Abstract
Background
In 2011, The Infectious Diseases Society of America released a clinical practice guideline (CPG) that recommended short-course antibiotic therapy and avoidance of fluoroquinolones for uncomplicated urinary tract infections (UTIs). Recommendations from this CPG were rapidly disseminated to clinicians via review articles, UpToDate, and the Centers for Disease Control and Prevention website; however, it is unclear if this CPG had an impact on national antibiotic prescribing practices.
Methods
We performed a retrospective cohort study of outpatient and emergency department visits within a commercial insurance database between January 1, 2009, and December 31, 2013. We included nonpregnant women aged 18–44 years who had an International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis code for a UTI with a concurrent antibiotic prescription. We performed interrupted time series analyses to determine the impact of the CPG on the appropriateness of the antibiotic agent and duration.
Results
We identified 654 432 women diagnosed with UTI. The patient population was young (mean age, 31 years) and had few comorbidities. Fluoroquinolones, nonfirstline agents, were the most commonly prescribed antibiotic class both before and after release of the guidelines (45% vs 42%). Wide variation was observed in the duration of treatment, with >75% of prescriptions written for nonrecommended treatment durations. The CPG had minimal impact on antibiotic prescribing behavior by providers.
Conclusions
Inappropriate antibiotic prescribing is common for the treatment of UTIs. The CPG was not associated with a clinically meaningful change in national antibiotic prescribing practices for UTIs. Further interventions are necessary to improve outpatient antibiotic prescribing for UTIs.
Keywords: antibiotic stewardship, antibiotics, fluoroquinolones, guidelines, urinary tract infection
Evaluating and improving antibiotic prescribing in outpatient settings is important. According to the Centers for Disease Control and Prevention, approximately 80% of antibiotic prescriptions are written in the outpatient setting, and 30% of all outpatient antibiotic prescriptions are inappropriate [1]. These inappropriate antibiotic prescriptions contribute to adverse events and increase health care costs [2]. In response, the National Action Plan for Combating Antibiotic-Resistant Bacteria targeted a 50% reduction in inappropriate outpatient antibiotic use by 2020 [3].
Clinical practice guidelines (CPGs) are an existing tool designed to improve patient care. Several CPGs have been specifically written to optimize antibiotic prescribing for common infectious (and noninfectious) conditions [4–6]. The Infectious Diseases Society of America (IDSA) published a CPG on the treatment of acute uncomplicated cystitis in women on March 1, 2011, focusing on avoidance of fluoroquinolones (FQs) for uncomplicated urinary tract infections (UTIs) and short courses of antibiotics [6]. This information was rapidly incorporated into UpToDate, the Centers for Disease Control and Prevention (CDC) website, and review articles in American Family Physician and the New England Journal of Medicine (NEJM) [7–10]. In 2015 and 2016, the American Board of Internal Medicine Foundation’s “Choosing Wisely” campaign, the American Urogynecologic Society, and the Food and Drug Administration (FDA) also released guidance to avoid FQ prescriptions for uncomplicated UTIs when reasonable alternatives exist [11, 12].
The aims of this study were to evaluate the impact of the IDSA CPG on national antibiotic prescribing practices for uncomplicated UTIs in younger women. Using a large administrative data set, we examined inappropriate antibiotic prescribing, as defined by antibiotic class and treatment duration, for time period before and after release of the IDSA CPG.
METHODS
Data Source and Study Design
We used the Truven Health Analytics MarketScan Commercial Claim and Encounters Database (MarketScan) database to perform a retrospective observational cohort study of uncomplicated UTIs in younger women treated as outpatients between January 1, 2009, and December 31, 2013. The MarketScan database contains administrative claims from all 50 states and approximately 100 different employer-sponsored health insurance plans. The database contains medical claims data from inpatient and outpatient sources and outpatient pharmacy claims. Encrypted patient-level identifiers allow enrollees to be tracked over time.
Case Selection and Definitions
We selected our inclusion criteria based on the IDSA uncomplicated UTI CPG [6]. Specifically, we queried the outpatient and pharmacy claims files for women age 18–44 years who had an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code of urinary tract infection (595.0), unspecified cystitis (595.9), or uncomplicated cystitis (599.0). We defined the index UTI date as the first service date coded for UTI. We searched office visit claims and non–office visit laboratory claims (eg, urinalysis without an associated visit). We required that women coded for UTI had an accompanying paid antibiotic prescription within +/- 5 days of the index UTI date with a days’ supply greater than 0 days. We required medical and pharmacy enrollment for at least 6 months before and 30 days after the index UTI date.
Exclusion Criteria
We conservatively excluded women aged 45 years or older to focus on the premenopausal population. We excluded women coded for the acute and semi-acute infectious conditions within 6 months before or 14 days after the index UTI date based on a study from Dubberke et al. [13]. We excluded women coded for several chronic conditions diagnosed from 6 months before to 1 month after the index UTI date (Supplementary Table 1). We specifically excluded chronic conditions diagnosed after the index UTI date to remove bias because these conditions were almost certainly present, but not yet diagnosed, on the index UTI date. As some conditions may not be coded, we also excluded individuals based on medications associated with the above conditions or with underlying urologic abnormalities within 6 months before the index UTI date (Supplementary Table 2). We also excluded women who received intravenous antibiotics as initial therapy, as these cases would not be consistent with uncomplicated infection. Subsequent UTIs were excluded from the analysis.
As the CPG was focused on nonpregnant women, we excluded pregnant women in the 6 months before or the 6 months after our index UTI date based on the study by Kuklina et al. (Supplementary Table 3) [14]. We also excluded women coded for a UTI who did not meet inclusion criteria (eg, UTI diagnosed during a hospitalization) within 6 months before the index UTI date as this may represent women with recurrent UTIs or providers may use prior culture results to inform empiric antibiotic selection for subsequent infections. Similarly, we required that all included women have at least a 6-month period free of a UTI before enrollment to further exclude women with recurrent UTIs. Finally, we excluded women with hospitalizations within 90 days before the index UTI date, as these health care exposures may have placed individuals at higher risk for infections with multidrug-resistant pathogens.
Patient Characteristics
We collected patient age and comorbidity information. Comorbidities identified in the 6 months before the index UTI were defined using the Elixhauser comorbidity algorithm.
Antibiotic Appropriateness Definitions
We defined antibiotic appropriateness based on the IDSA CPG in 2 ways: class and duration [6]. First, we evaluated appropriateness by antibiotic class. Appropriate antibiotics included firstline agents (fosfomycin, nitrofurantoin, and trimethoprim-sulfamethoxazole [TMP-SMX]); second-line agents (fluoroquinolones and β-lactam antibiotics) were considered inappropriate. Antibiotics not listed as first- or second-line antibiotics were categorized as “other.” The other category was then split into 2 separate categories: “other combined” and “other alone.” Other combined included other agents that were combined with a first- or second-line agent. Other alone included antibiotics without an accompanying first- or second line-agent. Other combined was not mutually exclusive, so a patient who received an antibiotic prescription for ciprofloxacin and azithromycin counted as an FQ prescription and an other combined prescription. Antibiotic prescriptions were considered the appropriate class if the claim was for a firstline therapy alone or a firstline therapy combined with an other antibiotic. This combination was conservatively deemed appropriate because we were unable to rule out the possibility that the other antibiotic was prescribed for a legitimate reason. However, prescriptions for 2 firstline therapies, a second-line therapy, or another other alone category were considered inappropriate.
Second, we evaluated antibiotic appropriateness by treatment duration according to the IDSA CPG. Appropriate prescription durations were defined as 1 day for fosfomycin, 3 days for FQ and TMP-SMX, 5 days for nitrofurantoin, and 3–7 days for β-lactams. All other antibiotic treatment durations were considered inappropriate. Antibiotic prescriptions without clearly established treatment durations for UTIs were excluded from these analyses. If the patient received 1 appropriate treatment duration and 1 inappropriate treatment duration, then the antibiotic treatment duration was considered inappropriate.
Statistical Analysis
We compared the demographic characteristics of patients before and after the release of the IDSA CPG. We plotted histograms of antibiotic treatment duration (in days’ supply) to evaluate variation in antibiotic prescribing by antibiotic agent. Rates of inappropriate antibiotic prescriptions by class and treatment duration before and after the CPG were compared via chi-square statistics. However, our main outcome was to evaluate temporal trends in the rates of inappropriate antibiotic prescriptions using interrupted time series regression models. Analyses were stratified by class and duration. We utilized the approach outlined by Bernal et al. to determine if guidelines were associated with the level and/or trend in rates of antibiotic prescribing [15]. To correct for the presence of autocorrelation, we used the Newey-West estimator to produce robust standard errors [16]. Several models for the interrupted time series analysis were explored, and the best-fitting one (based on Akaike information criterion and adjusted R2) was used in the final analysis. The Supplementary Data provide a more detailed summary of the regression model specifications. P values of less than .05 were considered statistically significant.
RESULTS
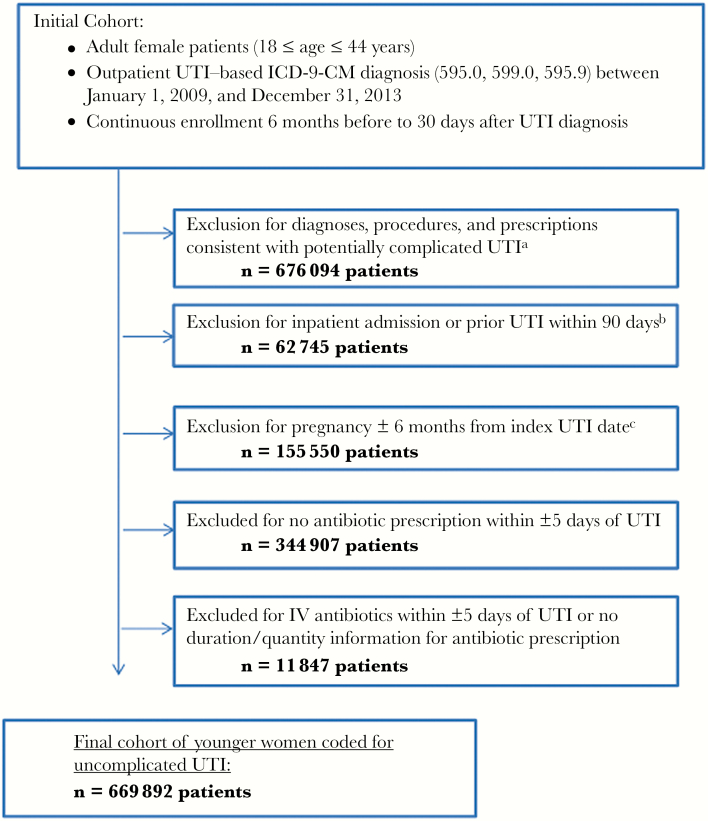
We identified 1 921 035 women aged 18–44 years with an ICD-9-CM diagnosis code for UTI between January 1, 2009, and December 31, 2013. After applying inclusion and exclusion criteria to exclude patients with recent hospitalizations, infections, procedures, urologic abnormalities, pregnancy, and significant underlying comorbidities, the final cohort consisted of 654 432 women coded for an index UTI (Figure 1). Patient characteristics before and after the guidelines were similar overall (Table 1).
Figure 1.
Cohort selection flow chart. aSee Supplementary Tables 1 and 2 for excluded conditions and medications. bThis exclusion criterion was applied to eliminate multidrug-resistant pathogens or empiric antibiotic selection based on prior cultures. cSee Supplementary Table 3 for codes used to exclude pregnancy. Abbreviations: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; IV, intravenous; UTI, urinary tract infection.
Table 1.
Characteristics of Women Aged 18–44 Years Coded for Uncomplicated Urinary Tract Infection With Concurrent Antibiotic Prescription(s)
| Before Guidelines (n = 307 609), No. (%) | After Guidelines (n = 346 823), No. (%) | |
|---|---|---|
| Age, mean (SD), y | 31.16 (8.14) | 30.18 (8.32) |
| Female sex | 307 609 (100) | 346 823 (100) |
| Comorbidities | ||
| Alcohol abuse | 728 (0.24) | 1079 (0.31) |
| Anemia deficiency | 824 (0.27) | 846 (0.24) |
| Arthritis (rheumatoid) and collagen vascular disease | 207 (0.07) | 276 (0.08) |
| Chronic blood loss anemia | 29 (0.01) | 48 (0.01) |
| Congestive heart failure | 17 (0.01) | 16 (0.00) |
| Chronic pulmonary disease | 1660 (0.54) | 1903 (0.55) |
| Coagulopathy | 63 (0.02) | 65 (0.02) |
| Depression | 4355 (1.42) | 5174 (1.49) |
| Drug abuse | 812 (0.26) | 1436 (0.41) |
| Hypertension | 3172 (1.03) | 3231 (0.93) |
| Hypothyroidism | 1732 (0.56) | 2083 (0.60) |
| Liver disease | 48 (0.02) | 61 (0.02) |
| Fluid and electrolyte disorders | 76 (0.02) | 80 (0.02) |
| Other neurological disorders | 498 (0.16) | 608 (0.18) |
| Obesity | 5313 (1.73) | 7370 (2.13) |
| Paralysis | 33 (0.01) | 42 (0.01) |
| Peripheral vascular disease | 17 (0.01) | 23 (0.01) |
| Psychoses | 6494 (2.11) | 7400 (2.13) |
| Pulmonary circulation disease | 46 (0.01) | 41 (0.01) |
| Valvular disease | 107 (0.03) | 116 (0.03) |
| Weight loss | 1340 (0.44) | 1485 (0.43) |
Data are reported as No. (%) unless otherwise specified.
In our cohort of 654 432 women, we identified a total of 665 120 antibiotic prescriptions. Fluoroquinolones represented 284 744 (43%) prescriptions and were the most commonly prescribed antibiotics both before and after the release of the IDSA CPG (Table 2). There was a slight reduction in the percentage of FQ (44% vs 42%) and trimethoprim-sulfamethoxazole (TMP-SMX; 28% vs 27%) prescriptions after the guidelines were released, whereas there were slight increases in the percentage of nitrofurantoin (22% vs 24%) prescriptions after the guideline release.
Table 2.
Frequency of Common Antibiotic Prescriptions Associated With Urinary Tract Infection Claims Before and After the Release of the IDSA Clinical Practice Guideline
| Antibiotic Class | Before IDSA Guideline (n = 313 405), No. (%) | After IDSA Guideline (n = 351 715), No. (%) | P Value |
|---|---|---|---|
| Fluoroquinolone | 138 033 (44.04) | 146 711 (41.71) | <.001 |
| Trimethoprim-sulfamethoxazole | 87 271 (27.85) | 96 622 (27.47) | <.001 |
| Nitrofurantoin | 68 639 (21.90) | 85 599 (24.34) | <.001 |
| Beta-lactams | 14 470 (4.62) | 18 752 (5.33) | <.001 |
| Fosfomycin | 55 (0.01) | 48 (0.01) | .194 |
| Other combined | 1193 (0.38) | 1297 (0.37) | .363 |
| Other alone | 862 (0.28) | 883 (0.25) | .045 |
The list is not exclusive and includes a combined category; therefore, the total will be slightly over 100%. “Other combined” was defined as nonlisted antibiotic prescriptions combined with a listed antibiotic or 2 nonlisted antibiotics. “Other alone” was defined as a single antibiotic prescription of a nonfirstline or non-second-line antibiotic agent.
Abbreviation: IDSA, Infectious Diseases Society of America.
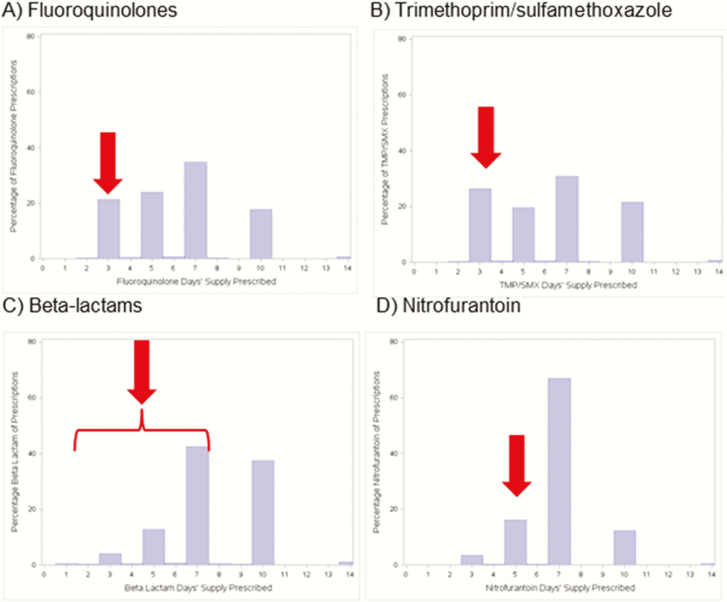
There was significant variation in the treatment duration for common antibiotic prescriptions for uncomplicated UTI (Figure 2). Providers tended to prescribe antibiotics for 3, 5, 7, or 10 days for first- and second-line UTI antibiotics, with the highest proportion at 7 days. Specifically, 35% of FQ prescriptions, 31% of TMP-SMX, 66% of nitrofurantoin, and 42% of beta-lactam antibiotics were prescribed for 7 days. Only 21% of FQ prescriptions, 26% of TMP-SMX prescriptions, and 16% of nitrofurantoin perceptions were for guideline-recommended durations.
Figure 2.
Antibiotic treatment duration for the most common antibiotics used to treat urinary tract infections. Duration truncated to 14 days. Appropriate treatment durations according to the clinical practice guideline are as follows: 3 days for fluoroquinolones, 3 days for trimethoprim/sulfamethoxazole, 3–7 days for beta-lactam antibiotics, and 5 days for nitrofurantoin; each of the guideline-endorsed treatment durations is denoted by a red arrow.
We observed modest improvements in antibiotic prescribing after the guideline release (Table 3). Importantly, nonfirstline agent prescriptions represented almost half of antibiotic prescriptions, and treatment durations were inappropriate about three-quarters of the time.
Table 3.
Appropriateness of Antibiotic Prescriptions Associated With Urinary Tract Infection Claims Before and After the Release of the IDSA Clinical Practice Guideline
| Antibiotic Prescribed | Before Guideline (n = 313 405), No. (%) |
After Guideline (n = 351 715), No. (%) |
P Value |
|---|---|---|---|
| Inappropriate agent | 153 123 (49.78) | 165 958 (47.85) | <.001 |
| Inappropriate duration | 238 016 (77.38) | 262 621 (75.72) | <.001 |
| Fluoroquinolones (non-3-d regimen) | 108 414/138 033 (78.54) | 115 838/146 711 (78.96) | .007 |
| Nitrofurantoin (non-5-d regimen) | 59 114/68 639 (86.12) | 70 778/85 599 (82.69) | <.001 |
| Trimethoprim/sulfamethoxazole (non-3-d regimen) | 65 821/87 354 (75.35) | 70 114/96 703 (72.50) | <.001 |
| Beta-lactams (non-3–7-d regimen) | 4955/11 678 (42.43) | 6126/15 718 (38.97) | <.001 |
“Inappropriate agent” was defined as a nonfirstline antibiotic prescription. “Inappropriate duration” was defined as a nonendorsed treatment duration.
Abbreviation: IDSA, Infectious Diseases Society of America.
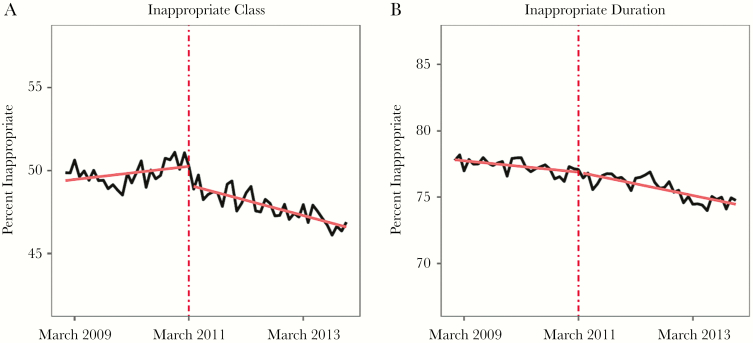
The interrupted time series analysis revealed that the IDSA CPG was associated with a statistically significant change in inappropriate prescribing by class by –0.110% per month (95% confidence interval [CI], –0.144 to –0.075; P < .01). Figure 3A depicts the rate of prescription of inappropriate class of antibiotics for treatment of uncomplicated UTI, along with the best-fitting interrupted time series model. Nonlinear regression discontinuity analysis models were also explored and are described in detail in the Supplementary Data (Supplementary Table 4 and Supplementary Figures 1 and 2).
Figure 3.
Interrupted time series analysis evaluating inappropriate antibiotic prescribing for uncomplicated urinary tract infections in younger women by class and treatment duration, before and after release of the Infectious Diseases Society of America clinical practice guideline in March 2011. The dashed red line corresponds to the release of the clinical practice guideline. The black lines demonstrate the percentage of inappropriate prescriptions. The solid red lines represent before and after trend lines from the time series analysis.
The IDSA CPG was also associated with a statistically significant change in the rate of antibiotic prescriptions of inappropriate duration for uncomplicated UTI by –0.037% per month (95% CI, –0.068 to –0.007; P < .05), as measured by the best-fitting interrupted time series model. Figure 3B depicts the rate of prescriptions of antibiotics for an inappropriate duration, along with the best-fitting interrupted time series model. Supplementary Table 5 summarizes the various regression models used to explore inappropriate duration.
DISCUSSION
In our cohort of more than 600 000 healthy women with uncomplicated UTIs, we observed that almost half of antibiotic prescriptions were for non-guideline-recommended antibiotics. Furthermore, >75% of antibiotic prescriptions for UTIs from 2009–2013 had treatment durations that were not consistent with a CPG endorsed by the CDC and recommended in UpToDate. We also found that CPGs were not associated with clinically relevant impact on provider antibiotic prescribing behavior.
These results are particularly worrisome given the recent FDA black box warning to clinicians to avoid FQs for uncomplicated infections. It is well established that FQs are associated with higher risks of infections and colonization with multidrug-resistant bacterial infections, including Clostridium difficile [17–20]. FQs also have other notable side effects, such as tendonitis, delirium, and cardiac complications [21–23]. However, FQs have been historically popular with clinicians, representing 49% of all antibiotic prescriptions in outpatient settings between 2002 and 2011 [24]. This high utilization places patients at high risk for adverse events and also likely facilitates the development of FQ-resistant pathogens [25, 26].
The publication of guideline recommendations may not be sufficient to meaningfully reduce inappropriate antibiotic use. Two smaller studies, involving a total of 3 outpatient clinics, also demonstrated poor adherence to the IDSA CPG for UTIs [27, 28]. Grigorian et al. evaluated 1546 visits for UTI in 2 private family medicine clinics associated with an academic medical center. They found that FQs were the most commonly prescribed antibiotic and that antibiotic treatment durations were frequently longer than recommended. Similarly, Kim et al. used chart review to evaluate 61 patients at a university-based internal medicine clinic; they found an overall guideline adherence rate of 34%. Notably, both studies were small, involved only academic medical center or faculty clinics, and performed less robust statistical analyses. In terms of other conditions, longitudinal data from UK primary care providers between 1995 and 2011 found mixed results from clinical practice guidelines alone, with antibiotic prescriptions for sore throat declining from 77% to 62% and antibiotic prescriptions for viral otitis media increasing from 77% to 85% [29]. A national analysis of outpatient antibiotic prescribing trends in the United States between 2006 and 2010 demonstrated a very slight reduction in antibiotic use [30]. More recent data from Express Scripts Holding Company, the largest pharmacy benefits manager in the United States, did not identify substantial reductions in outpatient antibiotic prescribing practices between 2013 and 2015 [31].
Specific interventions are likely required to improve antibiotic prescribing practices. National quality improvement initiatives have been released by the Joint Commission and the CDC to improve antibiotic prescribing practices in hospital settings [32, 33]. The CDC’s recently published core elements of outpatient antimicrobial stewardship highlight that clinics should demonstrate commitment to improve antibiotic prescribing practices by implementing at least 1 core policy to improve antibiotic prescribing practices, tracking and reporting antibiotic utilization, and providing education and expertise to clinicians in regards to antibiotic prescribing practices [34]. Active participation in antibiotic stewardship programs will be essential to improve antibiotic utilization, provide better patient care, and combat antimicrobial resistance.
Outpatient antimicrobial stewardship activities have demonstrated improvement in patient care. In a systematic review of outpatient antimicrobial stewardship interventions by Drekonja et al., the following antimicrobial stewardship interventions were effective: provider and/or patient education, provider feedback, guidelines, delayed prescribing, communication skills training, antibiotic restrictions, decision support tools, financial incentives, and rapid diagnostic tests [35]. Hecker et al. were able to improve adherence to UTI guidelines from 44% to 82% in the emergency department via an electronic UTI order set [36]. Other interventions have largely focused on reducing inappropriate antibiotic prescribing for upper respiratory tract infections. Meeker et al. reduced inappropriate antibiotic prescribing by 20% with a simple intervention: displaying a signed poster by providers committing to prescribing antibiotics appropriately [37]. This same group identified additional improvements in antibiotic prescribing by requiring providers to document justifications for antibiotic prescriptions that were outside of the guidelines and by providing benchmarking and feedback in regards to provider antibiotic prescribing performance [38]. However, it is unclear how well some of these interventions, which focus on eliminating inappropriate antibiotic prescribing for nonbacterial conditions, will be applicable to UTIs, for which providers should be focused on optimizing antibiotic selection and treatment duration.
Our study has several limitations. First, we used an insurance claims database, which precludes the ability to link antibiotic prescriptions to a provider or laboratory visit. We restricted antibiotic prescriptions to be temporally associated with the visit coded for UTI, so we assume that the diagnosis and antibiotic choice are linked. However, it is possible that the antibiotic was prescribed for a different condition. We believe this is unlikely given that >99.5% of antibiotic prescriptions captured in our data set involve classes commonly used to treat uncomplicated UTI. Second, ICD-9-CM diagnosis codes to identify UTIs have not been validated in the outpatient setting. It is possible that some of these visits coded for UTIs were for other conditions such as asymptomatic bacteriuria and pyelonephritis. However, we believe that these data demonstrate provider antibiotic selection for perceived uncomplicated UTIs based on coding. Furthermore, as pyelonephritis is much less frequent than UTI (with an estimated ratio of 1 case of pyelonephritis per 28 cases of cystitis), misclassification bias would likely be modest and stable over time [39]. Third, our analyses only captured antibiotic prescriptions with an associated ICD-9-CM code for UTI. Antibiotic prescriptions from providers who prescribed an antibiotic based on a telephone call but did not bill for the encounter were not captured in our database. These “phantom prescriptions” [40] may be different than those captured via laboratory claims and office visits in the database. Fifth, it is possible that antibiotic prescribing of providers has changed since the study period (2009–2013). We restricted the study period to earlier years to avoid later time periods that coincide with additional CPGs, such as the American Urologic Society and the American Board of Internal Medicine [12]. Sixth, we do not know provider rationale for antibiotic selection from the claims data. Some providers may have appropriately selected a second-line agent due to antibiotic allergies or local antibiotic resistance patterns. Seventh, although the CPG did not show a meaningful improvement in antibiotic prescribing during the study period, it is possible that we would find an impact if we expanded our time frame by several years.
CONCLUSIONS
The vast majority of antibiotic prescriptions for uncomplicated UTIs have been written for a non-guideline-endorsed treatment agent and/or duration. FQ, a nonfirstline agent with an FDA black box warning, was the most commonly prescribed antibiotic. Clinical practice guidelines endorsed and disseminated by the CDC, UpToDate, and an NEJM review article had no clinically relevant impact on antibiotic prescribing practices. Antimicrobial stewardship interventions are necessary to improve outpatient antibiotic prescribing for uncomplicated UTIs.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Author contributions. Conception and design: M.J. Durkin, M.A. Olsen; drafting of the article: M.J Durkin, M. Keller, J.H. Kwon, A.C. Miller, M.A. Olsen; critical revision of the article: M. Keller, A.M. Butler, J.H. Kwon, E.R. Dubberke, P.M. Polgreen, M.A. Olsen; final approval of the article: M.J. Durkin, M. Keller, A.M. Butler, J.H. Kwon, E.R. Dubberke, A.C. Miller, P.M. Polgreen, M.A. Olsen; statistical expertise: A.C. Miller, P.M. Polgreen, M.A. Olsen; obtaining of funding: M.J. Durkin, M.A. Olsen; administrative, technical, or logistic support: M. Keller, M.A. Olsen; collection and assembly of data: M. Keller, M.A. Olsen.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Financial support. The research in this publication was supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under award numbers KL2TR002346 and UL1 TR002345, the Agency for Healthcare Research and Quality (R24 HS19455), and the National Cancer Institute of the NIH (R24 HS19455).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315:1864–73. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2013. [Google Scholar]
- 3. White house national action plan for combating antibiotic-resistant bacteria Washington, DC: White House; 2015. https://www.cdc.gov/drugresistance/pdf/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed 28 March 2018.
- 4. Chow AW, Benninger MS, Brook I, et al. ; Infectious Diseases Society of America IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis 2012; 54:e72–e112. [DOI] [PubMed] [Google Scholar]
- 5. Wald ER, Applegate KE, Bordley C, et al. ; American Academy of Pediatrics Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics 2013; 132:e262–80. [DOI] [PubMed] [Google Scholar]
- 6. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52:e103–20. [DOI] [PubMed] [Google Scholar]
- 7. UpToDate. Acute uncomplicated cystitis in women https://www.uptodate.com/contents/acute-uncomplicated-cystitis-in-women?topicRef=8059&source=see_link. Accessed 28 March 2018.
- 8. Centers for Disease Control and Prevention. Antibiotic prescribing and use in doctor’s offices: adult treatment recommendations https://www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html. Accessed 2 November 2017.
- 9. Colgan R, Williams M. Diagnosis and treatment of acute uncomplicated cystitis. Am Fam Physician 2011; 84:771–6. [PubMed] [Google Scholar]
- 10. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med 2012; 366:1028–37. [DOI] [PubMed] [Google Scholar]
- 11. Food and Drug Administration. Drug Safety Communication. FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together http://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed 28 March 2018.
- 12. Choosing Wisely, an Initiative of the ABIM Foundation. Recommendations from the American Urogynecologic Society http://www.choosingwisely.org/clinician-lists/augs-fluoroquinolone-antibiotics-for-uncomplicated-utis/. Accessed 28 March 2018.
- 13. Dubberke ER, Olsen MA, Stwalley D, et al. Identification of medicare recipients at highest risk for Clostridium difficile infection in the US by population attributable risk analysis. PLoS One 2016; 11:e0146822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuklina EV, Whiteman MK, Hillis SD, et al. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Matern Child Health J 2008; 12:469–77. [DOI] [PubMed] [Google Scholar]
- 15. Lopez Bernal J, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2017; 46:348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newey WKW, Kenneth D. A simple, positive semi-definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica 1987; 55:703–8. [Google Scholar]
- 17. Gaynes R, Rimland D, Killum E, et al. Outbreak of Clostridium difficile infection in a long-term care facility: association with gatifloxacin use. Clin Infect Dis 2004; 38:640–5. [DOI] [PubMed] [Google Scholar]
- 18. Paterson DL. “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis 2004; 38(Suppl 4):S341–5. [DOI] [PubMed] [Google Scholar]
- 19. Wiener J, Quinn JP, Bradford PA, et al. Multiple antibiotic-resistant Klebsiella and Escherichia coli in nursing homes. JAMA 1999; 281:517–23. [DOI] [PubMed] [Google Scholar]
- 20. Weber SG, Gold HS, Hooper DC, et al. Fluoroquinolones and the risk for methicillin-resistant Staphylococcus aureus in hospitalized patients. Emerg Infect Dis 2003; 9:1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimatsu K, Subramaniam S, Sim H, Aronowitz P. Ciprofloxacin-induced tendinopathy of the gluteal tendons. J Gen Intern Med 2014; 29:1559–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhanel GG, Walkty A, Vercaigne L, et al. The new fluoroquinolones: a critical review. Can J Infect Dis 1999; 10:207–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tomé AM, Filipe A. Quinolones: review of psychiatric and neurological adverse reactions. Drug Saf 2011; 34:465–88. [DOI] [PubMed] [Google Scholar]
- 24. May L, Mullins P, Pines J. Demographic and treatment patterns for infections in ambulatory settings in the United States, 2006–2010. Acad Emerg Med 2014; 21:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanchez GV, Master RN, Karlowsky JA, Bordon JM. In vitro antimicrobial resistance of urinary Escherichia coli isolates among U.S. outpatients from 2000 to 2010. Antimicrob Agents Chemother 2012; 56:2181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cai T, Nesi G, Mazzoli S, et al. Asymptomatic bacteriuria treatment is associated with a higher prevalence of antibiotic resistant strains in women with urinary tract infections. Clin Infect Dis 2015; 61:1655–61. [DOI] [PubMed] [Google Scholar]
- 27. Grigoryan L, Zoorob R, Wang H, Trautner BW. Low concordance with guidelines for treatment of acute cystitis in primary care. Open Forum Infect Dis 2015; XXX(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim M, Lloyd A, Condren M, Miller MJ. Beyond antibiotic selection: concordance with the IDSA guidelines for uncomplicated urinary tract infections. Infection 2015; 43:89–94. [DOI] [PubMed] [Google Scholar]
- 29. Hawker JI, Smith S, Smith GE, et al. Trends in antibiotic prescribing in primary care for clinical syndromes subject to national recommendations to reduce antibiotic resistance, UK 1995–2011: analysis of a large database of primary care consultations. J Antimicrob Chemother 2014; 69:3423–30. [DOI] [PubMed] [Google Scholar]
- 30. Suda KJ, Hicks LA, Roberts RM, et al. Trends and seasonal variation in outpatient antibiotic prescription rates in the United States, 2006 to 2010. Antimicrob Agents Chemother 2014; 58:2763–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Durkin MJ, Jafarzadeh SR, Hsueh K, et al. Outpatient antibiotic prescription trends in the United States: a national cohort study. Infect Control Hosp Epidemiol 2018; 39:584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. The Joint Commission. New antimicrobial stewardship standard https://www.jointcommission.org/assets/1/6/New_Antimicrobial_Stewardship_Standard.pdf. Accessed 29 March 2018.
- 33. Centers for Disease Control and Prevention. The core elements of hospital antibiotic stewardship programs https://www.cdc.gov/antibiotic-use/healthcare/pdfs/core-elements.pdf. Accessed 29 March 2018. [DOI] [PMC free article] [PubMed]
- 34. Centers for Disease Control and Prevention. The core elements of outpatient antibiotic stewardship https://www.cdc.gov/getsmart/community/improving-prescribing/core-elements/core-outpatient-stewardship.html. Accessed 29 March 2018.
- 35. Drekonja DM, Filice GA, Greer N, et al. Antimicrobial stewardship in outpatient settings: a systematic review. Infect Control Hosp Epidemiol 2015; 36:142–52. [DOI] [PubMed] [Google Scholar]
- 36. Hecker MT, Fox CJ, Son AH, et al. Effect of a stewardship intervention on adherence to uncomplicated cystitis and pyelonephritis guidelines in an emergency department setting. PLoS One 2014; 9:e87899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meeker D, Knight TK, Friedberg MW, et al. Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med 2014; 174:425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA 2016; 315:562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ikäheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis 1996; 22:91–9. [DOI] [PubMed] [Google Scholar]
- 40. Riedle BN, Polgreen LA, Cavanaugh JE, et al. Phantom prescribing: examining the frequency of antimicrobial prescriptions without a patient visit. Infect Control Hosp Epidemiol 2017; 38:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.