Introduction
Metabolic syndrome (MetS) is a disorder encompassing multiple features associated with increased risk for type 2 diabetes mellitus, cardiovascular diseases, and premature mortality. MetS is prevalent in the younger population with reported rates of 8.6% in adolescents using the National Health and Nutrition Examination Survey (NHANES) data collected between 2001–2006 (approximately 1 in 10 children ages 12–19).1 Furthermore, MetS is increasing among adolescents and these risk factors which are present in adolescents and young adults are potentially associated with changes in the arteries that contribute to the development of atherosclerosis in adulthood.2
The consumption of processed foods is increasing among adolescents, which is associated with increased exposure to dietary advanced glycation end products (dAGEs). AGEs form in food during heating and cause oxidative stress and chronic inflammation leading to increased risk for metabolic and cardiovascular events.3 AGEs are products of the Maillard reaction, where sugar moieties in food react with proteins resulting in protein cross linking and product browning, together with formation of flavor and aroma compounds.4 Diets which contain more fat and meats contribute to more dAGEs, especially when cooked under dry heat.5 In a recent study, high dAGE intake was associated with higher risk of two components of MetS in adults, however this relationship has not been investigated in adolescents.3 Therefore, we utilized a nationally representative database with dietary data to estimate association of MetS with dAGE intake in this population. Our secondary aim was also to examine the association of dAGE consumption with the individual criterion of metabolic syndrome..
Material and Methods
The study sample included adolescents ages 12–19 years from the Continuous National Health and Nutrition Examination Survey (NHANES), a major program of the National Center for Health Statistics. It is a series of 2-year stratified, multistage probability surveys representative of the civilian, non-institutionalized US population, which include in-person interviews and examinations in mobile centers on a cross-sectional basis. The instruments included a health interview survey that collected information regarding socio-demographic characteristics, a health examination survey which comprised of anthropometry, BP measures and laboratory tests of the subjects obtained in a mobile examination center, and a nutrition questionnaire. We utilized data from years 2003–2004 and 2005–2006, as these were the years that the food frequency questionnaire was administered, which included data on frequency of consumption of multiple types of foods.
We calculated daily AGE intake per person by assigning an AGE score to each food item and then multiplying by the frequency of consumption. Frequencies of food consumption were calculated as 0 if food was consumed at the most once a month, 1 for at the most once a week, 2 for 2 times/week, 3.5 for 3–4 times/week, 5.5 for 5–6 times/week, 7.5 for daily consumption, 14 for 2 times or more/day. This was then normalized by total kilocalorie intake in order to standardize for the differences in portion sizes. The AGE score was adapted from a published database containing information on AGE content in more than 500 food items.5
As per The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III), Metabolic Syndrome is defined as having 3 or more of the following criteria: waist circumference greater than 102 cm in men or greater than 88 cm in women; serum level of triglycerides of 150 mg/dL or greater; high-density lipoprotein (HDL) cholesterol level of less than 40 mg/dL in men or less than 50 mg/dL in women; systolic/diastolic blood pressure of 130/85 mm Hg or greater or taking hypertension medications; or fasting plasma glucose level of 100 mg/dL. We excluded all adolescents who were on prescription medications of steroids, oral hypoglycemics and insulin to prevent confounding.
The patients’ baseline characteristics are presented as frequencies with percentages for categorical variables. We used survey logistical regression analysis to assess the relationship between dAGE intake and the odds of having MetS while adjusting for confounders. dAGE score were categorized into four quartiles: <2.73, 2.74–4.98, 4.99–8.68 and ≥8.68 U/kcal. Odds ratios (ORs) and 95% confidence interval (CI) values were calculated to detect MetS in adolescents and also to see the individual criterion associations. The model was adjusted for non-modifiable risk factors (age and gender) and family income. We performed all associations using designated weight values by NHANES to produce nationally representative estimates and considered a two-tailed p value ≤ 0.05 as statistically significant. We utilized SAS 9.4 (SAS Institute Inc. Cary, North Carolina) for all analyses.
Results
During the years 2003–2006, 46 million adolescents (ages 12–19) were analyzed. The study population characteristics with univariate analyses are presented in Table 1. The consumption of dAGEs was significantly higher in MetS adolescents than in non-MetS adolescents (33.1% vs. 20.3%, p = ?) even when dAGE was normalized to amount of kilocalories ingested. Those with MetS were more likely to be female (56.7% vs. 52.7%, P<.001), White (72.2 vs. 65.7%, P<.001), followed by Hispanic (18.6 vs. 14.6%, P<.001), and have lower family income (37.9% vs. 33.1% with an income >$65,000). They were more likely to have a daily dAGE score of >8.68 (33.1% vs. 20.3%, p< .001), with median dAGE score 5.1 (interquartile range 3.3–7.0) in participants with MetS vs. 4.85 (interquartile range 4.6–5.1) in participants without MetS. The highest quartile of dAGE was associated with an 83% increase in odds of having MetS (aOR 1.83; 95% CI 1.09–3.42; P=0.01) among adolescents after adjusting for demographic factors like gender, race and family income compared to that of the third quartile dAGE score of 4.99 – 8.68 (aOR 0.71; 95% CI 0.4–1.3; P=0.09) and second quartile dAGE score of 2.74 – 4.98 (aOR 0.83; 95% CI 0.3–2.3; P=0.51) with first quartile dAGE score of ≤2.73 as the reference. (Figure 1A) Additionally, we used survey logistic regression to see the association between the individual criteria of MetS and dAGE quartiles. After adjusting for confounders among adolescents, the highest quartile of dAGE was associated with an 82% increase in odds of having increased waist circumference (greater than 102 cm in men or greater than 88 cm in women) (aOR 1.82; 95% CI 1.16–2.85; P=0.01), 30% increase in odds of having increased serum triglycerides level (150 mg/dL or greater) (aOR 1.30; 95% CI 1.02–2.42; P=0.02) and 6% increase in odds of having low HDL (less than 40 mg/dL in men or less than 50 mg/dL in women) (aOR 1.06; 95% CI 1.01–1.24; P=0.02) whereas there was no statistically significant association between the highest quartile of dAGE and elevated blood pressure (130/85 mm Hg or greater )or taking hypertension medications (aOR 1.24; 95% CI 0.67–1.73; P=0.88) or increased fasting plasma glucose level (100 mg/dL or greater) (aOR 1.58; 95% CI 0.87–2.87; P=0.04). (Figure 1B)
Table 1.
Patient characteristics for adolescents with and without metabolic syndrome
| PATIENT CHARACTERISTICS | Adolescents with Metabolic Syndromea (N=1607634) (%) |
Adolescents without Metabolic Syndromea (N= 45045616) (%) |
P value |
|---|---|---|---|
| dAGE Quartile (U/kcal) | <.0001 | ||
| Median (IQR) | 5.18 (3.32–7.04) | 4.85 (4.59–5.10) | |
| ≤2.73 | 395463 (24.6) | 11423568 (25.36) | |
| 2.74 – 4.98 | 370242 (23.03) | 11959619 (26.55) | |
| 4.99 – 8.68 | 310327 (19.3) | 12509168 (27.77) | |
| >8.68 | 531602 (33.07) | 9153261 (20.32) | |
| Race (%) | <.0001 | ||
| White | 1159894 (72.15) | 29581937 (65.64) | |
| Black | 111733 (6.95) | 6557434 (14.57) | |
| Hispanic | 298768 (18.58) | 6348625 (14.11) | |
| Other | 37239 (2.32) | 2557620 (5.68) | |
| Gender (%) | <.0001 | ||
| Male | 695952 (43.29) | 21315585 (47.32) | |
| Female | 911682 (56.71) | 23730031 (52.68) | |
| Family Income (USD) | <.0001 | ||
| $0 - $34,999 | 593429 (37.93) | 17063279 (37.88) | |
| $35,000 - $44,999 | 114435 (7.31) | 4252306 (9.44) | |
| $45,000 - $64,999 | 339015 (21.67) | 6937024 (15.4) | |
| >$65,000 | 517541 (33.08) | 16793007 (37.28) |
Abbreviation: dAGE, dietary Advanced Glycation End Products
Data are presented as number (percentage) of patients unless otherwise indicated
Figure 1A:
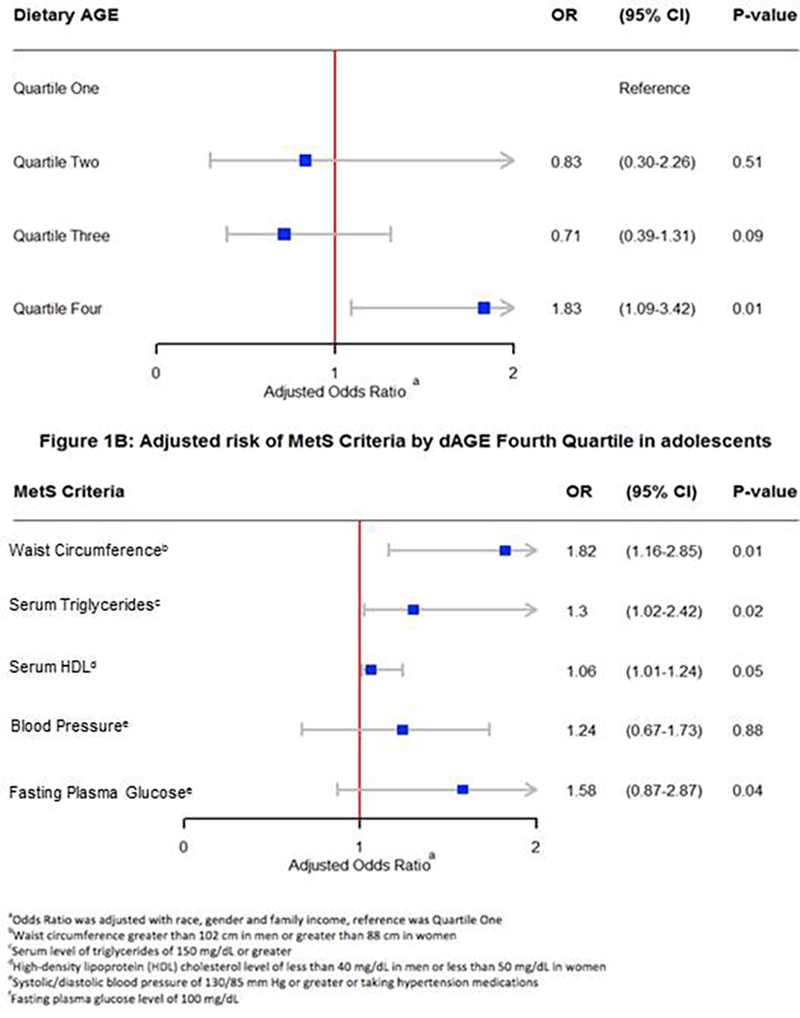
Adjusted risk of MetS by dAGE quartile in a adolescents Figure 1B: Adjusted risk of Criteria by dAGE Fourth quartile in a adolescents
Discussion
In this study, while we report an increased association of a very high dAGE intake (defined as the top quartile) and metabolic syndrome in adolescents. Furthermore, we demonstrate that very high dAGE intake was significantly associated with 3 out of 5 criteria for MetS, waist circumference, serum triglyceride level and HDL cholesterol level.Given the rising prevalence of MetS in adolescents, it is important to identify modifiable risk factors for MetS. A recent randomized controlled trial has shown improvement in metabolic parameters in participants randomized to a low AGE diet.7 Temperature and methods of cooking seem to be more critical to AGE formation than cooking time.8 Habitual preference of foods such as full-fat cheeses, meats, and highly processed foods in excess results in increased dAGE consumption, and therefore increasing the intake of fish, grains, low-fat milk products, fruits, and vegetables reduces consumption of dAGEs.9
We have found that those with MetS were more likely to be female, however other studies have reported that the prevalence is higher in boys than in girls (6.1% vs. 2.1%).2 This difference may be due to the exclusion of patients on pre-specified prescription medications. The differences in race is well established and likely related to genetic factors as demonstrated by family studies on variance in intra-abdominal fat.10 Although the NHANES has a large sample size and is a nationally representative sample of the US population allowing for power and true national generalizability, the limitations of the study include cross-sectional nature of the survey which precludes causal inference, possible bias in answering dietary questions and recall bias of the participants which could result in misclassification of exposure. Additionally, no information about the methods and duration of food preparation, which has a strong correlation with dAGE contents of food, was provided.
In conclusion, given that the daily AGE consumption in diet is an important predictor of MetS, educating adolescents and guardians about restricting dAGE intake in their daily diet and providing information on the methods of cooking to minimize dAGEs may be a simple but important public health method to reduce the rising prevalence of MetS in adolescents and thereby reduce future metabolic and cardiovascular disease. Moreover, a potential benefit of this knowledge is that it will enable adolescents to reduce a previously unrecognized dietary risk factor that contributes to MetS by reducing dAGE consumption associated with highly processed food and food prepared at higher temperatures under dry heat and for longer duration.
Footnotes
Disclosure of interest
The authors declare that they have no competing interest.
References:
- 1.Johnson WD, Kroon JJM, Greenway FL, Bouchard C, Ryan D, Katzmarzyk PT. Prevalence of Risk Factors for Metabolic Syndrome in Adolescents: National Health and Nutrition Examination Survey (NHANES), 2001–2006. Arch Pediatr Adolesc Med. 2009;163(4):371–377. [DOI] [PubMed] [Google Scholar]
- 2.Hadjiyannakis S The metabolic syndrome in children and adolescents. Paediatr Child Health. 2005;10(1):41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angoorani P, Ejtahed H-S, Mirmiran P, Mirzaei S, Azizi F. Dietary consumption of advanced glycation end products and risk of metabolic syndrome. Int J Food Sci Nutr. 2016;67(2):170–176. [DOI] [PubMed] [Google Scholar]
- 4.Henle T Protein-bound advanced glycation endproducts (AGEs) as bioactive amino acid derivatives in foods. Amino Acids. 2005;29(4):313–322. [DOI] [PubMed] [Google Scholar]
- 5.Uribarri J, Woodruff S, Goodman S, et al. Advanced Glycation End Products in Foods and a Practical Guide to Their Reduction in the Diet. J Am Diet Assoc. 2010;110(6):911–916. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A, Uribarri J. Dietary Advanced Glycation End Products and Their Potential Role in Cardiometabolic Disease in Children. Horm Res Paediatr. 2016;85(5):291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlassara H, Cai W, Tripp E, et al. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: a randomised controlled trial. Diabetologia. 2016;59(10):2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg T, Cai W, Peppa M, et al. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. 2004;104(8):1287–1291. [DOI] [PubMed] [Google Scholar]
- 9.Appel LJ, Moore TJ, Obarzanek E, et al. A Clinical Trial of the Effects of Dietary Patterns on Blood Pressure. N Engl J Med. 1997;336(16):1117–1124. [DOI] [PubMed] [Google Scholar]
- 10.BOUCHARD C, Despres J-P, MAURIÈGE P. Genetic and nongenetic determinants of regional fat distribution. Endocr Rev. 1993;14(1):72–93. [DOI] [PubMed] [Google Scholar]


