Abstract
Background: Tissue plasminogen activator (tPA) has been long approved as an efficacious treatment in patients with acute ischemic stroke (AIS); however, due to some serious complications, particularly intracranial hemorrhage (ICH), many physicians are still reluctant to use it liberally. This study sought to find potential prognostic factors in patients with AIS treated with tPA.
Methods: A retrospective, hospital-bases observational study was conducted. Consecutively, a total of 132 patients with AIS treated with intravenous tPA, form June 2011 to July 2015 were enrolled. Inclusion and exclusion criteria were based on updated guidelines. Probable prognostic variables were examined separately in three distinct groups; the occurrence of ICH within 24 hours after treatment, poor 3-month outcome on the basis of modified Rankin Scale (mRS) and 3-month mortality.
Results: Patients were 83 men (62.9%) and 49 women (37.1%) with a median age of 66 years [interquartile range (IQR)of 55-72]. Any type of hemorrhage, symptomatic hemorrhage [based on the European Cooperative Acute Stroke Study III (ECASS III) definition] within 24 hours posttreatment, poor 3-month outcome (mRS 3-6), and 3-month mortality were documented in 10.6%, 4.5%, 53.2%, and 23.6% of patients, respectively. Increased baseline blood glucose was a significant but dependent predictor of hemorrhage within the first 24 hours posttreatment. Dependent predictors of a 3-month poor outcome were high age, the National Institutes of Health Stroke Scale (NIHSS) at baseline, decreased admitting glomerular filtration rate (GFR), and the presence of atrial fibrillation (AF) rhythm, and ICH within 24 hours posttreatment. Only age [Odds ratio (OR) adjusted 1.05] and initial NIHSS (OR adjusted 1.23), however, were recognized as the independent variables in this regard. The only independent predictor of 3-month mortality was the initial NIHSS (OR adjusted 1.18).
Conclusion: According to the findings of the present study, advanced age and high baseline NIHSS are two independent prognostic factors in patients with AIS treated with tPA.
Key Words: Acute Stroke, Tissue Plasminogen Activator, Outcome, Risk Factors, Iran
Introduction
High prevalence of stroke and related disability have great devastating impact on the health status of general population.1 Providing immediate care in patients with cerebrovascular accidents could dramatically decrease associated consequences that usually cause permanent disability. Recent trials showed the superiority of endovascular therapy with a stent retriever to intravenous thrombolysis (IVT) in selected patients with acute ischemic stroke (AIS).2-6 Nevertheless, in developing countries, especially, with the lack of facilities, IVT still is the only available effective pharmacologic approach in selected patients with AIS.7-9
More than four fifths of stroke mortality in the world occur in developing countries.10 In Iran, stroke occurs about ten years earlier in comparison to most developed countries and its prevalence is 23-139 per 100000 populations.10-12
Noting the rapidly growing number of patients with stroke in our country and the need for studies focusing on potential prognostic factors in association with administration of tissue plasminogen activator (tPA) in such patients, the present work aimed to examine potential prognostic factors in Iranian patients with AIS who received intravenous tPA.
Materials and Methods
A total of 132 patients with AIS who received tPA in Tabriz Imam Reza teaching hospital, Iran, were enrolled in this retrospective hospital-bases observational study from January 2009 to March 2015. Inclusion and exclusion criteria were based on updated protocols published by the American Heart Association and the American Academy of Neurology.13
Data on demographic profile, risk factors, blood pressure at hospital arrival, laboratory tests, brain computed tomography (CT) scans, severity of initial stroke as assessed using the National Institutes of Health Stroke Scale (NIHSS), time of symptoms onset, and time of recombinant tPA (rtPA) administration were recorded. Brain CT scans were done for all patients just before and 24 hours after the treatment, and the presence of any hemorrhage or symptomatic intracranial hemorrhage (SICH) were recorded. The European Cooperative Acute Stroke Study III (ECASS III) definition for SICH was used (any new evidence of intracranial blood on imaging accompanied by a neurological deterioration of four or more points on the NIHSS score from baseline).
Functional outcome at 3 months was assessed using the modified Rankin Scale (mRS). Patients who were unable to attend were interviewed by phone. Possible prognostic factors were examined in three categories of intracranial hemorrhage (ICH) within the first 24 hours, poor 3-month outcome (mRS of 3-6), and 3-month mortality.
Written informed consent was obtained from each patient, and the Ethics Committee of Tabriz University of Medical Sciences approved the protocol of this study.
Data were shown as mean ± standard deviation (SD), median interquartile range (IQR), and frequency (%). The SPSS software (version 22, IBM Corporation, Armonk, NY, USA) was used for data analysis. The Kolmogorov-Smirnov test was employed to analyze the distribution of quantitative variables. Independent sample t, Mann-Whitney U, chi-square, and Fisher’s exact tests were used for analyses. The logistic regression analysis was used for multivariate study. The Kaplan-Meier plot was employed to examine survival. A P value of less than 0.05 was considered statistically significant.
Results
Patients were 83 men (62.9%) and 49 women (37.1%) with a median age of 66 years (IQR of 55-72). Medical history and clinical laboratory findings are summarized in table 1. The median (IQR) symptom-to-treatment interval was 143 (120-167) minutes. The median (IQR) baseline NIHSS was 14 (10-18). Intracranial bleeding occurred during the first 24-36 hours in 14 patients (10.6%), which was symptomatic in 6 patients (4.5%). The 3-month prognosis by using mRS was determined in 124 patients, among which poor prognosis (mRS 3-6) was documented in 66 patients (53.2%). The 3-month mortality rate in 127 patients was 23.6% (30 cases died). Study variables are compared between patients with and without ICH within the first 24 hours in table 2.
Table 1.
Medical history and clinical laboratory findings in admission
| Variable | n (%) |
|---|---|
| Hypertension | 79 (59.8) |
| Diabetes mellitus | 29 (22.0) |
| Smoking (current) | 27 (20.5) |
| Dyslipidemia | 16 (12.1) |
| Ischemic heart disease | 27 (20.5) |
| CABG | 9 (6.8) |
| AF | 30 (22.7) |
| Previous stroke | 16 (12.1) |
| Congestive heart disease | 11 (8.3) |
| Prior antiplatelet use | 32 (24.2) |
| Variable | Median (IQR) |
| Systolic blood pressure (mmHg) | 140 (125-155) |
| Diastolic blood pressure (mmHg) | 81 (80-92) |
| Serum hemoglobin (mg/dl) | 13.8 (12.5-14.9) |
| Platelet (× 1000/ml), | 209 (171-250) |
| INR (U) | 1.0 (1.00-1.07) |
| Serum glucose (mg/dl) | 136 (110-170) |
| GFR | 64 (48-80) |
CABG: coronary artery bypass grafting; IQR: Interquartile range; INR: international normalized ratio; GFR: glomerular filtration rate; AF: Atrial fibrillation
Table 2.
Study variables in patients with and without 24-hour intracranial hemorrhage (ICH)
| Variable |
ICH (n = 14)
|
ICH (n = 118)
|
P |
|---|---|---|---|
| n (%) | n (%) | ||
| Sex (Men) | 10 (71.4) | 73 (61.9) | 0.48 |
| Hypertension | 7 (50.0) | 72 (61.0) | 0.43 |
| Diabetes mellitus | 2 (14.3) | 27 (22.9) | 0.73 |
| Smoking (current) | 2 (14.3) | 25 (21.2) | 0.73 |
| Hypertriglyceridemia | 2 (14.3) | 14 (11.9) | 0.68 |
| Previous ischemic heart disease | 3 (21.4) | 24 (20.3) | 0.58 |
| Previous CABG | 2 (14.3) | 7 (5.9) | 0.24 |
| Previous AF | 5 (35.7) | 25 (21.2) | 0.31 |
| Previous stroke | 0 (0) | 16 (13.6) | 0.22 |
| Previous congestive heart disease | 0 (0) | 11 (10.7) | 0.42 |
| Antiplatelet drug | 2 (14.3) | 30 (33.3) | 0.50 |
| Variable | Median (IQR) | Median (IQR) | P |
| Age (year) | 65 (57-72) | 66 (54-72) | 0.97 |
| Systolic blood pressure (mmHg) | 140 (120-156) | 140 (125-157) | 0.89 |
| Diastolic blood pressure (mmHg) | 83 (73-91) | 81 (80-93) | 0.72 |
| Serum hemoglobin (mg/dl) | 13.3 (12.2-14.8) | 13.9 (12.5-14.9) | 0.41 |
| Platelet (× 1000/ml) | 203 (175-254) | 209 (170-251) | 0.63 |
| INR (U) | 1.00 (1.00-1.09) | 1.00 (1.00-1.07) | 0.92 |
| Serum glucose (mg/dl) | 137 (114-148) | 136 (109-179) | 0.04* |
| GFR (ml/minute/1.73 m2) | 66 (49-85) | 64 (48-80) | 0.12 |
| Symptom-to-treatment (minute) | 158 (123-180) | 142 (120-165) | 0.12 |
| Baseline NIHSS | 17.5 (14.5-19.5) | 13.5 (9-18) | 0.12 |
CABG: Coronary artery bypass grafting; ICH: Intracranial hemorrhage; GFR: Glomerular filtration rate; INR: International normalized ratio; NIHSS: The National Institutes of Health Stroke Scale; AF: Atrial fibrillation
P value of less than 0.05 which is statistically significant.
Accordingly, the admission blood glucose level was significantly higher in patients with intracranial bleeding.
Variables are compared between the two groups of with and without 3-month poor outcome in table 3. Accordingly, median age, mean baseline NIHSS, frequency of patients with atrial fibrillation (AF) rhythm, and frequency of patients with first 24-hour ICH were all significantly higher, and the baseline mean glomerular filtration rate (GFR) was significantly lower in patients with poor outcome. In multivariate study, however, only age [P = 0.04, Odds ratio (OR) = 1.05] and baseline NIHSS (P = 0.01, OR = 1.23) were independent predictors of 3-month poor outcome.
Table 3.
Study variables in patients with 3-month favorable outcome and bad prognosis
| Variable |
Favorable outcome (n = 58)
|
Bad prognosis (n = 66)
|
P |
|---|---|---|---|
| n (%) | n (%) | ||
| Sex (Men) | 38 (65.5) | 35 (59.1) | 0.46 |
| Hypertension | 31 (53.4) | 45 (68.2) | 0.09 |
| Diabetes mellitus | 11 (19.0) | 16 (24.2) | 0.48 |
| Smoking (current) | 12 (20.7) | 13 (19.7) | 0.89 |
| Hypertriglyceridemia | 8 (13.8) | 6 (9.1) | 0.41 |
| Previous ischemic heart disease | 12 (20.7) | 11 (16.7) | 0.57 |
| Previous CABG | 2 (3.4) | 5 (7.6) | 0.45 |
| Previous AF | 7 (12.1) | 20 (30.3) | 0.01* |
| Previous stroke | 7 (12.1) | 7 (10.6) | 0.80 |
| Previous congestive heart disease | 3 (5.7) | 6 (12.0) | 0.31 |
| Antiplatelet drug | 11 (23.9) | 18 (36.7) | 0.18 |
| 24-hour ICH | 2 (3.4) | 11 (6.7) | 0.02* |
| Variable | Median (IQR) | Median (IQR) | P |
| Age (year) | 60 (52-69) | 70 (59-75) | < 0.01* |
| Systolic blood pressure (mmHg) | 142 (128-160) | 140 (122-150) | 0.19 |
| Diastolic blood pressure (mmHg) | 85 (80-96) | 80 (79-90) | 0.22 |
| Serum hemoglobin (mg/dl) | 14.0 (13.0-14.6) | 13.6 (12.3-15.2) | 0.85 |
| Platelet (× 1000/ml) | 208 (182-248) | 212 (163-266) | 0.62 |
| INR (U) | 1.00 (1.00-1.07) | 1.00 (1.00-1.10) | 0.15 |
| Serum glucose (mg/dl) | 128 (105-149) | 140 (115-194) | 0.09 |
| GFR (ml/minute/1.73 m2) | 68 (55-87) | 60 (45-76) | 0.02* |
| Symptom-to-treatment (minute) | 128 (105-170) | 147 (130-165) | 0.11 |
| Baseline NIHSS | 10 (7-14) | 17 (13-19) | < 0.01* |
CABG: Coronary artery bypass grafting; ICH: Intracranial hemorrhage; INR: international normalized ratio; GFR: Glomerular filtration rate; NIHSS: The National Institutes of Health Stroke Scale; AF: Atrial fibrillation
P value of less than 0.05 which is statistically significant.
Predictors of 3-month mortality are set out in Table 4. Accordingly, median baseline NIHSS, previous AF rhythm, and the first 24-hour ICH significantly predicted mortality. The only independent predictor of 3-month mortality was the baseline NIHSS (P = 0.02, OR = 1.18).
Table 4.
Study variables in dead and alive patients 3 months posttreatment
| Variable |
Alive (n = 97)
|
Dead (n = 30)
|
P |
|---|---|---|---|
| n (%) | n (%) | ||
| Sex (Man) | 61 (62.9) | 18 (60.0) | 0.78 |
| Hypertension | 55 (56.7) | 22 (73.3) | 0.10 |
| Diabetes mellitus | 21 (21.6) | 7 (23.3) | 0.85 |
| Smoking (current) | 18 (18.6) | 8 (26.7) | 0.34 |
| Hypertriglyceridemia | 14 (14.4) | 2 (6.7) | 0.36 |
| Previous ischemic heart disease | 20 (20.6) | 5 (16.7) | 0.83 |
| Previous CABG | 4 (4.1) | 4 (13.3) | 0.09 |
| Previous AF | 16 (16.5) | 12 (40) | 0.01* |
| Previous stroke | 13 (13.4) | 2 (6.7) | 0.52 |
| Previous congestive heart disease | 8 (4.9) | 2 (9.5) | 0.63 |
| Antiplatelet drug | 25 (33.3) | 6 (26.1) | 0.51 |
| 24-hour ICH | 5 (5.2) | 9 (30.0) | < 0.01* |
| Variable | Median (IQR) | Median (IQR) | P |
| Age (year) | 65 (54-72) | 67 (58-75) | 0.09 |
| Systolic blood pressure (mmHg) | 140 (127-160) | 140 (122-151) | 0.53 |
| Diastolic blood pressure (mmHg) | 85 (80-95) | 80 (73-90) | 0.27 |
| Serum hemoglobin (mg/dl) | 13.8 (12.6-14.7) | 14.0 (12.0-15.1) | 0.89 |
| Platelet (× 1000/ml) | 219 (179-261) | 197 (154-238) | 0.45 |
| INR (U) | 1.00 (1.00-1.07) | 1.00 (1.00-1.11) | 0.82 |
| Serum glucose (mg/dl) | 136 (110-165) | 137 (113-202) | 0.24 |
| GFR (ml/minute/1.73 m2) | 64 (48-80) | 62 (48-80) | 0.41 |
| Symptom-to-treatment (minute) | 143 (120-167) | 148 (131-172) | 0.28 |
| Baseline NIHSS | 13 (8-18) | 16 (13-21) | 0.01* |
CABG: Coronary artery bypass grafting; ICH: Intracranial hemorrhage; INR: International normalized ratio; GFR: Glomerular filtration rate; NIHSS: The National Institutes of Health Stroke Scale; AF: Atrial fibrillation
P value of less than 0.05 which is statistically significant.
The related Kaplan-Meier plot of 3-month survival is depicted in figure 1.
Figure 1.
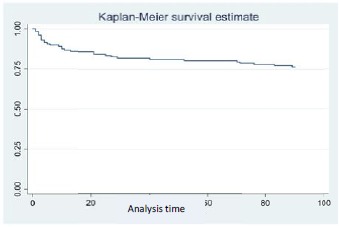
Kaplan-Meier plot for 3-month survival
Discussion
In the present study, potential prognostic factors in patients with AIS who received tPA were assessed. Any type of hemorrhage and SICH the first 24-36 hours was documented in 10.6% and 4.5% of these patients, respectively.
SICH in one of the devastating complications associated with thrombolytic therapy in patients with AIS, with an estimated incidence of 6% (maximum 23%) in other countries.14-21 Our patients had a lower rate of SICH compared with the National Institute of Neurological Disorders and Stroke (NINDS) rtPA trial (4.5% vs 6.4%) and the pooled analysis of 8 major randomized placebo-controlled trials of rtPA (alteplase) for acute stroke treatment (4.5% vs 5.2%); but this rate is higher in comparison with ECASS III trial (4.5% vs 2.4%).14,21,22 There are two other reports in regard to the rate of ICH from Iran. In the first report from Firoozgar hospital in Tehran, Iran, among 37 patients with acute stroke systematically thrombolysed with rtPA, rate of hemorrhagic transformations and symptomatic hemorrhage was 24% and 7%, respectively. They did not report mortality rate and late outcome.23 In another report from Ghaem hospital in Mashhad, Iran, none of their 14 patients had SICH.24 Age and stroke severity are important risk factors for SICH,25-27 and we think this difference is largely due to that patients they treated were significantly younger (mean age of 59 vs 65 years), and had a milder stroke severity (mean NIHSS of 14 vs 16).
According to the results of previous studies in this regard, various risk factors have been suggested in association with the development of ICH 24-36 hours after initiation of tPA in patients with AIS, including baseline NIHSS, intracranial edema/mass pressure effect, presence of hypodensity in pretreatment CT images, baseline hyperglycemia, more severe stroke, longer interval between symptoms and treatment, hypertension, low platelet count, previous cardiac disease, previous treatment with antiplatelet drugs, advance age, and a negative history of smoking.20,21,25-31 Among the mentioned variables, baseline serum glucose level was the only variable that was significantly associated with 24-hour ICH in the present study. This finding is in line with previous reports.17,20,31
In another section in the present study, factors in association with a 3-month poor outcome (mRS of 3-6) were investigated. In univariate analysis, advanced age, low baseline GFR, high baseline NIHSS, previous AF rhythm, and 24-hour ICH predicted bad outcome. In multivariate analysis, however, only age and baseline NIHSS were significant independent predictors in this regard. One of the interesting findings in the present work was an independent association found between baseline GFR and poor outcome. Reports are heterogeneous in this regard. Recently a meta-analysis by evaluating 7796 patients reported that renal dysfunction did not increase the risk of a poor outcome in patients who received thrombolysis;32 in another study, Gensicke, et al, Among 4,780 patients with AIS treated with tPA showed low GFR was independently associated with poor 3-month outcome and any GFR decrease by 10 ml/min/1.73 m2 increased the risk of poor outcome.33 The mechanism of how outcome after stroke is being affected by renal impairment is not so clear.
In line with a previous report,34 another significant but not independent variable in association with 3-month bad outcome in the present study was a positive AF rhythm. It should be born in mind that the presence of AF rhythm temporarily at admission is different from a condition in which AF is present chronically before the stroke. It has been suggested the prognosis is worse in the latter group, if the patient receives tPA.35 So, it is necessary to discriminate between these two conditions in future studies. On the other hand, emergence of AF rhythm in stroke has been found in a significant association with the patient’s age, with higher incidence in the older patients.36 Disappearing of this significant association in the multivariate model with including age may further corroborate such relationships between age and AF rhythm.
The 3-month mortality rate was 23.6% in the present study. Associated significant but not independent predictors were an increased baseline NIHSS, AF rhythm, and 24-hour ICH, with NIHSS as the only independent predictor in this regard in multivariate analysis. In similar studies, the mortality rate varies between 10% and 40% in such patients, and the related predictors have been reported to be the severity of stroke, previous patient disability, past medical status, age, sex and symptom-to-treatment interval.15,18,31,37-39
One of suggested factors affecting the association between response to tPA treatment is racial differences.40,41 Therefore, since the present study is the first one among Iranian population, which reports prognostic factors in AIS patients treated with tPA, the findings are unique but need to be confirmed in future studies.
Finally, despite the uniqueness of the present work among Iranians, a rather small sample size compared to some robust available reports in the literature should be acknowledged as a limitation; and this is an observational study and the associations reported in this article may not clarify the causations. Further studies with larger sample size are needed to better clarify prognostic factors in these patients.
Conclusion
According to the findings of the present study, advanced age and high baseline NIHSS are two independent prognostic factors in patients with AIS treated with intravenous (IVT) thrombolysis.
Acknowledgments
The authors would like to acknowledge Miss Rogayyeh Hassasi and the staff of Imam-Reza hospital Neurosciences Critical Care Unit (NeuroICU) for their outstanding help in conducting the research. This study was funded by Neuroscience Research Center (NSRC), Tabriz University of Medical Sciences.
Conflict of Interests
The authors declare no conflict of interest in this study.
Notes:
How to cite this article: Sadeghi-Hokmabadi E, Yazdchi M, Farhoudi M, Sadeghi H, Taheraghdam A, Rikhtegar R, et al. Prognostic factors in patients with acute ischemic stroke treated with intravenous tissue plasminogen activator: The first study among Iranian patients. Iran J Neurol 2018; 17(1): 31-7.
References
- 1.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: A randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282(21):2003–11. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 2.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 3.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 4.Molina CA, Chamorro A, Rovira A, de Miquel A, Serena J, Roman LS, et al. REVASCAT: A randomized trial of revascularization with SOLITAIRE FR device vs best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight-hours of symptom onset. Int J Stroke. 2015;10(4):619–26. doi: 10.1111/ijs.12157. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 6.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 7.Adams H, Adams R, Del ZG, Goldstein LB. Guidelines for the early management of patients with ischemic stroke: 2005 guidelines update a scientific statement from the Stroke Council of the American Heart Association/American Stroke Association. Stroke. 2005;36(4):916–23. doi: 10.1161/01.STR.0000163257.66207.2d. [DOI] [PubMed] [Google Scholar]
- 8.Adams HP Jr, Adams RJ, Brott T, del Zoppo GJ, Furlan A, Goldstein LB, et al. Guidelines for the early management of patients with ischemic stroke: A scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003;34(4):1056–83. doi: 10.1161/01.STR.0000064841.47697.22. [DOI] [PubMed] [Google Scholar]
- 9.Albers GW, Amarenco P, Easton JD, Sacco RL, Teal P. Antithrombotic and thrombolytic therapy for ischemic stroke: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):483S–512S. doi: 10.1378/chest.126.3_suppl.483S. [DOI] [PubMed] [Google Scholar]
- 10.Hosseini AA, Sobhani-Rad D, Ghandehari K, Benamer HT. Frequency and clinical patterns of stroke in Iran-Systematic and critical review. BMC Neurol. 2010;10:72. doi: 10.1186/1471-2377-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durai PJ, Padma V, Vijaya P, Sylaja PN, Murthy JM. Stroke and thrombolysis in developing countries. Int J Stroke. 2007;2(1):17–26. doi: 10.1111/j.1747-4949.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- 12.Azarpazhooh MR, Etemadi MM, Donnan GA, Mokhber N, Majdi MR, Ghayour-Mobarhan M, et al. Excessive incidence of stroke in Iran: Evidence from the Mashhad Stroke Incidence Study (MSIS), a population-based study of stroke in the Middle East. Stroke. 2010;41(1):e3–e10. doi: 10.1161/STROKEAHA.109.559708. [DOI] [PubMed] [Google Scholar]
- 13.Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 14.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 15.Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA. Intravenous tissue-type plasminogen activator for treatment of acute stroke: The Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA. 2000;283(9):1145–50. doi: 10.1001/jama.283.9.1145. [DOI] [PubMed] [Google Scholar]
- 16.Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, et al. Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768–74. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 17.Hill MD, Buchan AM. Thrombolysis for acute ischemic stroke: Results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ. 2005;172(10):1307–12. doi: 10.1503/cmaj.1041561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katzan IL, Furlan AJ, Lloyd LE, Frank JI, Harper DL, Hinchey JA, et al. Use of tissue-type plasminogen activator for acute ischemic stroke: The Cleveland area experience. JAMA. 2000;283(9):1151–8. doi: 10.1001/jama.283.9.1151. [DOI] [PubMed] [Google Scholar]
- 19.Sauser-Zachrison K, Shen E, Ajani Z, Neil WP, Sangha N, Gould MK, et al. Emergency care of patients with acute ischemic stroke in the Kaiser Permanente southern California integrated health system. Perm J. 2016;20(2):10–3. doi: 10.7812/TPP/15-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanne D, Kasner SE, Demchuk AM, Koren-Morag N, Hanson S, Grond M, et al. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: The Multicenter rt-PA Stroke Survey. Circulation. 2002;105(14):1679–85. doi: 10.1161/01.cir.0000012747.53592.6a. [DOI] [PubMed] [Google Scholar]
- 21.Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 22.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695–703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 23.Mehrpour M, Aghaei M, Motamed MR. Safety and feasibility of intravenous thrombolytic therapy in Iranian patients with acute ischemic stroke. Med J Islam Repub Iran. 2013;27(3):113–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Nikkhah K, Avan A, Shoeibi A, Azarpazhooh A, Ghandehari K, Foerch C, et al. Gaps and hurdles deter against following stroke guidelines for thrombolytic therapy in Iran: Exploring the problem. J Stroke Cerebrovasc Dis. 2015;24(2):408–15. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Cucchiara B, Kasner SE, Tanne D, Levine SR, Demchuk A, Messe SR, et al. Factors associated with intracerebral hemorrhage after thrombolytic therapy for ischemic stroke: Pooled analysis of placebo data from the Stroke-Acute Ischemic NXY Treatment (SAINT) I and SAINT II Trials. Stroke. 2009;40(9):3067–72. doi: 10.1161/STROKEAHA.109.554386. [DOI] [PubMed] [Google Scholar]
- 26.Lansberg MG, Albers GW, Wijman CA. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: A review of the risk factors. Cerebrovasc Dis. 2007;24(1):1–10. doi: 10.1159/000103110. [DOI] [PubMed] [Google Scholar]
- 27.Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: A secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32(2):438–41. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- 28.Bravo Y, Marti-Fabregas J, Cocho D, Rodriguez-Yanez M, Castellanos M, de la Ossa NP, et al. Influence of antiplatelet pre-treatment on the risk of symptomatic intracranial haemorrhage after intravenous thrombolysis. Cerebrovasc Dis. 2008;26(2):126–33. doi: 10.1159/000139659. [DOI] [PubMed] [Google Scholar]
- 29.Masrur S, Cox M, Bhatt DL, Smith EE, Ellrodt G, Fonarow GC, et al. Association of acute and chronic hyperglycemia with acute ischemic stroke outcomes post-thrombolysis: Findings from get with the guidelines-stroke. J Am Heart Assoc. 2015;4(10):e002193. doi: 10.1161/JAHA.115.002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uyttenboogaart M, Koch MW, Koopman K, Vroomen PC, De Keyser J, Luijckx GJ. Safety of antiplatelet therapy prior to intravenous thrombolysis in acute ischemic stroke. Arch Neurol. 2008;65(5):607–11. doi: 10.1001/archneur.65.5.noc70077. [DOI] [PubMed] [Google Scholar]
- 31.Wahlgren N, Ahmed N, Eriksson N, Aichner F, Bluhmki E, Davalos A, et al. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy (SITS-MOST) Stroke. 2008;39(12):3316–22. doi: 10.1161/STROKEAHA.107.510768. [DOI] [PubMed] [Google Scholar]
- 32.Hao Z, Yang C, Liu M, Wu B. Renal dysfunction and thrombolytic therapy in patients with acute ischemic stroke: A systematic review and meta-analysis. Medicine (Baltimore) 2014;93(28):e286. doi: 10.1097/MD.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gensicke H, Zinkstok SM, Roos YB, Seiffge DJ, Ringleb P, Artto V, et al. IV thrombolysis and renal function. Neurology. 2013;81(20):1780–8. doi: 10.1212/01.wnl.0000435550.83200.9e. [DOI] [PubMed] [Google Scholar]
- 34.Saposnik G, Gladstone D, Raptis R, Zhou L, Hart RG. Atrial fibrillation in ischemic stroke: Predicting response to thrombolysis and clinical outcomes. Stroke. 2013;44(1):99–104. doi: 10.1161/STROKEAHA.112.676551. [DOI] [PubMed] [Google Scholar]
- 35.Seet RC, Zhang Y, Wijdicks EF, Rabinstein AA. Relationship between chronic atrial fibrillation and worse outcomes in stroke patients after intravenous thrombolysis. Arch Neurol. 2011;68(11):1454–8. doi: 10.1001/archneurol.2011.248. [DOI] [PubMed] [Google Scholar]
- 36.Saposnik G, Cote R, Phillips S, Gubitz G, Bayer N, Minuk J, et al. Stroke outcome in those over 80: A multicenter cohort study across Canada. Stroke. 2008;39(8):2310–7. doi: 10.1161/STROKEAHA.107.511402. [DOI] [PubMed] [Google Scholar]
- 37.Aoki J, Kimura K, Sakamoto Y. Early administration of tissue-plasminogen activator improves the long-term clinical outcome at 5years after onset. J Neurol Sci. 2016;362:33–9. doi: 10.1016/j.jns.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Heuschmann PU, Kolominsky-Rabas PL, Roether J, Misselwitz B, Lowitzsch K, Heidrich J, et al. Predictors of in-hospital mortality in patients with acute ischemic stroke treated with thrombolytic therapy. JAMA. 2004;292(15):1831–8. doi: 10.1001/jama.292.15.1831. [DOI] [PubMed] [Google Scholar]
- 39.Strbian D, Meretoja A, Ahlhelm FJ, Pitkaniemi J, Lyrer P, Kaste M, et al. Predicting outcome of IV thrombolysis-treated ischemic stroke patients: The DRAGON score. Neurology. 2012;78(6):427–32. doi: 10.1212/WNL.0b013e318245d2a9. [DOI] [PubMed] [Google Scholar]
- 40.del Rio-Espinola A, Fernandez-Cadenas I, Giralt D, Quiroga A, Gutierrez-Agullo M, Quintana M, et al. A predictive clinical-genetic model of tissue plasminogen activator response in acute ischemic stroke. Ann Neurol. 2012;72(5):716–29. doi: 10.1002/ana.23664. [DOI] [PubMed] [Google Scholar]
- 41.Reed SD, Cramer SC, Blough DK, Meyer K, Jarvik JG. Treatment with tissue plasminogen activator and inpatient mortality rates for patients with ischemic stroke treated in community hospitals. Stroke. 2001;32(8):1832–40. doi: 10.1161/01.str.32.8.1832. [DOI] [PubMed] [Google Scholar]


