Abstract
Psoriasis is a chronic immune-mediated disease that represents a unique model for investigating inflammation at local and systemic levels. Bioactive lipid mediators (LMs) are potent compounds reported to play a role in the development and resolution of inflammation. Currently, it is not known to what extent these LMs are involved in psoriasis pathophysiology and related metabolic dysfunction. Here, we use targeted and untargeted liquid chromatography-tandem mass spectrometry approaches to quantify LMs in skin and peripheral blood from psoriasis patients and compared them with those of healthy individuals. Lesional psoriasis skin was abundant in arachidonic acid metabolites, as 8-, 12- and 15-hydroxyeicosatetraenoic acid, compared with adjacent nonlesional and skin from healthy individuals. Additionally, a linoleic acid-derived LM, 13-hydroxyoctadecadienoic acid, was significantly increased compared with healthy skin (607.9 ng/g vs. 5.4 ng/g, P = 0.001). These psoriasis skin differences were accompanied by plasma decreases in antioxidant markers, including glutathione, and impaired lipolysis characterized by lower concentrations of primary and secondary bile acids. In conclusion, our study shows that psoriasis skin and blood have disease-specific phenotype profiles of bioactive LMs represented by omega-6 fatty acid–oxidized derivatives. These findings provide insights into psoriasis pathophysiology that could potentially contribute to new biomarkers and therapeutics.
INTRODUCTION
Psoriasis comprises a group of immune-mediated inflammatory skin diseases that are closely correlated with metabolic and cardiovascular comorbidities (Ryan and Kirby, 2015). Psoriasis pathogenesis involves disbalance in effective anti-inflammatory and immune responses regulated by complex multisystemic interactions. However, the molecular basis for these biochemical abnormalities are poorly understood.
Omega-6 (or n-6) and omega-3 (or n-3) polyunsaturated fatty acids (PUFAs) are the main source of bioactive LMs stored in the skin. Linoleic (LA) and arachidonic (AA) acids are the most abundant omega-6 PUFAs in human epidermis (McCusker and Grant-Kels, 2010). Oxidized LMs derived from LA (known as OXLAMs) and AA (known as eicosanoids) have numerous inflammation-related functions (Strassburg et al., 2012). In turn, the enzymatic conversion of eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids by omega-3 PUFAs leads to the synthesis of a potent class of mediators known as specialized pro-resolving LMs (Zhang and Spite, 2012), which exert anti-inflammatory and immunomodulating effects (Serhan, 2007).
So far, there are no clinical trials investigating the bioactive lipid profile of psoriasis skin and peripheral blood from the same group of patients. Previous studies have identified a limited number of LMs in psoriatic skin (Camp et al., 1988; Hammarstrom et al., 1975) and peripheral blood (Corrocher et al., 1989) for eicosanoid presence, but comprehensive analysis has been completed only for healthy human skin (Kendall et al., 2015). Omega-3 and omega-6 PUFAs cannot be synthesized de novo by humans; therefore, the abundance of these omega-6/3 fatty acids and related metabolites in psoriatic skin is likely to be regulated by diet. Various clinical approaches have been used to determine whether changing the omega-6/omega-3 ratio by diet or pharmacological interventions might be beneficial in individuals with inflammation-associated conditions (Barden et al., 2016; Ramsden et al., 2012), as well as in healthy individuals (Markworth et al., 2016). Hence, we hypothesized that imbalance in these mediators could contribute to psoriasis pathogenesis.
However, some of these lipid autacoids, though extremely potent, are present only in minute concentrations (fmol/L–pmol/L range), which demands a highly sensitive diagnostic technique. Skin lipidomic analysis is suggested as being a fast and reliable tool for estimating and monitoring the progression of inflammatory skin diseases (Li et al., 2016). For this reason, we used LM metabololipidomics using highly sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) to identify bioactive LMs in human skin and peripheral blood of psoriasis patients and healthy individuals.
RESULTS
Study population
Demographic and clinical characteristics of the psoriasis (n = 60) and corresponding healthy (n = 30) study populations enrolled for plasma analysis are presented in Supplementary Table S1 online. Participants were white males without known history of tobacco or alcohol use and a mean age of 48 years.
A separate set of participants had paired lesional and nonlesional skin (n = 8), along with serum analysis, compared with corresponding healthy volunteers (n = 7) (see Supplementary Table S2 online). Recruitment and study design were based on the ongoing prospective observational study (Naik et al., 2015) (see Supplementary Materials online).
Metabolomic profiling of psoriatic and healthy plasma
Besides clinically evident local skin inflammation, psoriatic disease has a significant systemic inflammatory component, which was investigated by profiling circulating mediators attributed to inflammatory and metabolic dysfunction. To address this task, ultra-high performance LC-MS/MS combined with gas chromatography/MS was used. LC-MS/MS–based metabololipidomics was performed on plasma from 60 psoriasis patients and 30 corresponding healthy individuals. A total of 557 compounds of known identity were quantified, with 98 up-regulated and 52 down-regulated metabolites showing a level of significance less than or equal to 0.05. A complete list of metabolites identified in human plasma is available in the Supplementary Materials online.
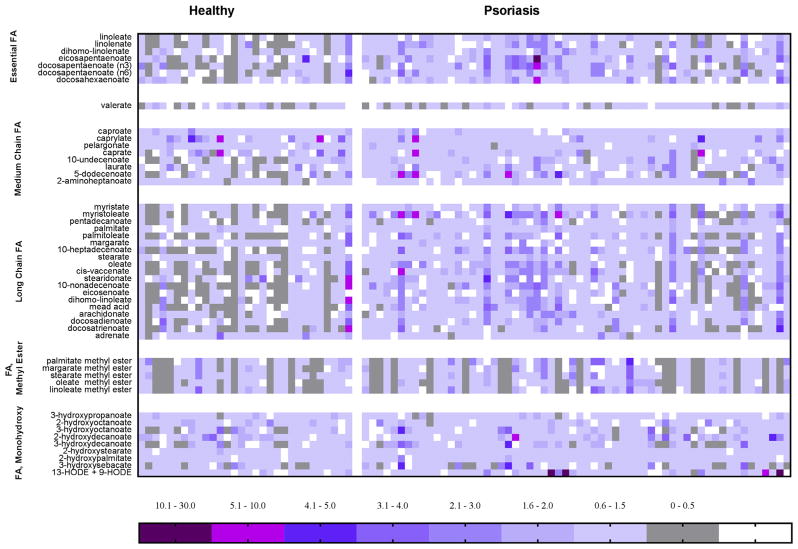
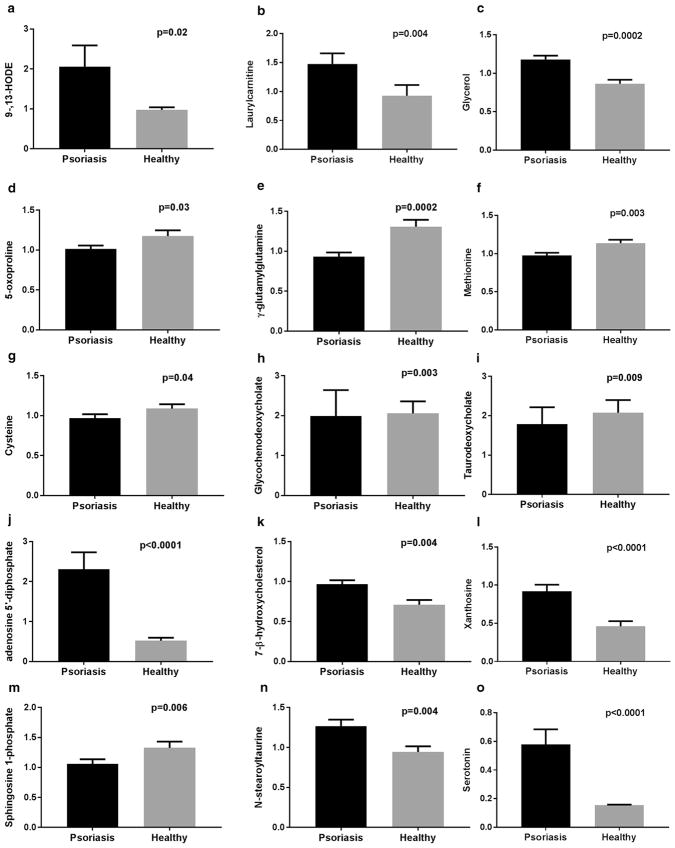
Higher presence of fatty acid monohydroxy and methyl ester forms was accompanied by medium-chain and long-chain fatty acids (Figure 1). One striking and consistent observation was a higher abundance of omega-3/6 PUFAs in plasma from patients with psoriasis. These differences were accompanied by a significant 2-fold increase in LA hydroxy fatty acids (9- and 13-hydroxyoctadecadienoic acid [HODE]) in psoriasis patients (Figure 2). AA-derived LMs, such as LTB4, PGE2, and 5-hydroxyeicosatetraenoic acid (HETE) were not significantly different between compared groups. These changes were accompanied by 1.4-fold (P < 0.01) elevation in glycerol and several acylcarnitines. Consequently, the key intracellular antioxidant markers of glutathione and γ-glutamyl were significantly decreased in psoriasis plasma. Because the production of glutathione and its pathway markers depends on methyl group donors, we expected to see a reduction in methionine and cysteine in psoriasis patients (Figure 2).
Figure 1. Heatmap of plasma biochemicals identified in psoriasis patients.
Heatmap represents plasma levels of different fatty acids based on the metabolomics scan of 30 healthy individuals and 60 psoriasis patients. Color plot is based on the mean levels of the metabolite within the studied cohort and described as scaled intensity.
Figure 2. Representative biochemical profile of psoriatic and healthy plasma.
Fold change of key metabolites, (a) 9- and 13-HODE, (b) lauroylcarnitine, (c) glycerol, (d) 5-oxoproline, (e) γ-glutamylglutamine, (f) methionine, (g) cysteine, (h) glycochenodeoxycholate, (i) taurodeoxycholate, (j) adenosine 5′-diphosphate, (k) 7-β-hydroxycholesterol, (l) xanthosine, (m) sphingosine-1-phosphate, (n) N-stearoyl taurine, and (o) serotonin, were identified after ultra-high performance liquid chromatography and tandem mass spectrometry combined with gas chromatography/mass spectrometry (see Materials and Methods section for details). Values represent the mean ± standard error of the mean. n = 60 for the psoriasis group, n = 30 for the healthy group. P-values were derived from the Welch t test. P < 0.05 was considered statistically significant.
Also, a dysfunctional bile acid profile in psoriasis patients was characterized by lower concentrations of the conjugated primary bile acids, glycocholate and glycochenodeoxycholate, along with such secondary bile acids as taurodeoxycholate and glycodeoxycholate (Figure 2). These changes contributed to the significant elevation in plasma cholesterol precursors, such as lathosterol and oxidized derivative 7-β-hydroxycholesterol, in the psoriasis group.
Consistent elevations in metabolites related to purine and pyrimidine nucleotide metabolism, such as adenosine 5′-diphosphate, was 4.5-fold (P < 0.01) higher in the psoriasis group (Figure 2). Also, the purine degradation (xanthosine) and the pyrimidine degradation products (5,6-dihydrothymine) were significantly higher in patients with psoriasis.
Some additional representatives of the endocannabinoid and sphingolipid metabolite classes were altered in patients with psoriasis (Figure 2). Excessive nucleotide accumulation and altered phospholipase activity might be responsible for the observed 1.3-fold (P < 0.05) N-stearoyl taurine and N-palmitoyl taurine increase in psoriasis plasma. This observation was also accompanied by accumulation of sphinganine, palmitoyl sphingomyelin, and stearoyl sphingomyelin, with a sphingosine 1-phosphate decrease in the psoriasis group. In addition, the 3.9-fold increase in serotonin level may be of particular interest because expression of its transporter protein, SERT, is altered in psoriatic lesions, which may contribute to inflammatory processes in the skin (Thorslund et al., 2013).
Identification of oxidized LMs in human psoriatic and healthy skin
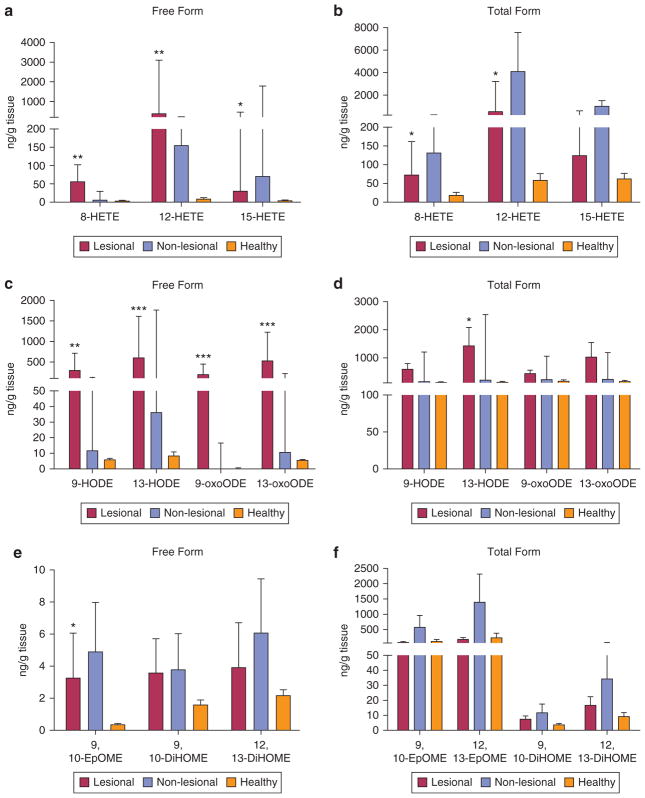
Considering the observed differences in omega-3/6 PUFA metabolism in psoriasis plasma compared with that of healthy volunteers, we hypothesized that psoriatic skin lesions and psoriatic nonlesional skin would have altered LM profiles. Identification and quantification of the reported mediators were done as free and total (sum of free and esterified) forms, as described in the Materials and Methods section (Figure 3).
Figure 3. Representative biochemical profile of psoriatic and healthy skin.
Lipid mediators, (a, c, e) free forms and (b, d, f) total (sum of free and esterified), derived from AA and LA were identified by liquid chromatography and tandem mass spectrometry (see Materials and Methods section for details). Results are expressed as ng/g tissue. Values represent the median (interquartile range). n = 8 for the psoriasis group, n = 7 for the healthy group. P-values were derived from the Mann-Whitney test. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 when comparing lesional with healthy skin data. DiHOME, dihydroxyoctadecenoate; EpOME, epoxyoctadecamonoenoic acid; HETE, hydroxyeicosatetraenoic acid; HODE, hydroxyoctadecadienoic acid; oxoODE, oxooctadecadienoic acid.
The most abundant oxylipins detected in the skin of psoriasis patients were monohydroxy derivatives from AA (5-, 8-, 9-, 11-, 12-, and 15-HETE) and LA (9- and 13-HODE) (see Supplementary Tables S3 and S4 online). All of these lipoxygenase products were abundant in lesional psoriasis skin, with distinct changes related to 8-HETE, 12-HETE, and 15-HETE monohydroxy fatty acids. Free 15-HETE levels were significantly higher in lesional skin compared with that of healthy participants (31.1 ng/g vs. 3.1 ng/g, respectively; P = 0.02) but relatively lower compared with nonlesional skin (31.1 ng/g vs. 71.7 ng/g, respectively; P = 0.34). In contrast, free 8- and 12-HETE levels were much higher in lesional psoriasis skin compared with healthy skin (395.0 ng/g vs. 3.1 ng/g, respectively; P = 0.004). Among measured oxylipins, differences in prostaglandins were related only to TXB2, the free form of which had higher levels in nonlesional skin of psoriasis participants compared with lesional skin (3.5 ng/g vs. 1.5 ng/g, respectively; P = 0.01) (see Supplementary Tables S3 and S4). Furthermore, a different pattern of changes was observed in relation to epoxide eicosatrienoic acid (EET) metabolites of AA. Total 5,6-EET levels were nonsignificantly higher in lesional psoriasis skin compared with nonlesional and healthy skin (see Supplementary Table S4). Another proinflammatory oxidized product of 5-HETE, 5-oxoETE, has been identified only in the total form, with slightly higher presence in lesional psoriasis skin.
LA-derived lipid autacoids were represented by several OXLAMs with proinflammatory effects. Significant accumulation of both the free and total forms of LA-derived HODEs was observed in lesional psoriasis skin (see Supplementary Tables S3 and S4). Specifically, 13-HODE levels were 2-fold higher than 9-HODE levels in the lesional skin and 113-fold higher compared with the free form in healthy skin (607.9 ng/g vs. 5.4 ng/g, respectively; P = 0.001) (see Supplementary Table S3). The specified mediators also differed between nonlesional and healthy skin. Epoxide and dihydroxy products of LA were represented by 9,10- and 12,13-epoxyoctadecamonoenoic acid and 9,10- and 12,13-dihydroxyoctadecenoate (i.e., DiHOME), the free and total forms of which were increased in both lesional and nonlesional skin. Oxidized forms of 9- and 13-HODE and 9- and 13-oxooctadecadienoic acid differed in the same pattern. (see Supplementary Table S3).
Omega-3 fatty acid metabolomes represented 34.9% of the completed LM profile and included analytes derived from α-LA, DHA, and EPA. Free 9-hydroxyoctadecatrienoic acid had an increased trend in psoriasis skin, whereas 13-hydroxyoctadecatrienoic acid did not differ significantly between the analyzed groups (see Supplementary Tables S3 and S4). The DHA pathway marker levels, such as 7-, 14-, 17-hydroxydocosahexaenoic acid (HDHA), were higher in psoriasis lesional skin compared with nonlesional and healthy skin, with predominant accumulation of total 14-HDHA (40.8 ng/g vs. 5.4 ng/g, P = 0.03) (see Supplementary Table S4). We found a higher detection rate of epoxydocosapentaenoic acid isomers, such as total forms of 16,17-epoxydocosapentaenoic acid and 19,20-epoxydocosapentaenoic acid, accumulated in psoriasis skin. The EPA-derived epoxide isomers, 14,15-epoxyeicosatetraenoic acid and 17,18- epoxyeicosatetraenoic acid, were mostly undetectable among all the groups (see Supplementary Tables S3 and S4).
Identification of oxylipins in psoriatic and healthy serum
Next, we investigated whether LMs identified in serum are representative of oxidized markers detected in psoriasis skin and plasma. In general, most of the omega-3/6 PUFA–derived metabolites found in the skin were also present in serum. However, unlike skin, LM concentrations were significantly different between psoriasis patients and corresponding healthy volunteers (see Supplementary Table S5 online). For example, oxidized AA hydroxy fatty acids, such as 11-, 12-, 15-HETE, were among the only LMs that changed significantly; however, these LMs were elevated in serum of healthy volunteers compared with psoriasis patients. Additionally, we identified the DHA-derived pro-resolving LM RvD2 (resolvin) in half of the psoriasis and healthy individuals. Overall, differences in each identified metabolite among groups were less significant in peripheral blood than reported for skin biospecimens, indicating that serum alone is not a reliable biomarker for the skin LM profile.
DISCUSSION
Psoriasis is a complex immune-mediated inflammatory disease. By applying targeted LM metabololipidomics, we identified a psoriasis-associated metabolomic profile with elevated levels of oxidized derivatives from LA and AA in psoriasis lesional skin and peripheral blood.
Skin changes
The omega-6 metabolome included LA- and AA-derived metabolites, which play an essential role in the epidermal barrier protective function (McCusker and Grant-Kels, 2010). LA and AA metabolites previously have been reported to be highly concentrated in healthy skin (Ziboh et al., 2000) and elevated in inflamed tissue samples (Wu et al., 2012). Specifically, these omega-6 PUFAs are metabolized by lipoxygenases (LOXs) 5-LOX, 12-LOX, and 15-LOX to generate hydroxy fatty acids such as 9- and 13-HODE, which were significantly elevated in psoriasis lesional skin and plasma, with a higher ratio of 13-HODE in free forms compared with the total pool (Figure 4). These oxidized LMs have heterogeneous biological effects in the skin epidermis. In particular, 13-HODE is considered to be anti-inflammatory (Miller and Ziboh, 1990) and maintains normal keratinocyte proliferation (Ogawa et al., 2011), whereas 9-HODE facilitates the release of different inflammatory cytokines (Hattori et al., 2008) and exhibits pro-atherogenic properties (Jira et al., 1998). Presumably, the amount of 13-HODE generated by the rapidly proliferating psoriatic epidermis is insufficient to suppress the hyperproliferation of psoriatic lesions. Moreover, oxidized forms of these LMs, 9- and 13-oxooctadecadienoic acid, were significantly elevated in psoriasis skin with the same predominant presence of 13-oxooctadecadienoic acid.
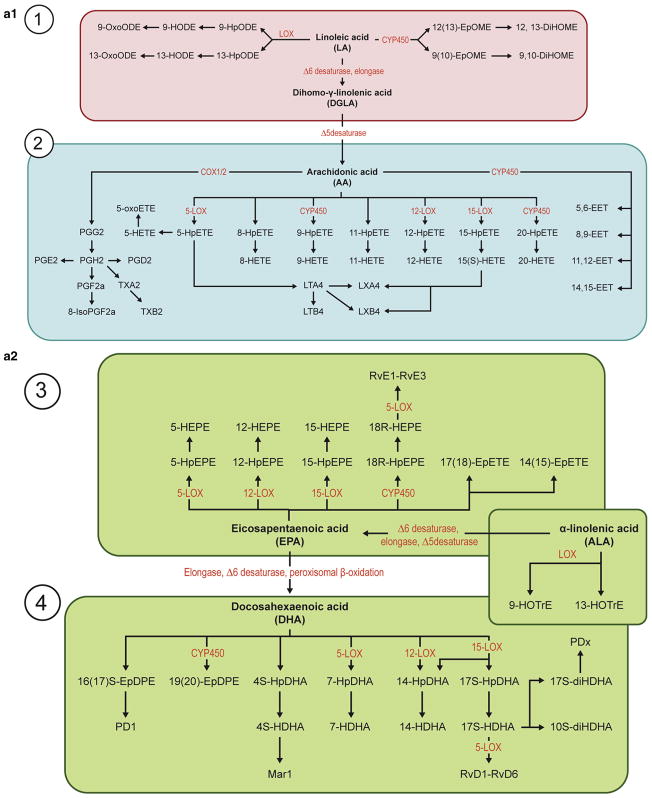
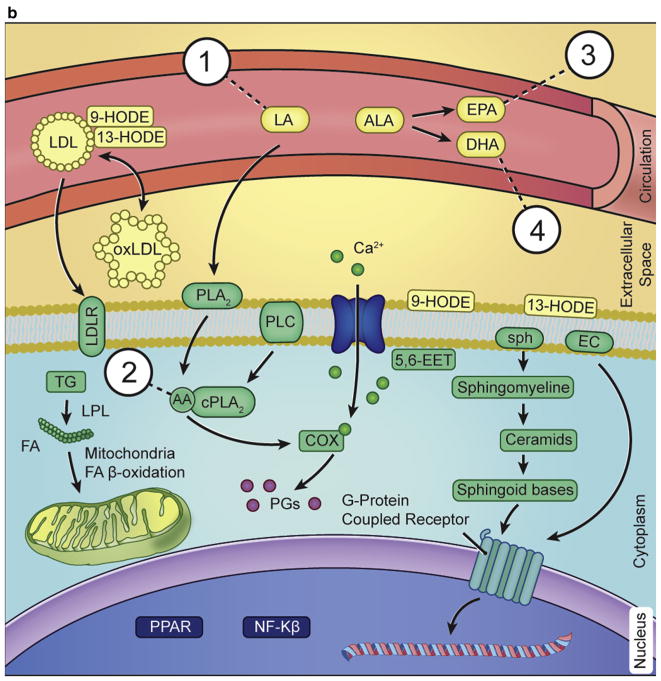
Figure 4. Explanatory outline of oxylipin biosynthesis and its estimated function in psoriasis.
Cutaneous fatty acids and bioactive lipid mediators contribute to the formation and maintenance of the epidermal barrier. These have been implicated in various inflammatory skin conditions, including psoriasis. Pathways involving lipid mediators in maintaining normal homeostasis in the skin and alteration in fatty acid composition and metabolism in psoriatic skin are shown. LA and ALA are the main sources of omega-6 and omega-3 fatty acids, respectively, which in humans are derived from the diet. Thus, dietary imbalance in polyunsaturated fatty acids (PUFAs) leads to an alteration in the metabolism of their derivatives, AA, EPA, and DHA. Furthermore, the metabolism of PUFA is a complex process involving several enzymes of desaturation, elongation, and β-oxidation, resulting in production of respective bioactive derivatives, as shown in section a1 and a2. (b) In membrane phospholipids, LA gives rise to AA, which in turn leads to an increase in COX and LOX enzyme production of AA-derived eicosanoids and a decrease in EPA/DHA-derived metabolites. This process culminates in class switching of lipid mediators, finally leading to an increase in proinflammatory cytokines and a decrease in pro-resolution lipid mediators, specialized pro-resolving mediators. As a consequence, PPARα gene expression is down-regulated, and NF-κβ gene expression is up-regulated, manifesting in corresponding protein synthesis. Psoriasis modulates eicosanoid synthesis, which is important in the formation of the lipid mediators that include specialized pro-resolving mediators, resolvins, and lipoxins. The rapidly proliferating psoriasis epidermis generates an excessive amount of ambiguously functioning mediators such as hydroxy fatty acids, proinflammatory 9-HODE and anti-inflammatory 13-HODE, which can be taken up by the circulating LDL. Additional uptake of HODEs from the skin, along with circulating LA, further increases oxidation and formation of oxidized LDL, which promotes atherogenesis. Other potential pathways of interest include those of sphingolipids and endocannabinoids metabolism, which also are modulated in psoriasis. AA, arachidonic acid; ALA, α-linolenic acid; COX, cyclooxygenase; CYP450, cytochrome P450; DHA, docosahexaenoic acid; DiHOME, dihydroxyoctadecenoate; EC, endocannabinoid; EDP, epoxydocosapentaenoic acid; EET, epoxide eicosatrienoic acid; EPA, eicosapentaenoic acid; EpETE, epoxyeicosatetraenoic acid; EpOME, epoxyoctadecamonoenoic acid; FA, fatty acid; HDHA, hydroxydocosahexaenoic acid; HEPE, hydroxyeicosapentaenoic acid; HETE, hydroxyeicosatetraenoic acid; HODE, hydroxyoctadecadienoic acid; HpETE, hydroperoxyeicosatetraenoic acid; LA, linoleic acid; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; LOP, lipoprotein lipase; LOX, lipoxygenase; LT, leukotriene; Mar, maresin; oxLDL, oxidized low-density lipoprotein; oxoODE, oxooctadecadienoic acid; PD/PDx, protectin; PG, prostaglandin; PLA2, phospholipase A2; PLC, phospholipase C; PPAR, peroxisome proliferator-activated receptor; Rv, resolvin; SPH, sphingolipid; TG, triglyceride.
Accumulation of AA pathway markers in lesional psoriasis skin is also in concordance with previous studies that reported different distributions of lipoxygenase products. Specifically, 12-HETE and 15-HETE are differently presented in skin layers, and this interaction might be compromised because of inflammation (Krieg and Furstenberger, 2014). 12-HETE is a potent chemoattractant with pro-inflammatory function (Camp et al., 1988), whereas 15-HETE can reduce inflammatory cell infiltration (Kragballe et al., 1986). Normal epidermis has a very active 12-LOX, which might explain the high presence of this mediator in lesional psoriasis skin as well (Dowd et al., 1987). The source of 15-HETE in psoriasis skin is not only from epidermis and infiltrating neutrophils, as for 12-HETE, but also from dermis. Dermis from nonlesional psoriasis skin produces less 15-HETE than normal dermis in vitro (Kragballe et al., 1986). Lesional skin from our psoriasis cohort was abundant in both monohydroxy fatty acids, with higher presence of 12-HETE. Prostaglandins, lipoxins, and leukotrienes previously reported in human skin (Kendall et al., 2015) were below the limit of quantitation based on our LC-MS/MS methods.
Further oxidation of LA and AA metabolites results in production of corresponding epoxide forms, such as LA-derived epoxyoctadecamonoenoic acid and EET from AA. Although the roles of these oxidized lipids in skin are incompletely understood, there are some reports showing their relevance to inflammatory responses (Spector et al., 2004). Epoxides of EET and keto acid of 5-HETE were present in all the investigated groups skin and serum, with a higher concentration of total form epoxides in lesional psoriasis skin. It has been shown that in the endothelium, EETs inhibit the cytokine-induced inflammatory response, increase Ca2+ uptake, and affect PGE2 production (Spector et al., 2004). Thus, the accumulation of total 5,6-EET in lesional skin may suggest a protective role in local skin inflammation. There is insufficient information about the levels of 5-oxo-ETE in body fluids or tissues, but some studies have suggested that this metabolite could possibly promote neutrophil infiltration and inflammation (Grant et al., 2009).
The omega-3 metabolome in psoriasis patients predominantly included DHA oxidized products, such as 14-HDHA and 17-HDHA. It is known that 17-HDHA is a pathway marker and precursor to D-series resolvins and protectins, which are involved in the resolution of inflammation and modulate innate and adaptive immunity (Chiurchiu et al., 2016; Serhan and Petasis, 2011). We did not measure a complete EPA and α-LA bioactive metabolome because of previous studies and unpublished data that suggested only minimal involvement of EPA and α-LA autacoid derivatives in healthy and inflamed skin biology (Kendall et al., 2015). These observations suggest that DHA-derived specialized pro-resolving mediators may play a role in psoriasis pathology. However, studies investigating the full DHA metabolome are warranted.
Blood changes
Circulating LA and HODE are esterified in cholesterol ester, triacylglycerol, and phospholipid components of lipoproteins, the main transportation of which in circulation is conducted through low-density lipoprotein (LDL) (Lrp1) and scavenger receptors (Jira et al., 1998). It is well known that the core of the LDL particle represents an assembly of LA esters of cholesterol, embedded in a monomolecular layer of phospholipids and a protein (apoB), with oxidized LDL expected to consist of OXLAMs (Spiteller, 1998). Although studies investigating oxidized lipids in psoriasis patients are scarce, oxidized LDL has been shown to accumulate in psoriasis lesional skin (Tekin et al., 2007), along with Lrp1 and apoB protein level increases (Swindell et al., 2015). However, diet supplementation with the intermediary long-chain omega-3 docosapentaenoic acid and EPA reverse accumulation of 9- and 13-HODE and its oxidized products (Markworth et al., 2016), which could be a potential treatment strategy in the future.
The transportation and metabolism of these omega-6/3 long-chain PUFAs are generally regulated by adequate lipolysis rate and efficient β-oxidation that can be altered in specific disease states. Thus, markers of this system dysregulation, including glycerol, acylcarnitine, and bile acid derivatives, can be found during diseases with inflammatory and metabolic origins (Fujieda et al., 2013; Schooneman et al., 2013). It is well known that secreted phospholipase A2 catalyzes the hydrolysis of glycerophospholipids at the sn-2 position, releasing glycerol and free fatty acids with a related protein expression increase in psoriasis lesional skin (Swindell et al., 2015). In humans, higher acylcarnitine efflux from cells to blood is determined by impaired conjugation of free fatty acids with carnitine, which is necessary for transport of these lipids into the mitochondrial matrix for subsequent β-oxidation (Schooneman et al., 2013). We found elevations in circulating glycerol and acylcarnitine concentrations associated with psoriasis, which might be explained by higher free fatty acid release from cell storage and its impaired oxidation. Dysfunction of the antioxidant system has been also confirmed by our observation that key intracellular antioxidant markers (glutathione and γ-glutamyl) were decreased in psoriasis plasma. Subsequent deterioration of fatty acid transportation and its cell bioprocessing was observed in markers of bile acid malfunction. Reduction in circulating primary and secondary bile acids may be a result of multiple factors, including decreased intestinal reabsorption and reduced hepatic synthesis/conjugation. The observed changes in cholesterol precursors could be attributed to the reported cholesterol metabolism dysfunction associated with psoriasis (Mehta et al., 2012).
Besides the mentioned lipid classes, PUFAs can be endogenously converted to additional species of bioactive lipids, including lysolipid phosphate mediators and endocannabinoids (Kendall et al., 2015). Lysophosphatidic acid, sphingosine-1-phosphate, and several other related molecules constitute a family of lysolipid phosphates that function as receptor-active mediators with complex roles in cell growth, differentiation, apoptosis, and development (Tokumura, 2004). Sphingosine-1-phosphate is a pleiotropic bioactive lipid that is thought to be dysregulated in a variety of disease conditions. It has been reported that reduced sphingosine-1-phosphate levels, caused by excessive sphingosine-1-phosphate lyase activity, might be associated with the keratinocyte hyperproliferation observed in psoriasis (Kim et al., 2004; Checa et al., 2015).
The higher accumulation of certain circulating endocannabinoids detected in our psoriasis cohort might exert anti-inflammatory effects through acting as endogenous ligands for the cannabinoid receptors (Zurier and Burstein, 2016). Although the role of the endocannabinoid system in psoriasis is incompletely understood, it has been shown that some classes of these lipids can impair keratinocyte differentiation and induce apoptosis (Wilkinson and Williamson, 2007).
To our knowledge, this is the first comprehensive LC-MS/MS–based analysis of bioactive lipid autacoids in human psoriasis skin and peripheral blood compared with healthy volunteers. However, we acknowledge that the striking global differences in fatty acids and LMs could be a result of other factors and remain difficult to interpret further. Thus, studies with larger sample sizes and in vitro experiments are warranted to provide mechanistic evidence for the observed changes. We found that a disbalance in oxylipins and anti-oxidants resulted in targeted analytes significantly accumulating in lesional psoriasis skin. This study shows that psoriasis skin and peripheral blood are characterized by the increased abundance of oxidized LMs derived from omega-6 fatty acids. Of note, distribution of targeted LMs was different among skin and blood and did not depend on given systemic treatment. A possible explanation for this discrepancy is the specific bioactivity of any given LM in relation to cell and tissue demand. It is known that some classes of LMs are mostly secreted to circulation, whereas other are deposited in the skin. Thus, quantitation of both free and total lipid moieties in tissue and circulation provides comprehensive oxylipin signature profiles related to psoriasis. Moreover, increased lipolysis and β-oxidation observed in psoriasis patients might be explained by higher skin tissue demand in bioactive LMs to resolve inflammation.
In conclusion, our collective findings suggest that locally produced and circulating specific LMs have a role in modulating skin inflammation. These results identify specific metabolic abnormalities associated with psoriasis, which could potentially be used to develop targeted approaches.
MATERIALS AND METHODS
Study population
Plasma for the LM identification was derived from a consecutive sample of 90 participants: 60 psoriasis patients and 30 age- and sex-matched healthy volunteers (age range = 19–72 years) (see Supplementary Tables S1 and S2). All enrolled participants were part of a National Institutes of Health clinical study (10H-0126 and 13H-0065). A diagnosis of psoriasis was confirmed and quantified by a dermatologist using the Psoriasis Area Severity Index. Corresponding healthy volunteers were consecutively recruited from the same center to undergo the same testing as the psoriasis patients.
Skin and serum analysis have been performed on eight psoriasis patients and seven healthy volunteers (age range = 26–82 years) from the ongoing National Institutes of Health observational study of psoriasis and cardiometabolic diseases (see Supplementary Materials). All included study participants were not receiving any systemic anti-psoriasis treatments or topical therapy within 2 weeks before biopsy. Supplementation with omega-3 fatty acids was also considered in the final data interpretation. At baseline, 4-mm punch biopsy samples were obtained under local anesthesia from psoriatic acute plaque lesions and unaffected skin. Biopsy sites were selected based on active plaques and varied among study participants. However, biopsy samples of unaffected and healthy skin were predominantly from buttocks. Financial compensation was provided to study participants. Study approval was granted by the National Heart, Lung, and Blood Institute institutional review board in keeping with the Declaration of Helsinki. All study participants submitted written informed consent before enrollment.
Blood collection
Peripheral blood from the same study-enrolled participants was collected in either serum separator or EDTA tubes and centrifuged at 1,000g for 20 minutes. Obtained serum and plasma were immediately stored at −80°C until analysis. Different blood fractions have been analyzed because the presence of targeted metabolites in these two compartments is unknown.
LC-MS/MS–based lipidomics of psoriatic skin and serum
Punch biopsy samples were extracted using ice-cold methanol. A known amount of internal standard was added to each sample. To quantify concentrations of LMs in skin and plasma, lipid extracts were purified using solid-phase extraction and quantified using LC-MS/MS. The mass spectrometer was operated in electrospray negative ionization using scheduled multiple reaction monitoring, acquiring multiple reaction monitoring data for each analyte with the retention time window of 90 seconds. The analytes were quantified using multiple reaction monitoring. Detailed description of the experimental protocol is provided in the Supplementary Materials.
Metabolomic profiling of human psoriatic plasma
Samples were extracted and prepared for analysis using Metabolon’s (Research Triangle Park, NC) standard solvent extraction method. The extracted samples were split into equal parts for analysis on the gas chromatography/mass spectrometry and LC-MS/MS platforms, as previously described (Cheng et al., 2012). Identification of known chemical entities was based on comparison with metabolomic library entries of more than 2,000 commercially available purified standards. Additional presently unknown entities were identified by virtue of their recurrent nature. Detailed description of the experimental protocol is provided in the Supplementary Materials.
Statistical analysis
Statistical analyses were done with a single two-tailed unpaired Student t test for parametric variables and the Mann-Whitney t test for nonparametric variables. The Pearson chi-square test was used for categorical variables. For pairwise Metabolon data comparisons, the Welch t test was applied, along with the false discovery rate (Q-value) adjustment for considering multiple comparisons (see Supplementary Materials). Analysis was performed using Stata/IC 12.0 (StataCorp, College Station, TX). P-value of 0.05 or less was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Justin Rodante and the clinical staff of the National Heart, Lung, and Blood Institute Section of Inflammation and Cardiometabolic Diseases for obtaining clinical data. This work was supported by the Intramural research funding of the National Heart, Lung, and Blood Institute and Z01-HL06193-3 grant to NNM. Studies in the CER laboratory reported here were supported by the Intramural programs of the National Institute on Aging and National Institute on Alcohol Abuse and Alcoholism.
Abbreviations
- AA
arachidonic acid
- DHA
docosahexaenoic acid
- EET
epoxide eicosatrienoic acid
- EPA
eicosapentaenoic acid
- HDHA
hydroxydocosahexaenoic acid
- HETE
hydroxyeicosatetraenoic acid
- HODE
hydroxyoctadecadienoic acid
- LA
linoleic acid
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- LDL
low-density lipoprotein
- LM
lipid mediator
- LOX
lipoxygenase
- OXLAM
oxidized linoleic acid metabolite
- PUFA
polyunsaturated fatty acid
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest. NNM is a full-time US Government employee and receives research grants to the National Heart, Lung, and Blood Institute from AbbVie, Janssen, Celgene, and Novartis.
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2018.02.003.
References
- Barden AE, Moghaddami M, Mas E, Phillips M, Cleland LG, Mori TA. Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot Essent Fatty Acids. 2016;107:24–9. doi: 10.1016/j.plefa.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Camp RD, Cunningham FM, Fincham NJ, Greaves MW, Kobza Black A, Mallet AI, et al. Psoriatic skin lesions contain a novel lipid neutrophil chemokinetic compound which is distinct from known chemoattractant eicosanoids. Br J Pharmacol. 1988;94:1043–50. doi: 10.1111/j.1476-5381.1988.tb11620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checa A, Xu N, Sar DG, Haeggstrom JZ, Stahle M, Wheelock CE. Circulating levels of sphingosine-1-phosphate are elevated in severe, but not mild psoriasis and are unresponsive to anti-TNF-alpha treatment. Sci Rep. 2015;5:12017. doi: 10.1038/srep12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Joyce A, Yates K, Aouizerat B, Sanyal AJ. Metabolomic profiling to identify predictors of response to vitamin E for non-alcoholic steatohepatitis (NASH) PLoS One. 2012;7(9):e44106. doi: 10.1371/journal.pone.0044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurchiu V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, et al. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med. 2016;8(353):353ra111. doi: 10.1126/scitranslmed.aaf7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrocher R, Ferrari S, de Gironcoli M, Bassi A, Olivieri O, Guarini P, et al. Effect of fish oil supplementation on erythrocyte lipid pattern, malondialdehyde production and glutathione-peroxidase activity in psoriasis. Clin Chim Acta. 1989;179:121–31. doi: 10.1016/0009-8981(89)90158-7. [DOI] [PubMed] [Google Scholar]
- Dowd PM, Black AK, Woollard PW, Greaves MW. Cutaneous responses to 12-hydroxy-5,8,10,14-eicosatetraenoic acid (12-HETE) and 5,12-dihydroxyeicosatetraenoic acid (leukotriene B4) in psoriasis and normal human skin. Arch Dermatol Res. 1987;279:427–34. doi: 10.1007/BF00412586. [DOI] [PubMed] [Google Scholar]
- Fujieda Y, Manno A, Hayashi Y, Rhodes N, Guo L, Arita M, et al. Inflammation and resolution are associated with upregulation of fatty acid beta-oxidation in Zymosan-induced peritonitis. PLoS One. 2013;8(6):e66270. doi: 10.1371/journal.pone.0066270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant GE, Rokach J, Powell WS. 5-Oxo-ETE and the OXE receptor. Prostaglandins Other Lipid Mediat. 2009;89(3–4):98–104. doi: 10.1016/j.prostaglandins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarstrom S, Hamberg M, Samuelsson B, Duell EA, Stawiski M, Voorhees JJ. Increased concentrations of nonesterified arachidonic acid, 12L-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin E2, and prostaglandin F2alpha in epidermis of psoriasis. Proc Natl Acad Sci USA. 1975;72:5130–4. doi: 10.1073/pnas.72.12.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Obinata H, Ogawa A, Kishi M, Tatei K, Ishikawa O, et al. G2A plays proinflammatory roles in human keratinocytes under oxidative stress as a receptor for 9-hydroxyoctadecadienoic acid. J Invest Dermatol. 2008;128:1123–33. doi: 10.1038/sj.jid.5701172. [DOI] [PubMed] [Google Scholar]
- Jira W, Spiteller G, Carson W, Schramm A. Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients. Chem Phys Lipids. 1998;91:1–11. doi: 10.1016/s0009-3084(97)00095-9. [DOI] [PubMed] [Google Scholar]
- Kendall AC, Pilkington SM, Massey KA, Sassano G, Rhodes LE, Nicolaou A. Distribution of bioactive lipid mediators in human skin. J Inveset Dermatol. 2015;135:1510–20. doi: 10.1038/jid.2015.41. [DOI] [PubMed] [Google Scholar]
- Kim DS, Kim SY, Kleuser B, Schafer-Korting M, Kim KH, Park KC. Sphingosine-1-phosphate inhibits human keratinocyte proliferation via Akt/protein kinase B inactivation. Cell Signal. 2004;16:89–95. doi: 10.1016/s0898-6568(03)00114-1. [DOI] [PubMed] [Google Scholar]
- Kragballe K, Desjarlais L, Duell EA, Voorhees JJ. In vitro synthesis of 12-hydroxyeicosatetraenoic acid is increased in uninvolved psoriatic epidermis. J Invest Dermatol. 1986;87:47–52. doi: 10.1111/1523-1747.ep12523561. [DOI] [PubMed] [Google Scholar]
- Krieg P, Furstenberger G. The role of lipoxygenases in epidermis. Biochim Biophys Acta. 2014;1841:390–400. doi: 10.1016/j.bbalip.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Li S, Ganguli-Indra G, Indra AK. Lipidomic analysis of epidermal lipids: a tool to predict progression of inflammatory skin disease in humans. Expert Rev Proteomics. 2016;13:451–6. doi: 10.1080/14789450.2016.1177462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markworth JF, Kaur G, Miller EG, Larsen AE, Sinclair AJ, Maddipati KR, et al. Divergent shifts in lipid mediator profile following supplementation with n-3 docosapentaenoic acid and eicosapentaenoic acid. FASEB J. 2016;30:3714–25. doi: 10.1096/fj.201600360R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker MM, Grant-Kels JM. Healing fats of the skin: the structural and immunologic roles of the omega-6 and omega-3 fatty acids. Clin Dermatol. 2010;28:440–51. doi: 10.1016/j.clindermatol.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Mehta NN, Li R, Krishnamoorthy P, Yu Y, Farver W, Rodrigues A, et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis. 2012;224:218–21. doi: 10.1016/j.atherosclerosis.2012.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CC, Ziboh VA. Induction of epidermal hyperproliferation by topical n-3 polyunsaturated fatty acids on guinea pig skin linked to decreased levels of 13-hydroxyoctadecadienoic acid (13-hode) J Invest Dermatol. 1990;94:353–8. doi: 10.1111/1523-1747.ep12874482. [DOI] [PubMed] [Google Scholar]
- Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, et al. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol. 2015;35:2667–76. doi: 10.1161/ATVBAHA.115.306460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa E, Owada Y, Ikawa S, Adachi Y, Egawa T, Nemoto K, et al. Epidermal FABP (FABP5) regulates keratinocyte differentiation by 13(S)-HODE-mediated activation of the NF-kappaB signaling pathway. J Invest Dermatol. 2011;131:604–12. doi: 10.1038/jid.2010.342. [DOI] [PubMed] [Google Scholar]
- Ramsden CE, Ringel A, Feldstein AE, Taha AY, MacIntosh BA, Hibbeln JR, et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids. 2012;87(4–5):135–41. doi: 10.1016/j.plefa.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33:41–55. doi: 10.1016/j.det.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;62(1):1–8. doi: 10.2337/db12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Ann Rev Immunol. 2007;25:101–37. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Petasis NA. Resolvins and protectins in inflammation-resolution. Chem Rev. 2011;111:5922–43. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Progr Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- Spiteller G. Linoleic acid peroxidation—the dominant lipid peroxidation process in low density lipoprotein—and its relationship to chronic diseases. Chem Phys Lipids. 1998;95:105–62. doi: 10.1016/s0009-3084(98)00091-7. [DOI] [PubMed] [Google Scholar]
- Strassburg K, Huijbrechts AM, Kortekaas KA, Lindeman JH, Pedersen TL, Dane A, et al. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis: application in cardiac surgery. Anal Bioanal Chem. 2012;404:1413–26. doi: 10.1007/s00216-012-6226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR, Remmer HA, Sarkar MK, Xing X, Barnes DH, Wolterink L, et al. Proteogenomic analysis of psoriasis reveals discordant and concordant changes in mRNA and protein abundance. Genome Med. 2015;7:86. doi: 10.1186/s13073-015-0208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin NS, Tekin IO, Barut F, Sipahi EY. Accumulation of oxidized low-density lipoprotein in psoriatic skin and changes of plasma lipid levels in psoriatic patients. Mediators Inflamm. 2007;2007:78454. doi: 10.1155/2007/78454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorslund K, Amatya B, Dufva AE, Nordlind K. The expression of serotonin transporter protein correlates with the severity of psoriasis and chronic stress. Arch Dermatol Res. 2013;305:99–104. doi: 10.1007/s00403-012-1303-8. [DOI] [PubMed] [Google Scholar]
- Tokumura A. Metabolic pathways and physiological and pathological significances of lysolipid phosphate mediators. J Cell Biochem. 2004;92:869–81. doi: 10.1002/jcb.20147. [DOI] [PubMed] [Google Scholar]
- Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci. 2007;45:87–92. doi: 10.1016/j.jdermsci.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Wu T, Xie C, Han J, Ye Y, Weiel J, Li Q, et al. Metabolic disturbances associated with systemic lupus erythematosus. PLoS Pne. 2012;7(6):e37210. doi: 10.1371/journal.pone.0037210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MJ, Spite M. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu Rev Nutr. 2012;32:203–27. doi: 10.1146/annurev-nutr-071811-150726. [DOI] [PubMed] [Google Scholar]
- Ziboh VA, Miller CC, Cho Y. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: generation of antiinflammatory and anti-proliferative metabolites. Am J Clin Nutr. 2000;71(1 Suppl):361s–6s. doi: 10.1093/ajcn/71.1.361s. [DOI] [PubMed] [Google Scholar]
- Zurier RB, Burstein SH. Cannabinoids, inflammation, and fibrosis. FASEB J. 2016;30:3682–9. doi: 10.1096/fj.201600646R. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.