Abstract
It has been well established that hypoxia significantly increases both cellular and tumor resistance to ionizing radiation. Hypoxia associated radiation resistance has been known for some time but there has been limited success in sensitizing cells to radiation under hypoxic conditions. These studies show that, when irradiated with low linear energy transfer (LET) gamma-rays, poly (ADP-ribose), polymerase (PARP), Fanconi Anemia (FANC), and mutant Chinese Hamster Ovary (CHO) cells respond similarly to the non-homologous end joining (NHEJ) and the homologous recombination (HR) repair mutant CHO cells. Comparable results were observed in cells exposed to 13 keV/μm carbon ions. However, when irradiated with higher LET spread out Bragg peak (SOBP) carbon ions, we observed a decrease in the oxygen enhancement ratio (OER) in all the DNA of repair mutant cell lines. Interestingly, PARP mutant cells were observed as having the largest decrease in OER. Finally, these studies show a significant increase in the relative biological effectiveness (RBE) of high LET SOBP carbon and iron ions in HR and PARP mutants. There was also an increase in the RBE of NHEJ mutants when irradiated to SOBP carbon and iron ions. However, this increase was lower than in other mutant cell lines. These findings indicate that high LET radiation produces unique types of DNA damage under hypoxic conditions and PARP and HR repair pathways play a role in repairing this damage.
Keywords: ionizing radiation, DNA repair, LET, OER, RBE
1. Introduction
Radiation-induced DNA damage results in chromosome aberrations, mutation, transformation, and cell death [1]. Ionizing radiation produces a variety of DNA damage, including, but not limited to: DNA double strand breaks; single strand breaks; base damages; and, crosslinks [2]. Due to the destructive nature of these DNA lesions, these cells have developed specific repair pathways to fix radiation induced DNA damage. Double strand breaks are the most lethal form of DNA damage and are primarily repaired by the non-homologous end joining (NHEJ) and homologous recombination (HR) repair pathways. These repair pathways are cell cycle dependent, with NHEJ functioning in G1/S/G2 and HR only functioning in S/G2. The loss of these repair pathways results in hypersensitivity to ionizing radiation and other DNA damaging agents [3,4]. Unrepaired or improperly repaired damage results in the formation of chromosome aberrations. The formation of dicentrics, translocations, and interstitial deletions, also results in the formation of micronuclei [5]. It has been well established that micronuclei can be utilized as a marker of radiation damage and radiation sensitivity [6,7,8,9].
The biological effects of ionizing radiation are heavily dependent on the presence of oxygen. In fact, the main mechanism of how low linear energy transfer (LET) radiation induces damage is through the formation of radical oxygen species [10,11]. The absence of oxygen in irradiated matter dramatically decreases damaging effects of radiation. Oxygen molecules chemically fix the DNA lesions produced by ionizing radiation. The degree of sensitization associated with oxygen is known as the oxygen enhancement ratio (OER). Typically, OER values are similar between all types of low LET radiation when using cell survival as an endpoint [12,13,14,15]. Additionally, it has been reported that OER is dependent on both LET and the presence or absence of DNA repair pathways [16]. As the LET of the radiation increases, OER values typically decrease [17,18]. High LET radiation such as alpha particles have been shown to have OER values of almost 1. This indicates that oxygen has almost no effect on cellular sensitivity to radiation [19].
High LET radiation, such as accelerated carbon–ions and the high-energy nuclei component of galactic cosmic rays, induce more biological effects, per absorbed dose, when compared to low LET radiation. High LET radiation is densely ionizing, which results in complex DNA damages that is not only difficult to repair, but may also require multiple DNA repair pathways to repair. As a result, high LET radiation has a higher RBE (Relative Biological Effectiveness) than low LET radiation. The loss of the NHEJ repair pathway results in high LET having a similar RBE as low LET radiation [20,21]. This suggests that the NHEJ repair pathway contributes to the repair of both low and high LET radiation damage. High LET radiation may produce DNA damage that oxygen reacts to differently and can potentially require different DNA repair pathways when the cell is under hypoxic conditions.
This study aims to investigate the role of various DNA repair pathways in response to DNA damage produced by high LET radiation under hypoxic condition. To do this, Chinese hamster ovary (CHO) cell lines with DNA repair defects in seven genes and four different radiation qualities were selected; cell survival was analyzed following their exposure to various LET radiation under aerobic and hypoxic conditions.
2. Results
2.1. Cell Survival
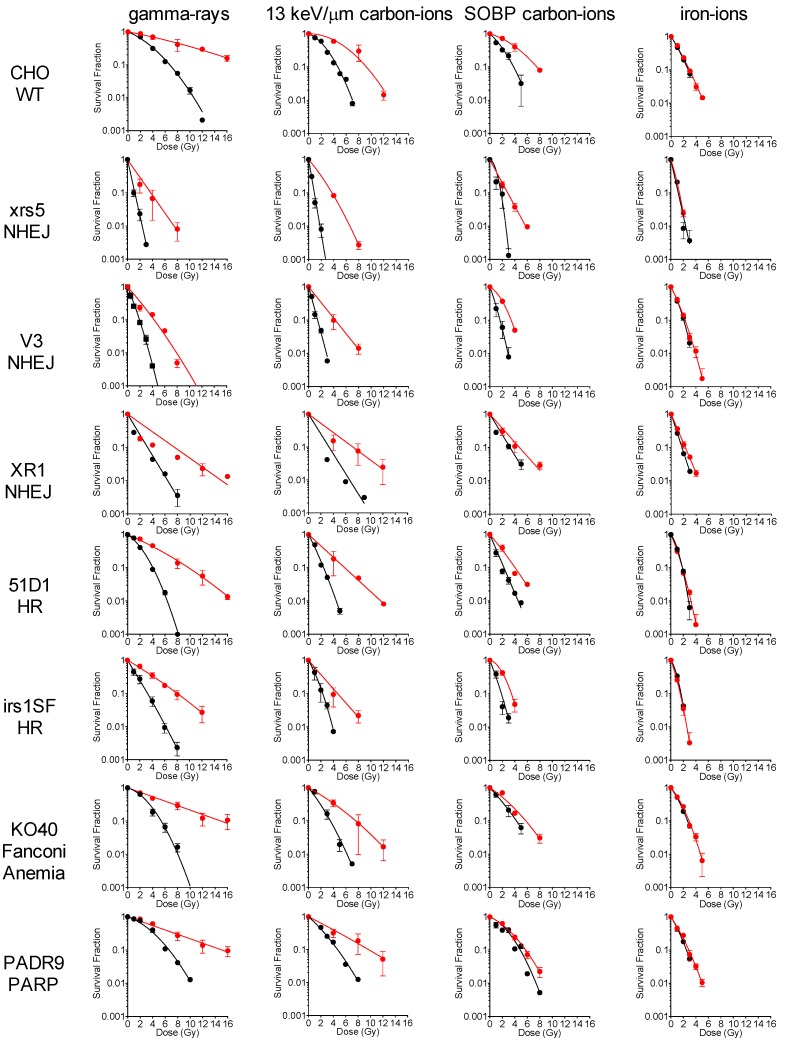
To determine the effects of hypoxia on the radiosensitivity of wild type and various DNA repair deficient cells, a variety of cells were irradiated with varying qualities of radiation under aerobic and hypoxic conditions. When exposed to low LET gamma-rays, there was a significant decrease in the radiation sensitivity of all cell lines under hypoxic conditions (Figure 1). It was observed that gamma-rays and low LET 13 keV/µm carbon ions showed a similar loss of radiation sensitivity under hypoxic conditions (Figure 1). When exposed to higher LET SOBP (Spread Out Bragg Peak) carbon ion radiation, a larger decrease in hypoxia associated radiation resistance in the HR and PARP (poly(ADP-ribose) polymerase) mutants was observed—as compared to the NHEJ and WT (wild type) cell lines (Figure 1). Finally, when cells were irradiated with high LET iron ions, the hypoxia associated radiation resistance was lost in all cell lines (Figure 1). Regression curves were determined for each cell line and a survival fraction of 2 Gy (SF2) was calculated for each radiation condition (Table 1). SF2 data further supported the conclusions drawn from the survival curves in Figure 1.
Figure 1.
Cell survival curves generated for gamma-rays, carbon ion, carbon ion SOBP (Spread Out Bragg Peak), and iron ions irradiation under aerobic and hypoxic conditions. Black circles indicate the aerobic condition and red circles indicate the hypoxic condition. Error bars represent the standard error of the means. At least three independent experiments were carried out.
Table 1.
SF2, survival fraction at 2 Gy, for different qualities of radiation.
| CHO | xrs5 | V3 | XR1 | 51D1 | irs1SF | PADR9 | KO40 | ||
|---|---|---|---|---|---|---|---|---|---|
| gamma-rays | aerobic | 0.631 | 0.020 | 0.092 | 0.238 | 0.426 | 0.250 | 0.657 | 0.618 |
| hypoxic | 0.840 | 0.291 | 0.362 | 0.543 | 0.676 | 0.595 | 0.737 | 0.735 | |
| carbon-ions 13 keV/μm | aerobic | 0.538 | 0.006 | 0.041 | 0.250 | 0.154 | 0.147 | 0.423 | 0.302 |
| hypoxic | 0.898 | 0.322 | 0.340 | 0.527 | 0.452 | 0.375 | 0.638 | 0.603 | |
| carbon-ions SOBP | aerobic | 0.371 | 0.060 | 0.054 | 0.241 | 0.131 | 0.081 | 0.551 | 0.372 |
| hypoxic | 0.710 | 0.201 | 0.369 | 0.379 | 0.339 | 0.428 | 0.583 | 0.520 | |
| iron-ions 200 keV/μm | aerobic | 0.201 | 0.021 | 0.110 | 0.070 | 0.074 | 0.042 | 0.174 | 0.204 |
| hypoxic | 0.222 | 0.025 | 0.138 | 0.131 | 0.081 | 0.037 | 0.223 | 0.248 |
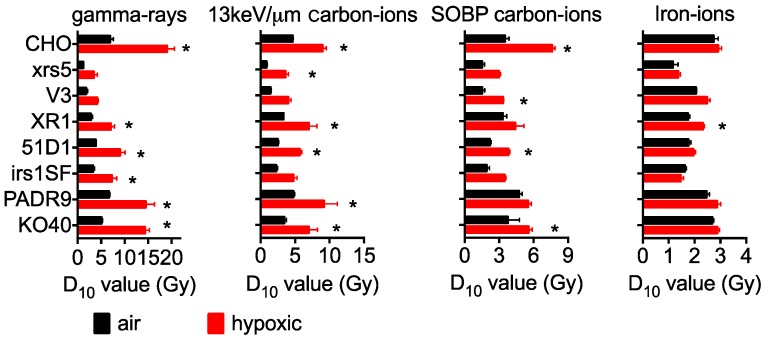
Additionally, linear quadratic regression was utilized to calculate the D10 values for wild type and DNA repair deficient cell lines (Figure 2). As shown previously, NHEJ mutants were the most radiation sensitive cells, followed by HR mutants, PARP, and FANCG (Fanconi Anemia complementation group G) mutants, with the CHO of wild type being the most radioresistant [22]. Hypoxic conditions resulted in an increased radioresistance when exposed to gamma-rays, carbon ion LET 13 keV/μm, and carbon ion SOBP—not, however, for iron ions.
Figure 2.
D10 values calculated from survival curves in different qualities of radiation. D10 values are the mean ± standard error of the means. * Indicates statistically significant differences between aerobic and hypoxic irradiation conditions (p < 0.05).
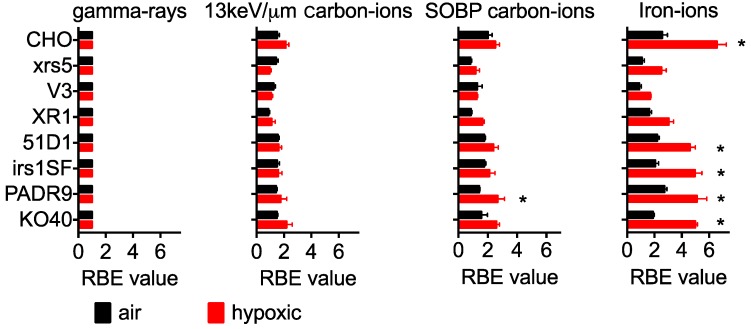
2.2. RBE in Aerobic Condition
RBE values were separately calculated for both aerobic and hypoxic conditions. Under aerobic conditions, the RBE values for CHO wild type cells increased with the increasing LET; the max RBE observed was 2.5 when exposed to iron ions (Figure 3). The three NHEJ repair deficient cells showed almost no change in RBE as the LET increased. RBE values were approximately 1, except for XR1 cells, which had an RBE of 1.85 for iron ions. HR deficient cells showed a slight increase in RBE, but not to the same extent as the wild type cells. When exposed to iron ions, the RBE values were observed at 2.20 and 1.98 for 51D1 and irs1SF, respectively. KO40 cells showed a similar trend to the HR repair deficient cells. PADR9, however, showed a similar trend to the wild type cells. PADR9 had an RBE value of 2.66 when irradiated to iron ions, which was slightly higher than in wild type cells.
Figure 3.
RBE values calculated from survival curves in different qualities of radiation. RBE values are the mean ± standard error of the means. * Indicates statistically significant differences between aerobic and hypoxic irradiation conditions (p < 0.05).
2.3. RBE for Hypoxic Condition
RBE values obtained under hypoxic conditions displayed some interesting trends (Figure 3). As the LET increased, the RBE values increased more significantly under hypoxic conditions than under aerobic conditions. Wild type cells had an RBE value of over 2 when irradiated with 13 keV/μm carbon ions and further increased to 6.52 when irradiated with iron ions. While the NHEJ deficient cells showed almost no increased RBE value under aerobic conditions, increased RBE values were observed when irradiated with SOBP carbon ions and iron ions. xrs5, V3, and XR1 had observed RBE values of 2.70, 1.89, and 3.32 for iron ion irradiation, respectively. HR deficient cells, KO40, and PADR9 cells showed similar changes in RBE to wild type cells. Hypoxic RBE values were greater than 2 for SOBP carbon ions and 5 for iron ions for all cell lines. These values were statistically significant when compared to the air RBE values of SOBP carbon ion exposed cells.
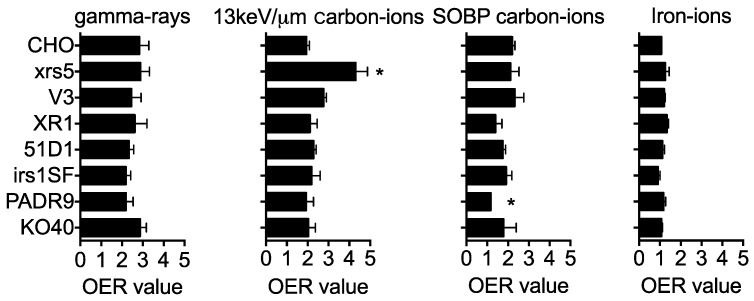
2.4. OER
To determine the effect of oxygen on radiation sensitivity, OER values were calculated from the D10 values. When exposed to gamma-rays, hypoxic conditions resulted in the radiation resistance of both the wild type and DNA repair deficient cell lines (Figure 4). OER values for wild type cells decreased as the LET increased, ranging from 2.83 with gamma radiation to 1 for iron–ions. Two of the NHEJ repair deficient cells (V3 and XR1) showed similar patterns to the wild type cells; whereas, xrs5 cells showed a statistically significant difference in OER value when compared to wild type cells exposed to 13 keV/μm carbon ions. The HR, PARP, and FANCG mutants showed a similar trend to the wild type cells when irradiated with low LET radiation. The main difference arose when cells were irradiated with SOBP carbon ions. Several of the DNA repair mutants had lower OER values than wild type cells when irradiated with SOBP carbon ions. The largest difference was observed in HR and PARP mutants. PARP deficient mutants showed the most statistically significant difference when compared to the wild type controls. These cell-line specific differences were not observed when cells were irradiated with 200 keV/μm iron ions.
Figure 4.
OER values calculated from survival curves in different qualities of radiation. RBE values are the mean ± standard error of the means. * Indicates statistically significant differences from wild type data (p < 0.05).
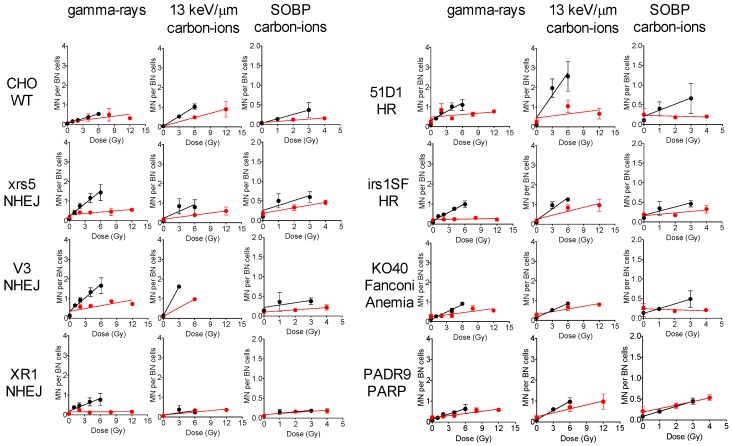
2.5. Micronuclei Formation
To further investigate the effects of oxygen on radiation sensitivity, we analyzed the formation of micronuclei in wild type and DNA repair mutants irradiated with gamma-rays, 13 keV/μm carbon ions, and SOBP carbon ions under both aerobic and hypoxic conditions (Figure 5 and Figure 6). When irradiated with gamma-rays under aerobic conditions, all cell lines had a dose-dependent increase in the observed micronuclei. DNA repair mutants had an increase in the number of micronuclei when compared to wild type cells. NHEJ mutants were observed to have had the highest number of micronuclei. All cell lines showed a statistically significant decrease in observed micronuclei when irradiated with gamma-rays under hypoxic conditions. Both the NHEJ and HR mutants showed the largest decrease in micronuclei formation when exposed to hypoxic conditions. Similar trends were observed when cells were irradiated with 13 keV/μm carbon ions, except for XR1, KO40 and PADR9 cells. These cells showed smaller differences in the number of micronuclei formed under aerobic and hypoxic irradiation conditions, as compared to the wild type cells. When irradiated with SOBP carbon ions, it was observed that XR1, KO40, and PADR9 cells did not experience a decrease in micronuclei formation under aerobic, as compared to, hypoxic conditions. This supports the lower OER values observed in Figure 4.
Figure 5.
Micronuclei formation assay after different qualities of irradiation in the presence or absence of oxygen. Black circles indicate aerobic condition and the red circles indicate hypoxic condition. All experiments were carried out three times independently.
Figure 6.
Representative images of micronuclei formation. (A) Unirradiated binucleated CHO cell without micronuclei. (B) 6 Gy of gamma-ray irradiated binucleated CHO cell with a micronucleus. The arrow indicates the micronucleus.
3. Discussion
Our findings suggest that the repair of high LET radiation-induced damage under hypoxic conditions requires not only the HR repair pathway, but also PARP. These findings are potentially of interest due to the hypoxic nature of tumors. One of the leading reasons for radiotherapy failure is tumor hypoxia [23]. It is quite common to find a portion of cells within a tumor to be either acutely or chronically hypoxic. One study showed that 70% of head and neck tumors and 63% of breast tumors were hypoxic and that tumor hypoxia resulted in high rates of radiotherapy failure [24]. Additionally, recent studies have shown that cancer stem cells colonize these hypoxic regions and have the potential to repopulate a tumor if it is not targeted with adequate radiation [25,26,27]. Traditionally, dose fractionation has been used to overcome this acute and chronic hypoxia via reoxygenation of the tumor core [28,29,30]. The issue with dose fractionation is tumor repopulation between fractionations [31,32,33]. In addition to dose fractionation, radiation sensitizers and high LET radiation has been utilized to overcome tumor hypoxia with limited success [34,35,36,37,38]. It has been reported that the inhibition of DNA repair pathways reduces the OER of gamma-rays, which we observed in Figure 4 [16].
We confirmed these findings, showing that the inhibition of all DNA repair pathways enhanced the effect of radiation under hypoxic conditions (Figure 1). Most notably, the inhibition of DNA-PKcs, XRCC4, and PARP showed the largest increase in radiation sensitivity under hypoxic conditions (Figure 1, Table 1). These findings indicate that the quality of radiation played a large role in OER. As the LET increased, we observed a decrease in hypoxia associated radiation resistance. It has been well established that, as LET increases, the complexity of radiation-induced damage increases [39]. Based on our data, we demonstrate a notable decrease in OER in HR and PARP mutant cell lines with adjuvant SOBP carbon ion irradiation and concurrent hypoxia. Higher LET radiation induces an increase in both the amount and type of DNA damage, which requires more than the NHEJ pathway to repair. It has been reported that PARP is required for a fully functional HR response [40,41,42,43]. These reports, taken in combination with our data, suggest that under hypoxic conditions, higher LET radiation produces complex DNA damage, which requires a functional HR pathway to fully repair. The reduction in OER was observed in all cell lines when LET reached its maximum biological effectiveness of above 100 keV/μm for iron ions. This observation supports the earlier finding that high LET iron ions do not rely on the presence of oxygen to cause DNA damage and cell death [44,45]. Despite the increased sensitivity of cells exposed to iron ions, this type of radiation is not clinically relevant. Of clinical significance is the findings observed in cells exposed to SOBP carbon ions, which are currently in use at 10 facilities [46]. It is interesting to note that mutations in XRCC4, HR, and PARP showed the highest sensitization under hypoxic conditions when irradiated with SOBP carbon ions. Specifically, PARP mutations had the highest RBE value and the lowest OER value in the cell lines tested. These findings indicate that PARP inhibition mitigates hypoxia associated radiation resistance. These findings in SOBP carbon ion therapy further supports the use of PARP inhibitors when combined with radiation therapy [47].
Our study suggests that high LET carbon–ion radiation therapy can be enhanced by adding an HR inhibitor, or specifically, a PARP inhibitor [48]. The addition of a PARP inhibitor sensitizes the entire tumor, including the hypoxic core. By overcoming the radioresistant hypoxic regions of a tumor via PARP inhibitors, this may introduce a potential reduction in overall fractionated dose, or conveniently no dosing at all. Given the added complexity, it is still unknown whether these findings would translate into an in vivo model. Additionally, further research into what role PARP plays in the repair of complex DNA damage caused by high LET radiation is needed. Whether PARP inhibitors are more effective when combined with high LET radiation than they are with low LET gamma or proton therapy also needs to be determined. It is worthy to mention that this investigation is limited to in vitro cell culture systems with two endpoints and also that in vivo studies should be conducted to determine the effectiveness of PARP inhibitors as a hypoxia radiation sensitizer in vivo.
4. Materials and Methods
4.1. Cell Culture
CHO wild type (CHO 10B2), and DNA repair deficient CHO mutant xrs5 (Ku80) [49], XR-1 (XRCC4) [50], and PADR9 (PARP) [51] were kindly supplied by Dr. Joel Bedford of Colorado State University (Fort Collins, CO, USA). DNA repair deficient CHO mutants, V3 (DNA-PKcs) [52], 51D1 (Rad51D) [53], irs1SF (XRCC3) [54], and KO40 (FANCG) [55] were kindly supplied by Dr. Larry Thompson at the Lawrence Livermore National Laboratory (Livermore, CA, USA). Cells were maintained in Alpha MEM (Hyclone, ThermoFisher, Waltham, MA, USA) with 10% heat inactivated Fetal Bovine Serum (Sigma, St. Louis, MO, USA), antibiotics (Anti-Anti; Invitrogen, Grand Island, NY, USA), and were cultured in 37 °C incubators with 5% CO2 and humidity.
Hypoxic conditions were maintained as previously published [56,57]. Hypoxia was achieved using the AnaeroPack system (Mitsubishi Gas Chemical, Tokyo, Japan) [58]. The cell cultures were placed into an airtight container with AnaeroPack oxygen absorbing and CO2 generating agents to reduce the O2 concentration to less than 1%. The cell cultures were treated in this hypoxic chamber for three hours at 37 °C before irradiation.
4.2. Irradiation
Gamma-ray irradiation was performed at Colorado State University with a J.L. Shepherd Model Mark I-68 nominal 6000 Ci 137Cs irradiator (J.L. Shepherd and Associates, San Fernando, CA, USA) at room temperature (20 °C) [59,60]. The dosage rate was 2.5 Gy/min for cell survival and micronuclei experiments. Particle-based irradiation experiments were carried out at the National Institute of Radiological Sciences (NIRS) in Chiba, Japan. Carbon ions and iron ions were accelerated to 290 and 500 MeV/n, respectively, using the Heavy Ion Medical Accelerator in Chiba (HIMAC) [60]. Specifics regarding the beam characteristics of the particle radiation, biological irradiation procedures, and dosimetry have been depicted previously [21,61,62]. Carbon ions were accelerated at 290 MeV/n of initial energy with 13 keV/μm on entrance or spread out with a ridge filter for 6 cm width of SOBP (spread out Bragg peak) [60]. The monolayer cell culture was irradiated at the center (50 keV/μm of average LET) within the SOBP at a distance of 119 mm from the entrance [57]. Monoenergetic 500 MeV/n iron ions which have a LET value of 200 keV/μm on entrance. Dose rates for carbon and iron ions irradiation were set at 1 Gy/min.
4.3. Cell Survival Colony Formation Assay and RBE, OER, and SF2 Calculation
After irradiation, cells were trypsinized and plated to form colonies. Colonies were fixed and stained 8 days later using 100% ethanol followed by 0.1% crystal violet. Macroscopic colonies containing more than 50 cells were marked as survivors [63]. Cell survival curves were drawn from cell survival fraction by Graphpad Prism 6 (GraphPad, La Jolla, CA, USA) with linear quadratic regression model. D10 values (radiation dose to achieve 10% cell survival) were obtained from regression curves. RBE and OER values were calculated from D10 values. Gamma-rays were used as a standard radiation for the RBE calculation. SF2 values were calculated from a regression model.
4.4. Micronuclei Formation Assay
Cells were irradiated and cultured in 4 μg/mL of Cytochalasin B (Sigma, St Louis, MO, USA) for 22 h [64]. Harvested cells were suspended in 5 mL of 75 mM KCl solution, centrifuged, and fixed in 3:1 methanol acetic acid solution and formaldehyde (ThermoFisher, Waltham, MA, USA). Cells were dropped onto slides and allowed to air dry at room temperature. Slides were stained in 5% Giemsa solution in GURR solution (Invitrogen, Grand Island, NY, USA) for 5 min. A total of 300 binucleated cells were scored per treatment dosage to obtain micronuclei per binucleated cells.
4.5. Statistics
All experiments were carried out at least two times and error bars indicate standard error of the means. Data was analyzed using Prism 6 software for one-way ANOVA analysis. p-values < 0.05 were categorized as significant differences.
5. Conclusions
A hallmark of hypoxia is radiation resistance. In this study we have shown that DNA repair deficient cells are more sensitive to high LET radiation under hypoxic conditions than in wild type controls. Interestingly, PARP deficient mutants showed similar OER values to HR mutants. These mutants had lower OER values than wild type controls when irradiated with carbon ion SOBP. Additionally, significantly higher RBE values were observed in HR, Fanconi Anemia, and PARP deficient cells with iron ion irradiation. NHEJ deficient cells also showed increased RBE values under hypoxic irradiation conditions. This study suggests that DNA repair inhibition may be a potential strategy for increasing the effectiveness of carbon ion radiotherapy when targeting the hypoxic regions of a tumor.
Acknowledgments
We thank NIRS-HIMAC for hadron radiation experiment supports.
Abbreviations
| CHO | Chinese Hamster Ovary |
| D10 | Dose to achieve 10% cell survival |
| SF2 | Survival Fraction at 2 Gy |
| LET | Linear Energy Transfer |
| RBE | Relative Biological Effectiveness |
| OER | Oxygen Enhancement Ratio |
| NIRS | National Institute of Radiological Sciences |
| HIMAC | Heavy Ion Medical Accelerator in Chiba |
| SOBP | Spread Out Bragg peak |
| ANOVA | Analysis of valiance |
| PARP | Poly (ADP-ribose) polymerase |
| FANC | Fanconi Anemia |
| NHEJ | Non Homologous End Joining |
| HR | Homologous Recombination |
Author Contributions
T.A.K. conceived and designed the experiments; I.M.C., C.S., J.S.H., V.A.S., S.S., H.Y., H.H., T.A.K. performed the experiments; I.M.C., M.U., A.F., T.A.K. analyzed the data; M.U., H.H., D.J.C., A.F., T.A.K. contributed reagents/materials/analysis tools; I.M.C., T.A.K. wrote the paper.
Funding
This research was partially supported by Colorado State University College Research Council Grant (T.A.K.), Japan Ministry of Education, Culture, Sports, Science and Technology (MEXT) Grants-in-Aid for Scientific Research on Innovative Areas, Grant Number (15H05935 and JP15K21745, A.F.), National Institute of Health (NIH) by (CA162804. D.J.C.) and Akiko Ueno Radiobiology Research Fund (T.A.K.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ruhm W., Woloschak G.E., Shore R.E., Azizova T.V., Grosche B., Niwa O., Akiba S., Ono T., Suzuki K., Iwasaki T., et al. Dose and dose-rate effects of ionizing radiation: A discussion in the light of radiological protection. Radiat. Environ. Biophs. 2015;54:379–401. doi: 10.1007/s00411-015-0613-6. [DOI] [PubMed] [Google Scholar]
- 2.Morgan W.F., Day J.P., Kaplan M.I., McGhee E.M., Limoli C.L. Genomic instability induced by ionizing radiation. Radiat. Res. 1996;146:247–258. doi: 10.2307/3579454. [DOI] [PubMed] [Google Scholar]
- 3.Nagasawa H., Latt S.A., Lalande M.E., Little J.B. Effects of X-irradiation on cell-cycle progression, induction of chromosomal aberrations and cell killing in ataxia telangiectasia (AT) fibroblasts. Mutat. Res. 1985;148:71–82. doi: 10.1016/0027-5107(85)90209-X. [DOI] [PubMed] [Google Scholar]
- 4.Cornforth M.N., Bedford J.S. X-Ray-Induced Breakage and Rejoining of Human Interphase Chromosomes. Science. 1983;222:1141–1143. doi: 10.1126/science.6648528. [DOI] [PubMed] [Google Scholar]
- 5.Norppa H., Falck G.C.M. What do human micronuclei contain? Mutagenesis. 2003;18:221–233. doi: 10.1093/mutage/18.3.221. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins S.R., McGregor G.A., Murray J.M., Downs J.A., Savic V. Novel synthetic lethality screening method identifies TIP60-dependent radiation sensitivity in the absence of BAF180. DNA Repair. 2016;46:47–54. doi: 10.1016/j.dnarep.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 7.Vandevoorde C., Depuydt J., Veldeman L., De Neve W., Sebastia N., Wieme G., Baert A., De Langhe S., Philippe J., Thierens H., et al. In vitro cellular radiosensitivity in relationship to late normal tissue reactions in breast cancer patients: A multi-endpoint case-control study. Int. J. Radiat. Biol. 2016;92:823–836. doi: 10.1080/09553002.2016.1230238. [DOI] [PubMed] [Google Scholar]
- 8.Vandevoorde C., Vral A., Vandekerckhove B., Philippe J., Thierens H. Radiation Sensitivity of Human CD34(+) Cells Versus Peripheral Blood T Lymphocytes of Newborns and Adults: DNA Repair and Mutagenic Effects. Radiat. Res. 2016;185:580–590. doi: 10.1667/RR14109.1. [DOI] [PubMed] [Google Scholar]
- 9.Morales A.G., Pezuk J.A., Brassesco M.S., de Oliveira J.C., Queiroz R.G.D., Machado H.R., Carlotti C.G., Neder L., de Oliveira H.F., Scrideli C.A., et al. BUB1 and BUBR1 inhibition decreases proliferation and colony formation, and enhances radiation sensitivity in pediatric glioblastoma cells. Child’s Nerv. Syst. 2013;29:2241–2248. doi: 10.1007/s00381-013-2175-8. [DOI] [PubMed] [Google Scholar]
- 10.Ward J.F. DNA Damage Produced by Ionizing-Radiation in Mammalian-Cells: Identities, Mechanisms of Formation, and Reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- 11.Riley P.A. Free-Radicals in Biology—Oxidative Stress and the Effects of Ionizing-Radiation. Int. J. Radiat. Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 12.Hamada N., Imaoka T., Masunaga S., Ogata T., Okayasu R., Takahashi A., Kato T.A., Kobayashi Y., Ohnishi T., Ono K., et al. Recent Advances in the Biology of Heavy-Ion Cancer Therapy. J. Radiat. Res. 2010;51:365–383. doi: 10.1269/jrr.09137. [DOI] [PubMed] [Google Scholar]
- 13.Okayasu R., Okada M., Okabe A., Noguchi M., Takakura K., Takahashi S. Repair of DNA damage induced by accelerated heavy ions in mammalian cells proficient and deficient in the non-homologous end-joining pathway. Radiat. Res. 2006;165:59–67. doi: 10.1667/RR3489.1. [DOI] [PubMed] [Google Scholar]
- 14.Radford I.R. The Level of Induced DNA Double-Strand Breakage Correlates with Cell Killing after X-Irradiation. Int. J. Radiat. Biol. 1985;48:45–54. doi: 10.1080/09553008514551051. [DOI] [PubMed] [Google Scholar]
- 15.Hauth F., Toulany M., Zips D., Menegakis A. Cell-line dependent effects of hypoxia prior to irradiation in squamous cell carcinoma lines. Clin. Transl. Radiat. Oncol. 2017;5:12–19. doi: 10.1016/j.ctro.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward J.F. The Complexity of DNA-Damage—Relevance to Biological Consequences. Int. J. Radiat. Biol. 1994;66:427–432. doi: 10.1080/09553009414551401. [DOI] [PubMed] [Google Scholar]
- 17.Scifoni E., Tinganelli W., Weyrather W.K., Durante M., Maier A., Kramer M. Including oxygen enhancement ratio in ion beam treatment planning: Model implementation and experimental verification. Phys. Med. Biol. 2013;58:3871–3895. doi: 10.1088/0031-9155/58/11/3871. [DOI] [PubMed] [Google Scholar]
- 18.Wenzl T., Wilkens J.J. Modelling of the oxygen enhancement ratio for ion beam radiation therapy. Phys. Med. Biol. 2011;56:3251–3268. doi: 10.1088/0031-9155/56/11/006. [DOI] [PubMed] [Google Scholar]
- 19.Barendsen G.W. Possibilities for the application of fast neutrons in radiotherapy: Recovery and oxygen enhancement of radiation induced damage in relation to linear energy transfer. Eur. J. Cancer. 1966;2:333–345. doi: 10.1016/0014-2964(66)90046-6. [DOI] [PubMed] [Google Scholar]
- 20.Weyrather W.K., Ritter S., Scholz M., Kraft G. RBE for carbon track-segment irradiation in cell lines of differing repair capacity. Int. J. Radiat. Biol. 1999;75:1357–1364. doi: 10.1080/095530099139232. [DOI] [PubMed] [Google Scholar]
- 21.Cartwright I.M., Bell J.J., Maeda J., Genet M.D., Romero A., Fujii Y., Fujimori A., Kitamuta H., Kamada T., Chen D.J., et al. Effects of targeted phosphorylation site mutations in the DNA-PKcs phosphorylation domain on low and high LET radiation sensitivity. Oncol. Lett. 2015;9:1621–1627. doi: 10.3892/ol.2015.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagasawa H., Chen D.J., Strniste G.F. Response of X-ray-sensitive CHO mutant cells to gamma radiation. I. Effects of low dose rates and the process of repair of potentially lethal damage in G1 phase. Radiat. Res. 1989;118:559–567. doi: 10.2307/3577413. [DOI] [PubMed] [Google Scholar]
- 23.Barker H.E., Paget J.T.E., Khan A.A., Harrington K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence (vol 15, pg 409, 2015) Nat. Rev. Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh J.C., Lebedev A., Aten E., Madsen K., Marciano L., Kolb H.C. The Clinical Importance of Assessing Tumor Hypoxia: Relationship of Tumor Hypoxia to Prognosis and Therapeutic Opportunities. Antioxid. Redox Sign. 2014;21:1516–1554. doi: 10.1089/ars.2013.5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visvader J.E., Lindeman G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 26.Pajonk F., Vlashi E., McBride W.H. Radiation resistance of cancer stem cells: The 4 R’s of radiobiology revisited. Stem Cells. 2010;28:639–648. doi: 10.1002/stem.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soeda A., Park M., Lee D., Mintz A., Androutsellis-Theotokis A., McKay R.D., Engh J., Iwama T., Kunisada T., Kassam A.B., et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28:3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 28.Moulder J.E., Rockwell S. Hypoxic fractions of solid tumors: Experimental techniques, methods of analysis, and a survey of existing data. Int. J. Radiat. Oncol. Biol. Phys. 1984;10:695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- 29.Brown J.M., Lemmon M.J. Tumor Hypoxia Can Be Exploited to Preferentially Sensitize Tumors to Fractionated-Irradiation. Int. J. Radiat. Oncol. 1991;20:457–461. doi: 10.1016/0360-3016(91)90057-B. [DOI] [PubMed] [Google Scholar]
- 30.Matsuo M., Krishna M., Tanaka H., Yamaguchi T., Mitchell J.B. Reoxygenation-Based Radiation Therapy Improve the Tumor Control. Int. J. Radiat. Oncol. 2017;99:E608. doi: 10.1016/j.ijrobp.2017.06.2063. [DOI] [Google Scholar]
- 31.Withers H.R., Taylor J.M.G., Maciejewski B. The Hazard of Accelerated Tumor Clonogen Repopulation during Radiotherapy. Acta Oncol. 1988;27:131–146. doi: 10.3109/02841868809090333. [DOI] [PubMed] [Google Scholar]
- 32.Kim J.J., Tannock I.F. Repopulation of cancer cells during therapy: An important cause of treatment failure. Nat. Rev. Cancer. 2005;5:516–525. doi: 10.1038/nrc1650. [DOI] [PubMed] [Google Scholar]
- 33.Shuryak I., Hall E.J., Brenner D.J. Dose dependence of accelerated repopulation in head and neck cancer: Supporting evidence and clinical implications. Radiother. Oncol. 2018;127:20–26. doi: 10.1016/j.radonc.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Overgaard J., Horsman M.R. Modification of hypoxia-induced radioresistance in tumors by the use of oxygen and sensitizers. Semin. Radiat. Oncol. 1996;6:10–21. doi: 10.1016/S1053-4296(96)80032-4. [DOI] [PubMed] [Google Scholar]
- 35.Tepper J.E., Wang A.Z. Improving local control in rectal cancer: Radiation sensitizers or radiation dose? J. Clin. Oncol. 2010;28:1623–1624. doi: 10.1200/JCO.2009.26.9787. [DOI] [PubMed] [Google Scholar]
- 36.Carreau A., El Hafny-Rahbi B., Matejuk A., Grillon C., Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 2011;15:1239–1253. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhani N., Fyles A., Hedley D., Milosevic M. The Clinical Significance of Hypoxia in Human Cancers. Semin. Nucl. Med. 2015;45:110–121. doi: 10.1053/j.semnuclmed.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Baumann R., Depping R., Delaperriere M., Dunst J. Targeting hypoxia to overcome radiation resistance in head & neck cancers: Real challenge or clinical fairytale? Expert Rev. Anticancer Ther. 2016;16:751–758. doi: 10.1080/14737140.2016.1192467. [DOI] [PubMed] [Google Scholar]
- 39.Prise K.M., Folkard M., Newman H.C., Michael B.D. Effect of radiation quality on lesion complexity in cellular DNA. Int. J. Radiat. Biol. 1994;66:537–542. doi: 10.1080/09553009414551581. [DOI] [PubMed] [Google Scholar]
- 40.Niedzwiedz W., Mosedale G., Johnson M., Ong C.Y., Pace P., Patel K.J. The Fanconi anaemia gene FANCC promotes homologous recombination and error-prone DNA repair. Mol. Cell. 2004;15:607–620. doi: 10.1016/j.molcel.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto K., Ishiai M., Matsushita N., Arakawa H., Lamerdin J.E., Buerstedde J.M., Tanimoto M., Harada M., Thompson L.H., Takata M. Fanconi anemia FANCG protein in mitigating radiation- and enzyme-induced DNA double-strand breaks by homologous recombination in vertebrate cells. Mol. Cell Biol. 2003;23:5421–5430. doi: 10.1128/MCB.23.15.5421-5430.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helleday T., Bryant H.E., Schultz N. Poly(ADP-ribose) polymerase (PARP-1) in homologous recombination and as a target for cancer therapy. Cell Cycle. 2005;4:1176–1178. doi: 10.4161/cc.4.9.2031. [DOI] [PubMed] [Google Scholar]
- 43.Schultz N., Lopez E., Saleh-Gohari N., Helleday T. Poly(ADP-ribose) polymerase (PARP-1) has a controlling role in homologous recombination. Nucleic Acids Res. 2003;31:4959–4964. doi: 10.1093/nar/gkg703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hada M., Georgakilas A.G. Formation of clustered DNA damage after high-LET irradiation: A review. J. Radiat. Res. 2008;49:203–210. doi: 10.1269/jrr.07123. [DOI] [PubMed] [Google Scholar]
- 45.Hirayama R., Uzawa A., Obara M., Takase N., Koda K., Ozaki M., Noguchi M., Matsumoto Y., Li H.Z., Yamashita K., et al. Determination of the relative biological effectiveness and oxygen enhancement ratio for micronuclei formation using high-LET radiation in solid tumor cells: An in vitro and in vivo study. Mutat. Res. Genet Toxicol. Environ. Mutagen. 2015;793:41–47. doi: 10.1016/j.mrgentox.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Ebner D.K., Kamada T. The Emerging Role of Carbon-Ion Radiotherapy. Front. Oncol. 2016;6:140. doi: 10.3389/fonc.2016.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drean A., Lord C.J., Ashworth A. PARP inhibitor combination therapy. Crit. Rev. Oncol. Hematol. 2016;108:73–85. doi: 10.1016/j.critrevonc.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 48.To C., Kim E.H., Royce D.B., Williams C.R., Collins R.M., Risingsong R., Sporn M.B., Liby K.T. The PARP inhibitors, veliparib and olaparib, are effective chemopreventive agents for delaying mammary tumor development in BRCA1-deficient mice. Cancer Prev. Res. 2014;7:698–707. doi: 10.1158/1940-6207.CAPR-14-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeggo P.A., Kemp L.M. X-ray-sensitive mutants of Chinese hamster ovary cell line. Isolation and cross-sensitivity to other DNA-damaging agents. Mutat. Res. 1983;112:313–327. doi: 10.1016/0167-8817(83)90026-3. [DOI] [PubMed] [Google Scholar]
- 50.Stamato T.D., Weinstein R., Giaccia A., Mackenzie L. Isolation of cell cycle-dependent gamma ray-sensitive Chinese hamster ovary cell. Somat. Cell Genet. 1983;9:165–173. doi: 10.1007/BF01543175. [DOI] [PubMed] [Google Scholar]
- 51.Witmer M.V., Aboul-Ela N., Jacobson M.K., Stamato T.D. Increased sensitivity to DNA-alkylating agents in CHO mutants with decreased poly(ADP-ribose) polymerase activity. Mutat. Res. 1994;314:249–260. doi: 10.1016/0921-8777(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 52.Whitmore G.F., Varghese A.J., Gulyas S. Cell cycle responses of two X-ray sensitive mutants defective in DNA repair. Int. J. Radiat. Biol. 1989;56:657–665. doi: 10.1080/09553008914551881. [DOI] [PubMed] [Google Scholar]
- 53.Hinz J.M., Tebbs R.S., Wilson P.F., Nham P.B., Salazar E.P., Nagasawa H., Urbin S.S., Bedford J.S., Thompson L.H. Repression of mutagenesis by Rad51D-mediated homologous recombination. Nucleic Acids Res. 2006;34:1358–1368. doi: 10.1093/nar/gkl020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuller L.F., Painter R.B. A Chinese hamster ovary cell line hypersensitive to ionizing radiation and deficient in repair replication. Mutat. Res. 1988;193:109–121. doi: 10.1016/0167-8817(88)90041-7. [DOI] [PubMed] [Google Scholar]
- 55.Tebbs R.S., Hinz J.M., Yamada N.A., Wilson J.B., Salazar E.P., Thomas C.B., Jones I.M., Jones N.J., Thompson L.H. New insights into the Fanconi anemia pathway from an isogenic FancG hamster CHO mutant. DNA Repair. 2005;4:11–22. doi: 10.1016/j.dnarep.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 56.Obata A., Kasamatsu S., Lewis J.S., Furukawa T., Takamatsu S., Toyohara J., Asai T., Welch M.J., Adams S.G., Saji H., et al. Basic characterization of 64Cu-ATSM as a radiotherapy agent. Nucl. Med. Biol. 2005;32:21–28. doi: 10.1016/j.nucmedbio.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 57.McMillan D.D., Maeda J., Bell J.J., Genet M.D., Phoonswadi G., Mann K.A., Kraft S.L., Kitamura H., Fujimori A., Yoshii Y., et al. Validation of 64Cu-ATSM damaging DNA via high-LET Auger electron emission. J. Radiat. Res. 2015;56:784–791. doi: 10.1093/jrr/rrv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seko Y., Tobe K., Ueki K., Kadowaki T., Yazaki Y. Hypoxia and hypoxia/reoxygenation activate Raf-1, mitogen-activated protein kinase kinase, mitogen-activated protein kinases, and S6 kinase in cultured rat cardiac myocytes. Circ. Res. 1996;78:82–90. doi: 10.1161/01.RES.78.1.82. [DOI] [PubMed] [Google Scholar]
- 59.Yu H., Haskins J.S., Su C., Allum A., Haskins A.H., Salinas V.A., Sunada S., Inoue T., Aizawa Y., Uesaka M., et al. In vitro screening of radioprotective properties in the novel glucosylated flavonoids. Int. J. Mol. Med. 2016;38:1525–1530. doi: 10.3892/ijmm.2016.2764. [DOI] [PubMed] [Google Scholar]
- 60.Maeda J., Cartwright I.M., Haskins J.S., Fujii Y., Fujisawa H., Hirakawa H., Uesaka M., Kitamura H., Fujimori A., Thamm D.H., et al. Relative biological effectiveness in canine osteosarcoma cells irradiated with accelerated charged particles. Oncol. Lett. 2016;12:1597–1601. doi: 10.3892/ol.2016.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki M., Kase Y., Yamaguchi H., Kanai T., Ando K. Relative biological effectiveness for cell-killing effect on various human cell lines irradiated with heavy-ion medical accelerator in Chiba (HIMAC) carbon-ion beams. Int. J. Radiat. Oncol. Biol. Phys. 1999;48:241–250. doi: 10.1016/S0360-3016(00)00568-X. [DOI] [PubMed] [Google Scholar]
- 62.Kamada T., Tsujii H., Tsuji H., Yanagi T., Mizoe J.E., Miyamoto T., Kato H., Yamada S., Morita S., Yoshikawa K., et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J. Clin. Oncol. 2002;20:4466–4471. doi: 10.1200/JCO.2002.10.050. [DOI] [PubMed] [Google Scholar]
- 63.Maeda J., Roybal E.J., Brents C.A., Uesaka M., Aizawa Y., Kato T.A. Natural and glucosyl flavonoids inhibit poly(ADP-ribose) polymerase activity and induce synthetic lethality in BRCA mutant cells. Oncol. Rep. 2014;31:551–556. doi: 10.3892/or.2013.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su C., Allum A.J., Aizawa Y., Kato T.A. Novel glyceryl glucoside is a low toxic alternative for cryopreservation agent. Biochem. Biophys. Res. Commun. 2016;476:359–364. doi: 10.1016/j.bbrc.2016.05.127. [DOI] [PubMed] [Google Scholar]