ER stress-associated apoptosis (8).
In a cancer context, vesicular trafficking and ER stress are considered major contributors to carcinogenesis (9,10). Functional characterization of the CS-related SEC23B p.Val594Gly variant in a non-malignant thyroid cell line, revealed that this mutation results in increased cell invasive potential, and ER stress-mediated cell colony formation, survival and growth (2). We have also previously reported that germline heterozygous SEC23B variants exist in up to 4% of individuals with apparently sporadic thyroid cancer from The Cancer Genome Atlas (TCGA) (2). These deleterious SEC23B variants are associated with a significantly elevated age-adjusted standardized incidence ratio (SIR) of thyroid cancer compared to the US general population (SIR 242.6; 95% CI 150.4–371.8; P = 10−11).
While our previous study provided genetic and functional evidence that SEC23B is associated with a cancer phenotype, the underlying process(s) remain unknown. Since the cancer-promoting heterozygous p.Val594Gly variant we studied did not affect SEC23B protein levels (2) as is observed in CDA II (11), we predicted that we are dealing with ‘change-of-function’ effects. In this context, the identification of the underlying processes leading to neoplastic transformation could point towards SEC23B-associated signaling pathways that are at play in thyroid cancer, or more generically in the process of carcinogenesis. Therefore, we sought to address the hypothesis that mutant SEC23B has potential non-canonical roles that could contribute to cancer-predisposition.
Results
Mutant SEC23B can exist in the nucleolus independent of COPII
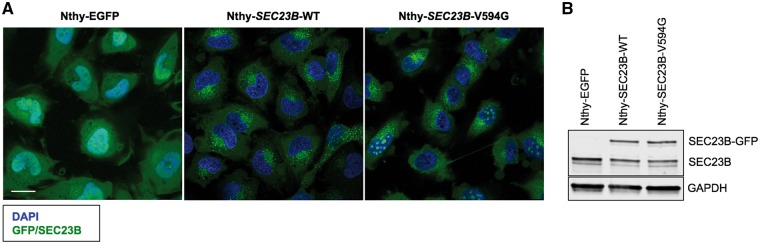
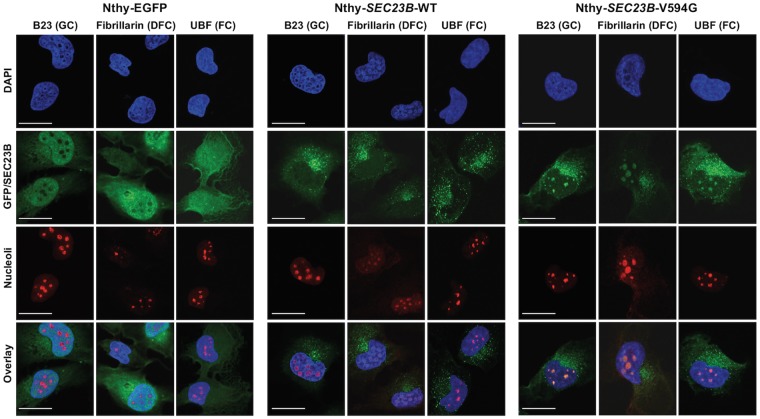
To functionally characterize the SEC23B p.Val594Gly identified in a CS family with predominant thyroid cancer, we utilized a non-malignant thyroid cell line model. By expressing EGFP-tagged wildtype or p.Val594Gly SEC23B (Nthy-SEC23B-WT or Nthy-SEC23B-V594G, respectively) in the parental thyroid follicule epithelial cell line Nthy-ori 3–1, we recapitulated the heterozygous state observed in the germline hereditary cancer context. As a control, we also expressed EGFP alone in the same parental cell line to generate Nthy-EGFP cells. The Nthy-SEC23B-WT cells showed normal ER-Golgi speckled expression pattern, whereas the Nthy-EGFP cells showed typical diffuse and non-specific global expression of GFP. Notably, we observed that subpopulations of Nthy-SEC23B-V594G mutant cells showed aberrant aggregation of SEC23B (2) and mislocalization within the nucleus (Fig. 1). Immunofluorescence staining of nucleolar markers representing the granular component (GC), dense fibrillar component (DFC) and fibrillar center (FC) confirmed localization of mutant SEC23B within all regions of cell nucleoli (Fig. 2).
Figure 1.
Mutant Nthy-SEC23B-V594G thyroid cells show aberrant aggregation of SEC23B within the nucleus. (A) Non-malignant thyroid Nthy-ori 3-1 cells were stably transduced with EGFP, wildtype SEC23B and mutant c.1781T>G (p.Val594Gly) SEC23B (the latter two fused with EGFP). Nthy-EGFP cells show typical diffuse expression of EGFP. Wildtype cells (Nthy-SEC23B-WT) show a speckled expression pattern typical of ER-Golgi proteins, while a subset of mutant cells (Nthy-SEC23B-V594G) show aberrant aggregation of SEC23B. Confocal images were taken using a TCS SP8 confocal microscope (Leica, Buffalo Grove, IL). Blue, DAPI; green, SEC23B-EGFP or EGFP. Scale bars, 25 μm. (B) Protein levels of transduced SEC23B-GFP, endogenous SEC23B and GAPDH (loading control) from the stable cell lines. The Nthy-EGFP cell line is used as a control to indicate baseline levels of SEC23B in the cell line (no SEC23B is introduced in these cells).
Figure 2.
Mutant SEC23B p.V594G protein can localize to nucleolar subcompartments of thyroid cells. Immunofluorescence staining of stably transduced Nthy-ori 3-1 thyroid cells with nucleolar protein markers. Abbreviations are as follows: GC, granular component; DFC, dense fibrillar component; FC, fibrillar center. Scale bars represent 25 µm.
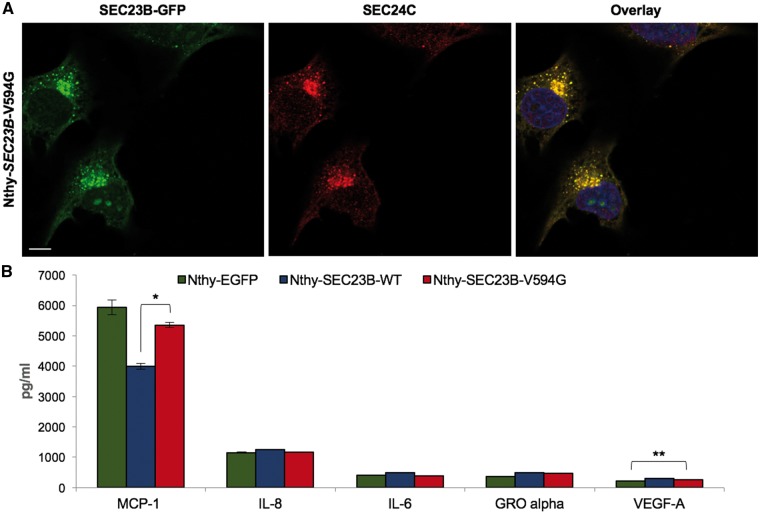
SEC23B is traditionally known to be exclusively localized to the ER-Golgi as a component of the coat protein complex II (COPII)-coated vesicles for ER to Golgi protein transport. We show that mutant SEC23B nucleolar presence is independent of SEC24, the COPII inner coat binding partner of SEC23 (12), and SEC31, the COPII outer coat binding partner of SEC23 (13) (Fig. 3A andSupplementary Material, Fig. S1). We next evaluated global secretory function in Nthy-EGFP, Nthy-SEC23B-WT and Nthy-SEC23B-V594G cells. However, we did not observe notable differences suggestive of gross secretory defects among these cell lines (Fig. 3B andSupplementary Material, Table S1). These data suggest that mutant SEC23B can exist in the nucleolus independent of COPII and without compromising global secretory function, supporting possible COPII-independent roles.
Figure 3.
SEC23B p.V594G can exist in the nucleolus independent of canonical COPII localization and function. (A) Immunofluorescence staining of Nthy-SEC23B-V594G cells with anti-SEC24C antibody and DAPI, followed by imaging with confocal microscopy. Scale bars represent 25 µm. (B) Multiplex analysis of secretome analytes extracted from supernatants derived from Nthy-EGFP, Nthy-SEC23B-WT and Nthy-SEC23B-V594G thyroid cells. Data represent analytes with concentrations ≥100 pg/ml. All other analytes are displayed in Supplementary Material, Table S1. Values represent two technical replicates for which data represent mean values ± SEM. **P < 0.01 and *P < 0.05 (two-sided Student’s t test).
Global transcriptomic analysis reveals ER stress adaptation phenotype in Nthy-SEC23B-V594G thyroid cells
Our data thus far revealed that mutant SEC23B can localize to the nucleolus independent of COPII. The nucleolus is considered as a cellular stress sensor and a central hub for orchestrating the stress response (14,15). We have previously reported that treatment of Nthy-SEC23B-WT and Nthy-SEC23B-V594G cells with the ER stress-inducing agents Thapsigargin and Tunicamycin resulted in increased colony formation and growth of mutant cells relative to wildtype (2). Since ER stress and downstream unfolded protein response (UPR) activation are at the crossroad of multiple hallmarks of cancer (10), we utilized RNA sequencing as an unbiased exploratory approach to determine signaling pathways that could explain the observed cellular phenotypes.
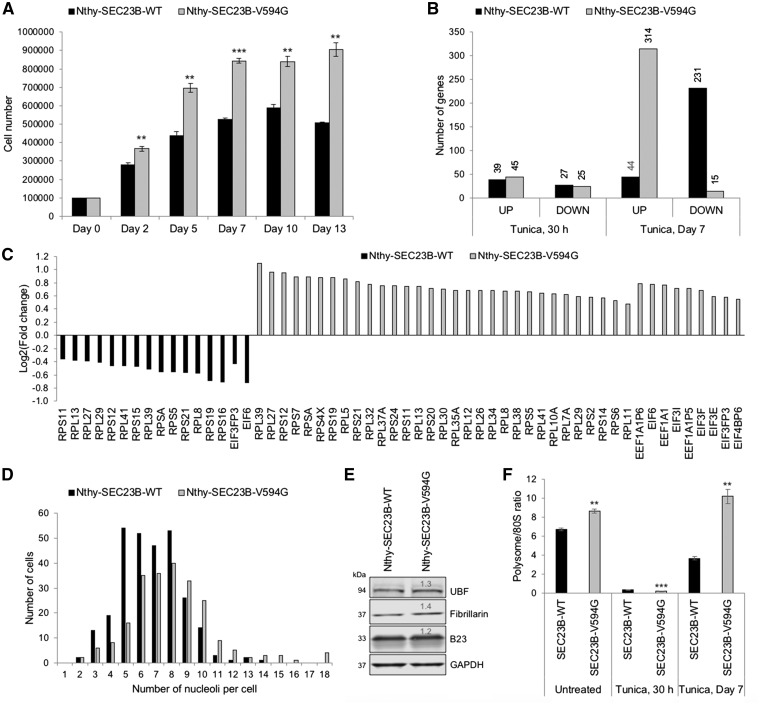
Analysis was done on total RNA isolated from Nthy-SEC23B-WT and Nthy-SEC23B-V594G cells, with and without Tunicamycin, after treatment for 30 h and 7 days. These timepoints represent an early response to ER stress (t = 30 h), and a later timepoint (t = 7 days) when genotype-specific pro-tumorigenic phenotypes are readily evident (Fig. 4A). At both timepoints, we compared the treatment response within each genotype to identify differentially expressed genes (FDR-corrected P < 0.05). With Tunicamycin treatment, the phenotypic differences were reflected in the direction of expression of differentially expressed genes in a time-dependent manner. At t = 30 h following treatment, both wildtype and mutant cells show a similar trend and number of differentially expressed genes (66 vs. 70 total, 27 vs. 25 underexpressed, respectively). At day 7, we observed an increase in the number of differentially expressed genes compared to the t = 30 h timepoint, and an inverse treatment response (231 vs. 44 underexpressed, 15 vs. 314 overexpressed, respectively) between wildtype and mutant cells (Fig. 4B).
Figure 4.
Global transcriptomic and polysome profiling analyses reveal altered translational capacity. (A) Wildtype Nthy-SEC23B-WT and mutant Nthy-SEC23B-V594G cells were seeded and allowed to grow overnight before treating with 0.1 μg/ml Tunicamycin. Cells were counted in triplicates at the represented timepoints. RNAseq analysis was done from cells harvested at t = 30 h (no differences in growth between wildtype and mutant) and at day 7 when differences are evident. **P < 0.01, ***P < 0.001 (two-sided Student’s t test). (B) Global changes in gene expression between Nthy-SEC23B-WT and Nthy-SEC23B-V594G cells after treatment with 0.1 µg/ml Tunicamycin for 30 h or 7 days. Tunica, tunicamycin; UP, differentially overexpressed genes; DOWN, differentially underexpressed genes. (C) Plot representing Log2 fold changes of ribosomal protein and eukaryotic translation initiation and elongation factors with differential expression within the EIF2 pathway. Canonical pathway analysis was done through Ingenuity Pathway Analysis (IPA). (D) Wildtype Nthy-SEC23B-WT and mutant Nthy-SEC23B-V594G cells were fixed and immunofluorescently stained with Nucleophosmin/B23 to count nucleoli. Approximately 250 cells were counted per genotype. Mutant cells show an increased number of nucleoli per cell (P < 0.001, two-sided Student’s t test). Representative of two independent biological replicates. (E) Western blot analysis of representative nucleolar markers show increased nucleolar mass in mutant cells compared to wildtype. GAPDH is used as a loading control for normalization. Quantification values are relative to wildtype. (F) Polysome profiling of untreated and Tunicamycin-treated (0.1 µg/ml) wildtype Nthy-SEC23B-WT and mutant Nthy-SEC23B-V594G cells. Mutant cells show apparent enhanced adaptation to the ER stress stimulus at day 7, evident through an increased polysome/80S ratio compared to wildtype. Values represent three technical replicates for which data represent mean values ± SEM. Comparisons are done between wildtype and mutant cells within each condition. ***P < 0.001, **P < 0.01 (two-sided Student’s t test).
To determine biological pathways that could be impacted by the differentially expressed genes, we used Ingenuity Pathway Analysis (IPA). We specifically looked for shared pathways impacted by Tunicamycin treatment across the two genotypes at each timepoint. At t = 30 h, top pathways include upregulated unfolded protein response and endoplasmic reticulum stress pathway, as appropriate, indicating both wildtype and mutant cells are responding to the ER stress stimulus (Supplementary Material, Table S2). At day 7, top pathways include EIF2 signaling and mitochondrial dysfunction/oxidative phosphorylation, with completely inverse direction of response between wildtype and mutant cells (Supplementary Material, Table S3). We also observed an overexpression of genes involved in the mTOR pathway, reflecting the increased growth phenotype in mutant cells. Interestingly, although not statistically significant, while wildtype cells show a persistent upregulation of the unfolded protein response, mutant cells show underexpression of genes within this pathway. Thus, our data here corroborate the ER stress adaptation phenotype in SEC23B-mutant cells.
Altered EIF2 signaling pathway and translational capacity in Nthy-SEC23B-V594G thyroid cells
Given our initial observations that SEC23B-V594G can localize to the nucleoli (Figs 1 and 2), we were intrigued by the observation that the top impacted pathway at day 7 is the EIF2 signaling pathway. This pathway includes a set of genes encoding ribosomal proteins and eukaryotic translation initiation and elongation factors (Fig. 4C). Indeed, the earliest hallmark of cancer cells was described as an increase in the size and number of nucleoli, the cells’ ribosome biogenesis factories (16). To functionally interrogate whether the observed gene expression changes in the EIF2 signaling pathway could be translated into observable cellular phenotypic differences, we first investigated nucleolar number and mass. We observed an increased mean number of nucleoli per cell in Nthy-SEC23B-V594G mutant cells, with a significant number of cells showing 10 or more nucleoli relative to Nthy-SEC23B-WT cells (Fig. 4D). We also confirmed an overall increase in nucleolar mass in mutant relative to wildtype cells (Fig. 4E).
To ultimately determine whether such changes in nucleoli and the EIF2 signaling pathway are contributing to differences in ribosome number and/or translation status, we performed ribosome profiling. This method provides a snapshot of ribosome subunit distribution (40S, 60S and 80S monosomes, and polysome-bound 80S), with a decreased polysome/80S ratio indicating impaired translation initiation (17). In the absence of ER stress, we note a baseline increase in the polysome/80S ratio in mutant cells. In the presence of ER stress and activation of the UPR, global protein synthesis is typically attenuated to alleviate the ER load, in addition to other pro-survival mechanisms (18). Expectedly, both wildtype and mutant cells show suppression of the polysome/80S ratio after 30 h of Tunicamycin treatment (Fig. 4F andSupplementary Material, Fig. S2). Interestingly, the mutant cells show a more severe suppression at this timepoint, noted by an increased free 80S fraction and decreased polysome fraction (Supplementary Material, Fig. S2). At day 7 post Tunicamycin treatment, mutant cells show a higher polysome/80S ratio (at least equivalent to baseline levels) relative to wildtype (Fig. 4F), further mirroring the observed gene expression and cellular phenotypic differences at this timepoint.
Mutant SEC23B impacts ribosomal DNA (rDNA) transcription
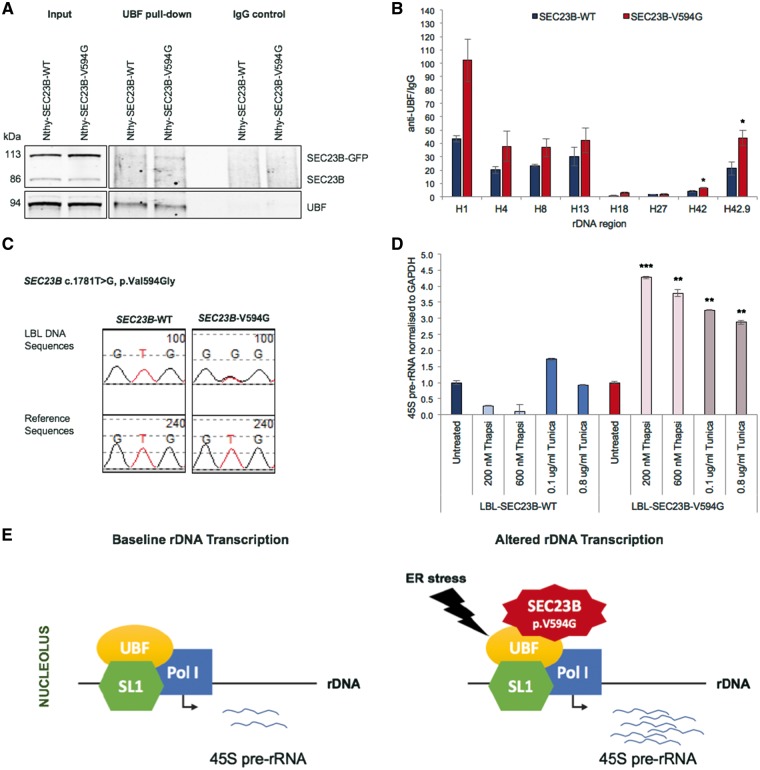
Given our functional data suggesting a role of SEC23B in protein translation and the ribosome biogenesis pathway, we next sought to determine whether mutant SEC23B preferentially localized to specific nucleolar subcompartments with ER stress, thus homing in on more specific functions. Cell nucleoli morphologically consist of three distinguishable regions: the granular component (GC), dense fibrillar component (DFC) and fibrillar center (FC) (19). These nucleolar subcompartments represent the sites of progressive stages of ribosomal RNA (rRNA) transcription from ribosomal DNA (rDNA), rRNA transcript processing and ribosome assembly prior to export to the cytoplasm (20). Immunofluorescence staining revealed non-preferential co-localization of mutant SEC23B with all three nucleolar subcompartments in the presence of ER stress (Fig. 5), suggesting a potential multi-faceted role in the ribosome biogenesis pathway. We then show through immunoprecipitation that mutant SEC23B binds to Upstream Binding Transcription Factor (UBF) (Fig. 6A), a key protein of the transcription pre-initiation complex, mediating RNA polymerase I (Pol I) recruitment to rDNA promoter regions (21). Importantly, we show through chromatin immunoprecipitation (ChIP) analysis enhanced UBF binding to rDNA loci in mutant cells, with significant differential UBF binding between wildtype and mutant cells at the region upstream of the promoter (H42) and promoter (H42.9) region (Fig. 6B andSupplementary Material, Fig. S3). These data provide one potential mechanism of how mutant SEC23B could be impacting the ribosome biogenesis and downstream signaling pathways.
Figure 5.
Mutant SEC23B p.V594G protein localizes to all nucleolar subcompartments in the presence of ER stress. Confocal microscopy images of Nthy-SEC23B-V594G cells stained with subnucleolar proteins after ER stress treatments (0.1 μg/ml Tunicamycin, day 7). Images were taken using a TCS SP8 confocal microscope (Leica). GC, granular component; DFC, dense fibrillar component; FC, fibrillar center. Blue, DAPI; green, SEC23B-EGFP; red, nucleolar subcompartment. Scale bars, 25 μm.
Figure 6.
Mutant SEC23B impacts rDNA transcription. (A) Immunoblot analysis of lysates immunoprecipitated using UBF antibody in Tunicamycin-treated Nthy-SEC23B-WT and Nthy-SEC23B-V594G cells (0.1 µg/ml, day 7). Mutant SEC23B p.V594G could be pulled down by UBF antibody. Data representative of two independent biological replicates. (B) ChIP analysis showing enrichment of UBF binding at different rDNA loci normalized to IgG negative control pull-down. DNA was quantitated by qPCR with primer sets specific for different rDNA regions (see Materials and Methods). Values represent three independent biological replicates for which data represent mean values ± SEM. *P < 0.05 (two-sided Student’s t test). (C) Genotyping of patient-derived lymphoblastoid cell line (LBL) pools. Each genotypic group includes five LBLs with either wildtype or p.V594G mutant SEC23B, respectively (see Materials and Methods). Chromatograms show heterozygous mutant state in mutant cells. (D) qRT-PCR analysis shows increased 45S pre-rRNA transcript expression in mutant cells relative to wildtype after treatment with different doses of Thapsigargin and Tunicamycin (day 5). Treatment experiments with Thapsigargin and Tunicamycin were performed independent of each other. Values are first normalized to GAPDH and then normalized to the untreated condition within each genotype. Representative of three technical replicates for which data represent mean values ± SEM. Significance comparisons are done across genotypes at each treatment condition. ***P < 0.001, **P < 0.01 (two-sided Student’s t test). (E) Proposed model of how mutant SEC23B could impact rDNA transcription. UBF, Upstream Binding Transcription Factor; Pol I, RNA Polymerase I; SL1, TATA-box binding protein associated factor.
SEC23B-ER stress-ribosome biogenesis axis is pertinent in Cowden syndrome patients
To determine whether our observations are pertinent in Cowden syndrome patients, we generated pools of control or patient-derived lymphoblastoid cell lines (LBLs, see Materials and Methods) that are wildtype for SEC23B (LBL-SEC23B-WT) or harboring SEC23B p.V594G heterozygous (LBL-SEC23B-V594G) mutation, respectively (Fig. 6C). We then utilized 45S pre-ribosomal RNA (pre-rRNA) transcript levels as a read-out for rDNA transcription. As expected, downstream 45S pre-rRNA transcript expression was increased in mutant LBL-SEC23B-V594G cells relative to wildtype in the presence of different doses of ER stress drugs Thapsigargin and Tunicamycin (Fig. 6D). These results validate our in vitro cell line observations that in the presence of ER stress, mutant SEC23B p.V594G is associated with enhanced ribosome biogenesis, which may be mediated at least in part through enhanced rDNA transcription (Fig. 6E).
Discussion
Our observations reveal mutant SEC23B p.V594G could localize to nucleoli, with multiple lines of evidence suggesting the observed ER stress-mediated tumorigenic cellular phenotypes are COPII-independent and downstream of the ribosome biogenesis pathway. Compared to wildtype SEC23B, mutant SEC23B cells showed increased expression of ribosomal protein genes and translation-related factors, and enhanced translational capacity in the presence of ER stress. To home in on the underlying mechanism, we sought to determine whether SEC23B localizes to particular nucleolar subcompartments. However, we found that SEC23B exists in all three nucleolar subcompartments, hinting at more generalized roles in the ribosome biogenesis pathway. We show one possible example by which mutant SEC23B binds to UBF to enhance downstream rDNA transcription.
Intriguingly, the ribosome biogenesis pathway is known to be orchestrated at different stages by multiple tumor suppressor and oncogenic proteins (22). Relevant to Cowden syndrome (CS), the tumor suppressor PTEN, known to be mutated in the germline of ∼25% of CS/CS-like individuals, has been shown to be a master regulator of the ribosome biogenesis pathway, among many signaling pathways. Indeed, we and others have primarily shown that PTEN, classically believed to be an exclusively cytoplasmic protein, also exists and functions within the nucleus (23–27). PTEN has also been shown to exist in the nucleolus, impacting nucleolar morphology (28) and repressing Pol I-mediated transcription of rDNA (29). More recently, Liang et al. (30) showed that a specific PTEN isoform, dubbed PTENβ, localizes predominantly to the nucleolus, dephosphorylates nucleolin and negatively regulates rDNA transcription and cellular proliferation. The involvement of both PTEN and SEC23B in the ribosome biogenesis pathway could create functional crosstalk between both proteins. Indeed, since both PTEN and SEC23B are Cowden syndrome susceptibility genes, it is intriguing to find that these two apparently and functionally disparate genes, encode proteins with converging and similar downstream effects within the ribosome biogenesis pathway. The functional interplay between PTEN and SEC23B warrants further investigation, with broader implications on cancer risk in CS patients.
Our observations also offer an important glimpse into heritable gene mutations interacting with the environment (e.g. exposure to ER stress) to result in a particular disease phenotype (here, cancer)—and hence, genetic predisposition to cancer. While SEC23B wildtype and mutant cells show some baseline differences such as SEC23B localization, nucleolar number/mass and translational capacity, the introduction of the stress stimuli result in more prominent phenotypic differences. While SEC23B wildtype cells activate expected pro-apoptotic signaling pathways, SEC23B p.V594G mutant cells adapt to the chronic stress and reflect multiple tumorigenic phenotypes. Ultimately, it may be informative to investigate the tumorigenic effects of SEC23B in the context of other germline heterozygous variants we previously identified (2) and in other cancer types relevant to Cowden syndrome (3). This is particularly intriguing since even in the somatic cancer context, copy number amplifications in SEC23B are observed in Cowden syndrome component cancers (3), such as breast, endometrial and colorectal cancers and melanoma. Interestingly, this preliminary data also reveal a significant tendency towards co-occurrence of somatic PTEN and SEC23B alterations in the CS-related apparently sporadic cancers derived from The Cancer Genome Atlas, TCGA (Supplementary Material, Fig. S4). The latter data further support potential PTEN-SEC23B crosstalk in cancer. Relatedly, whether increased copy number results in increased expression or tilting COPII complex-related SEC23B stoichiometry towards non-canonical function is one possible hypothesis worth testing in the future.
SEC23B has not been previously reported to exist or function outside of its role in COPII-mediated anterograde protein transport at the ER-Golgi interface. Interestingly, one study has shown that Sec13, a component of the outer COPII coat, can also shuttle to the nucleus, with a subpopulation stably interacting with the nuclear pore complex (NPC) protein Nup96 (31). A genetic in vivo model developed years later demonstrated the distinct roles of Sec13 at both compartments, COPII and the NPC (32). Loss of COPII function results in digestive organ defects, whereas loss of NPC function resulted in retinal lamination defects. These data provide evidence for existing dual and tissue/context-specific roles of COPII proteins. Whether mutant SEC23B functions as a stress sensor or effector warrants further experimentation. Importantly, it will also be crucial to determine whether wildtype SEC23B plays a baseline role in the ribosome biogenesis pathway, with the mutant p.Val594G cells reflecting exaggerated effects within this pathway. This hypothesis is currently under investigation.
SEC23B has been classically and extensively studied in the context of CDA II (6–8,33). But even in the anemia field, despite extensive efforts, it remains unknown what the cargo(es) responsible for CDA II is(are), nor the precise mechanism. Indeed, it is thought that the cell cycle and cytokinesis of erythroid cells are dependent on sufficient SEC23B protein (6). We speculate that the loss of non-canonical SEC23B function could also be, at least in part, contributing to the pathobiology of this disease. In parallel, ribosomopathies characterized by ribosomal haploinsufficiency have been implicated in multiple congenital disorders with component anemias (e.g. Diamond-Blackfan anemia, DBA). Intriguingly, patients with ribosomal dysfunction have also been reported to have significantly increased lifetime risks of certain cancers (34,35). Such a paradoxical transition from a hypoproliferative phenotype (anemia, cytopenia) to a hyperproliferative state (cancer) is known in the hematology world as ‘Dameshek’s riddle,’ which speculates that the initial insult resulting in ribosomal haploinsufficiency could select for cells that acquire compensatory mutations to resist and bypass impaired proliferation (survival of the fittest) (36). Therefore, relevant to SEC23B-related CDA II and CS-related cancers, we question whether such downstream ribosome biogenesis regulation is at the heart of such disparate phenotypes. Overall, we report that SEC23B has largely unexplored non-canonical function, particularly within the ribosome biogenesis pathway (Fig. 7). This has far-reaching implications relevant to cancer and beyond that, CDAII and normal cell biology.
Figure 7.
Non-canonical COPII-independent Role of mutant SEC23B in the ribosome biogenesis pathway.
Materials and Methods
Cell lines and culture conditions
The Nthy-ori 3-1 human thyroid follicular epithelium cell line (catalog number EC90011609, lot number 09C008, passage number 16, purchased in 2014 from Sigma-Aldrich, St. Louis, MO) was cultured in RPMI-1640 supplemented with 2 mM glutamine and 10% fetal bovine serum (FBS). Immortalized lymphoblastoid cell lines (LBLs) derived from patients or controls were generated by the Genomic Medicine Biorepository of the Genomic Medicine Institute of the Cleveland Clinic (Cleveland, OH) according to standard procedures and subsequently maintained in RPMI-1640 supplemented with 20% fetal bovine serum (FBS) and 1% penicillin/streptomycin. LBL pools were generated by co-culturing an equal number of cells from each patient or control. All cell lines were maintained at 37°C and 5% CO2 culture conditions and tested negative upon routine mycoplasma testing at the Eng lab (luminescence ratios < 0.9) using the MycoAlert Mycoplasma Detection Kit (Lonza, Allendale, NJ). Cell lines used have not been listed as cells known to be misidentified according to the International Cell Line Authentication Committee (ICLAC) (37). The Nthy-ori 3-1 cell line was authenticated through STR PCR (AmpFLSTR SGM Plus PCR Amplification Kit, Life Technologies) by the European Collection of Cell Cultures (ECACC, original source of the cell line, test date 14/04/2009). To generate stable cell lines, retrovirally transduced Nthy-ori 3-1 cells (pool as no individual clones were isolated) were kept under 1 µg/ml Puromycin selection for >30 days prior to downstream interrogation. We used four different pools of cells at passage number 28 after selection. All experiments were conducted using cells at passage numbers between 29 and 42 (2).
Immunofluorescence
Cells were seeded on coverslips, grown overnight, and then fixed with 4% paraformaldehyde for 5 min at room temperature. We permeabilized the cells with 0.1% Triton-X for 4 min and blocked with 10% goat serum for 1 h, both at room temperature. Primary antibodies were diluted in 10% goat serum and incubated overnight at 4°C in a humid chamber. Primary antibodies used include anti-SEC24C rabbit monoclonal (Cell Signaling #14676) at 1:100, anti-SEC31A rabbit monoclonal (Cell Signaling #13466) at 1:400, anti-UBF mouse monoclonal (Santa Cruz sc-13125) at 1:200, anti-Nucleophosmin/B23 (Abcam ab10530) mouse monoclonal at 1:500, anti-Fibrillarin rabbit monoclonal (Cell Signaling # 2639) at 1:400 dilutions. We used the Alexa 555 goat anti-mouse (Cell Signaling #4409) or goat anti-rabbit (Cell Signaling #4413) at 1:1000 dilution for 1 h at room temperature. Coverslips were mounted using ProLong Gold Antifade mountant with DAPI (Invitrogen, Carlsbad, CA, USA). Slides were visualized and images obtained using a laser scanning confocal TCS SP8 microscope controlled by the Leica Application Suite Software (Leica, Buffalo Grove, IL, USA). All images were obtained using a 63× oil immersion objective and 405, 488 and 594 nm lasers.
Multiplex analysis of gross secretory function
Cells were seeded and allowed to grow for 36 h prior to isolating the supernatant, representing the secretome. Supernatants were centrifuged at 3000g for 5 min, aliquoted and shipped to Eve Technologies (Calgary, AB, Canada) for downstream analysis. We used the 64-Plex Human Cytokine Discovery Assay performed using the Bio-PlexTM 200 system (Bio-Rad Laboratories, Hercules, CA, USA) and a Milliplex Human Cytokine kit (Millipore, St. Charles, MO, USA). Samples were measured in duplicates for each analyte. Analytes of low concentrations that resulted in low signal were excluded from the analysis. This resulted in a total of 36 analyzable analytes (Supplementary Material, Table S1).
RNA isolation and RNA sequencing
Total RNA was extracted from untreated and Tunicamycin-treated Nthy-SEC23B WT and Nthy-SEC23B V594G cells using the RNeasy Mini kit (Qiagen, Germantown, MD, USA), and then purified using Turbo DNase treatment (Life Technologies, Grand Island, NY, USA). Long-term experiments (day 7 treatments) were done using three biological replicates for each genotype and at each time point. The 30-h exploratory experiment was done using one sample for each genotype at each time point. RNA quality was assessed via the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Sequencing libraries were prepared using the Illumina TruSeq Stranded Total RNA with Ribo Zero kit (Illumina, San Diego, CA, USA). Libraries were sequenced (100 bp, paired-end) using the Illumina HiSeq platform. Sequencing was performed at the Genomics Core of Case Western Reserve University School of Medicine (Cleveland, OH, USA) and the Broad Institute Genomic Services (Cambridge, MA, USA).
Read mapping and differential gene expression analysis
Read mapping and gene expression quantification were performed as we previously reported (38). Sequencing reads were aligned to the reference human transcriptome (Genome Reference Consortium human genome build 37, hg19) using Tophat/Bowtie and then assembled into RNA transcripts using the Cufflinks package (39,40). Following computation of FPKM (Fragments Per Kilobase of transcript per Million mapped reads) values, we calculated differential gene expression using Cuffdiff 2. We compared the treatment response per genotype (Tunicamycin relative to DMSO untreated mock control), at each time point (30 h and day 7). Transcripts were considered differentially expressed at q < 0.05 following Benjamini-Hochberg multiple testing correction of the original P values (39). We performed pathway analysis using Ingenuity Pathway Analysis (IPA, QIAGEN Bioinformatics, Redwood City, CA, USA). P values derived from IPA indicate significance after Benjamini-Hochberg multiple testing correction.
Quantification of nucleolar number and mass
We performed immunofluorescence staining of nucleophosmin/B23 (same as immunofluorescence protocol above) in Nthy-SEC23B-WT and Nthy-SEC23B-V594G cells. Nucleophosmin/B23 marks the granular component (GC) of nucleoli. DAPI staining was used to demarcate nuclei. Slides were visualized and images obtained using a Leica DMI3000B manual inverted microscope (Leica, Buffalo Grove, IL, USA). We counted on average approximately 250 cells per genotype, and counting was done on two independent biological replicates.
For quantification of nucleolar mass, we performed Western blot analysis. We extracted protein using the Mammalian Protein Extraction Reagent M-PER (Thermo Scientific Pierce, Rockford, IL, USA) supplemented with a cocktail of protease and phosphatase inhibitors (Sigma-Aldrich, St. Louis, MO, USA) and quantified through the BCA protein assay (Thermo Scientific Pierce). Cell lysates were separated by SDS-PAGE and transferred onto nitrocellulose membranes. We probed for anti-UBF mouse monoclonal (Santa Cruz sc-13125) at 1:500, anti-Nucleophosmin/B23 (Abcam ab10530) mouse monoclonal at 1:1000, anti-Fibrillarin rabbit monoclonal (Cell Signaling # 2639) at 1:1000 and anti-GAPDH rabbit monoclonal (Cell Signaling #2118) at 1:20 000 dilutions. Blots were scanned digitally and quantified using the Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE, USA).
Ribosome profiling
Ribosome fractionation was done as previously described (41). Cells were treated with 100 μg/ml Cycloheximide (Sigma-Aldrich) for 15 min at 37°C before harvesting. Whole cell lysates were prepared from lysis buffer containing 10 mM HEPES-KOH pH 7.9, 2.5 mM MgCl2, 100 mM KCl, 1 mM DTT, 0.1% NP-40, 100 Units/ml RNase inhibitor, and 100 μg/ml Cycloheximide. Cell suspensions were homogenized by passing through 25 gauge syringes. Cell debris was removed by centrifugation at 13 000 rpm for 10 min at 4°C. Polyribosomes, ribosomes, and subunits were resolved by loading equivalent optical density (OD260) units onto 10–50% sucrose gradients containing 10 mM HEPES-KOH pH 7.4, 2.5 mM MgCl2, 100 mM KCl and 1 mM DTT. Gradient centrifugation was done at 18 000 rpm for 16 h, using a Beckman SW32.1 rotor (Beckman Coulter, Brea, CA, USA). Gradients were collected using the ISCO Programmable Density Gradient system with continuous monitoring at 254 nm using an ISCO UA-6 absorbance detector (Teledyne ISCO, Lincoln, NE, USA). Data were recorded and processed with PeakTrak V1.1 (Teledyne ISCO). For each gradient, 24 fractions with 750 μl each were collected. Ribosome profiles were graphed using ORIGIN Graphing and Analysis software program (OriginLab, Northampton, MA, USA) and the area under the curve determined for 40S, 60S, 80S and polysome fraction peaks.
ER stress induction and assessment of cell viability
Cells were seeded and allowed to grow overnight before being treated with different doses of Tunicamycin or Thapsigargin (Sigma-Aldrich), as we have previously reported (2). At each timepoint, adherent thyroid cells were trypsinized, gently homogenized, and counted from three independent wells (at least in three technical replicates) using the Countess automated cell counter (Invitrogen). We used Trypan blue to account for dead cells and assess cell viability.
RNA extraction and 45S pre-rRNA qRT-PCR
Total RNA was extracted with the GeneJET RNA Purification Kit (Thermo Fisher Scientific, Waltham, MA, USA), purified with Turbo DNase treatment (Life Technologies), and reverse transcribed with Superscript III Reverse Transcriptase (Life Technologies), all according to standard protocols provided in each kit. Extracted RNA was kept on ice during cDNA preparation. Unused RNA was stored at −80°C. RNA quality and concentration were assessed using NanoDrop 1000 spectrophotometer, with all RNA samples yielding a A260/A280 ratio of ∼2. Primers for 45S pre-rRNA were derived from Liang et al. (30) (Supplementary Material, Table S4). We utilized GAPDH as a housekeeping gene for each sample. We quantified the cDNA with SYBR Green (Life Technologies). Standard cycling parameters were 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. We utilized the Applied Biosystems 7500 Real-Time PCR System. Results were analyzed by the standard ΔΔCT method.
Immunoprecipitation
Cells were pelleted and lysed with M-PER (Thermo Scientific Pierce) supplemented with a cocktail of protease and phosphatase inhibitors (Sigma-Aldrich). Protein lysates were collected by centrifugation at 13 000 rpm for 10 min at 4°C and pre-cleared by incubation with Thermo Protein A/G Dynabeads for 3 h at 4°C on a rotator. Pre-cleared protein lysates were quantified with the BCA Protein Assay Kit (Thermo Scientific Pierce), and 1 mg/ml lysates were prepared. We used anti-GFP (Abcam ab290), anti-SEC23B (Abcam ab151258) and anti-UBF (Santa Cruz sc-13125) antibodies for pull-down and immunoblotting at the recommended dilutions. Cell lysates were separated by SDS-PAGE and transferred onto nitrocellulose membranes. Blots were scanned digitally and quantified using the Odyssey Infrared Imaging System (Li-Cor Biosciences).
Chromatin immunoprecipitation (ChIP) analysis
We performed ChIP using the EZ-ChIP Immunoprecipitation kit (Millipore, Billerica, MA, USA), as we previously reported (42). We sheared chromatin using the S220 Focused-ultrasonicator (Covaris, Woburn, MA, USA). To immunoprecipitate cross-linked protein-DNA complexes, we used 5 µg anti-UBF mouse monoclonal (Santa Cruz sc-13125) and 1 µg normal mouse IgG (Cell Signaling #5415). Following kit protocol instructions, complexes were eluted, DNA-protein cross-links reversed and the DNA purified and collected. Primers for ribosomal DNA (rDNA) were derived from Grandori et al. (43) (Supplementary Material, Table S4). Input chromatin DNA and DNA from normal IgG antibody pull-down were used as controls with each primer pair. Fold enrichment was calculated relative to background pull-down with the non-specific negative control IgG antibody for each primer set.
Statistical analyses
Experimental data between wildtype and mutant cell lines are given as means ± standard error of the mean (SEM), with n corresponding to the number of experiments or biological replicates performed. The Student’s t test was used for significance testing as indicated in figure legends. All statistical tests were two-sided, and P values <0.05 deemed significant.
Data Availability
RNA-seq data have been deposited into NCBI Gene Expression Omnibus (GEO) with accession number GSE112661.
Supplementary Material
Acknowledgements
We are grateful to our patients who contributed to this study. We thank the Genomic Medicine Biorepository of the Cleveland Clinic Genomic Medicine Institute, and our database and clinical research teams. We also thank Dr. George Stark, Dr. Alan Tartakoff, Dr. Ying Ni, Dr. Madhav Sankunny and Dr. Ata Abbas for critical discussions, and Farshad Niazi for bioinformatic assistance. This work was supported in part by the National Cancer Institute [P01CA124570, R01CA118989], American Cancer Society [RPG-02-151-01-CCE, Clinical Research Professorship]; Breast Cancer Research Foundation; William Randolph Hearst Foundations; and Doris Duke Distinguished Clinical Scientist Award [all to C.E.]. This work utilized the Leica SP8 confocal microscope purchased with funding from National Institutes of Health (NIH) shared instrument grant (SIG) grant [1S10OD019972–01], and a Fusion Lumos instrument [1S10OD023436-01]. L.Y. is an Ambrose Monell Foundation Cancer Genomic Medicine Fellow at the Cleveland Clinic Genomic Medicine Institute and was an International Fulbright Science and Technology Doctoral Fellow at the Cleveland Clinic Genomic Medicine Institute and recipient of the Dr. Michael H. Fakih Pre-Doctoral Scholarship. C.E. is the Sondra J. and Stephen R. Hardis Chair of Cancer Genomic Medicine at the Cleveland Clinic and an ACS Clinical Research Professor.
Conflict of Interest statement: None declared.
Funding
Funding to pay the Open Access publication charges for this article was provided by the American Cancer Society.
References
- 1. Boycott K.M., Vanstone M.R., Bulman D.E., MacKenzie A.E. (2013) Rare-disease genetics in the era of next-generation sequencing: discovery to translation. Nat. Rev. Genet., 14, 681–691. [DOI] [PubMed] [Google Scholar]
- 2. Yehia L., Niazi F., Ni Y., Ngeow J., Sankunny M., Liu Z., Wei W., Mester J.L., Keri R.A., Zhang B.. et al. (2015) Germline heterozygous variants in SEC23B are associated with cowden syndrome and enriched in apparently sporadic thyroid cancer. Am. J. Hum. Genet., 97, 661–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tan M.H., Mester J.L., Ngeow J., Rybicki L.A., Orloff M.S., Eng C. (2012) Lifetime cancer risks in individuals with germline PTEN mutations. Clin. Cancer Res., 18, 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barlowe C., Orci L., Yeung T., Hosobuchi M., Hamamoto S., Salama N., Rexach M.F., Ravazzola M., Amherdt M., Schekman R. (1994) COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell, 77, 895–907. [DOI] [PubMed] [Google Scholar]
- 5. Fromme J.C., Orci L., Schekman R. (2008) Coordination of COPII vesicle trafficking by Sec23. Trends Cell Biol., 18, 330–336. [DOI] [PubMed] [Google Scholar]
- 6. Schwarz K., Iolascon A., Verissimo F., Trede N.S., Horsley W., Chen W., Paw B.H., Hopfner K.P., Holzmann K., Russo R.. et al. (2009) Mutations affecting the secretory COPII coat component SEC23B cause congenital dyserythropoietic anemia type II. Nat. Genet., 41, 936–940. [DOI] [PubMed] [Google Scholar]
- 7. Bianchi P., Fermo E., Vercellati C., Boschetti C., Barcellini W., Iurlo A., Marcello A.P., Righetti P.G., Zanella A. (2009) Congenital dyserythropoietic anemia type II (CDAII) is caused by mutations in the SEC23B gene. Hum. Mutat., 30, 1292–1298. [DOI] [PubMed] [Google Scholar]
- 8. Tao J., Zhu M., Wang H., Afelik S., Vasievich M.P., Chen X.W., Zhu G., Jensen J., Ginsburg D., Zhang B. (2012) SEC23B is required for the maintenance of murine professional secretory tissues. Proc. Natl. Acad. Sci. U S A, 109, E2001–E2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldenring J.R. (2013) A central role for vesicle trafficking in epithelial neoplasia: intracellular highways to carcinogenesis. Nat. Rev. Cancer, 13, 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Urra H., Dufey E., Avril T., Chevet E., Hetz C. (2016) Endoplasmic reticulum stress and the hallmarks of cancer. Trends Cancer, 2, 252–262. [DOI] [PubMed] [Google Scholar]
- 11. Punzo F., Bertoli-Avella A.M., Scianguetta S., Della Ragione F., Casale M., Ronzoni L., Cappellini M.D., Forni G., Oostra B.A., Perrotta S. (2011) Congenital dyserythropoietic anemia type II: molecular analysis and expression of the SEC23B gene. Orphanet. J. Rare Dis., 6, 89.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bi X., Corpina R.A., Goldberg J. (2002) Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature, 419, 271–277. [DOI] [PubMed] [Google Scholar]
- 13. Bi X., Mancias J.D., Goldberg J. (2007) Insights into COPII coat nucleation from the structure of Sec23.Sar1 complexed with the active fragment of Sec31. Dev. Cell, 13, 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boulon S., Westman B.J., Hutten S., Boisvert F.M., Lamond A.I. (2010) The nucleolus under stress. Mol. Cell, 40, 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grummt I. (2013) The nucleolus-guardian of cellular homeostasis and genome integrity. Chromosoma, 122, 487–497. [DOI] [PubMed] [Google Scholar]
- 16. Ruggero D. (2012) Revisiting the nucleolus: from marker to dynamic integrator of cancer signaling. Sci. Signal., 5, pe38.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghosh A., Jindal S., Bentley A.A., Hinnebusch A.G., Komar A.A. (2014) Rps5-Rps16 communication is essential for efficient translation initiation in yeast S. cerevisiae. Nucleic Acids Res., 42, 8537–8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hetz C. (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol., 13, 89–102. [DOI] [PubMed] [Google Scholar]
- 19. Lam Y.W., Trinkle-Mulcahy L., Lamond A.I. (2005) The nucleolus. J. Cell Sci., 118, 1335–1337. [DOI] [PubMed] [Google Scholar]
- 20. Boisvert F.M., van Koningsbruggen S., Navascues J., Lamond A.I. (2007) The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol., 8, 574–585. [DOI] [PubMed] [Google Scholar]
- 21. Bywater M.J., Pearson R.B., McArthur G.A., Hannan R.D. (2013) Dysregulation of the basal RNA polymerase transcription apparatus in cancer. Nat. Rev. Cancer, 13, 299–314. [DOI] [PubMed] [Google Scholar]
- 22. Quin J.E., Devlin J.R., Cameron D., Hannan K.M., Pearson R.B., Hannan R.D. (2014) Targeting the nucleolus for cancer intervention. Biochim. Biophys. Acta, 1842, 802–816. [DOI] [PubMed] [Google Scholar]
- 23. Weng L.P., Brown J.L., Eng C. (2001) PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum. Mol. Genet., 10, 599–604. [DOI] [PubMed] [Google Scholar]
- 24. Weng L.P., Smith W.M., Brown J.L., Eng C. (2001) PTEN inhibits insulin-stimulated MEK/MAPK activation and cell growth by blocking IRS-1 phosphorylation and IRS-1/Grb-2/Sos complex formation in a breast cancer model. Hum. Mol. Genet., 10, 605–616. [DOI] [PubMed] [Google Scholar]
- 25. Lobo G.P., Waite K.A., Planchon S.M., Romigh T., Houghton J.A., Eng C. (2008) ATP modulates PTEN subcellular localization in multiple cancer cell lines. Hum. Mol. Genet., 17, 2877–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen W.H., Balajee A.S., Wang J., Wu H., Eng C., Pandolfi P.P., Yin Y. (2007) Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell, 128, 157–170. [DOI] [PubMed] [Google Scholar]
- 27. Bassi C., Ho J., Srikumar T., Dowling R.J., Gorrini C., Miller S.J., Mak T.W., Neel B.G., Raught B., Stambolic V. (2013) Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science, 341, 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li P., Wang D., Li H., Yu Z., Chen X., Fang J. (2014) Identification of nucleolus-localized PTEN and its function in regulating ribosome biogenesis. Mol. Biol. Rep., 41, 6383–6390. [DOI] [PubMed] [Google Scholar]
- 29. Zhang C., Comai L., Johnson D.L. (2005) PTEN represses RNA Polymerase I transcription by disrupting the SL1 complex. Mol. Cell Biol., 25, 6899–6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liang H., Chen X., Yin Q., Ruan D., Zhao X., Zhang C., McNutt M.A., Yin Y. (2017) PTENbeta is an alternatively translated isoform of PTEN that regulates rDNA transcription. Nat. Commun., 8, 14771.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Enninga J., Levay A., Fontoura B.M. (2003) Sec13 shuttles between the nucleus and the cytoplasm and stably interacts with Nup96 at the nuclear pore complex. Mol. Cell. Biol., 23, 7271–7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niu X., Hong J., Zheng X., Melville D.B., Knapik E.W., Meng A., Peng J. (2014) The nuclear pore complex function of Sec13 protein is required for cell survival during retinal development. J. Biol. Chem., 289, 11971–11985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khoriaty R., Vasievich M.P., Jones M., Everett L., Chase J., Tao J., Siemieniak D., Zhang B., Maillard I., Ginsburg D. (2014) Absence of a red blood cell phenotype in mice with hematopoietic deficiency of SEC23B. Mol. Cell Biol., 34, 3721–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vlachos A., Rosenberg P.S., Atsidaftos E., Alter B.P., Lipton J.M. (2012) Incidence of neoplasia in Diamond Blackfan anemia: a report from the Diamond Blackfan Anemia Registry. Blood, 119, 3815–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Narla A., Ebert B.L. (2010) Ribosomopathies: human disorders of ribosome dysfunction. Blood, 115, 3196–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Keersmaecker K., Sulima S.O., Dinman J.D. (2015) Ribosomopathies and the paradox of cellular hypo- to hyperproliferation. Blood, 125, 1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Capes-Davis A., Theodosopoulos G., Atkin I., Drexler H.G., Kohara A., MacLeod R.A., Masters J.R., Nakamura Y., Reid Y.A., Reddel R.R.. et al. (2010) Check your cultures! A list of cross-contaminated or misidentified cell lines. Int. J. Cancer, 127, 1–8. [DOI] [PubMed] [Google Scholar]
- 38. Tilot A.K., Bebek G., Niazi F., Altemus J.B., Romigh T., Frazier T.W., Eng C. (2016) Neural transcriptome of constitutional Pten dysfunction in mice and its relevance to human idiopathic autism spectrum disorder. Mol. Psychiatry, 21, 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trapnell C., Hendrickson D.G., Sauvageau M., Goff L., Rinn J.L., Pachter L. (2013) Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol., 31, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D.R., Pimentel H., Salzberg S.L., Rinn J.L., Pachter L. (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc., 7, 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Komar A.A., Gross S.R., Barth-Baus D., Strachan R., Hensold J.O., Goss Kinzy T., Merrick W.C. (2005) Novel characteristics of the biological properties of the yeast Saccharomyces cerevisiae eukaryotic initiation factor 2A. J. Biol. Chem., 280, 15601–15611. [DOI] [PubMed] [Google Scholar]
- 42. Nizialek E.A., Sankunny M., Niazi F., Eng C. (2016) Cancer-predisposition gene KLLN maintains pericentric H3K9 trimethylation protecting genomic stability. Nucleic Acids Res., 44, 3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grandori C., Gomez-Roman N., Felton-Edkins Z.A., Ngouenet C., Galloway D.A., Eisenman R.N., White R.J. (2005) c-Myc binds to human ribosomal DNA and stimulates transcription of rRNA genes by RNA polymerase I. Nat. Cell Biol., 7, 311–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data have been deposited into NCBI Gene Expression Omnibus (GEO) with accession number GSE112661.