Abstract
Mentha canadensis L. has important economic value for its abundance in essential oils. Menthol is the main component of M. canadensis essential oils, which is certainly the best-known monoterpene for its simple structure and wide applications. However, the regulation of menthol biosynthesis remains elusive in M. canadensis. In this study, transcriptome sequencing of M. canadensis with MeJA treatment was applied to illustrate the transcriptional regulation of plant secondary metabolites, especially menthol biosynthesis. Six sequencing libraries were constructed including three replicates for both control check (CK) and methyl jasmonate (MeJA) treatment and at least 8 Gb clean bases was produced for each library. After assembly, a total of 81,843 unigenes were obtained with an average length of 724 bp. Functional annotation indicated that 64.55% of unigenes could be annotated in at least one database. Additionally, 4430 differentially expressed genes (DEGs) with 2383 up-regulated and 2047 down-regulated transcripts were identified under MeJA treatment. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment indicated that “Monoterpenoid biosynthesis” was one of the most significantly enriched pathways in metabolism. Subsequently, DEGs involved in JA signal transduction, transcription factors, and monoterpene biosynthesis were analyzed. 9 orthologous genes involved in menthol biosynthesis were also identified. This is the first report of a transcriptome study of M. canadensis and will facilitate the studies of monoterpene biosynthesis in the genus Mentha.
Keywords: Mentha canadensis L., transcriptome sequencing, JA signaling, transcription factors, menthol biosynthesis
1. Introduction
The genus Mentha has important economic value for its abundance in essential oils that are widely used in the flavor, fragrance, and aromatherapy industries [1]. Monoterpenes are the major constituents of essential oils, which represent a large and diverse class of volatile C10 isoprenoids. The biosynthesis of monoterpenes originated in the upstream methylerythritol phosphate (MEP) pathway, then catalyzed by a series of enzymes. The catalytic mechanisms of monoterpene biosynthetic enzymes have been extensively studied in peppermint (Mentha × piperita L.) and spearmint (Mentha spicata L.), two well-known Mentha plants that have been employed as model systems for the study of monoterpene biosynthesis [2,3,4,5,6,7]. In genus Mentha, a variety of monoterpenes including (−)-menthol, (+)-neomenthol, (+)-isomenthol, (+)-carvone, (+)-menthofuran, and so on, are synthesized and stored in the peltate glandular trichomes on the aerial surfaces of the plant [8,9]. The oil compositions vary in different Mentha species and are regulated by transcriptional abundance, catalytic properties of enzyme catalysts, and cell type-specific epigenetic processes [9].
Mentha canadensis L., as the largest cultivated aromatic plant in the world, is mainly cultivated in China. Not only in the aromatic industry, M. canadensis is also widely used as a medicinal plant in traditional Chinese medicine [10]. The main component of M. canadensis essential oils is menthol which accounts for more than 78% of the total essential oils’ components [11]. Menthol is certainly the best-known monoterpene for its simple structure and wide applications. The earlier studies of M. canadensis mainly focused on the identification of active compounds and activity assays [12,13,14]. Since the absence of sequence data, the molecular mechanism of menthol biosynthesis in M. canadensis is still not clear. Only three menthol biosynthetic genes have been cloned in M. canadensis, including the Geranyl diphosphate synthase large subunit (GPPS-l), Geranyl diphosphate synthase small subunit (GPPS-s), and (−)-Limonene synthase (LS) [15].
The phytohormone jasmonate (JA) is an important regulator in plant responses to biotic and abiotic stresses as well as other metabolic pathways by extensive reprogramming of gene expression [16,17]. It is also an efficient elicitor of plant secondary metabolite production [18]. A lot of plant active ingredients including artemisinin, vinblastine, nicotine, taxol and ginsenoside may be transcriptional regulated by JA signal [19]. In Artemisia annua, high content of artemisinin could be produced in response to JA elicitor [20]. Some JA-responsive transcription factors (TFs) such as AaWRKY1, AaERF1, and AaERF2 could regulate the transcription of artemisinin biosynthetic genes and increase the accumulation of artemisinin [21,22,23]. So far, little is known about JA signal response and its effect on monoterpene biosynthesis in M. canadensis.
The transcriptome sequencing technology is a useful method to carry out genome-wide gene discovery and expression profiling for its high-throughput and accuracy. It has been widely used to explore plants’ physiological mechanism at the molecular level, such as model plants Arabidopsis thaliana [24] and Oryza sativa [25], as well as other non-model plants, such as Brassica napus [26], A. annua [27], and Salvia miltiorrhiza [28]. Using transcriptome sequencing, genome-wide changes in gene expression patterns under different treatment, such as hormone treatment, biotic and abiotic stress, could be easily accessed. For example, JA-mediated transcriptional reprogramming has been studied using RNA-seq in many plants, such as A. annua [27], Taraxacum koksaghyz [29], and Gentiana macrophylla [30].
In this study, high-throughput RNA-seq was applied to analyze the differential gene expression profiles of MeJA-treated M. canadensis versus the control. As a result, JA-responsive signal transduction genes, TFs, and monoterpene biosynthetic genes were identified. The results in this study would help us to further understand the reprogramming of JA-responsive gene expression in M. canadensis as well as facilitate studies of monoterpene biosynthesis in genus Mentha.
2. Results
2.1. Transcriptome Sequencing and De Novo Assembly of M. canadensis
Transcriptome sequencing was performed for M. canadensis under control check (CK) and MeJA treatment using Illumina HiSeq™ 4000. Six sequencing libraries were constructed including three replicates for both CK and MeJA treatment. As a result, each library produced at least 54 Mb clean reads and 8 Gb clean bases. The percentage of clean reads was more than 98% and Q20 percentage was at least 98.69% for the six libraries (Table 1). Then, clean reads of the six libraries were de novo assembled using Trinity program and finally 81,843 unigenes were generated with a GC percentage of 43.92%. The average length and N50 length for the assembled unigenes were 724 and 1126 bp, respectively (Table 2). The length distribution of the unigenes is indicated in Supplementary Figure S1 and 70.63% of all unigenes show lengths longer than 300 bp.
Table 1.
Summary of Illumina HiSeq4000 sequencing data.
| Samples a | Raw Reads | Clean Reads | Reads Length (bp) | Clean Bases | Q20 Percentage (%) b | GC Percentage (%) |
|---|---|---|---|---|---|---|
| CK-1 | 58,914,374 | 57,759,958 (98.04%) | 150 | 8,536,291,577 | 98.69 | 50.79 |
| CK-2 | 58,863,024 | 57,904,740 (98.37%) | 150 | 8,588,205,461 | 98.88 | 50.05 |
| CK-3 | 58,109,454 | 57,113,908 (98.29%) | 150 | 8,470,135,931 | 98.83 | 50.69 |
| MeJA-1 | 61,076,212 | 59,885,296 (98.05%) | 150 | 8,855,787,485 | 98.69 | 50.55 |
| MeJA-2 | 55,398,094 | 54,424,124 (98.24%) | 150 | 8,072,356,234 | 98.82 | 50.46 |
| MeJA-3 | 65,765,946 | 64,630,794 (98.27%) | 150 | 9,590,111,719 | 98.82 | 50.39 |
a CK and MeJA represent libraries constructed by CK and MeJA-treated samples, respectively. Numbers indicate three biological replicates; b Q20 percentage represents percentage of bases with a Phred value >20.
Table 2.
Statistics of assembly data.
| Total assembled bases | 59,279,270 |
| Unigene number | 81,843 |
| GC percentage (%) | 43.92 |
| N50 (bp) | 1126 |
| Average length (bp) | 724 |
2.2. Functional Annotation of Unigenes
For functional annotation analysis, all the assembled unigenes were searched against the public databases using BLAST. The results show that 52,700 (64.39%) unigenes could be annotated in Nr (non-redundant protein) database, 34,565 (42.23%) in Swissprot, 29,536 (36.09%) in KOG (Clusters of eukaryotic Orthologous Group), and 19,013 (23.23%) in KEGG (Kyoto Encyclopedia of Genes and Genomes). Taken together, there were 52,826 (64.55%) unigenes that could be annotated in at least one database (Table 3). GO (Gene Ontology), KOG and KEGG assignments were used to classify the function of the assembled unigenes of M. canadensis. For GO annotation, 4396, 4422 and 2566 unigenes were assigned to “biological process” category, “molecular function” category and “cellular component” category, respectively (Supplementary Figure S2). In the “molecular function” category, genes assigned to “binding” (2260) and “catalytic activity” (3326) accounted for the vast majority of this category. In the “biological process” category, “cellular process” (2891), “metabolic processes” (3447) and “single-organism process” (2344) were the main subcategories. For KOG classification, 29,536 unigenes were categorized into 25 KOG functional groups. Among them, “General functional prediction only” (9523) was the largest group, followed by “Signal transduction mechanisms” (5796) and “Posttranslational modification, protein turnover, chaperones” (4901) (Supplementary Figure S3). To further understand the biological functions of M. canadensis unigenes, KEGG pathway analysis was performed. As a result, a total of 10,365 unigenes could be mapped to 133 metabolic pathways. “Metabolism” (5104) was the largest category (Supplementary Figure S4).
Table 3.
Functional annotations of M. canadensis unigenes.
| Annotation Database | Number of Unigenes | Percentage (%) |
|---|---|---|
| Nr | 52,700 | 64.39 |
| Swissprot | 34,565 | 42.23 |
| KOG | 29,536 | 36.09 |
| KEGG | 19,013 | 23.23 |
| Annotated in at least one database | 52,826 | 64.55 |
| Total unigenes | 81,843 | 100 |
Nr: National Center for Biotechnology Information (NCBI) non-redundant protein database; KOG: eukaryotic Orthologous Group; KEGG: Kyoto Encyclopedia of Genes and Genomes.
2.3. Identification and Analysis of Differentially Expressed Genes (DEGs)
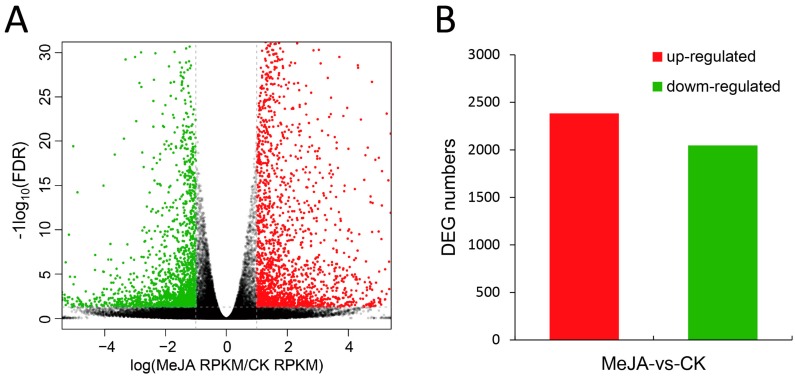
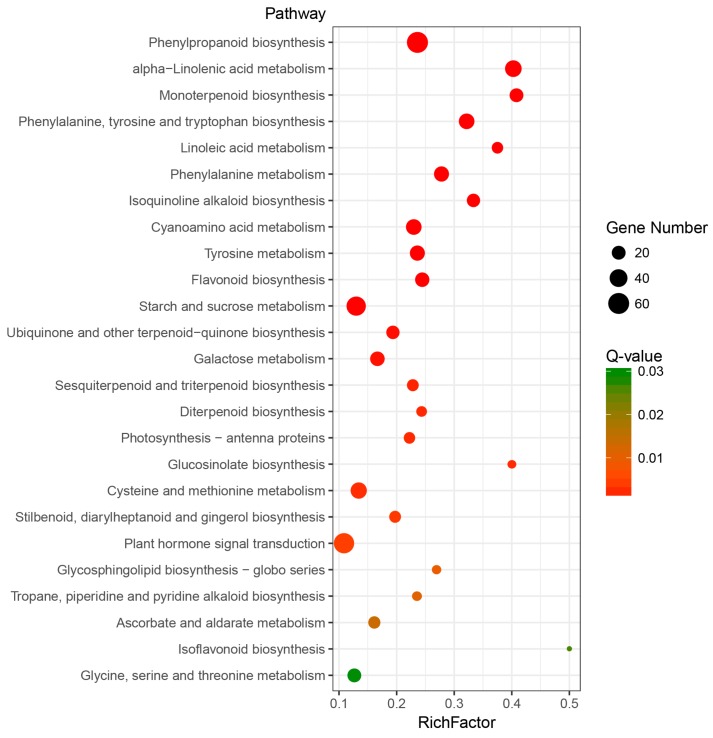
To identify differentially expressed genes under MeJA treatment, reads were mapped to the unigenes and reads per kb per million reads (RPKM) was used to measure the transcriptional levels. The Pearson correlation analysis indicated that there were high correlations between three biological replicates of both CK and MeJA treatment (Supplementary Figure S5). Then using a two-fold change of RPKM with False Discovery Rate (FDR) <0.05 as the cut-off criteria, 4430 differentially expressed genes (DEGs) were identified between CK and MeJA-treated samples in M. canadensis. Among them, 2383 DEGs were up-regulated and 2047 DEGs were down-regulated under MeJA treatment (Figure 1). To further explore the possible roles of the DEGs, GO and KEGG enrichment were conducted. For GO enrichment, 88, 27 and 6 GO terms were significantly enriched in “biological process”, “molecular function” and “cellular component” categories, respectively (Supplementary Table S1). “Terpene synthase activity” and “oxidoreductase activity” were the most significantly enriched terms in “molecular function”. In “biological process” category, biosynthetic processes involved in lipid, small molecule, dicarboxylic acid, phospholipid, terpene and sesquiterpene were the most significantly enriched terms. For KEGG pathway enrichment, 25 pathways were significantly enriched under MeJA treatment (Figure 2 and Supplementary Table S2). Of these, pathways involved in secondary metabolism accounted for a large part, including biosynthetic pathways of phenylpropanoid, terpenoid, alkaloid, flavonoid, glucosinolate and so on.
Figure 1.
Statistics of DEGs induced by MeJA in M. canadensis transcriptomes. (A) Volcano plots of the unigenes in the comparisons of MeJA-treated and CK samples; (B) DEG numbers in the comparisons of MeJA-treated and CK samples. RPKM: Reads per Kb per Million Reads, FDR: False Discovery Rate, DEG: Differentially Expressed Gene.
Figure 2.
KEGG pathway enrichment of DEGs induced by MeJA.
2.4. Identification and Expression Verification of JA Signal Pathway Genes under MeJA Treatment
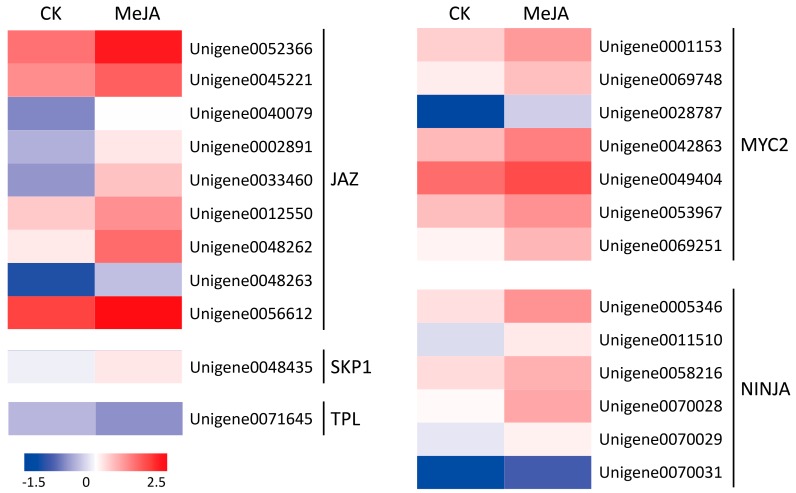
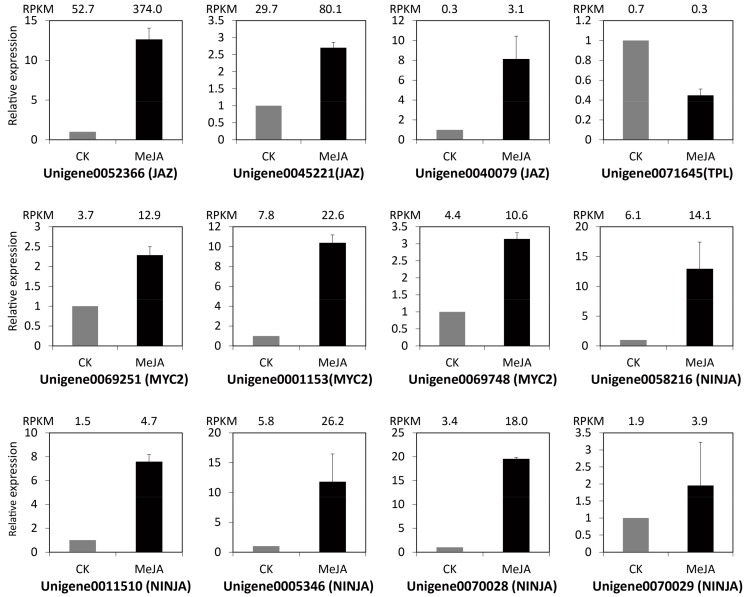
In Arabidopsis, the JA signal is perceived and transduced via the SCFCOI1-JAZ co-receptor complex. The SCFCOI1 complex includes COI1, ASK2, CULLIN1, Rbx, and E2. The JAZ repressors recruit the co-repressor complex consisting of Novel Interactor of JAZ (NINJA), TOPLESS (TPL), and histone deacetylase (HDA), interact with and repress the transcription activator bHLHzip transcription factor MYC2 (MYC2). The perception of JA-Ile by SCFCOI1 complex leads to degradation of JAZs via the 26S proteasome, then activate the downstream transcription factors of JA responses [17,31]. In this study, 24 DEGs associated with JA signal transduction were identified in M. canadensis. Of these, 23 DEGs showed up-regulation under MeJA treatment, which encode putative JAZ (9), MYC2 (7), NINJA (6), and S-phase kinase-associated protein 1 (SKP1) (1) (Figure 3). 12 of the 24 DEGs were selected for quantitative real-time PCR (qRT-PCR) to verify the reliability of the RNA-Seq data. The results showed that all the expression patterns of qRT-PCR were quite a good match with the RPKM results of RNA-Seq data (Figure 4). These validation results of qRT-PCR indicated that the RNA-Seq data were quite reliable.
Figure 3.
Heat maps of the DEGs in JA signal transduction pathway. JAZ, Jasmonate ZIM domain protein; MYC2, bHLHzip transcription factor MYC2; TPL, TOPLESS; NINJA, Novel interactor of JAZ; SKP1, S-phase kinase-associated protein 1.
Figure 4.
Expression validations of 12 selected JA signal transduction related genes in control and MeJA-treated samples using qRT-PCR. The RPKM values obtained from RNA-Seq data were indicated on the top of each graph.
2.5. Identification and Analysis of Differentially Expressed TFs under MeJA Treatment
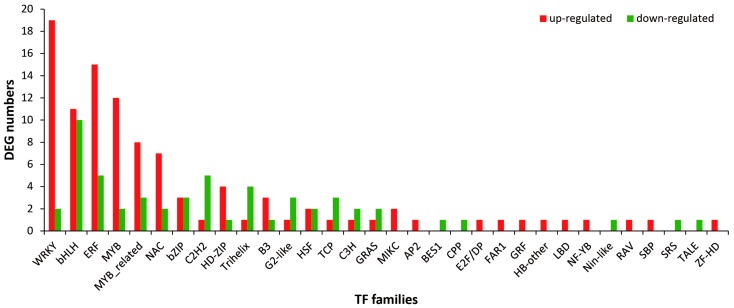
To further understand the transcriptional regulation of MeJA-treated M. canadensis, differentially expressed TFs were identified between CK and MeJA-treated samples. As a result, 1535 TFs belonged to 55 families were identified by annotating in PlantTFDB database. bHLH (basic/helix-loop-helix), ERF (ethylene-responsive factor), MYB_related (myeloblastosis DNA-binding related protein), C2H2 (C2H2 zinc-finger protein), MYB (myeloblastosis DNA-binding protein), WRKY (WRKY-type DNA binding protein), C3H (Cys3His zinc finger protein), NAC (NAC domain protein), bZIP (basic region/leucine zipper motif), and GRAS (Gibberellin-insensitive (GAI), Repressor of GA (RGA), and SCARECROW (SCR) protein) families were the top 10 largest TF families (Supplementary Table S3). Of the 1535 TFs, 157 belonged to 32 families were differentially expressed. WRKY, bHLH, ERF, MYB, MYB_related, NAC, bZIP, C2H2, HD-ZIP (Homeodomain-leucine zipper protein), and Trihelix families were the top 10 families that had the largest number of DEGs. Each of these 10 families had more than 5 DEGs and the WRKY and bHLH family were the largest differentially expressed families that both contained 21 DEGs (Figure 5 and Supplementary Table S3). Comparing the expression levels of these DEGs, 102 of the 157 DEGs were up-regulated and the other 55 were down-regulated. Considering the family distribution of the up-regulated and down-regulated DEGs, 19 families showed that the up-regulated unigenes were more abundant than the down-regulated ones, especially in the WRKY family, 19 unigenes were up-regulated and only 2 were down-regulated. On the contrary, there were 11 families that contained more down-regulated unigenes than up-regulated and 2 families contained the same number of up-regulated unigenes and down-regulated (Figure 5).
Figure 5.
Statistics of differentially expressed TFs under MeJA treatment.
2.6. Identification and Expression Verification of Monoterpenoids and Menthol Biosynthetic Genes under MeJA Treatment
In M. canadensis, monoterpenes are the major constituents of essential oils. In this study, the enrichment results of the KEGG database indicated that “Monoterpenoid biosynthesis” was one of the most significantly enriched pathways under MeJA treatment. 20 DEGs associated with monoterpenoid biosynthesis were identified, which consisted of genes encoding 10 terpene synthases (TPSs), 1 (−)-isopiperitenone reductase (IPR), 1 menthol dehydrogenase (MR), and 8 neomenthol dehydrogenases (NMRs). TPS is a multi-gene family that is widespread in plants, whose diversity and substrate complexity lead to the wide variety of terpenoids [32]. In this study, 10 differentially expressed TPSs were identified under MeJA treatment, 8 of which were up-regulated and the other 2 were down-regulated. The remaining 10 DEGs in “Monoterpenoid biosynthesis” were menthol/neomenthol biosynthesis related genes. Interestingly, the expression levels of the 10 DEGs were almost all up-regulated except for an NMR (Unigene0051701) under MeJA treatment (Figure 6).
Figure 6.
Heat maps of the DEGs in the monoterpenoid biosynthesis pathway. TPS, Terpene synthase; IPR, (−)-Isopiperitenone reductase; MR, (−)-Menthol dehydrogenase; NMR, (+)-Neomenthol dehydrogenase.
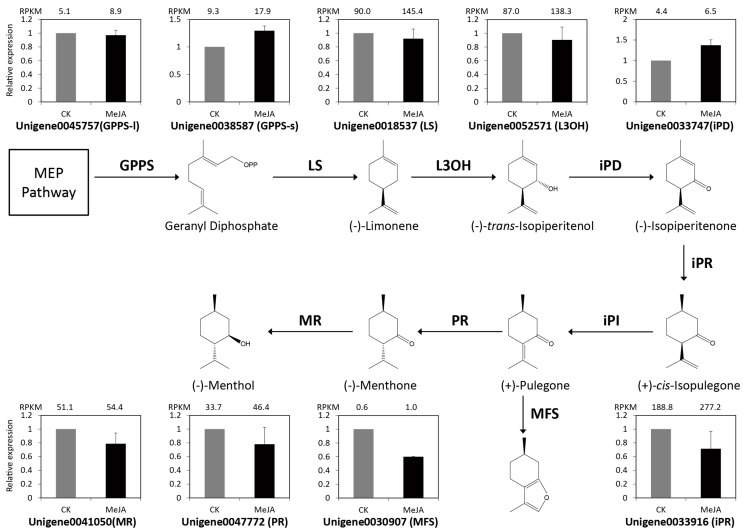
Menthol is the main constituent of monoterpenoids in M. canadensis, which is synthesized by a series of enzymatic reactions in the peltate glandular trichomes on the aerial surfaces of the plant. The biosynthetic pathway and enzyme catalysis mechanism of menthol has been extensively studied in peppermint and spearmint, two other species of genus Mentha [2,3,4,5,6]. Using the reference genes of peppermint and spearmint as queries, 9 orthologous genes were identified in M. canadensis (Supplementary Figure S6), including the previously reported GPPS-l, GPPS-s, and LS [15]. The other 6 genes that catalyze the biosynthesis of (−)-Menthol from (−)-Limonene were identified in M. canadensis for the first time. Considering the transcriptional levels of the 9 genes, although their RPKMs showed a certain level of increase after MeJA treatment, they did not reach significant levels above controls. Then, qRT-PCR was used to verify the expression of the 9 menthol biosynthetic genes (Figure 7). The results indicated that the expression levels of most genes were not significantly different between MeJA treatment and CK groups, which was consistent with the RNA-seq results. Among these genes, only Unigene0030907 (MFS) showed significant down-regulated expression after MeJA treatment. Unigene0038587 (GPPS-s) and Unigene0033747 (iPD) showed a certain degree of up-regulation in both qRT-PCR and RNA-Seq data.
Figure 7.
Expression validations of 9 menthol biosynthetic genes in control and MeJA-treated samples using qRT-PCR. The RPKM values obtained from RNA-Seq data are indicated on the top of each graph. GPPS, Geranyl diphosphate synthase; LS, (−)-Limonene synthase; L3OH, (−)-Limonene-3-hydroxylase; iPD, (−)-trans-Isopiperitenol dehydrogenase; iPR, (−)-Isopiperitenone reductase; iPI, (+)-cis-Isopulegone isomerase; PR, (+)-Pulegone reductase; MFS, Menthofuran synthase; MR, (−)-Menthol dehydrogenase.
3. Discussion
Transcriptome sequencing is an effective tool for large-scope gene identification and expression profiling analysis. In this study, high-throughput RNA-seq was applied to characterize the transcriptomes of M. canadensis treated with MeJA. Six sequencing libraries were constructed including three replicates for both CK and MeJA treatment and at least 8 Gb clean data was produced for each library. After assembly, a total of 81,843 unigenes were obtained with an average length of 724 bp. Functional annotation indicated that 64.55% of unigenes could be annotated in at least one database. This is the first reported transcriptome study of M. canadensis and will provide useful information for further study of this species.
Endogenous MeJA is believed to be a primary regulator of the JA signal pathway in plants. In Arabidopsis, the JA signal is perceived and transduced via the SCFCOI1-JAZ co-receptor complex. In the resting state, JAZ proteins interact with and repress downstream MYC2 to suppress of JA responses. In response to JA signal, JAZs are degraded by SCFCOI1-ubiquitin-proteasome, then MYC2 is released from the repressor complex to regulate gene expression [17,31]. In this transcriptome dataset, 24 DEGs associated with JA signal transduction were identified in M. canadensis. Of these, 23 DEGs showed up-regulation under MeJA treatment, encoding putative JAZ (9), MYC2 (7), TPL (1), NINJA (6), and SKP1 (1). qRT-PCR was also used to verify the reliability of the RNA-Seq data. These results suggested that application of exogenous MeJA may regulate the JA signaling pathway in M. canadensis.
TFs are sequence specific DNA-binding proteins that interact with the regulatory regions of the target genes and regulate their expression. Several transcriptome profiling studies have indicated that MeJA treatment triggers an extensive transcriptional reprogramming of metabolism [33,34,35]. A lot of TFs are elicited by MeJA and participate in regulation of specific metabolic processes [36]. Here, 157 differentially expressed TFs (102 up-regulated and 55 down-regulated) belonged to 32 families were identified by annotating in PlantTFDB database. It is worth noting that the WRKY family was the largest differentially expressed family containing 21 DEGs. It is also reported that more than 25% of WRKYs are induced in response to jasmonate in A. thaliana and Catharanthus roseus [37]. Of the 21 differentially expressed WRKYs, the number of up-regulated genes was much higher than that of down-regulated ones (19 vs. 2). Similar observations have been found in other plants. For example, 16 up-regulated and 3 down-regulated WRKY TFs were identified under MeJA treatment in A. annua [27]. In Lycoris aurea, 32 differentially expressed WRKY TFs were identified, of which 26 were up-regulated and only 6 were down-regulated [38]. The WRKY family is an important class of JA-responsive TFs that regulate plant secondary metabolism [39]. In A. annua, a WRKY TF AaWRKY1 was strongly induced by MeJA and regulated the expression of ADS, a key gene of artemisinin biosynthesis [21]. In Panax quinquefolius, a MeJA-responsive WRKY TF PqWRKY1 was isolated, which could positively regulate the biosynthesis of triterpene ginsenoside [40]. The large number of up-regulated WRKY TFs in M. canadensis suggests that they may function in JA-responsive transcriptional regulation of secondary metabolism.
Monoterpenes are the major constituents of M. canadensis essential oils. In this study, the KEGG pathway enrichment of DEGs indicated that “Monoterpenoid biosynthesis” was the most significantly enriched pathway under the treatment of MeJA. 20 DEGs associated with monoterpenoid biosynthesis were identified, which consisted of genes encoding TPS (10), IPR (1), MR (1), and NMR (8). Of these, genes encoding IPR, MR, and NMR were involved in menthol and neomenthol biosynthesis and interestingly, the expression levels of the 10 DEGs were almost all up-regulated under MeJA treatment. These results suggest that the biosynthesis of monoterpenes including menthol and neomenthol might be elicited by the MeJA signal in M. canadensis. The biosynthesis pathway of menthol has been extensively studied in peppermint and spearmint [2,3,4,5,6]. In this study, 9 orthologous genes were identified in M. canadensis including 6 new reported genes. However, expression analysis of these 9 genes in CK and MeJA-treated samples indicated that most of the gene expression levels did not change significantly after treatment. qRT-PCR results indicated that GPPS-s and iPD showed a certain degree of up-regulation and MFS was down-regulated. We speculate that only part of the menthol biosynthetic genes responds to the JA signal in M. canadensis. The response mechanism of menthol biosynthetic genes to JA signaling needs further study in M. canadensis, especially the possible DEGs including GPPS-s, iPD, and MFS. Another explanation might be that different secondary metabolic pathways are controlled by different regulatory modules. For example, in Medicago truncatula, genes involved in phenylpropanoid biosynthesis were transiently induced after application of MeJA at low concentrations (0.5–5 μmol/L), but triterpene biosynthetic genes were induced after 12–24 h at high concentrations (5–500 μmol/L) [41]. Many M. canadensis menthol biosynthetic genes might not respond to the MeJA signal under our treatment conditions (200 μmol/L, 24 h).
Transcriptional regulation is central to plant secondary metabolism. Compared with well-studied metabolic pathways such as flavonoids, the transcriptional regulation of terpene metabolism has been validated in a few studies. Several TFs have been identified from plants including A. annua (AaWRKY1, AaERF1, AaERF2, AabZIP1, and AaMYC2), Gossypium arboretum (GaWRKY1), Taxus chinensis (TaWRKY1), Hevea brasiliensis (EREBP1 and HbWRKY1), and O. sativa (OsTGAP1) that regulate terpene biosynthesis [21,22,23,42,43,44,45,46,47,48]. In Mentha species M. spicata, two TFs MsYABBY5 and MsMYB (M. spicata MYB DNA-binding protein) have been reported that negatively regulate monoterpene production [49,50]. In this study, homologous genes of the two TFs (Unigene0062650, homolog to MsYABBY5 and Unigene0030793, homolog to MsMYB) were identified in M. canadensis. Sequence alignment indicated that both genes had quite high similarities between M. spicata and M. canadensis (Supplementary Figure S7). Expression patterns deduced from RPKM values indicated that the expression of the two homologous genes declined to a certain extent under MeJA treatment (52.9-vs.-35.6 for Unigene0062650 and 0.72-vs.-0.38 for Unigene0030793), although the decline was not significantly (two-fold change with FDR <0.05). These results suggest that Unigene0062650 and Unigene0030793 might be MeJA-responsive TFs and similar negative regulation mechanism may also exist in M. canadensis.
4. Materials and Methods
4.1. Plant Material and JA Treatment
The M. canadensis used for this study was maintained at the Germplasm Nursery in the Institute of Botany, Jiangsu Province and Chinese Academy of Sciences, Nanjing, Jiangsu Province. Mint plants were planted in plastic pots containing a mixture of organic nutrient soil (XingNong Organic Fertilizer Co., Ltd., Zhenjiang, China) and vermiculite (3:1, v/v). The plants were cultured in an artificial climate chamber (Jiangnan, Ningbo, China) under 14 h light/10 h dark cycles (120 μmol/m2/s) with a constant temperature at 25 °C. For MeJA (Aladdin, Shanghai, China) treatment, plants were sprayed with 10 mL 200 μmol/L MeJA that dissolved with 2% ethanol (Sangon, Shanghai, China), and further wrapped in plastic wrap for 24 h to prevent water evaporation. 2% ethanol solution was used as control and the plants were also wrapped in plastic wrap. After 24 h, leaves of mint plants were harvested and frozen in liquid nitrogen and stored at −80 °C. Three biological replicates for both CK and MeJA treatment were performed.
4.2. RNA Extraction, cDNA Library Construction and Illumina Sequencing
Total RNA of the M. canadensis samples was extracted using RNAiso Plus (Takara, Dalian, China) according to the manufacturer’s instructions. The quality and concentration of RNAs were measured using a ND-2000 UV spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA) and Agilent 2100 (Agilent, Santa Clara, CA, USA). cDNA library construction and Illumina sequencing were performed by Gene Denovo Biotechnology Co. (Guangzhou, China). Briefly, mRNA was enriched from total RNA by Oligo(dT) beads and then fragmented into short fragments. The fragmented mRNA was reverse transcribed into cDNA with random primers and second-strand cDNA was subsequently synthesized. Then the cDNA fragments were purified, end repaired, poly(A) added, and ligated to sequencing adapters. After size selection by agarose gel electrophoresis, the ligation products were PCR amplified and sequenced using Illumina HiSeqTM 4000 (Illumina, San Diego, USA).
4.3. Transcriptome Assembly and Annotation
The raw reads obtained from RNA sequencing were filtered by trimming adapters and removing low quality reads (reads containing more than 10% of unknown nucleotides (N) or 40% of low quality (Q-value ≤ 10) bases) to obtain the high-quality clean reads. All data generated in this study have been deposited in the National Center for Biotechnology Information (NCBI) and can be accessed in the Short Read Archive (SRA) Sequence Database under accession number SRP132644. Then, Trinity program was used to perform de novo assembly of the clean data [51]. After assembly, unigenes were obtained and functional annotation was carried out using BLASTx program (http://www.ncbi.nlm.nih.gov/BLAST/) with an E-value threshold of 1 × 10−5. Public databases including the NCBI non-redundant protein database (Nr) (http://www.ncbi.nlm.nih.gov), the SwissProt database (http://www.expasy.ch/sprot), the KEGG (http://www.genome.jp/kegg), and the COG/KOG database (http://www.ncbi.nlm.nih.gov/COG) were used to annotate the M. canadensis unigenes. TFs were identified by aligning unigenes to the PlantTFDB database [52].
4.4. Differentially Expressed Genes Analysis
The abundances of unigenes were calculated and normalized to RPKM [53]. To identify DEGs between MeJA-treated samples and controls, the edgeR package (http://www.r-project.org/) was used. Genes with a fold change ≥2 and a FDR <0.05 in a comparison were identified as significant DEGs. Pathway enrichment analysis was conducted by comparing gene numbers of each pathway in DEGs to genome background. The significance test of enriched pathways was determined by calculating the p-value and FDR correction. Heatmaps of DEGs were generated using HemI [54].
4.5. Quantitative Real-Time PCR
qRT-PCR was conducted to verify the expression of selected genes in M. canadensis. Goldenstar™ RT6 cDNA Synthesis Kit (TsingKe Biotech, Nanjing, China) was used with 1 μg of total RNA to synthesize first-strand cDNA. The qRT-PCR reactions were carried out using the qTOWER2.2 Real-Time PCR Systems (Analytik, Jena, Germany). 2 × T5 Fast qPCR Mix Kit (SYBR Green I) (TsingKe Biotech, Nanjing, China) was used to prepare qRT-PCR reactions with 2 μL of diluted cDNA as a template. The reaction systems and steps were performed according to the manufacturer’s instructions. The M. canadensis actin gene was used as a control to normalize the relative expression levels of target genes. All results were representative of three independent experiments. Primers used for qRT-PCR were listed in Supplementary Table S4.
5. Conclusions
In this study, high-throughput RNA-seq was applied to characterize the transcriptomes of M. canadensis treated with MeJA. A total of 81,843 unigenes were obtained and 64.55% of which could be functionally annotated in at least one database. Additionally, 4430 DEGs with 2383 up-regulated and 2047 down-regulated transcripts were identified under MeJA treatment. A lot of unigenes associated with JA signal transduction were up-regulated, which suggested that application of exogenous MeJA may regulate the JA signaling pathway in M. canadensis. KEGG enrichment indicated that “Monoterpenoid biosynthesis” was one of the most significantly enriched pathways in metabolism. 9 orthologous genes involved in menthol biosynthesis were identified in M. canadensis and the response mechanism of menthol biosynthetic genes to JA signaling needs further study, especially the possible DEGs including GPPS-s, iPD, and MFS. 157 differentially expressed TFs belonged to 32 families were identified and the WRKY family was the largest differentially expressed family. The number of up-regulated WRKY TFs was much higher than that of down-regulated ones (19 vs. 2). The large number of up-regulated WRKY TFs in M. canadensis suggests that they may play important roles in JA-responsive transcriptional regulation of secondary metabolism. However, the regulation mechanism of JA signaling on development and metabolism of M. canadensis still requires further characterization and putative DEGs identified in this study might be important targets. This is the first reported transcriptome study of M. canadensis and will provide useful information for further study of this species.
Abbreviations
| MeJA | Methyl Jasmonate |
| CK | Control Check |
| MEP pathway | Methylerythritol Phosphate Pathway |
| GO | Gene Ontology |
| KOG | Eukaryotic Orthologous Group |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| DEGs | Differentially Expressed Genes |
| RPKM | Reads per Kb per Million Reads |
| FDR | False Discovery Rate |
| qRT-PCR | Quantitative Real-Time PCR |
| TF | Transcription Factor |
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/19/8/2364/s1.
Author Contributions
C.L. and W.L. conceived and designed the experiments. X.Y., H.L. and L.L. Y.C. performed the experiments. X.Q., H.F., Z.C. and D.X. analyzed the data. X.Q. wrote the paper.
Funding
The work was supported by the National Natural Science Foundation of China (30600051) and Fund of Jiangsu Key Laboratory for the Research and Utilization of Plant Resources (JSPKLB201811).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lange B.M., Ahkami A. Metabolic engineering of plant monoterpenes, sesquiterpenes and diterpenes-current status and future opportunities. Plant Biotechnol. J. 2013;11:169–196. doi: 10.1111/pbi.12022. [DOI] [PubMed] [Google Scholar]
- 2.Croteau R., Karp F., Wagschal K.C., Satterwhite D.M., Hyatt D.C., Skotland C.B. Biochemical characterization of a spearmint mutant that resembles peppermint in monoterpene content. Plant Physiol. 1991;96:744–752. doi: 10.1104/pp.96.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner G.W., Croteau R. Organization of monoterpene biosynthesis in Mentha, immunocytochemical localizations of geranyl diphosphate synthase, limonene-6-hydroxylase, isopiperitenol dehydrogenase, and pulegone reductase. Plant Physiol. 2004;136:4215–4227. doi: 10.1104/pp.104.050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croteau R.B., Davis E.M., Ringer K.L., Wildung M.R. (−)-Menthol biosynthesis and molecular genetics. Naturwissenschaften. 2005;92:562–577. doi: 10.1007/s00114-005-0055-0. [DOI] [PubMed] [Google Scholar]
- 5.Munoz-Bertomeu J., Ros R., Arrillaga I., Segura J. Expression of spearmint limonene synthase in transgenic spike lavender results in an altered monoterpene composition in developing leaves. Metab. Eng. 2008;10:166–177. doi: 10.1016/j.ymben.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Lange B.M., Mahmoud S.S., Wildung M.R., Turner G.W., Davis E.M., Lange I., Baker R.C., Boydston R.A., Croteau R.B. Improving peppermint essential oil yield and composition by metabolic engineering. Proc. Natl. Acad. Sci. USA. 2011;108:16944–16949. doi: 10.1073/pnas.1111558108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Champagne A., Boutry M. Proteomic snapshot of spearmint (Mentha spicata L.) leaf trichomes: A genuine terpenoid factory. Proteomics. 2013;13:3327–3332. doi: 10.1002/pmic.201300280. [DOI] [PubMed] [Google Scholar]
- 8.Lange B.M. Advances in Biochemical Engineering/Biotechnology. Springer; Berlin/Heidelberg, Germany: 2015. Biosynthesis and biotechnology of high-value p-menthane monoterpenes, including menthol, carvone, and limonene; pp. 319–353. [DOI] [PubMed] [Google Scholar]
- 9.Ahkami A., Johnson S.R., Srividya N., Lange B.M. Multiple levels of regulation determine monoterpenoid essential oil compositional variation in the mint family. Mol. Plant. 2015;8:188–191. doi: 10.1016/j.molp.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Chinese Pharmacopoeia Commission . The Pharmacopoeia of the People’s Republic of China. 2015 ed. Medical Science Press; Beijing, China: 2015. 377p. [Google Scholar]
- 11.Zhao D., Xu Y.W., Yang G.L., Husaini A.M., Wu W. Variation of essential oil of Mentha haplocalyx Briq. and Mentha spicata L. from China. Ind. Crops Prod. 2013;42:251–260. doi: 10.1016/j.indcrop.2012.06.010. [DOI] [Google Scholar]
- 12.Liang C.Y., Li W.L., Xia B., Liu Y., Yu X. Chemical composition of essential oils of two Mentha species. Chem. Nat. Compd. 2010;46:656–657. doi: 10.1007/s10600-010-9705-3. [DOI] [Google Scholar]
- 13.Cao G., Shan Q., Li X., Cong X., Zhang Y., Cai H., Cai B. Analysis of fresh Mentha haplocalyx volatile components by comprehensive two-dimensional gas chromatography and high-resolution time-of-flight mass spectrometry. Analyst. 2011;136:4653–4661. doi: 10.1039/c1an15616k. [DOI] [PubMed] [Google Scholar]
- 14.Dong W., Ni Y., Kokot S. A novel near-infrared spectroscopy and chemometrics method for rapid analysis of several chemical components and antioxidant activity of mint (Mentha haplocalyx Briq.) samples. Appl. Spectrosc. 2014;68:245–254. doi: 10.1366/13-07091. [DOI] [PubMed] [Google Scholar]
- 15.Wang H.T., Yu X., Liu Y., Liang C.Y., Li W.L. Analysis of genetic variability and relationships among Mentha L. using the limonene synthase gene, LS. Gene. 2013;524:246–252. doi: 10.1016/j.gene.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Pauwels L., Inzé D., Goossens A. Jasmonate-inducible gene: What does it mean? Trends Plant Sci. 2009;14:87–91. doi: 10.1016/j.tplants.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Wasternack C., Hause B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in annals of botany. Ann. Bot. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Geyter N., Gholami A., Goormachtig S., Goossens A. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. 2012;17:349–359. doi: 10.1016/j.tplants.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Afrin S., Huang J.J., Luo Z.Y. JA-mediated transcriptional regulation of secondary metabolism in medicinal plants. Sci. Bull. 2015;60:1062–1072. doi: 10.1007/s11434-015-0813-0. [DOI] [Google Scholar]
- 20.Maes L., Van Nieuwerburgh F.C., Zhang Y., Reed D.W., Pollier J., Vande Casteele S.R., Inzé D., Covello P.S., Deforce D.L., Goossens A. Dissection of the phytohormonal regulation of trichome formation and biosynthesis of the antimalarial compound artemisinin in Artemisia annua plants. New Phytol. 2011;189:176–189. doi: 10.1111/j.1469-8137.2010.03466.x. [DOI] [PubMed] [Google Scholar]
- 21.Ma D., Pu G., Lei C., Ma L., Wang H., Guo Y., Chen J., Du Z., Wang H., Li G., et al. Isolation and characterization of AaWRKY1, an Artemisia annua transcription factor that regulates the amorpha-4,11-diene synthase gene, a key gene of artemisinin biosynthesis. Plant Cell Physiol. 2009;50:2146–2161. doi: 10.1093/pcp/pcp149. [DOI] [PubMed] [Google Scholar]
- 22.Yu Z.X., Li J.X., Yang C.Q., Hu W.L., Wang L.J., Chen X.Y. The jasmonate-responsive AP2/ERF transcription factors AaERF1 and AaERF2 positively regulate artemisinin biosynthesis in Artemisia annua L. Mol. Plant. 2012;5:353–365. doi: 10.1093/mp/ssr087. [DOI] [PubMed] [Google Scholar]
- 23.Lu X., Jiang W., Zhang L., Zhang F., Zhang F., Shen Q., Wang G., Tang K. AaERF1 positively regulates the resistance to Botrytis cinerea in Artemisia annua. PLoS ONE. 2013;8:e57657. doi: 10.1371/journal.pone.0057657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eroglu S., Aksoy E. Genome-wide analysis of gene expression profiling revealed that COP9 signalosome is essential for correct expression of Fe homeostasis genes in Arabidopsis. Biometals. 2017;30:685–698. doi: 10.1007/s10534-017-0036-8. [DOI] [PubMed] [Google Scholar]
- 25.Kumar M., Gho Y.S., Jung K.H., Kim S.R. Genome-wide identification and analysis of genes, conserved between japonica and indica rice cultivars, that respond to low-temperature stress at the vegetative growth stage. Front. Plant Sci. 2017;8:1120. doi: 10.3389/fpls.2017.01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di F., Jian H., Wang T., Chen X., Ding Y., Du H., Lu K., Li J., Liu L. Genome-wide analysis of the PYL gene family and identification of PYL genes that respond to abiotic stress in Brassica napus. Genes. 2018;9:156. doi: 10.3390/genes9030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hao X., Zhong Y., Fu X., Lv Z., Shen Q., Yan T., Shi P., Ma Y., Chen M., Lv X., et al. Transcriptome analysis of genes associated with the artemisinin biosynthesis by jasmonic acid treatment under the light in Artemisia annua. Front. Plant Sci. 2017;8:971. doi: 10.3389/fpls.2017.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H., Wei T., Wang X., Zhang L., Yang M., Chen L., Song W., Wang C., Chen C. Transcriptome analyses from mutant Salvia miltiorrhiza reveals important roles for SmGASA4 during plant development. Int. J. Mol. Sci. 2018;19:2088. doi: 10.3390/ijms19072088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao X., Yan J., Lei J., Li J., Zhu J., Zhang H. De novo transcriptome sequencing of MeJA-induced Taraxacum koksaghyz Rodin to identify genes related to rubber formation. Sci. Rep. 2017;7:15697. doi: 10.1038/s41598-017-14890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao X., Guo X., Yang X., Wang H., Hua W., He Y., Kang J., Wang Z. Transcriptional responses and gentiopicroside biosynthesis in methyl jasmonate-treated Gentiana macrophylla seedlings. PLoS ONE. 2016;11:e0166493. doi: 10.1371/journal.pone.0166493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasternack C., Song S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transciption. J. Exp. Bot. 2017;68:1303–1321. doi: 10.1093/jxb/erw443. [DOI] [PubMed] [Google Scholar]
- 32.Tholl D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 2006;9:297–304. doi: 10.1016/j.pbi.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Pauwels L., Morreel K., Witte E.D., Lammertyn F., Montagu M.V., Boerjan W., Inzé D., Goossens A. Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc. Natl. Acad. Sci. USA. 2008;105:1380–1385. doi: 10.1073/pnas.0711203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L., Chen J., Zhou X., Chen X., Li Q., Tan H., Dong X., Xiao Y., Chen L., Chen W. Dynamic metabolic and transcriptomic profiling of methyl jasmonate-treated hairy roots reveals synthetic characters and regulators of lignan biosynthesis in Isatis indigotica Fort. Plant Biotechnol. J. 2016;14:2217–2227. doi: 10.1111/pbi.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao H., Nuruzzaman M., Xiu H., Huang J., Wu K., Chen X., Li J., Wang L., Jeong J.H., Park S.J., et al. Transcriptome analysis of methyl jasmonate-elicited Panax ginseng adventitious roots to discover putative ginsenoside biosynthesis and transport genes. Int. J. Mol. Sci. 2015;16:3035–3057. doi: 10.3390/ijms16023035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou M., Memelink J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol. Adv. 2016;34:441–449. doi: 10.1016/j.biotechadv.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Schluttenhofer C., Pattanaik S., Patra B., Yuan L. Analyses of Catharanthus roseus and Arabidopsis thaliana WRKY transcription factors reveal involvement in jasmonate signaling. BMC Genom. 2014;15:502. doi: 10.1186/1471-2164-15-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang R., Xu S., Wang N., Xia B., Jiang Y., Wang R. Transcriptome analysis of secondary metabolism pathway, transcription factors, and transporters in response to methyl jasmonate in Lycoris aurea. Front. Plant Sci. 2017;7:1971. doi: 10.3389/fpls.2016.01971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phukan U.J., Jeena G.S., Shukla R.K. WRKY transcription factors: Molecular regulation and stress responses in plants. Front. Plant Sci. 2016;7:760. doi: 10.3389/fpls.2016.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y., Niu Y., Xu J., Li Y., Luo H., Zhu Y., Liu M., Wu Q., Song J., Sun C., et al. Discovery of WRKY transcription factors through transcriptome analysis and characterization of a novel methyl jasmonate-inducible PqWRKY1 gene from Panax quinquefolius. Plant Cell Tissue Organ Cult. 2013;114:269–277. doi: 10.1007/s11240-013-0323-1. [DOI] [Google Scholar]
- 41.Suzuki H., Reddy M.S., Naoumkina M., Aziz N., May G.D., Huhman D.V., Sumner L.W., Blount J.W., Mendes P., Dixon R.A. Methyl jasmonate and yeast elicitor induce differential transcriptional and metabolic re-programming in cell suspension cultures of the model legume Medicago truncatula. Planta. 2005;220:696–707. doi: 10.1007/s00425-004-1387-2. [DOI] [PubMed] [Google Scholar]
- 42.Zhang F., Fu X., Lv Z., Lu X., Shen Q., Zhang L., Zhu M., Wang G., Sun X., Liao Z., et al. A basic leucine zipper transcription factor, AabZIP1, connects abscisic acid signaling with artemisinin biosynthesis in Artemisia annua. Mol. Plant. 2015;8:163–175. doi: 10.1016/j.molp.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Shen Q., Lu X., Yan T., Fu X., Lv Z., Zhang F., Pan Q., Wang G., Sun X., Tang K. The jasmonate-responsive AaMYC2 transcription factor positively regulates artemisinin biosynthesis in Artemisia annua. New Phytol. 2016;210:1269–1281. doi: 10.1111/nph.13874. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y.H., Wang J.W., Wang S., Wang J.Y., Chen X.Y. Characterization of GaWRKY1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-d-cadinene synthase-A. Plant Physiol. 2004;135:507–515. doi: 10.1104/pp.104.038612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C., Wu J., Mei X. Enhancement of taxol production and excretion in Taxus chinensis cell culture by fungal elicitation and medium renewal. Appl. Microbiol. Biotechnol. 2001;55:404–410. doi: 10.1007/s002530000567. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y.Y., Wang L.F., Dai L.J., Yang S.G., Tian W.M. Characterization of HbEREBP1, a wound-responsive transcription factor gene in laticifers of Hevea brasiliensis Muell. Arg. Mol. Biol. Rep. 2012;39:3713–3719. doi: 10.1007/s11033-011-1146-y. [DOI] [PubMed] [Google Scholar]
- 47.Zhou M., Wu L., Liang J., Shen C., Lin J. Expression analysis and functional characterization of a novel cold-responsive gene CbCOR15a from Capsella bursa-pastoris. Mol. Biol. Rep. 2012;39:5169–5179. doi: 10.1007/s11033-011-1313-1. [DOI] [PubMed] [Google Scholar]
- 48.Miyamoto K., Matsumoto T., Okada A., Komiyama K., Chujo T., Yoshikawa H., Nojiri H., Yamane H., Okada K. Identification of target genes of the bZIP transcription factor OsTGAP1, whose overexpression causes elicitor-induced hyperaccumulation of diterpenoid phytoalexins in rice cells. PLoS ONE. 2014;9:e105823. doi: 10.1371/journal.pone.0105823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Q., Reddy V.A., Panicker D., Mao H., Kumar N., Rajan C., Venkatesh P.N., Chua N., Sarojam R. Metabolic engineering of terpene biosynthesis in plants using a trichome-specific transcription factor MsYABBY5 from spearmint (Mentha spicata) Plant Biotechnol. J. 2016;14:1619–1632. doi: 10.1111/pbi.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reddy V.A., Wang Q., Dhar N., Kumar N., Venkatesh P.N., Rajan C., Panicker D., Sridhar V., Mao H.Z., Sarojam R. Spearmint R2R3-MYB transcription factor MsMYB negatively regulates monoterpene production and suppresses the expression of geranyl diphosphate synthase large subunit (MsGPPS.LSU) Plant Biotechnol. J. 2017;15:1105–1119. doi: 10.1111/pbi.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q., et al. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin J., Tian F., Yang D.C., Meng Y.Q., Kong L., Luo J., Gao G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017;45:D1040–D1045. doi: 10.1093/nar/gkw982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mortazavi A., Williams B.A., Mccue K., Schaeffer L., Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 54.Deng W., Wang Y., Liu Z., Cheng H., Xue Y. HemI: A toolkit for illustrating heatmaps. PLoS ONE. 2014;9:e111988. doi: 10.1371/journal.pone.0111988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.