Abstract
Here we report novel mutations in ABCA4 with the underlying phenotype in a large French cohort with autosomal recessive Stargardt disease. The DNA samples of 397 index subjects were analyzed in exons and flanking intronic regions of ABCA4 (NM_000350.2) by microarray analysis and direct Sanger sequencing. At the end of the screening, at least two likely pathogenic mutations were found in 302 patients (76.1%) while 95 remained unsolved: 40 (10.1%) with no variants identified, 52 (13.1%) with one heterozygous mutation, and 3 (0.7%) with at least one variant of uncertain significance (VUS). Sixty-three novel variants were identified in the cohort. Three of them were variants of uncertain significance. The other 60 mutations were classified as likely pathogenic or pathogenic, and were identified in 61 patients (15.4%). The majority of those were missense (55%) followed by frameshift and nonsense (30%), intronic (11.7%) variants, and in-frame deletions (3.3%). Only patients with variants never reported in literature were further analyzed herein. Recruited subjects underwent complete ophthalmic examination including best corrected visual acuity, kinetic and static perimetry, color vision test, full-field and multifocal electroretinography, color fundus photography, short-wavelength and near-infrared fundus autofluorescence imaging, and spectral domain optical coherence tomography. Clinical evaluation of each subject confirms the tendency that truncating mutations lead to a more severe phenotype with electroretinogram (ERG) impairment (p = 0.002) and an earlier age of onset (p = 0.037). Our study further expands the mutation spectrum in the exonic and flanking regions of ABCA4 underlying Stargardt disease.
Keywords: ABCA4, Stargardt disease, phenotype-genotype correlation
1. Introduction
Stargardt disease-1 (STGD1, Mendelian Inheritance in Man [MIM] 248,200) is a progressive autosomal recessive macular degeneration linked to pathogenic mutations in ABCA4 (MIM*601,691).
ABCA4 encodes the transmembrane protein ABCA4 (ATP-binding cassette (ABC), subfamily A, member 4), located in the outer segment disk membranes of cone and rod photoreceptor cells [1,2,3]. Its loss can lead to the development of several retinal disorders including STGD1, cone and cone-rod dystrophy, and retinitis pigmentosa [4,5,6,7,8,9,10,11].
Given the large number of variants reported in ABCA4 [12], most of them being polymorphisms, the identification of true disease-causing mutations is often challenging [5,13,14].
With the advent of new analytical approaches, such as next generation sequencing (NGS), the detection rates of ABCA4 mutations have greatly increased since its discovery in 1997. Nevertheless, homozygous or compound heterozygous mutations are regularly detected in no more than 70–75% of STGD1 patients, while a significant number of patients carry only a single ABCA4 mutation or none at all [15,16,17,18,19]. On the other hand, several deep intronic variants in non-coding regions were reported to segregate with STGD1 affecting correct splicing mechanisms [19,20,21,22,23].
Interestingly, all genotyping studies on ABCA4 report a broad mutation spectrum and a high allelic heterogeneity [6,12,24,25,26]. This phenomenon may be partially explained by the ethnic variability of the studied populations [12]. Therefore the need to screen large populations and to identify novel variants is still relevant [27,28] and can help not only to explore the genetic architecture of ABCA4 pathology, but also the genotype/phenotype correlation. Much effort today is directed towards a more specific correlation tailored on single variants [24,28,29,30,31], even though their low frequency makes it challenging. Reporting ABCA4 novel variants with a clear and definite correlation with STGD1 would also help the proper selection of patients for therapeutic clinical trials reaching a higher degree of confidence with molecular diagnosis [32,33].
To further explore the genetic characteristics of ABCA4 and broaden the spectrum of its pathogenic variants, we analyzed a large French cohort of 397 STGD1 patients using a combination of microarray analysis and Sanger sequencing. The purpose of this study was to report all the novel variants identified evaluating their pathogenicity with in silico analysis. The clinical features were also analyzed and correlated with the genetic results.
2. Results
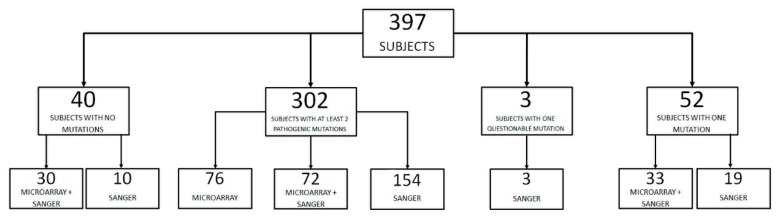
Sequencing of ABCA4 in our cohort of 397 STGD1 patients identified a large number of allelic variants. Microarray technology (ABCR600, ASPER Biotech, Inc., Tartu, Estonia) was used to screen 211 subjects for already known mutations, demonstrating at least two pathogenic variants in 76 of them. All the unsolved and the remaining cases were addressed to direct Sanger sequencing. At the end of the screening at least two likely pathogenic mutations were found in 302 patients (76.1%), while 95 remained unsolved: 40 (10.1%) with no variants identified, 52 (13.1%) with one heterozygous mutation, and 3 (0.7%) with at least one variant of uncertain significance (VUS) (Figure 1).
Figure 1.
Genotypic analysis of a cohort of patients with clinical diagnosis of Stargardt disease using microarray analysis and/or direct Sanger sequencing of exonic and exon-flanking regions of ABCA4.
2.1. Novel Variants
Sixty-three novel ABCA4 variants were identified in 63 index patients (30 males); 60 variants (in 61 subjects) were likely pathogenic or pathogenic. The other 3 variants (in 3 subjects) were VUS (Table 1, uncertain). CIC03678 was a carrier of two novel mutations, one likely pathogenic and one VUS.
Table 1.
Sixty-three novel variants in patients with Stargardt disease and their classification. Nucleotide positions and translation correspond to CCDS747.1 and NP_000341.2, respectively.
| Exon/Intron | Variant | Protein Change | Classification |
|---|---|---|---|
| 1 | c.53G>A | p.(Arg18Gln) | likely pathogenic |
| IVS5 | c.570+1_570+8del | p.? | pathogenic |
| 6 | c.686T>C | p.(Leu229Pro) | likely pathogenic |
| IVS7 | c.859−2A>G | p.? | pathogenic |
| 8 | c.902del | p.(Arg301Serfs*15) | pathogenic |
| 8 | c.972_973delinsAT | p.(Cys324 *) | pathogenic |
| 8 | c.978C>A | p.(Tyr326 *) | pathogenic |
| 8 | c.1019A>G | p.(Tyr340Cys) | likely pathogenic |
| 8 | c.1050del | p.(Ile351Leufs*23) | pathogenic |
| IVS8 | c.1099+1G>C | p.? | pathogenic |
| 9 | c.1201A>C | p.(Thr401Pro) | likely pathogenic |
| 10 | c.1252T>C | p.(Phe418Leu) | likely pathogenic |
| 10 | c.1301T>G | p.(Val434Gly) | likely pathogenic |
| 12 | c.1556G>A | p.(Cys519Tyr) | likely pathogenic |
| 12 | c.1648_1659del | p.(Gly550_Phe553del) | likely pathogenic |
| 12 | c.1706A>G | p.(Tyr569Cys) | likely pathogenic |
| 13 | c.1895T>A | p.(Ile632Asn) | likely pathogenic |
| 14 | c.2083G>C | p.(Val695Leu) | likely pathogenic |
| 15 | c.2169_2172dup | p.(Leu725Asnfs*42) | pathogenic |
| 15 | c.2299del | p.(Val767Serfs*20) | pathogenic |
| 16 | c.2443C>T | p.(Gln815 *) | pathogenic |
| 16 | c.2572dup | p.(Asp858Glyfs*27) | pathogenic |
| 19 | c.2868C>A | p.(Asn956Lys) | uncertain |
| 21 | c.3080A>G | p.(Tyr1027Cys) | likely pathogenic |
| 22 | c.3311T>C | p.(Leu1104Pro) | likely pathogenic |
| 23 | c.3409A>G | p.(Arg1137Gly) | uncertain |
| 25 | c.3682G>T | p.(Glu1228 *) | likely pathogenic |
| 25 | c.3811G>C | p.(Glu1271Asp) | likely pathogenic |
| 26 | c.3825G>C | p.(Lys1275Asn) | likely pathogenic |
| 27 | c.3966del | p.(Ala1324Argfs*65) | pathogenic |
| 27 | c.4061A>C | p.(His1354Pro) | likely pathogenic |
| IVS27 | c.4129−3C>A | p.? | likely pathogenic |
| 28 | c.4178_4192del | p.(Val1393_Phe1397del) | likely pathogenic |
| 29 | c.4324A>G | p.(Asn1442Asp) | likely pathogenic |
| 30 | c.4510_4535del | p.(Glu1504Profs*42) | pathogenic |
| 32 | c.4663_4664del | p.(Gln1555Glufs*41) | pathogenic |
| 33 | c.4689del | p.(Gly1564Glufs*17) | pathogenic |
| 33 | c.4696C>T | p.(Leu1566Phe) | likely pathogenic |
| 33 | c.4700C>T | p.(Pro1567Leu) | likely pathogenic |
| 34 | c.4775G>A | p.(Gly1592Asp) | likely pathogenic |
| 37 | c.5282C>G | p.(Pro1761Arg) | likely pathogenic |
| 38 | c.5332A>T | p.(Met1778Leu) | likely pathogenic |
| 38 | c.5342C>A | p.(Ala1781Glu) | likely pathogenic |
| 38 | c.5351T>C | p.(Leu1784Pro) | likely pathogenic |
| 38 | c.5384T>C | p.(Leu1795Ser) | likely pathogenic |
| 40 | c.5621T>C | p.(Leu1874Pro) | likely pathogenic |
| IVS42 | c.5898+2T>C | p.? | pathogenic |
| IVS42 | c.5899−3T>C | p.? | uncertain |
| 43 | c.5939C>T | p.(Thr1980Ile) | likely pathogenic |
| IVS43 | c.6005+1del | p.? | pathogenic |
| 44 | c.6110C>A | p.(Ala2037Asp) | likely pathogenic |
| 45 | c.6181_6184del | p.(Thr2061Serfs*53) | pathogenic |
| 45 | c.6191C>T | p.(Ala2064Val) | likely pathogenic |
| 45 | c.6250G>A | p.(Ala2084Thr) | likely pathogenic |
| 46 | c.6284A>G | p.(Asp2095Gly) | likely pathogenic |
| 47 | c.6394G>T | p.(Glu2132 *) | pathogenic |
| 47 | c.6454G>A | p.(Gly2152Ser) | likely pathogenic |
| 47 | c.6455G>T | p.(Gly2152Val) | likely pathogenic |
| 47 | c.6436_6437insT | p.(Gly2146Valfs*36) | pathogenic |
| 48 | c.6693del | p.(Ile2231Metfs*16) | pathogenic |
| 48 | c.6704C>G | p.(Ser2235 *) | pathogenic |
| 49 | c.6746C>A | p.(Ala2249Asp) | likely pathogenic |
| IVS49 | c.6816+1G>T | p.? | pathogenic |
p.?: Protein has not been analysed, an effect is expected but difficult to predict.
The 60 likely pathogenic or pathogenic variants comprised 33 missense and 6 nonsense variants, 11 deletions, 1 one-base pair (bp) duplication, 1 four-bp duplication, 1 insertion, and 7 variants presumably affecting splicing (5 single nucleotide substitutions, 1 single-bp deletion, and 1 eight-bp deletion) (Table 1), which co-segregated with the phenotype in tested available family members.
Co-segregation analysis allowed the identification of complex alleles with in cis mutations in 18 patients. Nine additional patients had more than two variations, but it was impossible to establish phase and determine each allele due to the lack of DNA from additional family members.
All genotypic information about patients and co-segregation analysis are reported in Table 2.
Table 2.
Sixty novel likely pathogenic mutations in 61 index patients with Stargardt disease and cosegregation analysis. Nucleotide positions and translation correspond to CCDS747.1 and NP_000341.2, respectively.
| Allele 1 | Allele 2 | Allele 1 or 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient ID | Family ID | Exon/Intron | Nucleotide Change | Protein Change | Exon/Intron | Nucleotide Change | Protein Change | Exon/Intron | Nucleotide Change | Protein Change | |
| CIC03734 | 1673 | Index | 1 | c.53G>A | p.(Arg18Gln) | 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||
| CIC03735 | 1673 | Affected brother | 1 | c.53G>A | p.(Arg18Gln) | 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||
| CIC06131 | 1673 | Unaffected father | 1 | c.53G>A | p.(Arg18Gln) | WILD TYPE | |||||
| CIC06908 | 3783 | Index | IVS5 | c.570+1_570+8del | p.? | 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||
| 45 | c.6148G>C [25] | p.(Val2050Leu) | |||||||||
| CIC08771 | 3783 | Unaffected mother | WILD TYPE | 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||||
| CIC08774 | 3783 | Unaffected brother | IVS5 | c.570+1_570+8del | - | WILD TYPE | |||||
| 45 | c.6148G>C [25] | p.(Val2050Leu) | |||||||||
| CIC07895 | 4412 | Index | 6 | c.686T>C | p.(Leu229Pro) | 24 | c.3602T>G [5] | p.(Leu1201Arg) | |||
| 40 | c.5621T>C | p.(Leu1874Pro) | |||||||||
| CIC07896 | 4412 | Unaffected mother | 6 | c.686T>C | p.(Leu229Pro) | WILD TYPE | |||||
| CIC07887 | 4407 | Index | 6 | c.686T>C | p.(Leu229Pro) | 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||
| 48 | c.6529G>A [35] | p.(Asp2177Asn) | |||||||||
| CIC07888 | 4407 | Unaffected mother | 6 | c.686T>C | p.(Leu229Pro) | WILD TYPE | |||||
| 48 | c.6529G>A [35] | p.(Asp2177Asn) | |||||||||
| CIC09848 | 5658 | Index | IVS7 | c.859−2A>G | - | 8 | c.872C>T [36] | p.(Pro291Leu) | |||
| 11 | c.1531C>T [18] | p.(Arg511Cys) | |||||||||
| CIC09849 | 5658 | Unaffected mother | WILD TYPE | 8 | c.872C>T [37] | p.(Pro291Leu) | |||||
| 11 | c.1531C>T [18] | p.(Arg511Cys) | |||||||||
| CIC09382 | 5385 | Index | 8 | c.978C>A | p.(Tyr326 *) | IVS42 | c.5898+2T>C | p.? | |||
| CIC06749 | 3669 | Index | 8 | c.902del | p.(Arg301Serfs*15) | 46 | c.6320G>A [26] | p.(Arg2107His) | |||
| CIC06088 | 3203 | Index | 8 | c.972_973delinsAT | p.(Cys324 *) | 23 | c.3386G>T [38] | p.(Arg1129Leu) | |||
| CIC08477 | 3203 | Unaffected father | 8 | c.972_973delinsAT | p.(Cys324 *) | WILD TYPE | |||||
| CIC08478 | 3203 | Unaffected mother | WILD TYPE | 23 | c.3386G>T [38] | p.(Arg1129Leu) | |||||
| CIC07725 | 4301 | Index | 8 | c.1019A>G | p.(Tyr340Cys) | 8 | c.1019A>G | p.(Tyr340Cys) | |||
| CIC07726 | 4301 | Unaffected father | 8 | c.1019A>G | p.(Tyr340Cys) | WILD TYPE | |||||
| CICO7727 | 4301 | Unaffected mother | WILD TYPE | 8 | c.1019A>G | p.(Tyr340Cys) | |||||
| CIC04235 | 2021 | Index | 8 | c.1050del | p.(Ile351Leufs*23) | 38 | c.5351T>C | p.(Leu1784Pro) | |||
| CIC04236 | 2021 | Unaffected sister | 8 | c.1050del | p.(Ile351Leufs*23) | WILD TYPE | |||||
| CIC07308 | 4026 | Index | 8 | c.1050del | p.(Ile351Leufs*23) | 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||
| CIC01080 | 659 | Index | 8 | c.1050del | p.(Ile351Leufs*23) | 19 | c.2819C>G [39] | p.(Pro940Arg) | |||
| 23 | c.3364G>A [6] | p.(Glu1122Lys) | |||||||||
| CIC03521 | 659 | Unaffected mother | WILD TYPE | 19 | c.2819C>G [39] | p.(Pro940Arg) | |||||
| 23 | c.3364G>A [6] | p.(Glu1122Lys) | |||||||||
| CIC03524 | 659 | Unaffected sister | WILD TYPE | WILD TYPE | |||||||
| CIC08372 | 4715 | Index | IVS8 | c.1099+1G>C | p.? | 22 | c.3322C>T [26] | p.(Arg1108Cys) | |||
| CIC05266 | 2672 | Index | 9 | c.1201A>C | p.(Thr401Pro) | 17 | c.2588G>C [25] | p.(Gly863Ala) | |||
| 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||||||||
| CIC05267 | 2672 | Unaffected mother | 9 | c.1201A>C | p.(Thr401Pro) | WILD TYPE | |||||
| CIC05268 | 2672 | Unaffected father | WILD TYPE | 17 | c.2588G>C [25] | p.(Gly863Ala) | |||||
| 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||||||||
| CIC02804 | 1037 | Index | 10 | c.1252T>C | p.(Phe418Leu) | IVS43 | c.6005+1del | p.? | 42 | c.5882G>A [25] | p.(Gly1961Glu) |
| CIC09625 | 5521 | Index | 10 | c.1301T>G | p.(Val434Gly) | 10 | c.1301T>G | p.(Val434Gly) | |||
| CIC09624 | 5521 | Affected brother | 10 | c.1301T>G | p.(Val434Gly) | 10 | c.1301T>G | p.(Val434Gly) | |||
| CIC09626 | 5521 | Unaffected father | 10 | c.1301T>G | p.(Val434Gly) | WILD TYPE | |||||
| CIC09628 | 5521 | Unaffected mother | WILD TYPE | 10 | c.1301T>G | p.(Val434Gly) | |||||
| CIC01301 | 784 | Index | 12 | c.1556G>A | p.(Cys519Tyr) | 22 | c.3292C>T [40] | p.(Arg1098Cys) | |||
| CIC09999 | 784 | Affected brother | 12 | c.1556G>A | p.(Cys519Tyr) | 22 | c.3292C>T [41] | p.(Arg1098Cys) | |||
| CIC06346 | 3356 | Index | 12 | c.1648_1659del | p.(Gly550_Phe553del) | 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||
| CIC08884 | 3356 | Unaffected father | 12 | c.1648_1659del | p.(Gly550_Phe553del) | WILD TYPE | |||||
| CIC08949 | 3356 | Unaffected mother | WILD TYPE | 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||||
| CIC04197 | 1992 | Index | 12 | c.1706A>G | p.(Tyr569Cys) | 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||
| CIC07749 | 4314 | Index | 13 | c.1895T>A | p.(Ile632Asn) | 33 | c.4700C>T | p.(Pro1567Leu) | 24 | c.3602T>G [5] | p.(Leu1201Arg) |
| CIC08724 | 4948 | Index | 14 | c.2083G>C | p.(Val695Leu) | 47 | c.6445C>T [6] | p.(Arg2149 *) | |||
| 44 | c.6079C>T [29] | p.(Leu2027Phe) | |||||||||
| CIC08723 | 4948 | Affected sister | 14 | c.2083G>C | p.(Val695Leu) | 47 | c.6445C>T [6] | p.(Arg2149 *) | |||
| 44 | c.6079C>T [29] | p.(Leu2027Phe) | |||||||||
| CIC08858 | 4948 | Unaffected mother | 14 | c.2083G>C | p.(Val695Leu) | WILD TYPE | |||||
| 44 | c.6079C>T [29] | p.(Leu2027Phe) | |||||||||
| CIC08859 | 4948 | Unaffected father | WILD TYPE | 47 | c.6445C>T [6] | p.(Arg2149 *) | |||||
| CIC07985 | 4793 | Index | 15 | c.2169_2172dup | p.(Leu725Asnfs*42) | 47 | c.6454G>A | p.(Gly2152Ser) | 44 | c.6079C>T [25] | p.(Leu2027Phe) |
| 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||||||||
| CIC06126 | 3227 | Index | 15 | c.2299del | p.(Val767Serfs*20) | 17 | c.2588G>C [25] | p.(Gly863Ala) | 40 | c.5603A>T [34] | p.(Asn1868Ile) |
| CIC09405 | 5401 | Index | 16 | c.2443C>T | p.(Gln815 *) | 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||
| CIC09407 | 5401 | Unaffected mother | WILD TYPE | 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||||
| CIC09408 | 5401 | Unaffected father | 16 | c.2443C>T | p.(Gln815 *) | WILD TYPE | |||||
| CIC00467 | 319 | Index | 16 | c.2572dup | p.(Asp858Glyfs*27) | 35 | c.4926C>G [42] | p.(Ser1642Arg) | |||
| 36 | c.5044_5058del [25] | p.(Val1682_Val1686del) | |||||||||
| CIC05014 | 319 | Unaffected sister | 16 | c.2572dup | p.(Asp858Glyfs*27) | WILD TYPE | |||||
| CIC05056 | 319 | Affected brother | 16 | c.2572dup | p.(Asp858Glyfs*27) | 35 | c.4926C>G [43] | p.(Ser1642Arg) | |||
| 36 | c.5044_5058del [25] | p.(Val1682_Val1686del) | |||||||||
| + CIC03678 | 1627 | Index | 19 | c.2868C>A | p.(Asn956Lys) | 43 | c.5939C>T | p.(Thr1980Ile) | |||
| CIC03679 | 1627 | Unaffected son | WILD TYPE | 43 | c.5939C>T | p.(Thr1980Ile) | |||||
| CIC04259 | 2036 | Index | 21 | c.3080A>G | p.(Tyr1027Cys) | 38 | c.5381C>A [24] | p.(Ala1794Asp) | |||
| CIC08538 | 4826 | Index | 22 | c.3311T>C | p.(Leu1104Pro) | IVS49 | c.6816+1G>A [6] | - | |||
| CIC04176 | 1973 | Index | 25 | c.3682G>T | p.(Glu1228*) | 22 | c.3322C>T [26] | p.(Arg1108Cys) | |||
| CIC02505 | 871 | Index | 25 | c.3811G>C | p.(Glu1271Gln) | 23 | c.3386G>T [38] | p.(Arg1129Leu) | |||
| CIC05899 | 3087 | Index | 25 | c.3811G>C | p.(Glu1271Gln) | 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||
| CIC07120 | 3909 | Index | 26 | c.3825G>C | p.(Lys1275Asn) | 22 | c.3322C>T [26] | p.(Arg1108Cys) | 40 | c.5603A>T [34] | p.(Asn1868Ile) |
| 46 | c.6320G>A [26] | p.(Arg2107His) | |||||||||
| CIC01750 | 1242 | Index | 27 | c.3966del | p.(Ala1324Argfs*65) | 35 | c.4918C>T [26] | p.(Arg1640Trp) | |||
| 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||||||||
| CIC02024 | 1242 | Affected sister | 27 | c.3966del | p.(Ala1324Argfs*65) | 35 | c.4918C>T [26] | p.(Arg1640Trp) | |||
| 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||||||||
| CIC07100 | 1242 | Unaffected father | WILD TYPE | 35 | c.4918C>T [26] | p.(Arg1640Trp) | |||||
| 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||||||||
| CIC07146 | 1242 | Unaffected mother | 27 | c.3966del | p.(Ala1324Argfs*65) | WILD TYPE | |||||
| CIC07744 | 4372 | Index | 27 | c.4061A>C | p.(His1354Pro) | 13 | c.1927G>A [44] | p.(Val643Met) | 8 | c.872C>T [37] | p.(Pro291Leu) |
| 24 | c.3602T>G [6] | p.(Leu1201Arg) | |||||||||
| CIC02690 | 961 | Index | IVS27 | c.4129−3C>A | p.? | IVS38 | c.5461-10T>C [45] | p. [Thr1821Valfs, Thr1821Aspfs] | |||
| 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||||||||
| CIC02691 | 961 | Unaffected mother | WILD TYPE | IVS38 | c.5461-10T>C [45] | p. [Thr1821Valfs, Thr1821Aspfs] | |||||
| 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||||||||
| CIC08194 | 4588 | Index | 28 | c.4178_4192del | p.(Val1393_Phe1397del) | 40 | c.5603A>T [34] | p.(Asn1868Ile) | 45 | c.6148G>C [25] | p.(Val2050Leu) |
| CIC07994 | 4463 | Index | 29 | c.4324A>G | p.(Asn1442Asp) | 3 | c.194G>A [46] | p.(Gly65Glu) | |||
| CIC07998 | 4463 | Unaffected mother | WILD TYPE | 3 | c.194G>A [46] | p.(Gly65Glu) | |||||
| CIC06727 | 3652 | Index | 30 | c.4510_4535del | p.(Glu1504Profs*42) | 28 | c.4139C>T [6] | p.(Pro1380Leu) | |||
| CIC06728 | 3652 | Unaffected father | WILD TYPE | 28 | c.4139C>T [6] | p.(Pro1380Leu) | |||||
| CIC06729 | 3652 | Unaffected brother | WILD TYPE | 28 | c.4139C>T [6] | p.(Pro1380Leu) | |||||
| CIC06730 | 3652 | Unaffected mother | 30 | c.4510_4535del | p.(Glu1504Profs*42) | WILD TYPE | |||||
| CIC08283 | 4647 | Index | 32 | c.4663_4664del | p.(Gln1555Glufs*41) | 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||
| CIC03252 | 1375 | Index | 33 | c.4689del | p.(Gly1564Glufs*17) | 17 | c.2588G>C [25] | p.(Gly863Ala) | |||
| 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||||||||
| CIC03253 | 1375 | Unaffected mother | WILD TYPE | 17 | c.2588G>C [25] | p.(Gly863Ala) | |||||
| 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||||||||
| CIC09219 | 5282 | Index | 33 | c.4696C>T | p.(Leu1566Phe) | 21 | c.3056C>T [26] | p.(Thr1019Met) | |||
| 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||||||||
| CIC10754 | 5282 | Unaffected mother | WILD TYPE | 21 | c.3056C>T [26] | p.(Thr1019Met) | |||||
| CIC10755 | 5282 | Unaffected father | 33 | c.4696C>T | p.(Leu1566Phe) | WILD TYPE | |||||
| 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||||||||
| CIC08932 | 5089 | Index | 34 | c.4775G>A | p.(Gly1592Asp) | 3 | c.288C>A [47] | p.(Asn96Lys) | |||
| 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||||||||
| ++ CIC08933 | 5089 | Unaffected father | WILD TYPE | WILD TYPE | |||||||
| CIC08934 | 5089 | Unaffected mother | WILD TYPE | 3 | c.288C>A [47] | p.(Asn96Lys) | |||||
| 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||||||||
| CIC07036 | 3867 | Index | 37 | c.5282C>G | p.(Pro1761Arg) | 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||
| CIC07960 | 4447 | Index | 37 | c.5282C>G | p.(Pro1761Arg) | 26 | c.3819dup [48] | p.(Leu1274Serfs*8) | |||
| 46 | c.6316C>T [25] | p.(Arg2106Cys) | |||||||||
| CIC07999 | 4447 | Unaffected sister | WILD TYPE | WILD TYPE | |||||||
| CIC08029 | 4447 | Unaffected mother | 37 | c.5282C>G | p.(Pro1761Arg) | WILD TYPE | |||||
| 46 | c.6316C>T [25] | p.(Arg2106Cys) | |||||||||
| CIC09601 | 5509 | Index | 37 | c.5282C>G | p.(Pro1761Arg) | 22 | c.3279C>A [18] | p.(Asp1093Glu) | |||
| 46 | c.6316C>T [25] | p.(Arg2106Cys) | |||||||||
| CIC09602 | 5509 | Unaffected daughter | 37 | c.5282C>G | p.(Pro1761Arg) | WILD TYPE | |||||
| 46 | c.6316C>T [25] | p.(Arg2106Cys) | |||||||||
| CIC07831 | 4373 | Index | 38 | c.5332A>T | p.(Met1778Leu) | 27 | c.3899G>A [44] | p.(Arg1300Gln) | |||
| CIC08439 | 4759 | Index | 38 | c.5332A>T | p.(Met1778Leu) | 47 | c.6394G>T | p.(Glu2132 *) | |||
| CIC08262 | 4633 | Index | 38 | c.5342C>A | p.(Ala1781Glu) | 3 | c.286A>G [49] | p.(Asn96Asp) | |||
| CIC08263 | 4633 | Unaffected mother | 38 | c.5342C>A | p.(Ala1781Glu) | WILD TYPE | |||||
| CIC09095 | 4633 | Unaffected father | WILD TYPE | 3 | c.286A>G [49] | p.(Asn96Asp) | |||||
| CIC08359 | 4702 | Index | 38 | c.5384T>C | p.(Leu1795Ser) | 17 | c.2588G>C [25] | p.(Gly863Ala) | 40 | c.5603A>T [34] | p.(Asn1868Ile) |
| CIC07436 | 4110 | Index | IVS42 | c.5898+2T>C | p.? | 40 | c.5642C>T [50] | p.(Ala1881Val) | |||
| CIC08523 | 4110 | Unaffected mother | WILD TYPE | 40 | c.5642C>T [50] | p.(Ala1881Val) | |||||
| CIC08524 | 4110 | Unaffected father | IVS42 | c.5898+2T>C | p.? | WILD TYPE | |||||
| CIC09857 | 5675 | Index | 44 | c.6110C>A | p.(Ala2037Asp) | IVS38 | c.5461−10T>C [45] | p. [Thr1821Valfs, Thr1821Aspfs] | |||
| 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||||||||
| CIC09858 | 5675 | Unaffected mother | 44 | c.6110C>A | p.(Ala2037Asp) | WILD TYPE | |||||
| CIC10452 | 5675 | Unaffected father | WILD TYPE | IVS38 | c.5461−10T>C [45] | p. [Thr1821Valfs, Thr1821Aspfs] | |||||
| 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||||||||
| CIC01199 | 719 | Index | 45 | c.6181_6184del | p.(Thr2061Serfs*53) | 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||
| CIC08054 | 719 | Affected sister | 45 | c.6181_6184del | p.(Thr2061Serfs*53) | 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||
| CIC08057 | 719 | Unaffected father | WILD TYPE | 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||||
| CIC8058 | 719 | Unaffected mother | 45 | c.6181_6184del | p.(Thr2061Serfs*53) | WILD TYPE | |||||
| CIC03710 | 1657 | Index | 45 | c.6191C>T | p.(Ala2064Val) | 30 | c.4537dup [44] | p.(Gln1513Profs*42) | |||
| 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||||||||
| CIC03711 | 1657 | Unaffected mother | 45 | c.6191C>T | p.(Ala2064Val) | WILD TYPE | |||||
| 40 | c.5603A>T [34] | p.(Asn1868Ile) | |||||||||
| CIC04571 | 2232 | Index | 45 | c.6250G>A | p.(Ala2084Thr) | 19 | c.2894A>G [25] | p.(Asn965Ser) | |||
| 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||||||||
| CIC04572 | 2232 | Unaffected mother | WILD TYPE | 19 | c.2894A>G [25] | p.(Asn965Ser) | |||||
| CIC04573 | 2232 | Unaffected maternal aunt | WILD TYPE | 19 | c.2894A>G [25] | p.(Asn965Ser) | |||||
| CIC07568 | 2232 | Unaffected father | 45 | c.6250G>A | p.(Ala2084Thr) | WILD TYPE | |||||
| 42 | c.5882G>A [25] | p.(Gly1961Glu) | |||||||||
| CIC07748 | 4312 | Index | 46 | c.6284A>G | p.(Asp2095Gly) | 22 | c.3323G>A [34] | p.(Arg1108His) | |||
| CIC09593 | 4312 | Unaffected mother | 46 | c.6284A>G | p.(Asp2095Gly) | WILD TYPE | |||||
| CIC09594 | 4312 | Unaffected father | WILD TYPE | 22 | c.3323G>A [34] | p.(Arg1108His) | |||||
| CIC09374 | 5379 | Index | 47 | c.6455G>T | p.(Gly2152Val) | 9 | c.1222C>T [43] | p.(Arg408*) | |||
| CIC09375 | 5379 | Unaffected mother | 47 | c.6455G>T | p.(Gly2152Val) | WILD TYPE | |||||
| CIC06396 | 3389 | Index | 47 | c.6436_6437insT | p.(Gly2146Valfs*36) | 47 | c.6436_6437insT | p.( Gly2146Valfs*36) | |||
| CIC00130 | 102 | Index | 48 | c.6693del | p.(Ile2231Metfs*16) | 44 | c.6079C>T [25] | p.(Leu2027Phe) | |||
| CIC00131 | 102 | Unaffected father | 48 | c.6693del | p.(Ile2231Metfs*16) | WILD TYPE | |||||
| CIC00132 | 102 | Unaffected mother | WILD TYPE | 44 | c.6079C>T [25] | p.(Leu2027Phe) | |||||
| CIC09114 | 5205 | Index | 48 | c.6704C>G | p.(Ser2235 *) | 21 | c.3113C>T [24] | p.(Ala1038Val) | |||
| CIC08581 | 4855 | Index | 49 | c.6746C>A | p.(Ala2249Asp) | 13 | c.1819G>A [40] | p.(Gly607Arg) | 42 | c.5882G>A [25] | p.(Gly1961Glu) |
| CIC08381 | 4722 | Index | IVS49 | c.6816+1G>T | p.? | 14 | c.2023G>A [38] | p.(Val675Ile) | |||
| CIC08383 | 4722 | Affected sister | IVS49 | c.6816+1G>T | p.? | 14 | c.2023G>A [38] | p.(Val675Ile) | |||
| CIC08402 | 4722 | Unaffected mother | WILD TYPE | 14 | c.2023G>A [38] | p.(Val675Ile) | |||||
+: CIC03678 is carrier of a variant of uncertain significance (c.2868C>A), and hence was excluded from genotype-phenotype analysis. ++: CIC08933, unaffected father of CIC08932 does not carry any likely pathogenic variant therefore variant c.4775G>A could be de novo or CIC08933 is not the biological father of CIC08932. p.?: Protein has not been analysed, an effect is expected but difficult to predict.
Four missense mutations (i.e., p.(Cys519Tyr), p.(Glu1271Gln), p.(Gly1592Asp), and p.(Asp2095Gly)) were analyzed also with the splicing predictor tools due to their proximity with a putative splice site. None of them was predicted to affect splicing significantly but they were predicted as disease-causing by the missense prediction in silico algorithms.
Among all novel mutations, p.(Tyr340Cys), p.(Val434Gly), and p.(Gly2146Valfs*36) were found at a homozygous state (Table 2).
CIC08932 inherited the complex allele p.(Asn96Lys; Gly1961Glu) from the unaffected mother, but the novel variant p.(Gly1592Asp) was not found in the unaffected father (Table 2). This variant could be de novo in the index patient or CIC08933 may not be the biological father. Unfortunately, it was impossible to get this information from the subject, and our informed consent did not cover single nucleotide polymorphisms analysis for paternity testing.
Three novel variants found in three index patients (p.(Asn956Lys), p.(Arg1137Gly), and c.5899−3T>C) were considered VUS. Conservation and pathogenic prediction data for each variant are reported in Supplementary Tables S1 and S2.
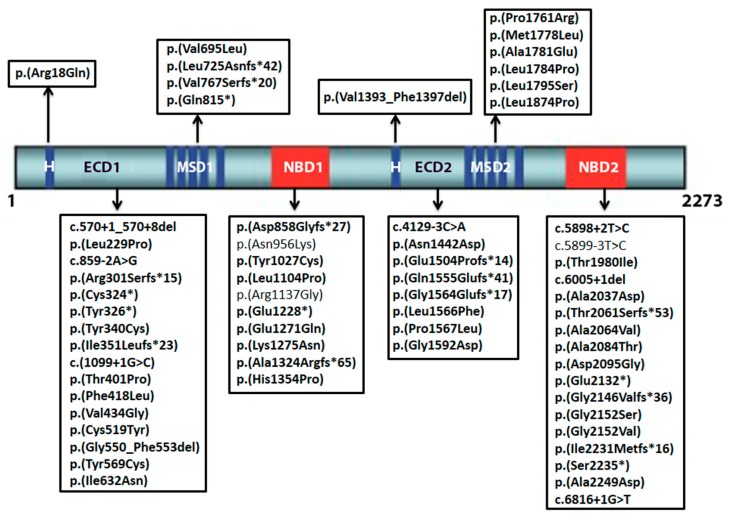
All novel variants found in the study and their respective position on ABCA4 are represented in Figure 2.
Figure 2.
Location of novel ABCA4 variants identified in the study. Nucleotide positions and translation correspond to CCDS747.1 and NP_000341.2, respectively. Only the three mutations of uncertain significance are not in bold letters; ECD1: first extracellular domain; NBD1: first nucleotide binding domain; MSD1: first membrane spanning domain; H: hydrophobic domain; ECD2: second extracellular domain; NBD2: second nucleotide binding domain; MSD2: second membrane spanning domain.
2.2. Genotype-Phenotype Correlation
Genotype-phenotype correlation analysis was performed on the 60 patients carrying at least one likely pathogenic variant on each allele. The three subjects carrying a VUS were excluded from the analysis. All clinical features are presented in the Supplementary Table S3.
Patients were divided in two genotype groups: (1) patients with at least one null mutation (NM), i.e., frame-shift or nonsense mutations, splicing variants affecting the first two nucleotides adjacent to an exonic sequence and/or other variants proven to cause a premature stop or incomplete formation of the whole protein in previous studies (e.g., c.5461−10T>C [51]); (2) patients with two or more missense variants (MM) [52].
The two genotypic groups were not statistically different for age (mean of 34.3 ± 17.8 years for NM group and 33.9 ± 18.3 years for MM group; p = 0.94) nor duration of disease (mean of 16.9 ± 14.6 years for NM group and 10.8 ± 10.9 years for MM group; p = 0.095).
Mean age of onset was 18.5 ± 12.59 years and 23.1 ± 14.8 years in NM and MM groups, respectively (p = 0.037).
Right eye (RE) and left eye (LE) did not differ significantly for best corrected visual acuity (BCVA) (p = 0.81), central retinal thickness (CRT) (p = 0.09), and macular volume (MV) (p = 0.137), therefore we chose to use data from the right eye for comparison between genotypic groups.
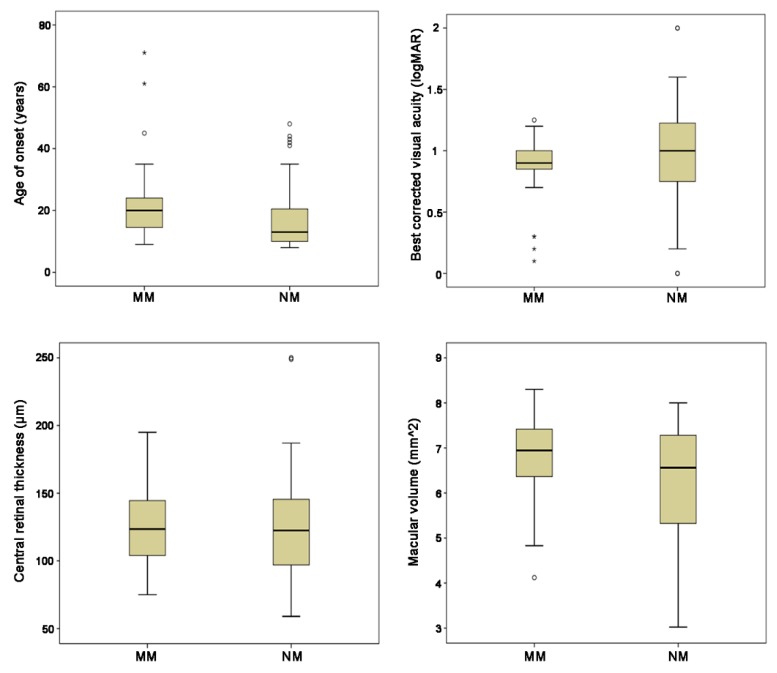
Null mutation and MM groups did not differ significantly for optical coherence tomography (OCT) parameters: mean CRT was 126.7 ± 45.3 µm and 122 ± 26.1 µm, respectively (p = 0.89), while mean MV was 6.2 ± 1.3 mm2 and 6.8 ± 1.1 mm2, respectively (p = 0.13 for MV). Mean BCVA was 0.97 ± 0.48 logMAR in the NM group and 0.85 ± 0.29 logMAR in the MM group (p = 0.26) (Figure 3).
Figure 3.
Box-plots showing the comparison of age of onset (AO), best corrected visual acuity (BCVA), central retinal thickness (CRT), and macular volume (MV) for the two genotype groups (patients with at least one null mutation (NM) and patients with two or more missense variants (MM)). The dark line in the middle of the boxes is the median; the bottom of the box indicates the 25th percentile while the top of the box represents the 75th percentile. The T-bars that extend from the boxes represent the minimum and maximum values when they are within 1.5 times the height of the box. The circles are outliers (i.e., values that do not fall in the T-bars). The stars are extreme outliers (i.e., values more than three times the height of the boxes). Only AO shows a statistically significant difference between NM and MM.
The phenotype was classified following all clinical criteria summarized in Table 3.
Table 3.
Clinical criteria used to classify patients in our study. FP: fundus photography; SW-AF: short-wavelength fundus autofluorescence; NIR-AF: near-infrared fundus autofluorescence; ERG: electroretinogram; OCT: optical coherence tomography; RPE: retinal pigment epithelium; EZ: ellipsoid zone; FS: foveal sparing.
| Groups/Stages | Criteria | Reference | |
|---|---|---|---|
| Age of onset | n.a. | Age at which visual loss was first noticed | Lois et al., 2001 [53] |
| FP | Stage 1 | Central macular atrophy with parafoveal or perifoveal flecks | Fishman GA et al., 1976 [54] |
| Stage 2 | Numerous flecks extended anterior to the vascular arcades and/or nasal to the optic disc | ||
| Stage 3 | Desorbed flecks with choriocapillaris atrophy within the macula | ||
| Stage 4 | Widespread RPE and chorioretinal atrophy throughout the fundus defined stage | ||
| SW-AF and NIR-AF | Group 1 | Central lesion with jagged border | Duncker et al., 2014 [55] |
| Group 2 | Lesion with extensive fundus changes | ||
| Group 3 | Central lesion with smooth border and hyperautofluorescent SW-AF and NIR-AF ring | ||
| Group 4 | Central lesion with smooth border and no hyperautofluorescent NIR-AF ring | ||
| Group 5 | Discrete central lesions better visualized in NIR-AF images | ||
| Peripapillary area preserved | No alterations within an eccentricity of 0.6 mm from the optic disc | Cideciyan et al., 2005 [56] | |
| Flecks in the peripapillary area | Presence of flecks within an eccentricity of 0.6 mm from the optic disc | ||
| Peripapillary area not preserved | Absence of EZ band and/or EPR atrophy within an eccentricity of 0.6 mm from the optic disc | ||
| ERG | I | Normal scotopic and full-field ERG | Lois et al., 2001 [53] |
| II | Loss of photopic function | ||
| III | Loss of both photopic and scotopic function | ||
| SW-AF and OCT | FS-YES | Foveal sparing | Fujinami et al., 2013 [38] |
| FS-NO | Early onset foveal atrophy | ||
| OCT | EZ Absent | EZ band loss | Parodi et al., 2015 [57] |
| EZ Disrupted | EZ band disorganization | ||
| EZ Preserved | Identification of EZ band | ||
| Genotype | NM | At least one null or splice variant is present | Fujinami et al., 2013 [52] |
| MM | Two or more missense variants are present | ||
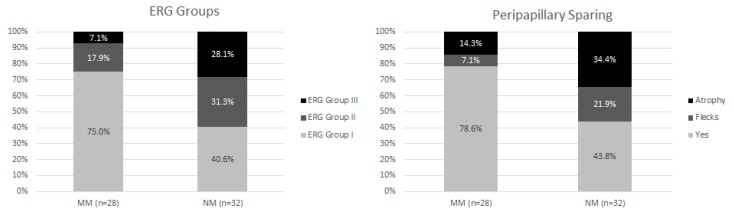
Fundus staging, short-wavelength fundus autofluorescence (SW-FAF), and near infrared fundus autofluorescence (IR-FAF) groups, foveal sparing, the presence of retinal pigment epithelium (RPE) atrophy and the integrity of the ellipsoid zone (EZ) band were not statistically different between the NM and MM groups (p = 0.315, p = 0.855, p = 0.441, p = 0.327, p = 0.089, respectively). However, electroretinogram (ERG) group distribution and the presence of autofluorescence peripapillary sparing significantly differed between the two groups (p = 0.002 and p = 0.003 respectively) (Figure 4).
Figure 4.
Repartition between the two genotypic subtypes for electroretinography (ERG) groups (on the left) and peripapillary sparing (on the right). For ERG classification, group I has abnormal multifocal (mf-) ERG with normal full-field (ff-) ERG; in group II there were mf-ERG abnormalities with cone dysfunction (assessed with light-adapted 30 Hz flicker and light-adapted 3.0); Group III has additional rod dysfunction (assessed using dark-adapted 0.01 and dark-adapted 10.0). Peripapillary area was considered speared (Yes in the graph) if no alterations were found in the fundus autofluorescence within 0.6 mm from the optic disc edge. This area was not considered speared when flecks or ellipsoid zone (EZ) and/or retinal pigment epithelium (RPE) atrophy were present (see Table 3 for classifications). Photoreceptors dysfunction (ERG groups II and III) and peripapillary area involved with the presence of flecks or atrophy are more prevalent in the group of patients with at least one null mutation.
3. Discussion
This work is a longitudinal study starting in 2007 which used a combination of microarray analysis and direct Sanger sequencing to identify ABCA4 mutations in a large French cohort with a clinical diagnosis of STGD1 disease. At that time targeted NGS and whole exome sequencing (WES) was not available in our laboratory. The rate of bi-allelic variants detected in the population using this approach was 75.6%, which is in accordance with previously reported data (about 75%) [15,16,17,18,19].
The authors believe that the same results would have been obtained using only direct Sanger sequencing on the entire cohort. Initially, patients were analyzed with microarray to evaluate the prevalence of known mutations, which was at this time commonly used to reduce screening costs. However, the high number of unsolved cases led us to further investigate the cohort with direct Sanger sequencing. The sole application of microarray was enough to solve 76 cases, which were not further investigated with Sanger technique. Indeed, this may have led to an underestimation of the prevalence of novel additional mutations and/or complex alleles in this cohort [28,58]. The relatively high number of unsolved cases may be related to a combination of different factors: (1) The clinical overlap of distinct genetic entities could have led to uncertainties in patients’ selection. Therefore the screening of other genes (e.g., ELOVL4, PROM1, PRPH2, etc.) responsible for overlapping phenotypes may help solving such cases; (2) Large rearrangements within the ABCA4 locus have been shown to occur in ~0.5% to ~2% of STGD1 cases in previous reports and they would not have been detected by our mutational screening [22,24]; (3) Mutations in noncoding regions of the ABCA4 gene locus have been proposed as a common source for a second causative mutation in patients with typical Stargardt phenotype. In particular, the occurrence of deep intronic variants in subjects with a single ABCA4 mutations may range from ~2% to ~18% in different reports [19,20,21,22,27,59]. This may have led not only to an underestimation of bi-allelic cases, but also of the number of novel variants found in the cohort, whose report was the main aim of this study. However, the number of novel variants affecting deep intronic regions should be relatively small. Schulz et al. [27] in a recent report screened for deep intronic variants 237 STGD1 patients showing a single ABCA4 variant. Among the cohort, ten different sequence variants were found, only two of which were not previously reported [27]. The addition of the first, the screening of selected noncoding regions known to be “hot spots” for pathogenic variants (e.g., intron 30 and 36 [27]), and second, whole genome sequencing (WGS) analysis, will be the next steps in order to raise the number of solved cases in our cohort.
Among the 305 patients with bi-allelic variants, we found a total of 240 disease-causing mutations, of which 60 (25%) were never described before (Table 2).
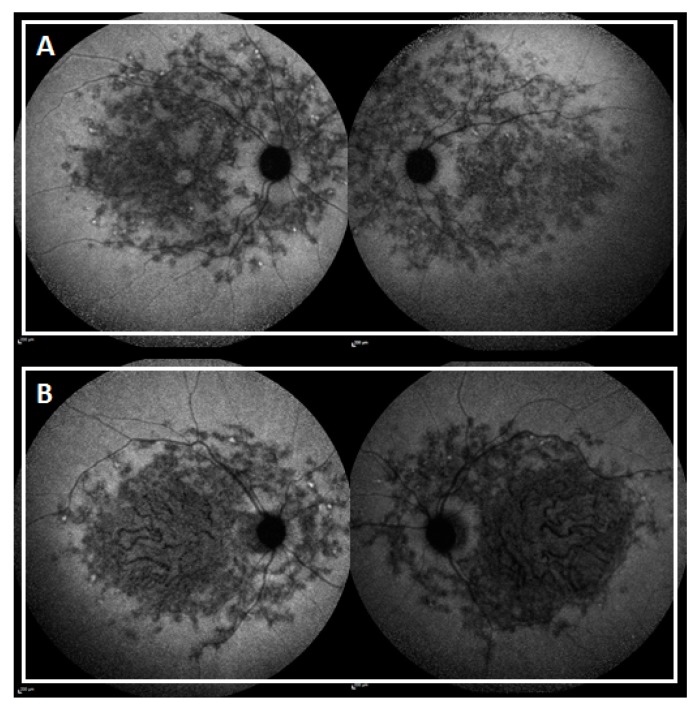
The high number of new variants found in this cohort may seem surprising considering that several studies had already been conducted on the western and central European populations [5,15,17,27,43]. These findings are probably related to the extensive allelic heterogeneity of ABCA4 and the possible contribution of ethnic differences [12]. Among the 61 patients with novel likely pathogenic or pathogenic variants, three subjects had eastern European origins and nine subjects had non-European origins. The novel variant p.(Pro1761Arg) is carried by three subjects with no ties of consanguinity and with non-European origins: Armenia (CIC07960), Turkey (CIC09601), and Algeria (CIC07036). This may suggest a higher prevalence of this mutation in the south Mediterranean area. Both CIC07960 and CIC09601 harbor the variant in cis with p.(Arg2106Cys). Interestingly, the two subjects share the same pattern distribution of the lesion, but CIC07960 has an earlier age of onset and an important foveal involvement, probably due to the associated truncating mutation p.(Leu1274Serfs*8) (Figure 5).
Figure 5.
Near infrared autofluorescence (SW-AF) of subjects CIC09601 (A) and CIC07960 (B).
The novel variant p.(Ile351Leufs*23) was carried by three unrelated French subjects indicating a possible higher prevalence in this population. While CIC01080 and CIC04235 share an early onset advanced disease with spread fundus atrophy, CIC07308 has a normal ff-ERG and no posterior atrophy probably due to the second mutation p.(Gly1961Glu), known for its “milder” pathologic effect [30,31].
Among the likely pathogenic novel variants three were homozygous: p.(Tyr340Cys), p.(Val434Gly), and p.(Gly2146Valfs*36).
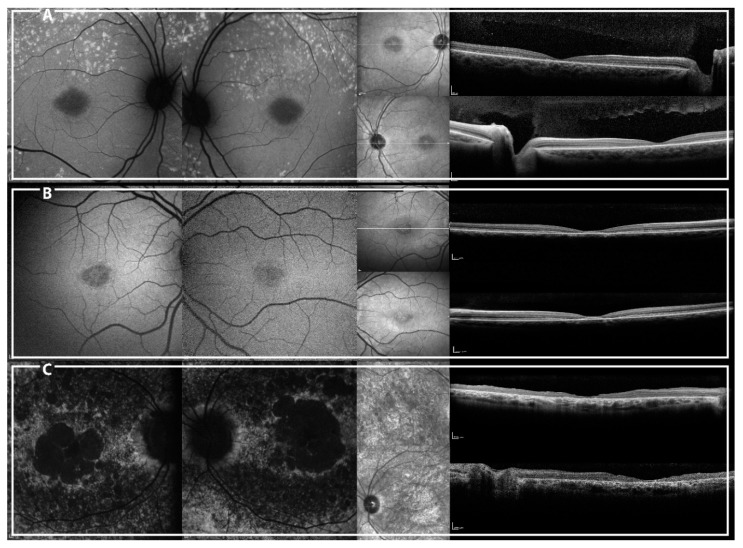
CIC07725, harboring p.(Tyr340Cys), had early-onset disease (nine years of age) with normal ff-ERG, centripetal spread of flecks and foveal EZ band involvement. CIC09625, harboring p.(Val434Gly) has an early onset disease (nine years of age) with a central lesion without flecks and with foveal sparing. Even though they share a similar age of onset and duration of the disease (one and two years, respectively), CIC09625 shows a “milder” phenotype (Figure 6). These two missense mutations may have a distinct impact on the protein function even though they are located in the same domain (first ABCA4 extracellular domain). Alternatively, genetic or environmental modifiers may explain the phenotypic variability.
Figure 6.
Short-wavelength autofluorescence (SW-AF) (left) and spectral domain optical coherence tomography (SD-OCT) central line (right) of patients CIC07725 (A), CIC09625 (B), and CIC06396 (C).
As expected, CIC06396, homozygous for p.(Gly2146Valfs*36), showed a more advanced phenotype with an early onset disease (nine years of age), extensive fundus RPE atrophy and photoreceptor impairment (Figure 6).
VUS identified in the cohort were two missense variants: p.(Asn956Lys), p.(Arg1137Gly), and one non-canonical splice-site variant (c.5899−3T>C) (Table 1 and Supplementary Table S2).
CIC05824 harbors the novel intronic variant c.5899−3T>C, which is not predicted to affect splicing by the algorithms used since the nucleotide is poorly evolutionarily conserved (Supplementary Table S2). Indeed, we cannot exclude the possibility that this variant may affect splicing in vivo since it has already been proven that ABCA4 can have non-canonical splice sites variant with important effects on protein [19,20,21,22,23,59].
The VUS p.(Asn956Lys) harbored by CIC03678 is predicted to be pathogenic only by SIFT and the AA is not conserved (Table 1, Supplementary Table S2). Asparagine and Lysine share similar characteristics of hydropathy and polarity. Unfortunately, although genetic analysis of CIC03679, unaffected son of CIC03678, revealed that he was not carrying p.(Asn956Lys), in absence of other family members available for co-segregation analysis, its significance still remains unclear.
Variant c.3409A>G, p.(Arg1137Gly) carried by CIC03545, is predicted to be pathogenic by SIFT and mutation taster although the residue was not conserved, being substituted by a Glutamine, a Threonine or a Lysine in the conservation analysis (Table 1, Supplementary Table S2). However, all these amino acids are polar while Glycine is not, hence an effect on the tridimensional structure of the protein cannot be completely excluded.
Several hypomorphic alleles have been associated with STGD1 [27,29]. Even though many of them were considered benign because of their high frequency, these alleles could be pathogenic when they are in trans with severe variants, explaining many of the unsolved cases with only one or no mutations detected on ABCA4 [27,29]. The presence of these alleles could help in classifying accompanying ABCA4 mutations as severe mutations and in genotype-phenotype correlations.
For example, Zernant et al. [29] recently reported that the hypormophic variant p.(Asn1868Ile) accounts for more than 50% of the missing causal alleles in monoallelic cases and about 80% of late-onset cases and distinguishes ABCA4-related disease from AMD [29]. In our sub-cohort of 61 patients, this variant appeared in 14 subjects. Most of these cases presented p.(Asn1868Ile) in cis with other disease causing mutations (12 subjects) and the phenotypes were typically consistent with the effect of the overall genotype with mainly early onset and severe disease as documented by Zernant et al. [29]. Only two subjects (CIC08283 and CIC04197) had p.(Asn1868Ile) alone, in trans with other pathogenic variants, resulting in a milder phenotype. Further functional analysis should be performed in order to confirm the pathogenicity of these variants.
Among the 63 patients carrying novel variants, we performed genotype-phenotype correlation on 60 of them. The three subjects carrying a VUS were excluded from the analysis. According to the genotype-phenotype model previously proposed [24], we observed an earlier age of onset and more diffuse photoreceptor dysfunction (assessed by ff-ERGs) in NM group. These findings are in accordance with the previous literature [12,52]. Regarding the peripapillary sparing, its loss was more frequent in the NM group which is also associated with a higher prevalence of non-group I ff-ERG consistent with previous publications reporting a loss of this sign in non-group I Stargardt disease [60].
Overall, in our group of 60 patients harboring novel likely pathogenic ABCA4 changes, we observed a tendency for a worse phenotype when a truncating variant is present.
In this report we wanted to prioritize the characterization of the patients carrying novel variants. Even though it is only a subgroup of subjects the results seem strong and consistent with previous literature [12] and they probably reflect the genotype-phenotype correlation of the entire cohort. Further analysis including a full clinical assessment of patients with known variants will be necessary to confirm these findings.
However, even with a large cohort of patients, it is impossible to establish a precise phenotype-genotype correlation only on the basis of the type of the mutation. In fact, two different missense mutations involving residues at different location with distinct effects on the quaternary structure of ABCA4 can produce very different phenotypes [12,61]. Nowadays, the main efforts are aimed to find correlations between specific variants and clinical phenotype although the rarity of many alleles makes this difficult. For this reason, we tried to provide an exhaustive clinical characterization of each subject showing at least one novel mutation among the two mutant alleles of ABCA4 (see Supplementary Table S3).
4. Materials and Methods
Patients with a presumed diagnosis of STGD1 disease were recruited at the Reference Center for rare diseases, Referet of the Quinze-Vingts hospital, Paris. Informed consent was obtained from each patient after explanation of the study and its potential outcome. The study protocol adhered to the tenets of the Declaration of Helsinki and was approved by a national ethics committee (CPP Ile de France V, Project number 06693, N◦EUDRACT 2006-A00347-44, 11 December 2006). All the patients and available family members were asked to donate a blood sample for genetic screening for ABCA4 mutations. Subjects with at least one novel variant were called back to undergo a complete phenotype analysis as described below.
4.1. Mutation Analysis
Total genomic DNA was extracted from peripheral whole blood samples by standard salting out procedures according to the manufacturer’s recommendation (Puregen Kit; Qiagen, Courtaboeuf, France). The first consecutive 211 subjects were screened for known ABCA4 mutations by microarray analysis on a commercially available microarray (ABCR600, ASPER Biotech, Inc., Tartu, Estonia) [48]. Among them, samples which were excluded for known variants were further investigated for variants in the coding exons and their flanking regions of ABCA4 by PCR and direct Sanger sequencing. The DNA of the other patients included in the study was directly sequenced by Sanger sequencing. The 50 coding exons and flanking intronic regions were amplified in 48 fragments (ABCA4 RefSeq NM_000350) using oligonucleotides reported in Supplementary Table S4, a commercially available polymerase (HotFire, Solis Biodyne, Tartu, Estonia), 1.5 mM MgCl2 at an annealing temperature of 58 °C for 1 min. The PCR products were enzymatically purified (ExoSAP-IT, USB Corporation, Cleveland, OH, USA, purchased from GE Healthcare, Orsay, France) sequenced and investigated as previously reported [62].
Nucleotide numbering reflects cDNA numbering with +1 corresponding to the A of the ATG translation initiation codon in the reference sequence, according to journal guidelines (www.hgvs.org/mutnomen).
All variants were classified following the American College of Medical Genetics and Genomics (ACMG) standards and guidelines and the Association of Molecular Pathology (AMP) Clinical Practice Guidelines and Reports based on previous publications (as compiled in the Human Gene Mutation Database (HGMD) [63] and in the Leiden Open (source) Variation Database (LOVD) [64]), population data, computational data and functional data. The Exome Aggregation Consortium (ExAC) database was used to check variant frequency.
Novel variants with unknown frequency or minor allele frequency (MAF) ≤0.05 were further tested using Alamut Visual software v. 2.7.1 where the in silico predictive programs PolyPhen2 (Polymorphism Phenotyping, http://genetics.bwh.harvard.edu/pph2/) [65], SIFT (Sorting Intolerant from Tolerant; http://sift.bii.a-star.edu.sg/) [66], Mutation Taster (http://www.mutationtaster.org/) [67] are implemented.
For splice site variants three different algorithms of prediction were used: MaxEntScan (http://genes.mit.edu/burgelab/maxent/Xmaxentscan_scoreseq.html) [68], NNSplice [69], and human splicing finder v 3.0 (http://www.umd.be/HSF3/) [70].
Evolutionary conservation was investigated using the 46-way Vertebrate Multiz Alignment and Conservation of the University of California Santa Cruz (UCSC) genome browser [71,72].
For missense mutations, an amino acid residue was considered highly conserved if the same residue was present in all species or was different in just one species among fishes or reptiles, moderately conserved if different in 2 to 5 species (included), and not conserved if different in more than 5 species or in at least one primate.
A novel sequence variant was considered pathogenic if it represented a nonsense variant or small insertion, deletion, or duplication inducing a frame-shift. In the case of a missense change, a novel sequence variant was considered to be likely pathogenic if it was either predicted to be deleterious by all three prediction algorithms or if it affected a highly or moderately evolutionary conserved amino acid residue and was predicted to be pathogenic by at least one algorithm mentioned above [73]. In all other cases, a variant was classified as a VUS.
The same criteria described above, but considering DNA sequence, were applied to study the conservation of nucleotide residues and pathogenicity of variants on putative or non-canonic splice sites (±10 bases from exon boundaries).
4.2. Phenotypic Analysis
Patients with novel ABCA4 variants underwent full ophthalmic examination with assessment of best corrected visual acuity (BCVA) with the Early Treatment Diabetic Retinopathy Study (ETDRS) chart, kinetic and static perimetry, and color vision with the desaturated Farnsworth Panel D-15. Full-field and multifocal electroretinography (ff-ERG and mf-ERG) were performed with DTL recording electrodes and incorporated ISCEV Standards (Espion E2; for full field ERG; Diagnosys, Lowell, MA, USA; and Veris II for multifocal ERG; EDI, Redwood City, CA, USA) [74,75]. Clinical assessment was completed with color fundus photograph (FP; Topcon, Tokyo, Japan), short-wavelength fundus autofluorescence (SW-AF), near-infrared fundus autofluorescence (NIR-AF), spectral domain optical coherence tomography (SD-OCT; Heidelberg retina angiograph [HRA] II or Spectralis HRA+OCT; Heidelberg Engineering, Dossenheim, Germany) after pupil dilatation with Tropicamide 1% and phenylephrine 2.5%.
The phenotype was classified following all clinical criteria summarized in Table 3. Color fundus photographs were acquired for each index patient and stratified in 4 groups according to a previously described classification [54].
A confocal scanning laser ophthalmoscope (Heidelberg Retina Angiograph [HRA] II; Heidelberg Engineering, Dossenheim, Germany) was used to acquire SW-AF images (488 nm excitation) and NIR-AF images (787 nm excitation). Thanks to the eye-tracking function of the HRA and averaging at least 30 single frames, 2 high-quality images (30° and 50° field of view respectively) for each modality were taken for each eye. Subsequently, we divided patients into 5 groups according to a previously described classification [55]: group 1: central lesion with jagged borders, group 2: central lesion with extensive fundus changes; group 3: central lesion with smooth borders and an hyperautofluorescent ring-like halo in SW-AF and NIR-AF; group 4: central lesion with smooth borders and no hyperautofluorescent NIR-AF ring; group 5: small discrete central lesion better visualized in NIR-AF. When RPE atrophy was present the measurement of the area was performed using RegionFinder software (Heidelberg Engineering, Dossenheim, Germany; software version 2.5.8.0) on SW-AF images [76]. The 50° images were used to establish the extension of fundus abnormalities and for evaluating the peripapillary area [56].
A single horizontal high-resolution OCT B-Scan (9 mm) was obtained together with a simultaneously acquired infra-red (IR) fundus image in a transverse plane through the fovea. This image was used to evaluate the preservation of the ellipsoid zone (EZ) through the fovea [57] and the possible foveal sparing from the disease [38]. A cube of OCT scans was then obtained in high-speed mode with the automated real-time mode activated and set to 10 (creating a mean image of ten repeated identical B-scans to improve signal-to-noise ratio). The cube scan protocol contains a density of 49 B-scan lines covering an area of 20° × 20° centered on the fovea. As previously described, a trained operator corrected any segmentation errors made by the software version with manual segmentation [77,78]. When the quality of segmentation was adequate, the central retinal thickness (CRT) and the total macular volume (TMV) were recorded and compared against the data of 30 normal subjects (15 subjects younger than 30 years and 15 subjects older; Supplementary Table S5).
All patients underwent electrophysiological assessment, including ff-ERG and mf-ERG, incorporating the minimum standards of the International Society for Clinical of Electrophysiology of Vision (ISCEV) including the following stimulations: dark adapted dim flash 0.01 candela second (cd·s/m2); dark-adapted bright flash 10.0 cd·s/m2; light adapted 3.0 cd·s/m2 30 Hz flicker ERG and light adapted 3.0 cd·s/m2 at 2 Hz. The patient data set were compared against those of 30 healthy subjects (15 younger than 30 years old and 15 older; Supplementary Table S1). The limits of ERG normality were defined for all the components of the ERG as the mean value ± 2 standard deviations. All the components of the ERG from each eye were taken into account when classifying patients into the three ERG groups defined by Lois et al. [53]: Group 1 has abnormal mf-ERG with normal ff-ERG; in Group 2 there were mf-ERG abnormalities with cone dysfunction (assessed with light-adapted 30 Hz flicker and light-adapted 3.0); Group 3 has additional rod dysfunction (assessed using dark-adapted 0.01 and dark-adapted 10.0). The overall classification was based on the more severe eye when the ERG group was different between eyes in the same patient.
4.3. Statistical Analysis
All data analysis was conducted using IBM SPSS Statistics software v. 21.0 (Chicago, IL, USA).
For BCVA, MV, and CRT we used the Wilcoxon signed-rank test to prove the agreement between eyes.
Differences between the two genotype groups were analyzed using the Wilcoxon-Mann-Whitney test for age of onset, age at visit, duration of the disease, BCVA, MV, and CRT. The Goodman-Kruskal Gamma test was used to analyze the difference between NM and MM groups for fundus stage, NIR-FAF, and IR-FAF groups, the presence of RPE atrophy, foveal sparing, peripapillary sparing, EZ band integrity, and ERG group distribution. For these descriptive variants, when there was discordance between the right and left eye, we considered the eye with the worse phenotype. A p value inferior to 0.05 was considered statistically significant.
5. Conclusions
Despite certain limitations in terms of detecting large CNVs (<1% of all disease-associated alleles) [79] or mutations in non-coding regions, and the lack of functional analysis, our study broadens the spectrum of ABCA4 mutations with 60 likely pathogenic or pathogenic variants, all associated with STGD1.
Acknowledgments
The authors thank Rand Wilcox Vanden Berg for writing assistance and proofreading. DNA samples included in this study originate from NeuroSensCol DNA bank, dedicated for research in neurosensory disorders (PI: JA Sahel, coPI I Audo, partner with CHNO des Quinze-Vingts, Inserm and CNRS).
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| STGD-1 | Stargardt disease-1 |
| ABCA4 | ATP-binding cassette, subfamily A, member 4 |
| BCVA | Best Corrected Visual Acuity |
| ff-ERG | Full field electroretinography |
| mf-ERG | Multi focal electroretinography |
| SW-AF | Short-wavelength autofluorescence |
| NIR-AF | Near-infrared Autofluorescence |
| SD-OCT | Spectral Domain Optical Coherence Tomography |
| ISCEV | International Society for Clinical of Electrophysiology of Vision |
| ACMG | American College of Medical Genetics and Genomics |
| AMP | Association of Molecular Pathology |
| HGMD | Human Gene Mutation Database |
| ExAC | Exome Aggregation Consortium |
| PolyPhen2 | Polymorphism Phenotyping |
| UCSC | University of California Santa Cruz |
| SIFT | Sorting intolerant from tolerant |
| RPE | Retinal pigment epithelium |
| CRT | Central retinal thickness |
| TMV | Total macular volume |
| VUS | Variant of uncertain significance |
| MAF | Minor allele frequency |
| NGS | Next-generation sequencing |
| WES | Whole exome sequencing |
| WGS | Whole genome sequencing |
| EZ | Ellipsoid zone |
| FP | Fundus photograph |
| FS | Foveal sparing |
| MM | At least one missense variant in the genotype |
| NM | At least one null variant in the genotype |
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/19/8/2196/s1.
Author Contributions
M.N., C.Z., and I.A. were involved in study design, collection and analysis of the data as well as drafting of the manuscript. A.A., S.M.-S., C.-M.D., C.M., C.M.E., and J.-A.S. were involved in study design, data collection, and critical review of the manuscript. F.B., V.D., C.A., C.C., and M.F. were involved in data collection and analysis.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Molday L.L., Rabin A.R., Molday R.S. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat. Genet. 2000;25:257–258. doi: 10.1038/77004. [DOI] [PubMed] [Google Scholar]
- 2.Maeda A., Maeda T., Golczak M., Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J. Biol. Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeda T., Maeda A., Matosky M., Okano K., Roos S., Tang J., Palczewski K. Evaluation of potential therapies for a mouse model of human age-related macular degeneration caused by delayed all-trans-retinal clearance. Investig. Ophthalmol. Vis. Sci. 2009;50:4917–4925. doi: 10.1167/iovs.09-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koenekoop R.K. The gene for Stargardt disease, ABCA4, is a major retinal gene: A mini-review. Ophthalmic Genet. 2003;24:75–80. doi: 10.1076/opge.24.2.75.13996. [DOI] [PubMed] [Google Scholar]
- 5.Maia-Lopes S., Aguirre-Lamban J., Castelo-Branco M., Riveiro-Alvarez R., Ayuso C., Silva E.D. ABCA4 mutations in Portuguese Stargardt patients: Identification of new mutations and their phenotypic analysis. Mol. Vis. 2009;15:584–591. [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis R.A., Shroyer N.F., Singh N., Allikmets R., Hutchinson A., Li Y., Lupski J.R., Leppert M., Dean M. Genotype/Phenotype analysis of a photoreceptor-specific ATP-binding cassette transporter gene, ABCR, in Stargardt disease. Am. J. Hum. Genet. 1999;64:422–434. doi: 10.1086/302251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-Mir A., Paloma E., Allikmets R., Ayuso C., del Rio T., Dean M., Vilageliu L., Gonzàlez-Duarte R., Balcells S. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat. Genet. 1998;18:11–12. doi: 10.1038/ng0198-11. [DOI] [PubMed] [Google Scholar]
- 8.Huang X., Yuan L., Xu H., Zheng W., Cao Y., Yi J., Guo Y., Yang Z., Li Y., Deng H. Identification of a Novel Mutation in the ABCA4 Gene in a Chinese Family with Retinitis Pigmentosa Using Exome Sequencing. Biosci. Rep. 2018 doi: 10.1042/BSR20171300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Audere M., Rutka K., Šepetiene S., Lāce B. Presentation of Complex Homozygous Allele in ABCA4 Gene in a Patient with Retinitis Pigmentosa. Case Rep. Ophthalmol. Med. 2015;2015:452068. doi: 10.1155/2015/452068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozet J.M., Gerber S., Ghazi I., Perrault I., Ducroq D., Souied E., Cabot A., Dufier J.L., Munnich A., Kaplan J. Mutations of the retinal specific ATP binding transporter gene (ABCR) in a single family segregating both autosomal recessive retinitis pigmentosa RP19 and Stargardt disease: Evidence of clinical heterogeneity at this locus. J. Med. Genet. 1999;36:447–451. [PMC free article] [PubMed] [Google Scholar]
- 11.Mullins R.F., Kuehn M.H., Radu R.A., Enriquez G.S., East J.S., Schindler E.I., Travis G.H., Stone E.M. Autosomal recessive retinitis pigmentosa due to ABCA4 mutations: Clinical, pathologic, and molecular characterization. Investig. Ophthalmol. Vis. Sci. 2012;53:1883–1894. doi: 10.1167/iovs.12-9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelis S.S., Bax N.M., Zernant J., Allikmets R., Fritsche L.G., den Dunnen J.T., Ajmal M., Hoyng C.B., Cremers F.P.M. In Silico Functional Meta-Analysis of 5,962 ABCA4 Variants in 3,928 Retinal Dystrophy Cases. Hum. Mutat. 2017;38:400–408. doi: 10.1002/humu.23165. [DOI] [PubMed] [Google Scholar]
- 13.Allikmets R. Simple and complex ABCR: Genetic predisposition to retinal disease. Am. J. Hum. Genet. 2000;67:793–799. doi: 10.1086/303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passerini I., Sodi A., Giambene B., Mariottini A., Menchini U., Torricelli F. Novel mutations in of the ABCR gene in Italian patients with Stargardt disease. Eye Lond. Engl. 2010;24:158–164. doi: 10.1038/eye.2009.35. [DOI] [PubMed] [Google Scholar]
- 15.Riveiro-Alvarez R., Lopez-Martinez M.-A., Zernant J., Aguirre-Lamban J., Cantalapiedra D., Avila-Fernandez A., Gimenez A., Lopez-Molina M.-I., Garcia-Sandoval B., Blanco-Kelly F., et al. Outcome of ABCA4 disease-associated alleles in autosomal recessive retinal dystrophies: Retrospective analysis in 420 Spanish families. Ophthalmology. 2013;120:2332–2337. doi: 10.1016/j.ophtha.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang F., Pan Z., Xu K., Tian L., Xie Y., Zhang X., Chen J., Dong B., Li Y. Screening of ABCA4 Gene in a Chinese Cohort with Stargardt Disease or Cone-Rod Dystrophy with a Report on 85 Novel Mutations. Investig. Ophthalmol. Vis. Sci. 2016;57:145–152. doi: 10.1167/iovs.15-18190. [DOI] [PubMed] [Google Scholar]
- 17.Bocquet B., Lacroux A., Surget M.-O., Baudoin C., Marquette V., Manes G., Hebrard M., Sénéchal A., Delettre C., Roux A.-F., et al. Relative frequencies of inherited retinal dystrophies and optic neuropathies in Southern France: Assessment of 21-year data management. Ophthalmic Epidemiol. 2013;20:13–25. doi: 10.3109/09286586.2012.737890. [DOI] [PubMed] [Google Scholar]
- 18.Zernant J., Schubert C., Im K.M., Burke T., Brown C.M., Fishman G.A., Tsang S.H., Gouras P., Dean M., Allikmets R. Analysis of the ABCA4 gene by next-generation sequencing. Investig. Ophthalmol. Vis. Sci. 2011;52:8479–8487. doi: 10.1167/iovs.11-8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zernant J., Xie Y.A., Ayuso C., Riveiro-Alvarez R., Lopez-Martinez M.-A., Simonelli F., Testa F., Gorin M.B., Strom S.P., Bertelsen M., et al. Analysis of the ABCA4 genomic locus in Stargardt disease. Hum. Mol. Genet. 2014;23:6797–6806. doi: 10.1093/hmg/ddu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun T.A., Mullins R.F., Wagner A.H., Andorf J.L., Johnston R.M., Bakall B.B., Deluca A.P., Fishman G.A., Lam B.L., Weleber R.G., et al. Non-exomic and synonymous variants in ABCA4 are an important cause of Stargardt disease. Hum. Mol. Genet. 2013;22:5136–5145. doi: 10.1093/hmg/ddt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauwens M., De Zaeytijd J., Weisschuh N., Kohl S., Meire F., Dahan K., Depasse F., De Jaegere S., De Ravel T., De Rademaeker M., et al. An augmented ABCA4 screen targeting noncoding regions reveals a deep intronic founder variant in Belgian Stargardt patients. Hum. Mutat. 2015;36:39–42. doi: 10.1002/humu.22716. [DOI] [PubMed] [Google Scholar]
- 22.Bax N.M., Sangermano R., Roosing S., Thiadens A.A.H.J., Hoefsloot L.H., van den Born L.I., Phan M., Klevering B.J., Westeneng-van Haaften C., Braun T.A., et al. Heterozygous deep-intronic variants and deletions in ABCA4 in persons with retinal dystrophies and one exonic ABCA4 variant. Hum. Mutat. 2015;36:43–47. doi: 10.1002/humu.22717. [DOI] [PubMed] [Google Scholar]
- 23.Sangermano R., Khan M., Cornelis S.S., Richelle V., Albert S., Garanto A., Elmelik D., Qamar R., Lugtenberg D., van den Born L.I., et al. ABCA4 midigenes reveal the full splice spectrum of all reported noncanonical splice site variants in Stargardt disease. Genome Res. 2018;28:100–110. doi: 10.1101/gr.226621.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maugeri A., van Driel M.A., van de Pol D.J., Klevering B.J., van Haren F.J., Tijmes N., Bergen A.A., Rohrschneider K., Blankenagel A., Pinckers A.J., et al. The 2588G-->C mutation in the ABCR gene is a mild frequent founder mutation in the Western European population and allows the classification of ABCR mutations in patients with Stargardt disease. Am. J. Hum. Genet. 1999;64:1024–1035. doi: 10.1086/302323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allikmets R., Singh N., Sun H., Shroyer N.F., Hutchinson A., Chidambaram A., Gerrard B., Baird L., Stauffer D., Peiffer A., et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat. Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 26.Rozet J.M., Gerber S., Souied E., Perrault I., Châtelin S., Ghazi I., Leowski C., Dufier J.L., Munnich A., Kaplan J. Spectrum of ABCR gene mutations in autosomal recessive macular dystrophies. Eur. J. Hum. Genet. 1998;6:291–295. doi: 10.1038/sj.ejhg.5200221. [DOI] [PubMed] [Google Scholar]
- 27.Schulz H.L., Grassmann F., Kellner U., Spital G., Rüther K., Jägle H., Hufendiek K., Rating P., Huchzermeyer C., Baier M.J., et al. Mutation Spectrum of the ABCA4 Gene in 335 Stargardt Disease Patients from a Multicenter German Cohort-Impact of Selected Deep Intronic Variants and Common SNPs. Investig. Ophthalmol. Vis. Sci. 2017;58:394–403. doi: 10.1167/iovs.16-19936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salles M.V., Motta F.L., Dias da Silva E., Varela P., Costa K.A., Filippelli-Silva R., Martin R.P., Chiang J.P.-W., Pesquero J.B., Sallum J.M.F. Novel Complex ABCA4 Alleles in Brazilian Patients with Stargardt Disease: Genotype-Phenotype Correlation. Investig. Ophthalmol. Vis. Sci. 2017;58:5723–5730. doi: 10.1167/iovs.17-22398. [DOI] [PubMed] [Google Scholar]
- 29.Zernant J., Lee W., Collison F.T., Fishman G.A., Sergeev Y.V., Schuerch K., Sparrow J.R., Tsang S.H., Allikmets R. Frequent hypomorphic alleles account for a significant fraction of ABCA4 disease and distinguish it from age-related macular degeneration. J. Med. Genet. 2017;54:404–412. doi: 10.1136/jmedgenet-2017-104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burke T.R., Fishman G.A., Zernant J., Schubert C., Tsang S.H., Smith R.T., Ayyagari R., Koenekoop R.K., Umfress A., Ciccarelli M.L., et al. Retinal phenotypes in patients homozygous for the G1961E mutation in the ABCA4 gene. Investig. Ophthalmol. Vis. Sci. 2012;53:4458–4467. doi: 10.1167/iovs.11-9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cella W., Greenstein V.C., Zernant-Rajang J., Smith T.R., Barile G., Allikmets R., Tsang S.H. G1961E mutant allele in the Stargardt disease gene ABCA4 causes bull’s eye maculopathy. Exp. Eye Res. 2009;89:16–24. doi: 10.1016/j.exer.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campa C., Gallenga C.E., Bolletta E., Perri P. The Role of Gene Therapy in the Treatment of Retinal Diseases: A Review. Curr. Gene Ther. 2017;17:194–213. doi: 10.2174/1566523217666171116170040. [DOI] [PubMed] [Google Scholar]
- 33.Lu L.J., Liu J., Adelman R.A. Novel therapeutics for Stargardt disease. Graefes Arch. Clin. Exp. Ophthalmol. 2017;255:1057–1062. doi: 10.1007/s00417-017-3619-8. [DOI] [PubMed] [Google Scholar]
- 34.Webster A.R., Héon E., Lotery A.J., Vandenburgh K., Casavant T.L., Oh K.T., Beck G., Fishman G.A., Lam B.L., Levin A., et al. An analysis of allelic variation in the ABCA4 gene. Investig. Ophthalmol. Vis. Sci. 2001;42:1179–1189. [PubMed] [Google Scholar]
- 35.Allikmets R., Shroyer N.F., Singh N., Seddon J.M., Lewis R.A., Bernstein P.S., Peiffer A., Zabriskie N.A., Li Y., Hutchinson A., et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 36.Ernest P.J.G., Boon C.J.F., Klevering B.J., Hoefsloot L.H., Hoyng C.B. Outcome of ABCA4 microarray screening in routine clinical practice. Mol. Vis. 2009;15:2841–2847. [PMC free article] [PubMed] [Google Scholar]
- 37.Jimenez-Rolando B., Noval S., Rosa-Perez I., Mata Diaz E., Del Pozo A., Ibañez C., Silla J.C., Montaño V.E.F., Martin-Arenas R., Vallespin E. Next generation sequencing in the diagnosis of Stargardt’s disease. Arch. Soc. Espanola Oftalmol. 2017 doi: 10.1016/j.oftale.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Fujinami K., Sergouniotis P.I., Davidson A.E., Wright G., Chana R.K., Tsunoda K., Tsubota K., Egan C.A., Robson A.G., Moore A.T., et al. Clinical and molecular analysis of Stargardt disease with preserved foveal structure and function. Am. J. Ophthalmol. 2013;156:487–501.e1. doi: 10.1016/j.ajo.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Souied E.H., Ducroq D., Rozet J.M., Gerber S., Perrault I., Munnich A., Coscas G., Soubrane G., Kaplan J. ABCR gene analysis in familial exudative age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2000;41:244–247. [PubMed] [Google Scholar]
- 40.Rivera A., White K., Stöhr H., Steiner K., Hemmrich N., Grimm T., Jurklies B., Lorenz B., Scholl H.P., Apfelstedt-Sylla E., Weber B.H. A comprehensive survey of sequence variation in the ABCA4 (ABCR) gene in Stargardt disease and age-related macular degeneration. Am. J. Hum. Genet. 2000;67:800–813. doi: 10.1086/303090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sangermano R., Bax N.M., Bauwens M., van den Born L.I., De Baere E., Garanto A., Collin R.W.J., Goercharn-Ramlal A.S.A., den Engelsman-van Dijk A.H.A., Rohrschneider K., et al. Photoreceptor Progenitor mRNA Analysis Reveals Exon Skipping Resulting from the ABCA4 c.5461-10T→C Mutation in Stargardt Disease. Ophthalmology. 2016;123:1375–1385. doi: 10.1016/j.ophtha.2016.01.053. [DOI] [PubMed] [Google Scholar]
- 42.Birch D.G., Peters A.Y., Locke K.L., Spencer R., Megarity C.F., Travis G.H. Visual function in patients with cone-rod dystrophy (CRD) associated with mutations in the ABCA4(ABCR) gene. Exp. Eye Res. 2001;73:877–886. doi: 10.1006/exer.2001.1093. [DOI] [PubMed] [Google Scholar]
- 43.Simonelli F., Testa F., Zernant J., Nesti A., Rossi S., Allikmets R., Rinaldi E. Genotype-phenotype correlation in Italian families with Stargardt disease. Ophthalmic Res. 2005;37:159–167. doi: 10.1159/000086073. [DOI] [PubMed] [Google Scholar]
- 44.Briggs C.E., Rucinski D., Rosenfeld P.J., Hirose T., Berson E.L., Dryja T.P. Mutations in ABCR (ABCA4) in patients with Stargardt macular degeneration or cone-rod degeneration. Investig. Ophthalmol. Vis. Sci. 2001;42:2229–2236. [PubMed] [Google Scholar]
- 45.Klevering B.J., Deutman A.F., Maugeri A., Cremers F.P.M., Hoyng C.B. The spectrum of retinal phenotypes caused by mutations in the ABCA4 gene. Graefes Arch. Clin. Exp. Ophthalmol. 2005;243:90–100. doi: 10.1007/s00417-004-1079-4. [DOI] [PubMed] [Google Scholar]
- 46.Fishman G.A., Stone E.M., Grover S., Derlacki D.J., Haines H.L., Hockey R.R. Variation of clinical expression in patients with Stargardt dystrophy and sequence variations in the ABCR gene. Arch. Ophthalmol. 1999;117:504–510. doi: 10.1001/archopht.117.4.504. [DOI] [PubMed] [Google Scholar]
- 47.Stenirri S., Fermo I., Battistella S., Galbiati S., Soriani N., Paroni R., Manitto M.P., Martina E., Brancato R., Allikmets R., et al. Denaturing HPLC profiling of the ABCA4 gene for reliable detection of allelic variations. Clin. Chem. 2004;50:1336–1343. doi: 10.1373/clinchem.2004.033241. [DOI] [PubMed] [Google Scholar]
- 48.Jaakson K., Zernant J., Külm M., Hutchinson A., Tonisson N., Glavac D., Ravnik-Glavac M., Hawlina M., Meltzer M.R., Caruso R.C., et al. Genotyping microarray (gene chip) for the ABCR (ABCA4) gene. Hum. Mutat. 2003;22:395–403. doi: 10.1002/humu.10263. [DOI] [PubMed] [Google Scholar]
- 49.Papaioannou M., Ocaka L., Bessant D., Lois N., Bird A., Payne A., Bhattacharya S. An analysis of ABCR mutations in British patients with recessive retinal dystrophies. Investig. Ophthalmol. Vis. Sci. 2000;41:16–19. [PubMed] [Google Scholar]
- 50.Testa F., Rossi S., Sodi A., Passerini I., Di Iorio V., Della Corte M., Banfi S., Surace E.M., Menchini U., Auricchio A., et al. Correlation between photoreceptor layer integrity and visual function in patients with Stargardt disease: Implications for gene therapy. Investig. Ophthalmol. Vis. Sci. 2012;53:4409–4415. doi: 10.1167/iovs.11-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aukrust I., Jansson R.W., Bredrup C., Rusaas H.E., Berland S., Jørgensen A., Haug M.G., Rødahl E., Houge G., Knappskog P.M. The intronic ABCA4 c.5461-10T>C variant, frequently seen in patients with Stargardt disease, causes splice defects and reduced ABCA4 protein level. Acta Ophthalmol. (Copenh.) 2017;95:240–246. doi: 10.1111/aos.13273. [DOI] [PubMed] [Google Scholar]
- 52.Fujinami K., Lois N., Mukherjee R., McBain V.A., Tsunoda K., Tsubota K., Stone E.M., Fitzke F.W., Bunce C., Moore A.T., et al. A longitudinal study of Stargardt disease: Quantitative assessment of fundus autofluorescence, progression, and genotype correlations. Investig. Ophthalmol. Vis. Sci. 2013;54:8181–8190. doi: 10.1167/iovs.13-12104. [DOI] [PubMed] [Google Scholar]
- 53.Lois N., Holder G.E., Bunce C., Fitzke F.W., Bird A.C. Phenotypic subtypes of Stargardt macular dystrophy-fundus flavimaculatus. Arch. Ophthalmol. 2001;119:359–369. doi: 10.1001/archopht.119.3.359. [DOI] [PubMed] [Google Scholar]
- 54.Fishman G.A. Fundus flavimaculatus. A clinical classification. Arch. Ophthalmol. 1976;94:2061–2067. doi: 10.1001/archopht.1976.03910040721003. [DOI] [PubMed] [Google Scholar]
- 55.Duncker T., Marsiglia M., Lee W., Zernant J., Tsang S.H., Allikmets R., Greenstein V.C., Sparrow J.R. Correlations among near-infrared and short-wavelength autofluorescence and spectral-domain optical coherence tomography in recessive Stargardt disease. Investig. Ophthalmol. Vis. Sci. 2014;55:8134–8143. doi: 10.1167/iovs.14-14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cideciyan A.V., Swider M., Aleman T.S., Sumaroka A., Schwartz S.B., Roman M.I., Milam A.H., Bennett J., Stone E.M., Jacobson S.G. ABCA4-associated retinal degenerations spare structure and function of the human parapapillary retina. Investig. Ophthalmol. Vis. Sci. 2005;46:4739–4746. doi: 10.1167/iovs.05-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parodi M.B., Iacono P., Triolo G., La Spina C., Zucchiatti I., Cicinelli M.V., Borrelli E., Manitto M.P., Martina E., Bandello F. Morpho-functional correlation of fundus autofluorescence in Stargardt disease. Br. J. Ophthalmol. 2015;99:1354–1359. doi: 10.1136/bjophthalmol-2014-306237. [DOI] [PubMed] [Google Scholar]
- 58.Ścieżyńska A., Oziębło D., Ambroziak A.M., Korwin M., Szulborski K., Krawczyński M., Stawiński P., Szaflik J., Szaflik J.P., Płoski R., et al. Next-generation sequencing of ABCA4: High frequency of complex alleles and novel mutations in patients with retinal dystrophies from Central Europe. Exp. Eye Res. 2016;145:93–99. doi: 10.1016/j.exer.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 59.Zaneveld J., Siddiqui S., Li H., Wang X., Wang H., Wang K., Li H., Ren H., Lopez I., Dorfman A., et al. Comprehensive analysis of patients with Stargardt macular dystrophy reveals new genotype-phenotype correlations and unexpected diagnostic revisions. Genet. Med. 2015;17:262–270. doi: 10.1038/gim.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burke T.R., Allikmets R., Smith R.T., Gouras P., Tsang S.H. Loss of peripapillary sparing in non-group I Stargardt disease. Exp. Eye Res. 2010;91:592–600. doi: 10.1016/j.exer.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahn J., Beharry S., Molday L.L., Molday R.S. Functional interaction between the two halves of the photoreceptor-specific ATP binding cassette protein ABCR (ABCA4). Evidence for a non-exchangeable ADP in the first nucleotide binding domain. J. Biol. Chem. 2003;278:39600–39608. doi: 10.1074/jbc.M304236200. [DOI] [PubMed] [Google Scholar]
- 62.Audo I., Manes G., Mohand-Saïd S., Friedrich A., Lancelot M.-E., Antonio A., Moskova-Doumanova V., Poch O., Zanlonghi X., Hamel C.P., et al. Spectrum of rhodopsin mutations in French autosomal dominant rod-cone dystrophy patients. Investig. Ophthalmol. Vis. Sci. 2010;51:3687–3700. doi: 10.1167/iovs.09-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stenson P.D., Ball E.V., Mort M., Phillips A.D., Shiel J.A., Thomas N.S.T., Abeysinghe S., Krawczak M., Cooper D.N. Human Gene Mutation Database (HGMD): 2003 update. Hum. Mutat. 2003;21:577–581. doi: 10.1002/humu.10212. [DOI] [PubMed] [Google Scholar]
- 64.Fokkema I.F.A.C., Taschner P.E.M., Schaafsma G.C.P., Celli J., Laros J.F.J., den Dunnen J.T. LOVD v.2.0: The next generation in gene variant databases. Hum. Mutat. 2011;32:557–563. doi: 10.1002/humu.21438. [DOI] [PubMed] [Google Scholar]
- 65.Adzhubei I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P., Kondrashov A.S., Sunyaev S.R. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sim N.-L., Kumar P., Hu J., Henikoff S., Schneider G., Ng P.C. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012;40:W452–W457. doi: 10.1093/nar/gks539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwarz J.M., Cooper D.N., Schuelke M., Seelow D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 68.Yeo G., Burge C.B. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J. Comput. Biol. 2004;11:377–394. doi: 10.1089/1066527041410418. [DOI] [PubMed] [Google Scholar]
- 69.Reese M.G., Eeckman F.H., Kulp D., Haussler D. Improved splice site detection in Genie. J. Comput. Biol. 1997;4:311–323. doi: 10.1089/cmb.1997.4.311. [DOI] [PubMed] [Google Scholar]
- 70.Desmet F.-O., Hamroun D., Lalande M., Collod-Béroud G., Claustres M., Béroud C. Human Splicing Finder: An online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casper J., Zweig A.S., Villarreal C., Tyner C., Speir M.L., Rosenbloom K.R., Raney B.J., Lee C.M., Lee B.T., Karolchik D., et al. The UCSC Genome Browser database: 2018 update. Nucleic Acids Res. 2017 doi: 10.1093/nar/gkx1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kent W.J. BLAT—The BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marmor M.F., Fulton A.B., Holder G.E., Miyake Y., Brigell M., Bach M., International Society for Clinical Electrophysiology of Vision ISCEV Standard for full-field clinical electroretinography (2008 update) Doc. Ophthalmol. 2009;118:69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- 75.Hood D.C., Bach M., Brigell M., Keating D., Kondo M., Lyons J.S., Palmowski-Wolfe A.M. ISCEV guidelines for clinical multifocal electroretinography (2007 edition) Doc. Ophthalmol. 2008;116:1–11. doi: 10.1007/s10633-007-9089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmitz-Valckenberg S., Brinkmann C.K., Alten F., Herrmann P., Stratmann N.K., Göbel A.P., Fleckenstein M., Diller M., Jaffe G.J., Holz F.G. Semiautomated image processing method for identification and quantification of geographic atrophy in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011;52:7640–7646. doi: 10.1167/iovs.11-7457. [DOI] [PubMed] [Google Scholar]
- 77.Strauss R.W., Muñoz B., Wolfson Y., Sophie R., Fletcher E., Bittencourt M.G., Scholl H.P.N. Assessment of estimated retinal atrophy progression in Stargardt macular dystrophy using spectral-domain optical coherence tomography. Br. J. Ophthalmol. 2016;100:956–962. doi: 10.1136/bjophthalmol-2015-307035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parker M.A., Choi D., Erker L.R., Pennesi M.E., Yang P., Chegarnov E.N., Steinkamp P.N., Schlechter C.L., Dhaenens C.-M., Mohand-Said S., et al. Test-Retest Variability of Functional and Structural Parameters in Patients with Stargardt Disease Participating in the SAR422459 Gene Therapy Trial. Transl. Vis. Sci. Technol. 2016;5:10. doi: 10.1167/tvst.5.5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee W., Xie Y., Zernant J., Yuan B., Bearelly S., Tsang S.H., Lupski J.R., Allikmets R. Complex inheritance of ABCA4 disease: Four mutations in a family with multiple macular phenotypes. Hum. Genet. 2016;135:9–19. doi: 10.1007/s00439-015-1605-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.