Abstract
Transforming growth factor (TGF)-β signaling is not only important in skeletal development, but also essential in bone remodeling in adult bone. The bone remodeling process involves integrated cell activities induced by multiple stimuli to balance bone resorption and bone formation. TGF-β plays a role in bone remodeling by coordinating cell activities to maintain bone homeostasis. However, mineral metabolism disturbance in chronic kidney disease (CKD) results in abnormal bone remodeling, which leads to ectopic calcification in CKD. High circulating levels of humoral factors such as parathyroid hormone, fibroblast growth factor 23, and Wnt inhibitors modulate bone remodeling in CKD. Several reports have revealed that TGF-β is involved in the production and functions of these factors in bone. TGF-β may act as a factor that mediates abnormal bone remodeling in CKD.
Keywords: bone remodeling, mineral metabolism disturbance, chronic kidney disease, humoral factors
1. Introduction
Transforming growth factor (TGF)-β superfamily molecules play critical roles in tissue development and differentiation in an autocrine/paracrine/endocrine manner [1]. In bone also, TGF-β family members are involved in the control of cell activities and metabolism throughout osteogenesis [2,3]. Intramembranous ossification and endochondral ossification are skeletal ossification processes that take place following condensation of mesenchymal stem cells in fetal skeletal development [4,5]. Intramembranous ossification occurs in some parts of the clavicles, neurocranium, and viscerocranium. During intramembranous ossification, mesenchymal stem cells differentiate directly into osteoblasts. Endochondral ossification takes place in the skull base and the posterior part of the skull, the axial skeleton, and the appendicular skeleton. During the endochondral ossification process, mesenchymal cells undergo chondrogenesis to form cartilage, which is later replaced by mineralized bone. TGF-β controls these processes. For example, TGF-β1-deficient mice display reduced bone growth and mineralization [6]. TGF-β2−/− and TGF-β3−/− double knockout mice display a lack of distal parts of the rib [7]. Especially, TGF-β2-null mice display severe skeletal abnormalities in both intramembranous and endochondral ossification [8].
Apart from bone development, TGF-β and its signaling are important for adult bone remodeling [9,10]. The skeleton in adults undergoes continuous renewal throughout life to maintain bone mass and bone strength to resist fracture. This process is critically dependent on the activities of two cell types and their interactions. Osteoclasts, which are derived from hematopoietic cells, resorb old bone matrix. Osteoblasts, which are derived from mesenchymal cells, deposit new bone matrix in the resorbed area and facilitate mineralization. TGF-β is intimately involved in each stage of these processes. TGF-β regulates recruitment of osteoclasts and osteoblasts, the crosstalk mediating bone remodeling, and the quality of bone matrix. Disturbance of the balance between bone resorption and bone formation underlies osteoporosis, which is characterized by reduced bone mass and deterioration of bone microarchitecture, resulting in increased fragility and fracture risk. In this review, we focus on the effect of TGF-β on bone remodeling and discuss the potential involvement of TGF-β action in the pathogenesis of bone abnormalities in chronic kidney disease (CKD).
2. TGF-β Exists in Bone Tissue and Is Implicated in Bone Metabolism
In mammals, the TGF-β family consists of three isoforms: TGF-β1, TGF-β2, and TGF-β3 [11]. All TGF-β isoforms are expressed in bones, especially in the perichondrium, periosteum, and epiphyseal growth plate [12,13,14]. As in other tissues, TGF-β in osteoblasts is synthesized as a large precursor molecule called pre-pro-TGF-β, which contains the signal peptide, latency associated peptide (LAP), and C-terminal sequence. The C-terminal amino acid sequence forms the active form of TGF-β (mature TGF-β) after modifications [15,16] in the bone matrix. The latent TGF-β binding protein (LTBP) binds with latent TGF-β to form a complex, which is secreted from osteoblastic cells. Parts of this complex interact with extracellular matrix (ECM) components including fibronectin, fibrillin 1, and integrin [17,18,19,20,21]. Upon cleavage of the LAP by osteoclasts, abundant mature TGF-β is released from the latent TGF-β complex and is activated in bone resorption lacunae [22,23].
2.1. TGF-β Acts on Osteoblasts
The released mature TGF-β recruits perivascular mesenchymal cells to the surface of resorption lacunae and differentiates them to osteoblasts. TGF-β binds two types of TGF-β receptors: type I (RI/ALK5) and type II (RII). TGF-β RII transphosphorylates TGF-β RI in association with ALK5 and phosphorylates receptor-regulated Smads (R-Smads) including Smad 2 and 3 [11]. R-Smads 1, 5, and 8 are also partially activated by TGF-β bound to ALK1 [24,25]. R-Smads are dissociated from the tetrameric receptor complex to form complexes with Smad 4, and translocate to the nucleus to regulate transcription of target genes to induce proliferation and early differentiation of osteoprogenitor cells. TGF-β1 increases the pool of osteoprogenitors by inducing chemotaxis and proliferation mediated via Smad signaling pathways [10]. In addition, TGF-β2 induces activation of the Extracellular Signal-regulated kinase (ERK) signal that stimulates cell proliferation to enrich osteoprogenitor cells [26]. After recruiting mesenchymal stem cells, TGF-β stimulates osteoprogenitor proliferation in part by promoting the degradation of p57kip2, which is a cell-cycle inhibitor [27].
Smad 3 signaling has the strongest effect on the differentiation of mesenchymal stem cells to osteoblasts induced by TGF-β. In vitro study indicates that Smad 3 overexpression in cultured osteoblastic MC-3T3E1 cells enhances the bone matrix protein levels, alkaline phosphatase activity, and mineralization [28]. TGF-β-induced non-Smad-dependent pathways (noncanonical signaling pathways) are also involved in osteoblast differentiation. When TGF-β binds the TGF-β receptor complex, the phosphorylated TGF-β activation kinase 1 (TAK1) and TAK1 binding protein 1 (TAB1) complex initiates activation of the mitogen-activated protein kinase (MAPK) signaling cascade. Phosphorylated MAP kinase kinase (MKK) p38 MAPK induces type I collagen expression [29]. Previous research indicates that osteocalcin, which is an extracellularly expressed protein that interacts with hydroxyl apatite, is also regulated via TGF-β through ERK and p38 MAPK activation [30]. Bone sialoprotein, which is a major glycoprotein, is regulated by TGF-β through β-catenin signaling [31]. Recently, it has also been reported that osteoblast-derived soluble factors, including TGF-β, indeed promote metastatic potential in prostate cancer cells, and the effect by TGF-β is, at least partly, mediated by noncanonical TRAF6-dependent signaling [32].
Smad-dependent and -independent pathways converge at the Runt-related transcription factor 2 (Runx2) gene to control mesenchymal cell differentiation [33]. Runx2, which is also called core-binding factor alpha subunit (cbfa1), is a master regulator of osteoblast differentiation. Runx2-deficient mice completely lack osteoblasts and mineralized matrix [34]. Runx2 directly regulates the expression of several genes including type I collagen, alkaline phosphatase, osteopontin, osteonectin, and osteocalcin, because Runx2 regulates the expression of osterix, the zinc-finger containing transcription factor that regulates the nuclear factor for activated T cells (NFAT2). Cooperation of NFAT2 and osterix is required for the expression of these target genes [35,36]. TGF-β increases Runx2 expression during the early differentiation of osteoblasts, accelerating the proliferation of osteoblasts.
The proliferated osteoblasts synthesize new extracellular matrices, including type I collagen, osteocalcin, osteopontin, alkaline phosphatase, and matrix metalloprotease-13 (MMP-13), on the bone surface [37]. In contrast, in the late differentiation stage of osteoblasts, TGF-β represses Runx2 gene expression and inhibits the terminal differentiation of osteoblasts. TGF-β-induced Smad 3 activation is involved in this pathway, which suppresses matrix mineralization via the terminal differentiation of osteoblasts [38].
Upon termination of bone matrix synthesis, osteoblasts undergo apoptosis or differentiate into osteocytes, the bone lining cells. Mature TGF-β controls osteoblast survival through blocking osteoblast apoptosis. Membrane-type MMP (MT1-MMP), which is produced by osteoblasts and activates latent TGF-β, is involved in osteoblast survival [39]. Another study revealed that the deletion of Smad 3 does not inhibit osteoblast terminal differentiation, but induces osteoblast differentiation to osteocytes. This finding indicates that Smad 3 signaling regulates the lifespan of osteoblasts and bone formation rate [40]. Therefore, TGF-β plays distinct roles at each stage of the osteoblast lifecycle.
2.2. TGF-β Acts on Osteoclasts
TGF-β promotes the migration of osteoclast precursors into bone, such as osteoblasts [41]. Bone marrow macrophages are osteoclast precursors, and they require the receptor activator of nuclear factor-κB ligand (RANKL) [42,43] and macrophage-colony stimulating factor (M-CSF) [44] for osteoclastogenesis. TGF-β acts directly on bone marrow macrophages and promotes osteoclastogenesis [45,46,47]. Yasui et al. [48] demonstrated that the TGF-β-induced molecular interaction between Smad 2/3 and TRAF 6 is critical for RANKL-induced osteoclastogenic signaling. In addition, RANKL and M-CSF are produced by osteoblasts. Therefore, the effects of TGF-β on osteoclasts are also derived from osteoblasts. TGF-β-stimulated osteoblasts express not only osteoblastic functional proteins including collagen type 1, alkaline phosphatase, and osteocalcin, but also osteoclast regulatory genes including M-CSF, RANKL, and osteoprotegerin (OPG) [45,49]. The effect of TGF-β on osteoclastogenesis is dose-dependent. Treatment with low-dose TGF-β enhances osteoclastogenesis by increasing M-CSF expression and prostaglandin production, as well as the RANKL to OPG ratio [50], whereas treatment with a high concentration represses M-CSF and RANKL expression while increasing OPG expression [51,52,53]. Because OPG is a high-affinity ligand for RANKL and acts as a soluble inhibitor of RANKL produced by osteoblasts, the effects of TGF-β on osteoclasts mediated by osteoblasts may serve as a negative feedback for bone remodeling.
2.3. TGF-β Regulates Bone Homeostasis Mediated by Osteocytes
Osteocytes are the most abundant bone cells embedded in the lacunae of the bone matrix. These cells are transformed from osteoblasts, and are speculated to function as a network of sensory cells mediating the effect of mechanical loading through the extensive lacuno-canalicular network. Because osteocytes stimulate osteoclast formation and activation through RANKL expression [54], osteocytes are able to induce and control bone remodeling.
In addition to regulating osteoclasts and osteoblasts, osteocytes also have direct resorbing capability, depositing new bone matrix surrounding the lacuno-canalicular network [55]. The purpose of this process is to maintain mineral homeostasis, the magnitude of fluid shear stress, and the mechanical properties of bone. Osteocytes drive several proteases including MMPs, cathepsin K, carbonic anhydrase 2, and tartrate-resistant acid phosphatase in perilacunar/canalicular remodeling [55,56,57,58]. TGF-β tightly regulates osteocyte MMP-13 expression. Moreover, TGF-β has been shown to be important for the stabilization of osteocytes [59]. Dying osteocytes express antiapoptotic and proapoptotic molecules, leading to the activation of osteoclast activity and bone resorption [60]. Targeted ablation of osteocytes by injection of diphtheria toxin in mice dramatically activates osteoclasts [61]. Osteocyte viability may reflect bone remodeling and integrity. TGF-β may be an important factor in maintaining bone homeostasis through regulating osteocyte function.
Given that TGF-β is an important regulator of bone remodeling and that high levels of TGF-β are found in CKD, TGF-β may be involved in bone abnormalities in CKD.
3. Abnormalities of Bone and Mineral Metabolism in Chronic Kidney Disease
Since the kidney plays important roles in mineral metabolism, disturbances in mineral and bone metabolism are common complications in CKD. These abnormalities were traditionally termed renal osteodystrophy (ROD), and have been renamed “Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD)” [62].
Along with a decreasing glomerular filtration rate (GFR), serum fibroblast growth factor 23 (FGF23) and sclerostin, which are derived from osteocytes, begin to increase prior to change in parathyroid hormone (PTH) and 1,25 dihydroxyvitamin D3 (1,25D) concentrations. The circulating levels of these proteins increase with an accompanying decline in GFR [63,64]. FGF23 is an endocrine hormone that regulates phosphate metabolism and accelerates degradation of 1,25D in impaired phosphate excretion as seen in CKD. Hyperparathyroidism, hyperphosphatemia, and decreased circulating levels of 1,25D are observed in advanced CKD.
In CKD, bone metabolism is aggravated in response to mineral metabolism disturbance. Because PTH stimulates the speed of osteoclastic bone resorption and remodeling, an extremely high turnover of bone exists in patients with advanced CKD on stable hemodialysis or with inadequate control of PTH. Extremely high bone turnover reduces bone mineral density (BMD) and worsens the bone microstructure, leading to fragility. Bone microstructure disturbance is associated with high blood levels of PTH [65,66,67]. High turnover of bone together with high blood PTH level is often accompanied by bone marrow fibrosis. On the other hand, marked decreases in both bone resorption and bone formation caused by suppressed PTH secretion or skeletal resistance to PTH under a uremic condition are found in low-turnover bone lesions in CKD [68,69,70]. Regardless of high or low bone turnover, abnormal bone turnover increases fracture risk in CKD patients [71,72,73,74].
Ectopic cardiovascular mineralization is frequently associated with decreasing BMD and/or bone turnover disturbance. Regarding vascular calcification, the mineralization process in blood vessels shows a pattern analogous to bone mineralization, in which not only the mineralization step, but also the matrix production is regulated [75]. There are two forms of vascular calcification in CKD. One is associated with atherosclerosis, which is the intimal calcification produced by the osteoblastic transformation of cells derived from smooth muscle cells and mesenchymal cells. The other is the transformation of medial vascular smooth muscle cells to osteo/chondrocytic cells. Both types of calcifications are linked to elevated Dickkopf-1 (Dkk1), sclerostin, and activin levels [75,76]. As mentioned above, CKD-MBD is a syndrome which could result in disorders of bone metabolism and/or the cardiovascular system. On the other hand, ROD is used to indicate bone morphologic changes in patients with CKD and is one measure of the skeletal disorder component of CKD-MBD [62].
In addition to mineral disturbance, TGF-β production is associated with CKD progression [77]. Circulating TGF-β levels is a reliable biomarker of CKD [78]. In acute and chronic kidney injury, increased TGF-β levels parallel the reduced expression of bone morphogenetic protein (BMP)-7, which is also a member of the TGF-β superfamily. BMP-7 is known to be an important inhibitor of vascular calcification [79]. The reduction of BMP-7 action on the vascular wall is associated with abnormal bone turnover [79].
4. Possible Role of TGF-β in Chronic Kidney Disease-Mineral and Bone Disorder
4.1. Renal Osteodystrophy and Resistance to PTH
The possible TGF-β involvements in abnormalities of bone and mineral metabolisms in CKD patients have been reported since the 1990s. Jiang et al. [80] investigated the plasma levels of TGF-β in hemodialysis patients and found that patients with ROD have significantly higher levels of TGF-β than in patients without ROD. They concluded that the pathological condition of ROD may stimulate overproduction of TGF-β in patients undergoing hemodialysis. Using immunohistochemistry, Duarte et al. [81] also demonstrated intense TGF-β expression in bone samples with osteitis fibrosa from patients with ROD, which is accompanied by extremely high bone turnover. A study conducted by Santos et al. [82] indicated that TGF-β expression in bone changes before and after parathyroidectomy. Because TGF-β modulates the functions of both osteoblasts and osteoclasts, these results are rational. However, humoral parameters, including PTH and alkaline phosphatase, were not found to be associated with blood TGF-β concentration in Jiang et al.’s study [80]. From the above reports, it remains unclear whether the serum concentration of TGF-β reflects bone turnover. In an in situ hybridization study using bone biopsy samples, Hoyland and Picton [83] demonstrated that TGF-β signals localize predominantly to osteoblasts, although the same signals are also observed in some osteocytes and osteoclasts, and the levels decrease in renal bone samples. In that study, the level of TGF-β expression depends on bone turnover; the lowest level of TGF-β in osteoblasts is observed in adynamic bone associated with low PTH levels. This observation is consistent with in vitro results that show that PTH increases TGF-β production in cultures of normal osteoblast-like cells [84].
Liu et al. [85] reported elevated expression of TGF-β1 mRNA and its receptor, as well as TGF-β signaling in jck mice, a genetic model of polycystic kidney disease with progressive decline in renal function. In these mice, bone turnover increases independently of detectable PTH change. Administration of 1D11, a neutralizing anti-TGF-β antibody, to jck mice suppresses osteoblast and osteoclast functions and reduces bone turnover independent of renal function and changes in serum indices. These findings suggest a direct effect of 1D11 on bone rather than a systemic effect. The mechanisms of the effects of 1D11 on osteoblast and osteoclast functions are elucidated by assessing intracellular signaling pathways. The phospho-Smad 2/Smad 2 ratio in osteoblasts increases in jck mice, and treatment with 1D11 significantly attenuates the increase.
Some reports reveal that there is interplay between TGF-β and Wnt signaling in osteoblasts. Using chemical kinase inhibitors, Zhou demonstrated that TGF-β stabilize β-catenin through both Smad3 and non-Smad pathways [31]. McCarthy and Centrella demonstrated that Wnt pathway induction stabilizes β-catenin and increases T cell factor/lymphoid enhance factor (TCF/LEF)-dependent gene expression in parallel with β-catenin-independent complex formation between TCF-4 and Runx2. They also presented that activation of Runx2 or TCF-4 coenhances TCF and Runx2 activity and increases TGF-β receptor I expression [86]. As described above, high TGF-β protein expression has been observed in high-turnover bones from patients with end-stage renal disease [81,82]. Existence of a positive regulatory loop between TGF-β and Wnt signaling may lead to the pathogenesis of high-turnover bone disease in CKD.
It has long been recognized that in CKD, the bone is resistant to the calcemic action of PTH. This phenomenon is seen in CKD patients [87] and also uremic rats [88,89,90]. One of the mechanisms is the downregulation of the PTH/PTH-related peptide (PTHrP) receptor mRNA in uremic rats compared to normal rats [91,92]. TGF-β attenuates PTH signaling in osteoblasts [93]. In cultured osteoblasts derived from fetal rat calvariae, TGF-β2 induces a decrease in the steady-state level of PTH/PTHrP receptor mRNA, resulting in decreased PTHrP receptor binding [93]. TGF-β binds to TGF-β receptor II (TBR2), leading to direct phosphorylation of the cytoplasmic domain of the PTH type I receptor (PTH1R). This reaction results in the formation and internalization of the TBR2 PTH1R complex [94]. Although the pathogenesis of skeletal resistance and the contributing factors remain unclear, the action of TGF-β on bone and/or osteoblasts may be involved in PTH resistance in CKD conditions such as the accumulation of uremic toxins [95,96,97]. Since PTH is a master regulator of bone turnover in CKD, these studies suggest that TGF-β may contribute to the pathogenesis of ROD.
4.2. High Serum FGF23 Level
As CKD progress, there is a progressive increase in serum FGF23 level, which may reach exceptionally high levels [64,98]. The blood FGF23 level is associated with increased mortality in CKD patients [99]. The regulation of FGF23 production is still incompletely understood. PTH stimulates FGF23 production [100,101]. This phenomenon is confirmed by the outcome of parathyroidectomy [100] and calcimimetic treatment [102], both of which decrease the circulating level of FGF23. Although FGF23 decreases the PTH level as a negative feedback mechanism under normal conditions [103,104], resistance to the action of FGF23 in both the parathyroid and kidney is found in advanced CKD due to downregulation of the FGF23 receptor complex, the klotho‒FGF receptor 1 (FGFR1) [105,106,107,108]. As a result, FGF23 production is maintained, resulting in the coexistence of high PTH and high FGF23 [100]. The mechanism of PTH-stimulated FGF23 production is, at least in part, mediated by activation of the orphan nuclear receptor Nurr1 [109]. On the other hand, 1,25D directly increases FGF23 transcription via vitamin D response element-mediated transcriptional activation and indirectly via extracellular signaling pathways, both of which are mediated by leptin and IL-6-induced STAT phosphorylation [110] or Nurr1 activation [109].
A recent study has found that TGF-β2 controls the production of FGF23 in UMR106 osteoblast-like cells [101]. The quantities of TGF-β-stimulated FGF23 gene and protein expression depend on the TGF-β concentration. The effect of TGF-β on FGF23 production is mediated by store-operated calcium entry (SOCE) through Orai1/STIM1 [101]. A SOCE inhibitor significantly blunts the induction of FGF23 synthesis via TGF-β2. A similar pathway is observed when activation of the inflammatory transcription factor NF-kB upregulates the Orai1/STIM1-mediated SOCE that triggers FGF23 production [111].
In uremic animals, renal FGF23 expression correlates with local TGF-β1 expression [112]. Smith et al. demonstrated that FGF23 augments FGFR4 activation and upregulation of the calcium transporter in the absence of klotho, leading to enhanced TGF-β1 autoinduction through increases of both intracellular calcium and mitochondrial reactive oxygen species [113]. Although klotho expression in bone is repressed in CKD [114], expression of FGFR4 in osteoblasts has been confirmed [115]. A feed-forward loop between TGF-β and FGF23 may exist in osteoblasts in CKD.
Moreover, reduction of 1,25D levels may contribute to the effect of TGF-β on FGF23 production in CKD. Active 1,25D inhibits downstream TGF-β signaling, due to vitamin D receptor (VDR)–phospho-Smad 3 complex formation. In CKD, reduced expression of VDR and decreased level of its ligand may contribute to hyperactive TGF-β signaling [116]. With the progression of renal dysfunction, the expression of FGF23, an important target of PTH and 1,25D, may be modulated by TGF-β, which may be involved in the pathogenesis of CKD-MBD.
4.3. Wnt Inhibitors
Wnt pathways increase the osteogenic commitment of bone marrow stem cells, enhance matrix formation, and decrease the apoptosis of osteoblasts and osteocytes. Wnt signaling is regulated by secreted decoy receptors (secreted frizzled-related protein, sfrp) or antagonists. Sclerostin and Dkk1 are Wnt antagonists.
In CKD patients, higher circulating sclerostin levels correlate with higher BMD and better bone microarchitecture [117,118]. Because sclerostin expression in bone increases in early CKD [119,120,121], the skeleton is speculated to be the source of increased circulating sclerostin in CKD. High sclerostin levels may reflect increased osteocyte numbers and skeletal mass [122,123]. Both serum and bone sclerostin levels correlate negatively with the histomorphometric parameters of bone turnover and osteoblastic number in dialysis patients [124,125]. In line with this observation, circulating sclerostin levels have been found to correlate negatively with biomarkers of bone formation [123,126] and resorption [123]. CKD patients with PTH within the normal range often have adynamic bone, with suppressed bone turnover. Adynamic bone or low-turnover bone may be caused by high levels of sclerostin.
PTH is known to be a regulator of SOST (the gene that encodes sclerostin9 expression) [127]. This PTH-mediated repression of SOST requires recruitment of MEF2 to a highly conserved regulatory region 35-kb downstream from the SOST gene [128]. Loots et al. [129] demonstrated the effect of TGF-β on SOST expression in UMR-106.01, a rat osteosarcoma cell line. A TGF-β isoform induces SOST expression, and a significantly higher level compared to that before induction is sustained. This induction involves Smad 3, and PTH antagonizes the TGF-β-stimulated SOST induction. Interestingly, TGF-β targets the ECR5 region, which is a distal enhancer of the SOST promoter. This enhancer that responds to PTH stimulation [128] contains a binding site for both the MEF2 family of transcription factors and Smad2/3. The above findings would suggest that TGF-β may cause repression of sclerostin expression in CKD, because high levels of PTH and TGF-β coexist in the setting of CKD. However, both serum and bone sclerostin levels are high in CKD.
On the other hand, Notsu et al. [130] reported that advanced glycation end product (AGE)-3 induces SOST expression and osteocyte apoptosis via upregulated TGF-β expression. Since AGEs accumulate in the CKD condition [131], the effect of AGE-3 on SOST expression through increased TGF-β may contribute to the high SOST level. Bone sclerostin expression is diminished in late-stage CKD compared to that in early-stage CKD [119,121]. Stage-dependent sclerostin expression may be reflected by the degree of TGF-β stimulation, because the PTH level is elevated in the late-stage, while the accumulation of AGEs occurs in all stages of CKD. Further in vivo and/or in vitro studies that assess the MEF2 transcriptional activity using models simulating CKD conditions are needed to elucidate the effect of TGF-β on sclerostin production in CKD.
Dkk1 is another Wnt inhibitor, mainly produced by osteoblasts and osteocytes [132]. In normal subjects, Dkk1 is expressed at low levels in the skin, placenta, prostate, kidney, and platelets [133]. Dkk1 expression is regulated by growth factors and hormones including calcitonin, morphogenetic proteins, PTH [134], and estrogens [135]. Although bone cells are regarded as the main producing tissue, Dkk1 expression increases during renal tubule epithelial proliferation and renal repair in early CKD [134].
Circulating Dkk1 levels are only minimally affected by gender, renal function, and age [118,136,137]. Contrary to sclerostin, the Dkk1 level does not correlate with BMD or bone histomorphometric parameters in CKD patients [125,138]. A negative correlation between blood Dkk1 level and the degree of atherosclerosis [136] or aorta calcification [137] has been observed.
Fang et al. [139] demonstrated the effect of neutralization of Dkk1 using a monoclonal antibody in ldlr−/− mice. These mice have reduced GFR to an extent similar to that of patients with stage 2 CKD. Tissue levels of Dkk1 are elevated in these mice. Neutralization of Dkk1 prevents osteochondrogenic transdifferentiation of vascular smooth muscle cells, vascular calcification, and renal osteodystrophy. However, elevated Dkk1 levels and downregulation of vascular klotho (both key findings in the abovementioned mouse model) are not observed in all patients with early-stage CKD [118,140]. The effect of Dkk1 neutralization on vascular calcification remains unclear.
Circulating activin increase is accompanied by increased activin production in the kidney under CKD conditions [141]. Activin, a member of the TGF-β superfamily, increases during development [142] and in injured kidneys [143]. Agapova et al. [141] demonstrated that a ligand trap for the activin type IIA receptor (ActRIIA) significantly decreases Dkk1 expression both in the kidney and circulating levels [141]. Interestingly, the ligand trap for ActRIIA restores phospho-Smad/3 in the aorta, leading to suppressed gene and protein expression of Runx2, a transcription factor associated with osteoblast transition. The mouse model of CKD with atherosclerosis (ldlr−/− mice) used by Agapova et al. [141] exhibits decreased ActRIIA expression due to overexpression of the circulating activin ligand, possibly through endocytosis and degradation. Their results suggest that ActRIIA signaling plays an important role in vascular smooth muscle cell differentiation and that high levels of Dkk1 and repression of ActRIIA signaling induce osteoblast transition from vascular smooth muscle cells without affecting serum phosphorus and PTH levels. Consequently, the activation of two factors involved in kidney development, activin and Dkk1, cooperates in CKD-induced vascular disease. The ligand trap for ActRIIA also inhibits osteoclasts to stimulate high bone turnover [144]. ActRIIA signaling not only stimulates vascular calcification and bone remodeling, but also induces left ventricular hypertrophy in an Alport syndrome model [145]. Thus, ActRIIA signaling may be the key factor that induces renal osteodystrophy, cardiovascular disease, and renal fibrosis.
5. Conclusions
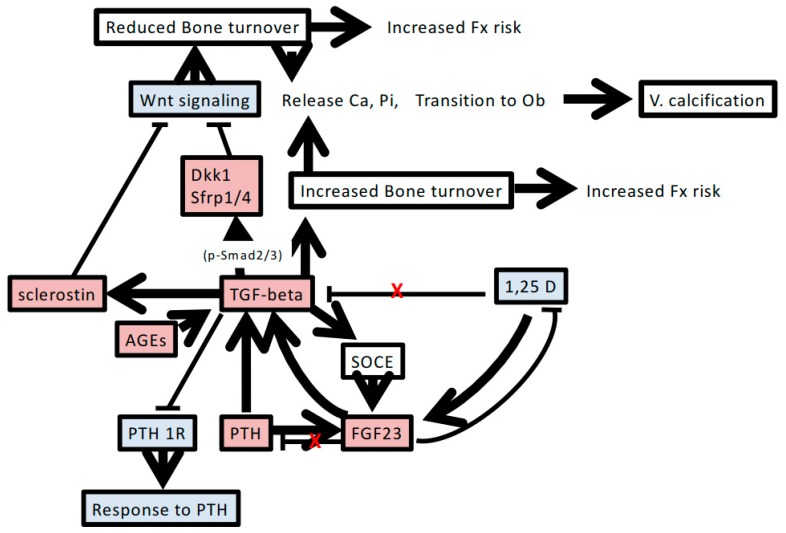
In Figure 1, we summarize the possible involvement of TGF-β in abnormalities of bone and mineral metabolism in CKD. Mineral metabolism disturbance caused by renal dysfunction is inherently complicated. The involvement of TGF-β in this setting seems to increase the complexity and promote the pathogenesis of CKD-MBD. The results of a series of studies on a ligand trap activin type IIA receptor are surprising, because treatment with the ligand trap restores bone turnover as well as protects against ectopic calcification and renal fibrosis. These reports suggest that apart from activin-A, other TGF-β superfamily factors that can enhance both Smad2/3 and Wnt signaling may be involved in the pathogenesis of CKD-MBD.
Figure 1.
Possible factors and pathways associated with the involvement of TGF-β in chronic kidney disease-mineral and bone disorder. Increasing TGF-β levels accompanied by renal dysfunction induces mineral metabolism disturbance and bone remodeling. The detailed mechanisms and interactions are described in the text. Red-shaded boxes indicate increased levels of factors or phenomena. Blue-shaded boxes indicate decreased levels of factors. Red X indicates regulatory disturbance in chronic kidney disease. T bar indicates inhibitory action of humoral factor. FGF23, fibroblast growth factor 23; PTH, parathyroid hormone; PTH1R, PTH type 1 receptor; 1,25D, 1,25 dihydroxyvitamin D3; AGEs, advanced glycation end products; Dkk1, Dickkopf-1; V. calcification, vascular calcification; Ob, osteoblasts; Ca, calcium; Pi, phosphorus; SOCE, store-operated calcium entry; Fx, fracture.
On the other hand, elucidation of the involvement of FGF23 in CKD-MBD has gradually increased our understanding of the pathogenesis of CKD-MBD. A recent study shows that FGF23 increases the expression of secreted frizzled-related protein 4 and Dkk1, and these increases are not ameliorated by PTH treatment [146]. Another report shows that FGF23 also regulates local bone mineralization in a vitamin D- and klotho-independent manner [147]. Knowing the detailed interactions between FGF23 and TGF-β would facilitate better understanding of the pathogenesis of CKD as well as the search for new therapeutic targets and optimal timing of intervention.
To better understand the pathogenesis of CKD-MBD, more studies are warranted to investigate the roles of TGF-β in CKD-MBD.
Abbreviations
| Runx2 | Runt-related transcription factor 2 |
| MMP | matrix metalloprotease |
| RANKL | receptor activator of nuclear factor-kappa B ligand |
| M-CSF | macrophage-colony stimulating factor |
| OPG | osteoprotegerin |
| ROD | renal osteodystrophy |
| CKD-MBD | chronic kidney disease-mineral and bone disorder |
| GFR | glomerular filtration rate |
| 1,25D | 1,25 dihydroxyvitamin D3 |
| BMD | bone mineral density |
| Dkk1 | Dickkopf-1 |
Author Contributions
Y.I., H.Y., and M.F. discussed the outline of the paper. Y.I. wrote a draft and M.F. and H.Y. revised the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fleisch M.C., Maxwell C.A., Barcellos-Hoff M.-H. The pleiotropic roles of transforming growth factor beta in homeostasis and carcinogenesis of endocrine organs. Endocr. Relat. Cancer. 2006;13:379–400. doi: 10.1677/erc.1.01112. [DOI] [PubMed] [Google Scholar]
- 2.Lehnert S.A., Akhurst R.J. Embryonic expression pattern of TGF beta type-1 RNA suggests both paracrine and autocrine mechanisms of action. Development. 1988;104:263–273. doi: 10.1242/dev.104.2.263. [DOI] [PubMed] [Google Scholar]
- 3.Horner A., Kemp P., Summers C., Bord S., Bishop N.J., Kelsall A.W., Coleman N., Compston J.E. Expression and distribution of transforming growth factor-β isoforms and their signaling receptors in growing human bone. Bone. 1998;23:5–102. doi: 10.1016/S8756-3282(98)00080-5. [DOI] [PubMed] [Google Scholar]
- 4.Berendsen A.D., Olsen B.R. Bone development. Bone. 2015;80:14–18. doi: 10.1016/j.bone.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long F., Ornitz D.M. Development of the endochondral skeleton. Cold Spring Harb. Perspect. Biol. 2013;5:a008334. doi: 10.1101/cshperspect.a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geiser A.G., Hummel C.W., Draper M.W., Henck J.W., Cohen I.R., Rudmann D.G., Donnelly K.B., Adrian M.D., Shepherd T.A., Wallace O.B., et al. A new selective estrogen receptor modulator with potent uterine antagonist activity, agonist activity in bone, and minimal ovarian stimulation. Endocrinology. 2005;46:4524–4535. doi: 10.1210/en.2005-0024. [DOI] [PubMed] [Google Scholar]
- 7.Dunker N., Krieglstein K. Tgfβ2−/− Tgfβ3−/− double knockout mice display severe midline fusion defects and early embryonic lethality. Anat. Embryol. 2002;206:73–83. doi: 10.1007/s00429-002-0273-6. [DOI] [PubMed] [Google Scholar]
- 8.Sanford L.P., Ormsby I., Gittenberger-de Groot A.C., Sariola H., Friedman R., Boivin G.P., Cardell E.L., Doetschman T. TGF beta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane J.L., Xian L., Cao X. Role of TGF-β signaling in coupling bone remodeling. Methods Mol. Biol. 2016;1344:287–300. doi: 10.1007/978-1-4939-2966-5_18. [DOI] [PubMed] [Google Scholar]
- 10.Tang Y., Wu X., Lei W., Pang L., Wan C., Shi Z., Zhao L., Nagy T.R., Peng X., Hu J., et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng X.H., Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 12.Sakou T., Onishi T., Yamamoto T., Nagamine T., Sampath T.K., Ten Dijke P. Localization of Smads, the TGF-β family intracellular signaling components during endochondral ossification. J. Bone Miner. Res. 1999;14:1145–1152. doi: 10.1359/jbmr.1999.14.7.1145. [DOI] [PubMed] [Google Scholar]
- 13.Serra R., Karaplis A., Sohn P. Parathyroid hormone-related peptide (PTHrP)-dependent and -independent effects of transforming growth factor β (TGF-β) on endochondral bone formation. J. Cell Biol. 1999;145:783–794. doi: 10.1083/jcb.145.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li T.F., O’Keefe R.J., Chen D. TGF-β signaling in chondrocytes. Front. Biosci. 2005;10:681–688. doi: 10.2741/1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentry L.E., Nash B.W. The pro domain of pre-pro-transforming growth factor beta 1 when independently expressed is a functional binding protein for the mature growth factor. Biochemistry. 1990;29:6851–6857. doi: 10.1021/bi00481a014. [DOI] [PubMed] [Google Scholar]
- 16.Derynck R., Jarrett J.A., Chen E.Y., Eaton D.H., Bell J.R., Assoian R.K., Roberts A.B., Sporn M.B., Goeddel D.V. Human transforming growth factor-β complementary DNA sequence and expression in normal and transformed cells. Nature. 1985;316:701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhry S.S., Cain S.A., Morgan A., Dallas S.L., Shuttleworth C.A., Kielty C.M. Fibrillin-1 regulates the bioavailability of TGFβ1. J. Cell Biol. 2007;176:355–367. doi: 10.1083/jcb.200608167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taipale J., Miyazono K., Heldin C.H., Keski-Oja J. Latent transforming growth factor-β1 associates to fibroblast extracellular matrix via latent TGF-β binding protein. J. Cell Biol. 1994;124:171–181. doi: 10.1083/jcb.124.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunes I., Gleizes P.E., Metz C.N., Rifkin D.B. Latent transforming growth factor-β binding protein domains involved in activation and transglutaminase-dependent cross-linking of latent transforming growth factor-β. J. Cell Biol. 1997;136:1151–1163. doi: 10.1083/jcb.136.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unsöld C., Hyytiäinen M., Bruckner-Tuderman L., Keski-Oja J. Latent TGF-β binding protein LTBP-1 contains three potential extracellular matrix interacting domains. J. Cell Sci. 2001;114:187–197. doi: 10.1242/jcs.114.1.187. [DOI] [PubMed] [Google Scholar]
- 21.Isogai Z., Ono R.N., Ushiro S., Keene D.R., Chen Y., Mazzieri R., Charbonneau N.L., Reinhardt D.P., Rifkin D.B., Sakai L.Y. Latent transforming growth factor β-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J. Biol. Chem. 2003;278:2750–2757. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- 22.Dallas S.L., Rosser J.L., Mundy G.R., Bonewald L.F. Proteolysis of latent transforming growth factor-β (TGF-β)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-β from bone matrix. J. Biol. Chem. 2002;277:21352–21360. doi: 10.1074/jbc.M111663200. [DOI] [PubMed] [Google Scholar]
- 23.Oreffo R.O., Mundy G.R., Seyedin S.M., Bonewald L.F. Activation of the bone-derived latent TGF β complex by isolated osteoclasts. Biochem. Biophys. Res. Commun. 1989;158:817–823. doi: 10.1016/0006-291X(89)92795-2. [DOI] [PubMed] [Google Scholar]
- 24.Van der Kraan P.M., Goumans M.J., Blaney Davidson E., Ten Dijke P. Age-dependent alteration of TGF-β signalling in osteoarthritis. Cell Tissue Res. 2012;347:257–265. doi: 10.1007/s00441-011-1194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van den Bosch M.H., Blom A.B., van Lent P.L., van Beuningen H.M., Davidson E.N.B., van der Kraan P.M., van den Berg W.B. Canonical Wnt signaling skews TGF-β signaling in chondrocytes towards signaling via ALK1 and Smad 1/5/8. Cell. Signal. 2014;26:951–958. doi: 10.1016/j.cellsig.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Lee S.W., Choi K.Y., Cho J.Y., Jung S.H., Song K.B., Park E.K., Choi J.-Y., Shin H.-I., Kim S.-Y., Woo K.-M., et al. TGF-β2 stimulates cranial suture closure through activation of the Erk-MAPK pathway. J. Cell Biochem. 2006;98:981–991. doi: 10.1002/jcb.20773. [DOI] [PubMed] [Google Scholar]
- 27.Urano T., Yashiroda H., Muraoka M., Tanaka K., Hosoi T., Inoue S., Ouchi Y., Tanaka K., Toyoshima H. p57(Kip2) is degraded through the proteasome in osteoblasts stimulated to proliferation by transforming growth factor β1. J. Biol. Chem. 1999;74:12197–12200. doi: 10.1074/jbc.274.18.12197. [DOI] [PubMed] [Google Scholar]
- 28.Sowa H., Kaji H., Yamaguchi T., Sugimoto T., Chihara K. Smad3 promotes alkaline phosphatase activity and mineralization of osteoblastic MC3T3-E1 cells. J. Bone Miner. Res. 2002;17:1190–1199. doi: 10.1359/jbmr.2002.17.7.1190. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.I., Kwak J.H., Zachariah M., He Y., Wang L., Choi M.E. TGF-β-activated kinase 1 and TAK1-binding protein 1 cooperate to mediate TGF-β1-induced MKK3-p38 MAPK activation and stimulation of type I collagen. Am. J. Physiol. Renal Physiol. 2007;292:F1471–F1478. doi: 10.1152/ajprenal.00485.2006. [DOI] [PubMed] [Google Scholar]
- 30.Lai C.F., Cheng S.L. Signal transductions induced by bone morphogenetic protein-2 and transforming growth factor-beta in normal human osteoblastic cells. J. Biol. Chem. 2002;277:15514–15522. doi: 10.1074/jbc.M200794200. [DOI] [PubMed] [Google Scholar]
- 31.Zhou S. TGF-β regulates β-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J. Cell Biochem. 2011;112:1651–1660. doi: 10.1002/jcb.23079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karlsson T., Sundar R., Widmark A., Landström M., Persson E. Osteoblast-derived factors promote metastatic potential in human prostate cancer cells, in part via non-canonical transforming growth factor β (TGFβ) signaling. Prostate. 2018;78:446–456. doi: 10.1002/pros.23489. [DOI] [PubMed] [Google Scholar]
- 33.Lee K.S., Hong S.H., Bae S.C. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-β and bone morphogenetic protein. Oncogene. 2002;21:7156–7163. doi: 10.1038/sj.onc.1205937. [DOI] [PubMed] [Google Scholar]
- 34.Otto F., Thornell A.P., Crompton T., Denzel A., Gilmour K.C., Rosewell I.R., Stamp G.W.H., Beddington R.S.P., Mundlos S., Olsen B.R., et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/S0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 35.Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J.M., Behringer R.R., de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/S0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 36.Koga T., Matsui Y., Asagiri M., Kodama T., de Crombrugghe B., Nakashima K., Takayanagi H. NFAT and Osterix cooperatively regulate bone formation. Nat. Med. 2005;11:880–885. doi: 10.1038/nm1270. [DOI] [PubMed] [Google Scholar]
- 37.Tuckermann J.P., Pittois K., Partridge N.C., Merregaert J., Angel P. Collagenase-3 (MMP-13) and integral membrane protein 2a (Itm2a) are marker genes of chondrogenic/osteoblastic cells in bone formation: Sequential temporal, and spatial expression of Itm2a, alkaline phosphatase, MMP-13, and osteocalcin in the mouse. J. Bone Miner. Res. 2000;15:1257–1265. doi: 10.1359/jbmr.2000.15.7.1257. [DOI] [PubMed] [Google Scholar]
- 38.Alliston T., Choy L., Ducy P., Karsenty G., Derynck R. TGF-β-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001;20:2254–2272. doi: 10.1093/emboj/20.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karsdal M.A., Larsen L., Engsig M.T., Lou H., Ferreras M., Lochter A., Delaissé J.M., Foged N.T. Matrix metalloproteinase-dependent activation of latent transforming growth factor-β controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J. Biol. Chem. 2002;277:44061–44067. doi: 10.1074/jbc.M207205200. [DOI] [PubMed] [Google Scholar]
- 40.Borton A.J., Frederick J.P., Datto M.B., Wang X.F., Weinstein R.S. The loss of Smad 3 results in lower rate of bone formation and osteopenia through dysregulation of osteoblast differentiation and apoptosis. J. Bone Miner. Res. 2001;16:1754–1764. doi: 10.1359/jbmr.2001.16.10.1754. [DOI] [PubMed] [Google Scholar]
- 41.Pilkington M.F., Sims S.M., Dixon S.J. Transforming growth factor-beta induces osteoclast ruffling and chemotaxis: Potential role in osteoclast recruitment. J. Bone Miner. Res. 2001;16:1237–1247. doi: 10.1359/jbmr.2001.16.7.1237. [DOI] [PubMed] [Google Scholar]
- 42.Suda T., Takahashi N., Udagawa N., Jimi E., Gillespie M.T., Martin T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 43.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 44.Pixley F.J., Stanley E.R. CSF-1 regulation of the wandering macrophage: Complexity in action. Trends Cell Biol. 2004;4:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 45.Kaneda T., Nojima T., Nakagawa M., Ogasawara A., Kaneko H., Sato T., Mano H., Kumegawa M., Hakeda Y. Endogenous production of TGF-β is essential for osteoclastogenesis induced by a combination of receptor activator of NF-κB ligand and macrophage-colony-stimulating factor. J. Immunol. 2000;165:4254–4263. doi: 10.4049/jimmunol.165.8.4254. [DOI] [PubMed] [Google Scholar]
- 46.Galvin R.J.S., Gatlin C.L., Horn J.W., Fuson T.R. TGF-β enhances osteoclast differentiation in hematopoietic cell cultures stimulated with RANKL and M-CSF. Biochem. Biophys. Res. Commun. 1999;265:233–239. doi: 10.1006/bbrc.1999.1632. [DOI] [PubMed] [Google Scholar]
- 47.Fuller K., Lean J.M., Bayley K.E., Wani M.R., Chambers T.J. A role for TGFbeta(1) in osteoclast differentiation and survival. J. Cell Sci. 2000;113:2445–2453. doi: 10.1242/jcs.113.13.2445. [DOI] [PubMed] [Google Scholar]
- 48.Yasui T., Kadono Y., Nakamura M., Oshima Y., Matsumoto T., Masuda H., Hirose J., Omata Y., Yasuda H., Imamura T., et al. Regulation of RANKL-induced osteoclastogenesis by TGF-β through molecular interaction between Smad3 and Traf6. J. Bone Miner. Res. 2011;26:1447–1456. doi: 10.1002/jbmr.357. [DOI] [PubMed] [Google Scholar]
- 49.Mohammad K.S., Chen C.G., Balooch G., Stebbins E., McKenna C.R., Davis H., Niewolna M., Peng X.H., Nguyen D.H.N., Ionova-Martin S.S., et al. Pharmacologic inhibition of the TGF-β type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS ONE. 2009;4:e5275. doi: 10.1371/journal.pone.0005275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karst M., Gorny G., Galvin R.J.S., Oursler M.J. Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-β regulation of osteoclast differentiation. J. Cell Physiol. 2004;200:99–106. doi: 10.1002/jcp.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murakami T., Yamamoto M., Yamamoto M., Ono K., Nishikawa M., Nagata N., Motoyoshi K., Akatsu T. Transforming growth factor-β1 increases mRNA levels of osteoclastogenesis inhibitory factor in osteoblastic/stromal cells and inhibits the survival of murine osteoclast-like cells. Biochem. Biophys. Res. Commun. 1998;252:747–752. doi: 10.1006/bbrc.1998.9723. [DOI] [PubMed] [Google Scholar]
- 52.Thirunavukkarasu K. Stimulation of osteoprotegerin (OPG) gene expression by transforming growth factor-β (TGF-β): Mapping of the OPG promoter region that mediates TGF-β effects. J. Biol. Chem. 2001;276:36241–36250. doi: 10.1074/jbc.M104319200. [DOI] [PubMed] [Google Scholar]
- 53.Subramaniam M., Hawse J.R., Bruinsma E.S., Grygo S.B., Cicek M., Oursler M.J., Spelsberg T.C. TGFβ inducible early gene-1 directly binds to, and represses, the OPG promoter in osteoblasts. Biochem. Biophys. Res. Commun. 2010;392:72–76. doi: 10.1016/j.bbrc.2009.12.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka K., Yamaguchi Y., Hakeda Y. Isolated chick osteocytes stimulate formation and bone-resorbing activity of osteoclast-like cells. J. Bone Miner. Metab. 1995;13:61–70. doi: 10.1007/BF01771319. [DOI] [Google Scholar]
- 55.Qing H., Bonewald L.F. Osteocyte remodeling of the perilacunar and pericanalicular matrix. Int. J. Oral Sci. 2009;1:59–65. doi: 10.4248/ijos.09019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kogawa M., Wijenayaka A.R., Ormsby R.T., Thomas G.P., Anderson P.H., Bonewald L.F., Findlay D.M., Atkins G.J. Sclerostin regulates release of bone mineral by osteocytes by induction of carbonic anhydrase 2. J. Bone Miner. Res. 2013;28:2436–2448. doi: 10.1002/jbmr.2003. [DOI] [PubMed] [Google Scholar]
- 57.Qing H., Ardeshirpour L., Pajevic P.D., Dusevich V., Jahn K., Kato S., Wysolmerski J., Bonewald L.F. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J. Bone Miner. Res. 2012;27:1018–1029. doi: 10.1002/jbmr.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wysolmerski J.J. Osteocytes remove and replace perilacunar mineral during reproductive cycles. Bone. 2013;54:230–236. doi: 10.1016/j.bone.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simpson A.E., Stoddart M.J., Davies C.M., Jähn K., Furlong P.I., Gasser J.A., Jones D.B., Noble B.S., Richards R.G. TGF β3 and loading increases osteocyte survival in human cancellous bone cultured ex vivo. Cell Biochem. Funct. 2009;27:23–29. doi: 10.1002/cbf.1529. [DOI] [PubMed] [Google Scholar]
- 60.Verborgt O., Tatton N.A., Majeska R.J., Schaffler M.B. Spatial distribution of Bax and Bcl-2 in osteocytes after bone fatigue: Complementary roles in bone remodeling regulation? J. Bone Miner. Res. 2002;17:907–914. doi: 10.1359/jbmr.2002.17.5.907. [DOI] [PubMed] [Google Scholar]
- 61.Tatsumi S., Ishii K., Amizuka N., Li M., Kobayashi T., Kohno K., Ito M., Takeshita S., Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Moe S., Drüeke T., Cunningham J., Goodman W., Martin K., Olgaard K., Ott S., Sprague S., Lameire N., Eknoyan G. Kidney Disease: Improving Global Outcomes (KDIGO). Definition, evaluation, and classification of renal osteodystrophy: A position statement from kidney disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 63.Evenepoel P., Meijers B., Viaene L., Bammens B., Claes K., Kuypers D., Vanderschueren D., Vanrenterghem Y. Fibroblast growth factor-23 in early chronic kidney disease: Additional support in favor of a phosphate-centric paradigm for the pathogenesis of secondary hyperparathyroidism. Clin. J. Am. Soc. Nephrol. 2010;5:1268–1276. doi: 10.2215/CJN.08241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Isakova T., Wahl P., Vargas G.S., Gutiérrez O.M., Scialla J., Xie H., Appleby D., Nessel L., Bellovich K., Chen J., et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nickolas T.L., Stein E.M., Dworakowski E., Nishiyama K.K., Komandah-Kosseh M., Zhang C.A., McMahon D.J., Liu X.S., Boutroy S., Cremers S., et al. Rapid cortical bone loss in patients with chronic kidney disease. J. Bone Miner. Res. 2013;28:1811–1820. doi: 10.1002/jbmr.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Russo C.R., Taccetti G., Caneva P., Mannarino A., Maranghi P., Ricca M. Volumetric bone density and geometry assessed by peripheral quantitative computed tomography in uremic patients on maintenance hemodialysis. Osteoporos. Int. 1998;8:443–448. doi: 10.1007/s001980050089. [DOI] [PubMed] [Google Scholar]
- 67.Paranhos-Neto F.P., Lima G.A.C., Silva L.C., Madeira M., Vieira L.N., Mendonça L.M.C., Lima I.C.B., Delgado A.G., Leite M., Jr., Gomes C.P., et al. HR-pQCT detects alterations in bone microstructure in men with CKD stages 3 and 4, which are influenced by hormonal changes and body composition. Clin. Nephrol. 2018;89:10–17. doi: 10.5414/CN109006. [DOI] [PubMed] [Google Scholar]
- 68.Fukagawa M., Kazama J.J., Shigematsu T. Skeletal resistance to PTH as a basic abnormality underlying uremic bone disease. Am. J. Kidney Dis. 2001;38(Suppl. 1):S152–S155. doi: 10.1053/ajkd.2001.27426. [DOI] [PubMed] [Google Scholar]
- 69.Iwasaki Y., Yamato H., Nii-Kono T., Fujieda A., Uchida M., Hosokawa A., Motojima M., Fukagawa M. Insufficiency of PTH action on bone in uremia. Kidney Int. 2006;70:S34–S36. doi: 10.1038/sj.ki.5001600. [DOI] [PubMed] [Google Scholar]
- 70.Bover J., Ureña P., Brandenburg V., Goldsmith D., Ruiz C., DaSilva I., Bosch R.J. Adynamic bone disease: From bone to vessels in chronic kidney disease. Semin. Nephrol. 2014;34:626–640. doi: 10.1016/j.semnephrol.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 71.Rudser K.D., de Boer I.H., Dooley A., Young B., Kestenbaum B. Fracture risk after parathyroidectomy among chronic kidney disease. J. Am. Soc. Nephrol. 2007;18:2401–2407. doi: 10.1681/ASN.2007010022. [DOI] [PubMed] [Google Scholar]
- 72.Maruyama Y., Taniguchi M., Kazama J.J., Yokoyama K., Hosoya T., Yokoo T., Shigematsu T., Iseki K., Tsubakihara Y. A higher serum alkaline phosphatase is associated with the incidence of hip fracture and mortality among patients receiving hemodialysis in Japan. Nephrol. Dial. Transplant. 2014;29:1532–1538. doi: 10.1093/ndt/gfu055. [DOI] [PubMed] [Google Scholar]
- 73.Atsumi K., Kushida K., Yamazaki K., Shimizu S., Ohmura A., Inoue T. Risk factors for vertebral fracture in renal osteodystrophy. Am. J. Kidney Dis. 1999;33:287–293. doi: 10.1016/S0272-6386(99)70302-1. [DOI] [PubMed] [Google Scholar]
- 74.Iwasaki Y., Kazama J.J., Yamato H., Fukagawa M. Changes in chemical composition of cortical bone associated with bone fragility in rat model with chronic kidney disease. Bone. 2011;48:1260–1267. doi: 10.1016/j.bone.2011.03.672. [DOI] [PubMed] [Google Scholar]
- 75.Brandenburg V.M., D’Haese P., Deck A., Mekahli D., Meijers B., Neven E., Evenepoel P. From skeletal to cardiovascular disease in 12 steps-the evolution of sclerostin as a major player in CKD-MBD. Pediatr. Nephrol. 2016;31:195–206. doi: 10.1007/s00467-015-3069-7. [DOI] [PubMed] [Google Scholar]
- 76.Seifert M.E., de las Fuentes L., Rothstein M., Dietzen D.J., Bierhals A.J., Cheng S.C., Ross W., Windus D., Dávila-Román V.G., Hruska K.A. Effects of phosphate binder therapy on vascular stiffness in early-stage chronic kidney disease. Am. J. Nephrol. 2013;38:158–167. doi: 10.1159/000353569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loeffler I., Wolf G. Transforming growth factor-β and the progression of renal disease. Nephrol. Dial. Transplant. 2014;29(Suppl. 1):i37–i45. doi: 10.1093/ndt/gft267. [DOI] [PubMed] [Google Scholar]
- 78.Seifert M.E., de las Fuentes L., Rothstein M., Dietzen D.J., Bierhals A.J., Cheng S.C., Ross W., Windus D., Dávila-Román V.G., Hruska K.A. Circulating transforming growth factor-β1 levels and the risk for kidney disease in African Americans. Kidney Int. 2009;76:72–80. doi: 10.1038/ki.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mathew S., Davies M., Lund R., Saab G., Hruska K.A. Function and effect of bone morphogenetic protein-7 in kidney bone and the bone-vascular links in chronic kidney disease. Eur. J. Clin. Investig. 2006;36(Suppl. 2):43–50. doi: 10.1111/j.1365-2362.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- 80.Jiang X., Kanai H., Shigehara T., Maezawa A., Yano S., Naruse T. Metabolism of transforming growth factor-beta in patients receiving hemodialysis especially those with renal osteodystrophy. Ren. Fail. 1998;20:135–145. doi: 10.3109/08860229809045096. [DOI] [PubMed] [Google Scholar]
- 81.Duarte M.E., Carvalho E.F., Cruz E.A., Lucena S.B., Andress D.L. Cytokine accumulation in osteitis fibrosa of renal osteodystrophy. Braz. J. Med. Biol. Res. 2002;35:25–29. doi: 10.1590/S0100-879X2002000100004. [DOI] [PubMed] [Google Scholar]
- 82.Santos F.R., Moysés R.M., Montenegro F.L., Jorgetti V., Noronha I.L. IL-1beta, TNF-alpha, TGF-beta, and bFGF expression in bone biopsies before and after parathyroidectomy. Kidney Int. 2003;63:899–907. doi: 10.1046/j.1523-1755.2003.00835.x. [DOI] [PubMed] [Google Scholar]
- 83.Hoyland J.A., Picton M.L. Cellular mechanisms of renal osteodystrophy. Kidney Int. 1999;56:S8–S13. doi: 10.1046/j.1523-1755.1999.07312.x. [DOI] [PubMed] [Google Scholar]
- 84.Hughes D.E., Boyce B.F. Apoptosis in bone physiology and disease. Mol. Pathol. 1997;50:132–137. doi: 10.1136/mp.50.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu S., Song W., Boulanger J.H., Tang W., Sabbagh Y., Kelley B., Gotschall R., Ryan S., Phillips L., Malley K., et al. Role of TGF-β in a mouse model of high turnover renal osteodystrophy. J. Bone Miner. Res. 2014;29:1141–1157. doi: 10.1002/jbmr.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McCarthy T.L., Centrella M. Novel links among Wnt and TGF-β signaling and Runx2. Mol. Endocrinol. 2010;24:587–597. doi: 10.1210/me.2009-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Massry S.G., Coburn J.W., Lee D.B.M., Towsey J., Kleeman C. Skeletal resistance to parathyroid hormone in renal failure. Study in 105 human subjects. Ann. Intern. Med. 1973;78:357–364. doi: 10.7326/0003-4819-78-3-357. [DOI] [PubMed] [Google Scholar]
- 88.Rodriguez M., Martin-Malo A., Martinez M.E., Torres A., Felsenfeld A.J., Llach F. Calcemic response to parathyroid hormone in renal failure: Role of phosphorus and its effect on calcitriol. Kidney Int. 1991;40:1055–1062. doi: 10.1038/ki.1991.314. [DOI] [PubMed] [Google Scholar]
- 89.Bover J., Jara A., Trinidad P., Rodriguez M., Martin Malo A., Felsenfeld A.J. The calcemic response to PTH in the rat: Effect of elevated PTH levels and uremia. Kidney Int. 1994;46:310–317. doi: 10.1038/ki.1994.276. [DOI] [PubMed] [Google Scholar]
- 90.Rodriguez M., Felsenfeld A.J., Llach F. Calcemic response to parathyroid hormone in renal failure: Role of calcitriol and the effect parathyroidectomy. Kidney Int. 1991;40:1063–1068. doi: 10.1038/ki.1991.315. [DOI] [PubMed] [Google Scholar]
- 91.Urena P., Kubrusly M., Mannstradt M., Hruby M., Tan M.T.T., Silve C., Lacour B., Abou-Samra A., Segre G., Drüeke T. The renal PTH/PTHrP receptor is downregulated in rats with chronic renal failure. Kidney Int. 1994;45:605–611. doi: 10.1038/ki.1994.79. [DOI] [PubMed] [Google Scholar]
- 92.Urena P., Mannstadt M., Hruby M., Ferreira A., Schmitt F., Silve C., Ardaillou R., Lacour B., Abou-Samra A.B., Segre G.V., et al. Parathyroidectomy does not prevent the renal PTH/PTHrP receptor down regulation in uremic rats. Kidney Int. 1995;47:1797–1805. doi: 10.1038/ki.1995.248. [DOI] [PubMed] [Google Scholar]
- 93.Jongen J.W., Willemstein-Van Hove E.C., Van der Meer J.M., Bos M.P., Juppner H., Segre G.V., Abou-Samra A.B., Feyen J.H.M., Herrmann-Erlee M.P.M. Down-regulation of the receptor for parathyroid hormone (PTH) and PTH-related peptide by transforming growth factor-beta in primary fetal rat osteoblasts. Endocrinology. 1995;136:3260–3266. doi: 10.1210/endo.136.8.7628359. [DOI] [PubMed] [Google Scholar]
- 94.Qiu T., Wu X., Zhang F., Clemens T.L., Wan M., Cao X. TGF-beta type II receptor phosphorylates PTH receptor to integrate bone remodelling signalling. Nat. Cell Biol. 2010;12:224–234. doi: 10.1038/ncb2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nii-Kono T., Iwasaki Y., Uchida M., Fujieda A., Hosokawa A., Motojima M., Yamato H., Kurokawa K., Fukagawa M. Indoxyl sulfate induces skeletal resistance to parathyroid hormone in cultured osteoblastic cells. Kidney Int. 2007;71:738–743. doi: 10.1038/sj.ki.5002097. [DOI] [PubMed] [Google Scholar]
- 96.Tanaka H., Iwasaki Y., Yamato H., Mori Y., Komaba H., Watanabe H., Maruyama T., Fukagawa M. p-Cresyl sulfate induces osteoblast dysfunction through activating JNK and p38 MAPK pathways. Bone. 2013;56:347–354. doi: 10.1016/j.bone.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Yano S., Yamaguchi T., Kanazawa I., Ogawa N., Hayashi K., Yamauchi M., Sugimoto T. The uraemic toxin phenylacetic acid inhibits osteoblastic proliferation and differentiation: An implication for the pathogenesis of low turnover bone in chronic renal failure. Nephrol. Dial. Transplant. 2007;22:3160–3165. doi: 10.1093/ndt/gfm455. [DOI] [PubMed] [Google Scholar]
- 98.Westerberg P.A., Linde T., Wikström B., Ljunggren O., Stridsberg M., Larsson T.E. Regulation of fibroblast growth factor-23 in chronic kidney disease. Nephrol. Dial. Transplant. 2007;22:3202–3207. doi: 10.1093/ndt/gfm347. [DOI] [PubMed] [Google Scholar]
- 99.Isakova T., Xie H., Yang W., Xie D., Anderson A.H., Scialla J., Wahl P., Gutiérrez O.M., Steigerwalt S., He J., et al. Chronic Renal Insufficiency Cohort (CRIC) Study Group. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lavi-Moshayoff V., Wasserman G., Meir T., Silver J., Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am. J. Physiol. Ren. Physiol. 2010;299:F882–F889. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 101.Feger M., Hase P., Zhang B., Hirche F., Glosse P., Lang F., Föller M. The production of fibroblast growth factor 23 is controlled by TGF-β2. Sci. Rep. 2017;7:4982. doi: 10.1038/s41598-017-05226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koizumi M., Komaba H., Nakanishi S., Fujimori A., Fukagawa M. Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol. Dial. Transplant. 2012;27:784–790. doi: 10.1093/ndt/gfr384. [DOI] [PubMed] [Google Scholar]
- 103.Hu M.C., Shiizaki K., Kuro-o M., Moe O.W. Fibroblast growth factor 23 and Klotho: Physiology and pathophysiology of an endocrine network of mineral metabolism. Annu. Rev. Physiol. 2013;75:503–533. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ben-Dov I.Z., Galitzer H., Lavi-Moshayoff V., Goetz R., Kuro-o M., Mohammadi M., Sirkis R., Naveh-Many T., Silver J. The parathyroid is a target organ for FGF23 in rats. J. Clin. Investig. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Galitzer H., Ben-Dov I.Z., Silver J., Naveh-Many T. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77:211–218. doi: 10.1038/ki.2009.464. [DOI] [PubMed] [Google Scholar]
- 106.Canalejo R., Canalejo A., Martinez-Moreno J.M., Rodriguez-Ortiz M.E., Estepa J.C., Mendoza F.J., Munoz-Castaneda J.R., Shalhoub V., Almaden Y., Rodriguez M. FGF23 fails to inhibit uremic parathyroid glands. J. Am. Soc. Nephrol. 2010;21:1125–1135. doi: 10.1681/ASN.2009040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Komaba H., Goto S., Fujii H., Hamada Y., Kobayashi A., Shibuya K., Tominaga Y., Otsuki N., Nibu K., Nakagawa K., et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010;77:232–238. doi: 10.1038/ki.2009.414. [DOI] [PubMed] [Google Scholar]
- 108.Hu M.C., Shi M., Zhang J., Quiñones H., Kuro-o M., Moe O.W. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240–1251. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meir T., Durlacher K., Pan Z., Amir G., Richards W.G., Silver J., Naveh-Many T. Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int. 2014;86:1106–1115. doi: 10.1038/ki.2014.215. [DOI] [PubMed] [Google Scholar]
- 110.Saini R.K., Kaneko I., Jurutka P.W., Forster R., Hsieh A., Hsieh J.C., Haussler M.R., Whitfield G.K. 1,25-dihydroxyvitamin D-3 regulation of fibroblast growth factor-23 expression in bone cells: Evidence for primary and secondary mechanisms modulated by leptin and interleukin-6. Calcif. Tissue Int. 2013;92:339–353. doi: 10.1007/s00223-012-9683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang B., Yan J., Umbach A.T., Fakhri H., Fajol A., Schmidt S., Salker M.S., Chen H., Alexander D., Spichtig D., et al. NFκB-sensitive Orai1 expression in the regulation of FGF23 release. J. Mol. Med. 2016;94:557–566. doi: 10.1007/s00109-015-1370-3. [DOI] [PubMed] [Google Scholar]
- 112.Mace M.L., Gravesen E., Nordholm A., Hofman-Bang J., Secher T., Olgaard K., Lewin E. Kidney fibroblast growth factor 23 does not contribute to elevation of its circulating levels in uremia. Kidney Int. 2017;92:165–178. doi: 10.1016/j.kint.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 113.Smith E.R., Holt S.G., Hewitson T.D. FGF23 activates injury-primed renal fibroblasts via FGFR4-dependent signalling and enhancement of TGF-β autoinduction. Int. J. Biochem. Cell Biol. 2017;92:63–78. doi: 10.1016/j.biocel.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 114.Komaba H., Kaludjerovic J., Hu D.Z., Nagano K., Amano K., Ide N., Sato T., Densmore M.J., Hanai J.I., Olauson H., et al. Klotho expression in osteocytes regulates bone metabolism and controls bone formation. Kidney Int. 2017;92:599–611. doi: 10.1016/j.kint.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 115.Cool S., Jackson R., Pincus P., Dickinson I., Nurcombe V. Fibroblast growth factor receptor 4 (FGFR4) expression in newborn murine calvaria and primary osteoblast cultures. Int. J. Dev. Biol. 2002;46:519–523. [PubMed] [Google Scholar]
- 116.Zerr P., Vollath S., Palumbo-Zerr K., Tomcik M., Huang J., Distler A., Beyer C., Dees C., Gela K., Distler O., et al. Vitamin D receptor regulates TGF-β signalling in systemic sclerosis. Ann. Rheum. Dis. 2015;74:e20. doi: 10.1136/annrheumdis-2013-204378. [DOI] [PubMed] [Google Scholar]
- 117.Cejka D., Jäger-Lansky A., Kieweg H., Weber M., Bieglmayer C., Haider D.G., Diarra D., Patsch J.M., Kainberger F., Bohle B., et al. Sclerostin serum levels correlate positively with bone mineral density and microarchitecture in haemodialysis patients. Nephrol. Dial. Transplant. 2012;27:226–230. doi: 10.1093/ndt/gfr270. [DOI] [PubMed] [Google Scholar]
- 118.Thambiah S., Roplekar R., Manghat P., Fogelman I., Fraser W.D., Goldsmith D., Hampson G. Circulating sclerostin and Dickkopf-1 (DKK1) in predialysis chronic kidney disease (CKD): Relationship with bone density and arterial stiffness. Calcif. Tissue Int. 2012;90:473–480. doi: 10.1007/s00223-012-9595-4. [DOI] [PubMed] [Google Scholar]
- 119.Sabbagh Y., Graciolli F.G., O’Brien S., Tang W., dos Reis L.M., Ryan S., Phillips L., Boulanger J., Song W., Bracken C., et al. Repression of osteocyte Wnt/β-catenin signaling is an early event in the progression of renal osteodystrophy. J. Bone Miner. Res. 2012;27:1757–1772. doi: 10.1002/jbmr.1630. [DOI] [PubMed] [Google Scholar]
- 120.De Oliveira R.B., Graciolli F.G., dos Reis L.M., Cancela A.L., Cuppari L., Canziani M.E., Carvalho A.B., Jorgetti V., Moysés R.M. Disturbances of Wnt/β-catenin pathway and energy metabolism in early CKD: Effect of phosphate binders. Nephrol. Dial. Transplant. 2013;28:2510–2517. doi: 10.1093/ndt/gft234. [DOI] [PubMed] [Google Scholar]
- 121.Graciolli F.G., Neves K.R., Barreto F., Barreto D.V., Dos Reis L.M., Canziani M.E., Sabbagh Y., Carvalho A.B., Jorgetti V., Elias R.M., et al. The complexity of chronic kidney disease-mineral and bone disorder across stages of chronic kidney disease. Kidney Int. 2017;91:1436–1446. doi: 10.1016/j.kint.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 122.Mödder U.I., Hoey K.A., Amin S., McCready L.K., Achenbach S.J., Riggs B.L., Melton L.J., III, Khosla S. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J. Bone Miner. Res. 2011;26:373–379. doi: 10.1002/jbmr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ishimura E., Okuno S., Ichii M., Norimine K., Yamakawa T., Shoji S., Nishizawa Y., Inaba M. Relationship between serum sclerostin, bone metabolism markers, and bone mineral density in maintenance hemodialysis patients. J. Clin. Endocrinol. Metab. 2014;99:4315–4320. doi: 10.1210/jc.2014-2372. [DOI] [PubMed] [Google Scholar]
- 124.De Oliveira R.A., Barreto F.C., Mendes M., dos Reis L.M., Castro J.H., Britto Z.M., Marques I.D., Carvalho A.B., Moysés R.M., Jorgetti V. Peritoneal dialysis per se is a risk factor for sclerostin-associated adynamic bone disease. Kidney Int. 2015;87:1039–1045. doi: 10.1038/ki.2014.372. [DOI] [PubMed] [Google Scholar]
- 125.Cejka D., Herberth J., Branscum A.J., Fardo D.W., Monier-Faugere M.C., Diarra D., Haas M., Malluche H.H. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin. J. Am. Soc. Nephrol. 2011;6:877–882. doi: 10.2215/CJN.06550810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Viaene L., Behets G.J., Claes K., Meijers B., Blocki F., Brandenburg V., Evenepoel P., D’Haese P.C. Sclerostin: Another bone-related protein related to all-cause mortality in haemodialysis? Nephrol. Dial. Transplant. 2013;28:3024–3030. doi: 10.1093/ndt/gft039. [DOI] [PubMed] [Google Scholar]
- 127.Keller H., Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37:148–158. doi: 10.1016/j.bone.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 128.Leupin O., Kramer I., Collette N.M., Loots G.G., Natt F., Kneissel M., Keller H. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J. Bone Miner. Res. 2007;22:1957–1967. doi: 10.1359/jbmr.070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Loots G.G., Keller H., Leupin O., Murugesh D., Collette N.M., Genetos D.C. TGF-β regulates sclerostin expression via the ECR5 enhancer. Bone. 2012;50:663–669. doi: 10.1016/j.bone.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Notsu M., Kanazawa I., Takeno A., Yokomoto-Umakoshi M., Tanaka K.I., Yamaguchi T., Sugimoto T. Advanced glycation end product 3 (AGE3) increases apoptosis and the expression of sclerostin by stimulating TGF-β expression and secretion in osteocyte-like MLO-Y4-A2 cells. Calcif. Tissue Int. 2017;100:402–411. doi: 10.1007/s00223-017-0243-x. [DOI] [PubMed] [Google Scholar]
- 131.Miyata T., Wada Y., Cai Z., Iida Y., Horie K., Yasuda Y., Maeda K., Kurokawa K., van Ypersele de Strihou C. Implication of an increased oxidative stress in the formation of advanced glycation end products in patients with end-stage renal failure. Kidney Int. 1997;51:1170–1181. doi: 10.1038/ki.1997.160. [DOI] [PubMed] [Google Scholar]
- 132.Baron R., Kneissel M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 133.Ke H.Z., Richards W.G., Li X., Ominsky M.S. Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr. Rev. 2012;33:747–783. doi: 10.1210/er.2011-1060. [DOI] [PubMed] [Google Scholar]
- 134.Kramer I., Loots G.G., Studer A., Keller H., Kneissel M. Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J. Bone Miner. Res. 2010;25:178–189. doi: 10.1359/jbmr.090730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fujita K., Roforth M.M., Demaray S., McGregor U., Kirmani S., McCready L.K., Peterson J.M., Drake M.T., Monroe D.G., Khosla S. Effects of estrogen on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in postmenopausal women. J. Clin. Endocrinol. Metab. 2014;99:E81–E88. doi: 10.1210/jc.2013-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Register T.C., Hruska K.A., Divers J., Bowden D.W., Palmer N.D., Carr J.J., Wagenknecht L.E., Hightower R.C., Xu J., Smith S.C., et al. Plasma Dickkopf1 (DKK1) concentrations negatively associate with atherosclerotic calcified plaque in African-Americans with type 2 diabetes. J. Clin. Endocrinol. Metab. 2013;98:E60–E65. doi: 10.1210/jc.2012-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Szulc P., Schoppet M., Rachner T.D., Chapurlat R., Hofbauer L.C. Severe abdominal aortic calcification in older men is negatively associated with DKK1 serum levels: The STRAMBO study. J. Clin. Endocrinol. Metab. 2014;99:617–624. doi: 10.1210/jc.2013-3201. [DOI] [PubMed] [Google Scholar]
- 138.Hampson G., Edwards S., Conroy S., Blake G.M., Fogelman I., Frost M.L. The relationship between inhibitors of the Wnt signalling pathway (Dickkopf-1(DKK1) and sclerostin), bone mineral density, vascular calcification and arterial stiffness in post-menopausal women. Bone. 2013;56:42–47. doi: 10.1016/j.bone.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 139.Fang Y., Ginsberg C., Seifert M., Agapova O., Sugatani T., Register T.C., Freedman B.I., Monier-Faugere M.C., Malluche H., Hruska K.A. CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. J. Am. Soc. Nephrol. 2014;25:1760–1763. doi: 10.1681/ASN.2013080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lindberg K., Olauson H., Amin R., Ponnusamy A., Goetz R., Taylor R.F., Mohammadi M., Canfield A., Kublickiene K., Larsson T.E. Arterial klotho expression and FGF23 effects on vascular calcification and function. PLoS ONE. 2013;8:e60658. doi: 10.1371/journal.pone.0060658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Agapova O.A., Fang Y., Sugatani T., Seifert M.E., Hruska K.A. Ligand trap for the activin type IIA receptor protects against vascular disease and renal fibrosis in mice with chronic kidney disease. Kidney Int. 2016;89:1231–1243. doi: 10.1016/j.kint.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maeshima A., Nojima Y., Kojima I. The role of the activin-follistatin system in the developmental and regeneration processes of the kidney. Cytokine Growth Factor Rev. 2001;12:289–298. doi: 10.1016/S1359-6101(01)00010-7. [DOI] [PubMed] [Google Scholar]
- 143.Ying S.Y. Inhibins, activins, and follistatins: Gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr. Rev. 1988;9:267–293. doi: 10.1210/edrv-9-2-267. [DOI] [PubMed] [Google Scholar]
- 144.Sugatani T., Agapova O.A., Fang Y., Berman A.G., Wallace J.M., Malluche H.H., Faugere M.C., Smith W., Sung V., Hruska K.A. Ligand trap of the activin receptor type IIA inhibits osteoclast stimulation of bone remodeling in diabetic mice with chronic kidney disease. Kidney Int. 2017;91:86–95. doi: 10.1016/j.kint.2016.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Williams M.J., Sugatani T., Agapova O.A., Fang Y., Gaut J.P., Faugere M.C., Malluche H.H., Hruska K.A. The activin receptor is stimulated in the skeleton, vasculature, heart, and kidney during chronic kidney disease. Kidney Int. 2018;93:147–158. doi: 10.1016/j.kint.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carrillo-López N., Panizo S., Alonso-Montes C., Román-García P., Rodríguez I., Martínez-Salgado C., Dusso A.S., Naves M., Cannata-Andía J.B. Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int. 2016;90:77–89. doi: 10.1016/j.kint.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 147.Murali S.K., Roschger P., Zeitz U., Klaushofer K., Andrukhova O., Erben R.G. FGF23 regulates bone mineralization in a 1,25(OH)2D3 and Klotho-independent manner. J. Bone Miner. Res. 2016;31:129–142. doi: 10.1002/jbmr.2606. [DOI] [PubMed] [Google Scholar]