Abstract
Significance:
Objectively measured limitations in daytime activity levels appear to be inextricably linked with sleep disturbances in retinitis pigmentosa (RP) patients, as well as associated with unemployment status and central vision loss. Innovative interventional strategies should be developed to help improve these issues and overall quality of life for RP patients.
Purpose:
Novel sensor devices are emerging as valuable tools to objectively assess behavior. We used validated measures of wrist accelerometry to determine relationships between sleep, vision, and physical activity in RP subjects.
Methods:
For one-week, 33 RP adults wore a wrist Actiwatch to detect movement during the day (average total activity counts) and disturbed sleep at night. They completed ETDRS visual acuity testing, Pelli-Robson contrast sensitivity, Goldmann V4e visual fields, sleep diaries, and validated questionnaires to assess their sleep and general health.
Results:
Greater wake after sleep onset time measured with actigraphy (i.e., sleep disruption) (P=0.01), loss of visual acuity (P=0.009), and non-employment/student status (P=0.002) were all significant predictors of reduced daytime average total activity counts in a multiple linear regression model, after adjusting for contrast sensitivity as a cooperative suppressor variable (P=0.01)(R2=0.54). Fragmentation measured with actigraphy (i.e., restlessness during sleep)(P=0.069) and decreased sleep quality ratings reported upon awakening by the participants in a sleep diary (P=0.055) were each marginally associated with reduced daytime average total activity counts, while non-employment/student status, reduced visual acuity, and contrast sensitivity were still significant predictors. Objective and subjective measures of sleep or daytime activity were not statistically significantly correlated (P>0.05).
Conclusions:
We find non-employment/student status and sleep disturbances appear to be related to reduced daytime activity levels in adults with central vision loss due to RP. These findings underscore the importance of developing and evaluating interventions to help RP patients maintain engagement in productive activities and improve their disturbed sleep.
Keywords: retinitis pigmentosa, actigraphy, sleep, physical activity, employment, visual function, low vision
Wearable sensor devices are important tools to non-invasively monitor individuals’ behavior in real world environments during typical activities of daily living. For the past 40 years, actigraphy has been widely used as a valuable objective measure to quantify both sleep behavior1 and daytime activity2–3 in both research and clinical settings. This widely accepted measurement tool uses validated algorithms to analyze data collected by a triaxial accelerometer, often as an actiwatch worn on the wrist to measure sleep and on the hip or wrist to measure physical activity. While actigraphy is often used to measure physical activity with the accelerometer worn on the waist and validated algorithms have been developed to estimate metabolic equivalents via hip worn devices, when used to measure both sleep and physical activity in the same study, a single device is often worn on the wrist to improve adherence. Waist worn devices have not been found to accurately measure sleep,4 whereas several studies have demonstrated the utility of estimating daytime physical activity (albeit less accurately than waist worn measures) via wrist worn accelometry.5,7 Several previous studies have used actigraphy or accelerometers to document reduced physical activity levels in patients with increasing vision loss due to glaucoma,10 diabetic retinopathy,11 age-related macular degeneration12–13 dual sensory (hearing and vision) impairment,14 due to any cause in children15 or resulting in visual acuity worse than 20/40,16 20/500,17 or visual field loss.18 Other previous studies of actigraphy in subjects with vision loss have found evidence for sleep abnormalities among those with total blindness,19–20 children with optic nerve hypoplasia,21 or students at a school for the blind.22
Retinitis pigmentosa is a devastating retinal disease that involves progressive visual function loss and leads to increased difficulty with completing important activities of daily living,23 with the potential to affect both daytime activity levels and sleep. More severe vision loss in retinitis pigmentosa has been previously associated with greater impairment of mobility in studies involving either self-reports or performance measures in controlled laboratory or real world settings.24–28 A recent study of self-reported and objectively measured activity using actigraphy in visually impaired individuals found that subjective and objective measures were not in agreement.29 Subjective reports of mobility may be influenced by several factors, such as the patient’s psychosocial status or mental components, such as cognitive load, reliance on memory, information processing, concentration, attention, self-perception of one’s abilities, and preference for devices, which are all non-observable parameters, and may impact mobility.30 However, a previous study that evaluated mobility performance in retinitis pigmentosa did not find a significant correlation with personality traits or locus of control,27 although this study did not evaluate other factors that may potentially influence subjective reports of mobility. Therefore the extent to which subjective and objective measures of mobility are correlated in retinitis pigmentosa and whether they can be used equivalently is unknown. Our PubMed search for studies involving the use of objectively measured activity using actigraphy or accelerometers revealed no previous publications that involved retinitis pigmentosa patients.
Difficulty performing important day-to-day tasks was the most prominent quality of life issue among retinitis pigmentosa patients who participated in qualitative interviews.31 In particular, retinitis pigmentosa subjects previously self-reported that several of the most difficult activities are related to mobility, such as orienting in poor light, avoiding peripheral obstacles, and using public transport, especially among those with longer duration of visual loss and/or more severe vision loss.23 Difficulty with orientation and mobility may cause people with retinitis pigmentosa to restrict their activities. A previous survey administered in the Republic of Korea showed that retinitis pigmentosa patients were significantly more likely to be physically inactive than the general population.32 A recent survey in the United States found that 43% of retinitis pigmentosa patients were insufficiently active, which was significantly related to reduced scores on the NEI Visual Function Questionnaire-25 (VFQ-25) for overall visual function and peripheral vision.33 In addition, decreased physical functioning on the SF-36 questionnaire was significantly associated with unemployment in an retinitis pigmentosa population.33 It has been previously reported that retinitis pigmentosa patients have difficulty adjusting to vocational (work, school or home) environments, possibly reflecting a decrease in the perceived quality of work performance, job satisfaction, and interest.34
Both statutory retirement35 and lack of lifestyle regularity or regimented behavior for the timing of activities of daily living36 have been previously correlated with subjective sleep disturbances in older adults. In a previous study, nearly two-thirds of retinitis pigmentosa patients (i.e., 21 of 33) self-reported poor sleep quality on a validated sleep questionnaire,37 the Pittsburgh Sleep Quality Index, and another study that used this same questionnaire found reduced sleep quality, increased sleep latency, shorter sleep duration, and more need for sleep aid medication when compared to age-matched individuals with normal vision.38 Legally blind retinitis pigmentosa subjects had more daytime sleepiness, reduced alertness and more disturbed nighttime sleep measured with polysomnography than individuals with normal retinal function.39 However, objective measures of sleep outside of a laboratory using actigraphy are currently lacking in retinitis pigmentosa patients. Pain is often correlated with sleep disturbance and activity restriction, but it is currently not well understood whether people with retinitis pigmentosa tend to experience bodily pain related to mobility incidents, although has been reported that they experience headaches.40
One aim of the present study was to explore the relationships between daytime activity levels and several other variables, such as visual function loss, employment status, sleep disturbances, and physical pain in a cohort of study participants with retinitis pigmentosa. In addition, we were interested in evaluating and comparing both objective and subjective measures of sleep and daytime activity. We hypothesized that these aspects may be interrelated and a single study in which all of these factors were evaluated could be helpful to gain a better understanding of issues faced by retinitis pigmentosa patients, which could be used to guide the development of novel interventional strategies.
METHODS
The protocol for the study was approved by the Institutional Review Board (IRB) of the Johns Hopkins University and followed the tenets of the Declaration of Helsinki. Informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study.
The subjects in this study included 33 individuals with retinitis pigmentosa whose ages ranged from 20 to 78 years (mean 54 years). About half (48.5%) of the participants were women (n=16 of 33). The subjects were not recruited on the premise of having difficulties with activity or sleep, but rather only on the basis of their diagnosis of retinitis pigmentosa and to be involved in a study of day-to-day vision fluctuations. The majority of the subjects were recruited through the clinical practices of low vision optometrists and retinal specialists at the Johns Hopkins Wilmer Eye Institute, while the remaining subjects self-referred after learning of the study through online listings. Individuals were eligible for the study if they had a confirmed diagnosis of retinitis pigmentosa, were over age 18, and experienced any degree of vision loss.
The tests to assess the subjects’ level of visual function consisted of best-corrected Early Treatment Diabetic Retinopathy Study charts for binocular visual acuity (ETDRS; Lighthouse International, New York, NY, USA) at 3 meters or closer if fewer than 10 letters were read, binocular Pelli-Robinson contrast sensitivity (Metropia Ltd., Harlow, UK) at 1 meter, and Goldmann visual field with the V4e target in each eye. All vision tests were administered by a single examiner (AKB).
A triaxial wrist accelerometer (Actiwatch L; Mini Mitter Co., Inc.; A Respironics Inc., Co.; Bend, OR, USA) was used to assess the parameters of activity (average total activity counts) and sleep disruption in the retinitis pigmentosa subjects. Subjects wore the actiwatch continuously on the non-dominant wrist during the daytime and night for a one-week period. We acquired data with a 60 second sampling rate via the Actiwatch and used the software’s “medium” sensitivity setting and validated algorithm to establish sleep and wake epochs.41 We derived three parameters for analysis from these data: daytime activity level, sleep fragmentation and wake after sleep onset time. We followed published guidelines for actigraphy monitoring and data scoring for estimating the primary sleep period, using the automated software to define the sleep period, which was reviewed and hand corrected as needed by a trained scorer in conjunction with participant sleep logs data indicating bed and wake times.42 We used total activity counts averaged during the non-primary sleep period as an index of overall daytime activity level, similar to other studies.43,44 The Actiwatch utilizes a piezoelectric sensor to detect vertical accelerations at the wrist between 0.5 and 2.0g with a frequency response range between 0.35–7.5Hz. Activity counts from the device reflect the peak acceleration detected over each epoch and are used in determining sleep and wake intervals. To measure sleep fragmentation, a measure of brief arousals during the sleep period, we used the software’s calculated fragmentation index; i.e., a sum of: [proportion of minutes moving] + [proportion of # of 1-minute increments without motion vs. # of continuous blocks of immobility]. We measured wake after sleep onset time (WASO) as derived by the Actiwatch scoring algorithm, which is a measure of the amount of time awake in the middle of the night after initial sleep onset.
Subjects self-completed the Short Form Health Survey (SF-36) to assesses overall general health based on eight subscales, including vitality, physical functioning, bodily pain, general health perceptions, physical role functioning, emotional role functioning, social role functioning, and mental health.45 Subjects also self-completed the Pittsburgh Sleep Quality Index questionnaire.46 and the Beck Depression Inventory.47 Only 15% of subjects had moderate depression and none were severely depressed; given our relatively small sample size and infrequency of depression, this variable was not included in the analyses. In addition, sleep diaries were completed by the subjects during the same week when they wore the Actiwatch. They were asked to rate their sleep quality on a scale of 0 to 10 each morning upon waking, with a score of 0 indicating the worst sleep quality (i.e., shallow, unrefreshing), while a score of 10 indicated the best sleep quality (i.e., deep and refreshing). They also recorded the amount of time they were awake in the middle of the night after falling asleep initially (i.e., wake after sleep onset). All participants in the study were compliant with wearing the Actiwatch and completing the sleep diary during the one-week period (i.e., no missing data).
Simple Pearson’s correlation coefficients (r) were used to explore the relationships between variables. Simple and multivariate linear regression analyses were used to assess the relationships between daytime average total activity counts measured by actigraphy and the other variables using Stata/IC version 13.1 (Stata Corp., College Station, TX, USA). Shapiro-Wilk testing was used to confirm normality of the variables used in the linear regression analyses. We analyzed the GVF data for eye with the larger diameter and used the log of the diameter since it has been documented in several longitudinal, natural history studies that the remaining viable retinal area declines over time according to a negative exponential function.48 The first multivariate regression model compared the primary outcome of daytime activity level to variables that are modifiable (i.e., employment/student status and presence of pain), then any significant covariates were included in a multivariate model with non-modifiable variables (i.e., age, visual acuity, contrast sensitivity, visual field). Non-significant variables in that model were dropped sequentially until the multivariate model contained only significant variables, which were then included in a multivariate analysis with each of the sleep measures.
RESULTS
Predictors of Daytime Total Activity Counts
Table 1 displays the descriptive statistics and results for simple correlations (r) between daytime average total activity counts and all other study variables. Decreased daytime average total activity counts were significantly related to reduced binocular visual acuity (P =0.03), Goldmann visual field log diameter in the better eye (P =0.009), SF-36 pain score (P =0.027), subjective sleep quality ratings from subjects’ diaries (P=0.028), wake after sleep onset time (sleep disruption) from actigraphy (P=0.025), sleep fragmentation score from actigraphy (restlessness)(P=0.028) and non-employment/student status (P=0.004). Roughly half of the study participants (48%) reported having pain in the last four weeks as per the SF-36 questionnaire. The SF-36 pain subscale was not significantly related to age (P=0.59). Other subscales in the SF-36 questionnaire and age were not significantly associated with daytime activity level measured by actigraphy.
Table 1.
Descriptive statistics (mean, SD, range) and results of simple correlations (r coefficients) for daytime average total activity counts versus other covariates
| Mean | SD | Range | Coefficient (r) | P-value | |
|---|---|---|---|---|---|
| Visual acuity OU (logMAR) | 0.34 | 0.54 | -0.12, 2.0 | -0.38 | 0.032* |
| Pelli-Robson contrast OU (logCS) | 1.18 | 0.67 | 0, 2.0 | 0.12 | 0.50 |
| Goldmann visual field diameter | 47° | 49° | 0, 149° | 0.45 | 0.009* |
| Employed/Student | 36%/10% | 0.51 | 0.0035* | ||
| Pittsburgh Sleep Quality Index | 6 | 3 | 1–13 | -0.02 | 0.92 |
| Sleep Quality Ratings | 6.7 | 1.7 | 2.9, 9.3 | 0.39 | 0.028* |
| Wake After Sleep Onset (disruption) | 70 | 21 | 37, 127 | -0.39 | 0.025* |
| Fragmentation (sleep restlessness) | 36 | 12 | 14, 62 | -0.38 | 0.028* |
| SF-36 pain score | 86 | 18 | 45, 100 | 0.43 | 0.027* |
| SF-36 physical function | 87 | 18 | 40, 100 | 0.13 | 0.52 |
| SF-36 physical role | 73 | 30 | 0, 100 | -0.04 | 0.85 |
| SF-36 emotional role | 83 | 31 | 0, 100 | -0.02 | 0.92 |
| SF-36 energy | 65 | 22 | 15, 100 | -0.001 | 0.996 |
| SF-36 emotional | 72 | 22 | 16, 100 | -0.09 | 0.68 |
| SF-36 social functioning | 79 | 28 | 0, 100 | -0.003 | 0.99 |
| SF-36 general health | 78 | 14 | 50, 100 | -0.04 | 0.85 |
| Age (years) | 53.5 | 15.6 | 20, 78 | -0.23 | 0.20 |
*=statistically significant (P<0.05)
In a multiple linear regression model, employment/student status (P=0.049) was a significant predictor of daytime average total activity counts, but presence of bodily pain was not (P=0.30) (R2=0.25). In another multivariate regression, employment/student status (P=0.025) was a significant predictor of daytime average total activity counts, while binocular visual acuity (P=0.062) was marginally significant, and age (P=0.61), and Goldmann visual field log diameter (P=0.27) were not significantly associated with daytime activity, after adjusting for contrast sensitivity as a significant cooperative suppressor of visual acuity (P=0.02). A multivariate regression model that only included employment/student status (P=0.005), visual acuity (P=0.015) and contrast sensitivity (P=0.03) found that all three were significant predictors of daytime activity (R2=0.41), with cooperative (reciprocal) suppression occurring for visual acuity and contrast sensitivity, as they were highly correlated with each other (r=−0.76; P<0.001). The relationship between daytime activity level and employment/student status is depicted in Figure 1A.
Figure 1.
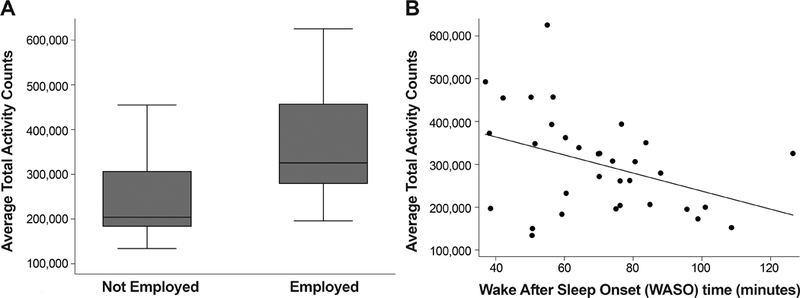
Box plot of (A) average total activity counts measured with actigraphy for subjects who were employed/student versus not employed/student (p=0.0035). A scatterplot shows the significant relationship between (B) average total activity counts measured with actigraphy versus wake after sleep onset (sleep disruptions); the drawn line represents a linear regression line (p=0.025). In the box plot, the bottom and top of the box are the 25th and 75th percentile (i.e. the upper and lower quartiles, respectively) and the band near the middle of the box is the 50th percentile (i.e. the median). The ends of the whiskers represent the lowest datum within 1.5 times the interquartile range of the lower quartile, and the highest datum still within 1.5 times the interquartile range of the upper quartile.
Next we assessed whether objective or subjective measures of sleep disturbance were significantly related to daytime average total activity counts, when adjusting for employment/student status, visual acuity and contrast sensitivity in multivariate regression models. Greater wake after sleep onset time measured with actigraphy (i.e., sleep disruption) (P=0.01), loss of visual acuity (P=0.009), contrast sensitivity (P=0.01), and non-employment/student status (P=0.002) were all significant predictors of reduced daytime average total activity counts in a multiple linear regression model (R2=0.54). The relationship between daytime activity level and wake after sleep onset time (sleep disruptions) is shown in Figure 1B. Fragmentation measured with actigraphy (i.e., restlessness during sleep) (P=0.069) was marginally associated with reduced daytime average total activity counts, while non-employment/student status (P=0.004), reduced visual acuity (P=0.03), and contrast sensitivity (P=0.047) were still significant predictors. Sleep quality rating reported upon awakening by the participants in a sleep diary (P=0.055) was also marginally associated with daytime average total activity counts after adjusting for employment/student status (P=0.025), visual acuity (P=0.02) and contrast sensitivity (P=0.04). However, the Pittsburgh Sleep Quality Index (P=0.92) was not significantly associated with daytime average total activity counts after adjusting for employment/student status (P=0.009), visual acuity (P=0.02) and contrast sensitivity (P=0.03).
Table 2 shows the simple correlations between the variables that were significantly related to daytime average total activity counts (from Table 1). Reduced Goldmann visual field log diameter (P=0.003) and bodily pain (P=0.002) were each independently related to non-employment/non-student status. SF-36 bodily pain was significantly independently associated with visual acuity loss (P=0.02), increased fragmentation (restlessness) during sleep (P=0.04), greater wake after sleep onset time (sleep disruptions) measured by actigraphy (P=0.01) and reduced sleep quality ratings in participants’ diaries (P=0.046). The sleep measures were not significantly correlated with visual loss (P>0.05), as shown in Table 2.
Table 2.
Results of simple correlations (r coefficients) among covariates that were significantly related to daytime average total activity counts.
| VA OU | GVF diam | Employed | WASO | Fragmentation | Pain | |
|---|---|---|---|---|---|---|
| Sleep Quality Ratings | 0.06 | 0.22 | 0.29 | -0.20 | -0.17 | 0.39 |
| P=0.73 | P=0.22 | P=0.13 | P=0.27 | P=0.35 | P=0.046* | |
| SF-36 Pain score | -0.45 | 0.37 | 0.56 | -0.47 | -0.40 | |
| P=0.02* | P=0.057 | P=0.002* | P=0.01* | P=0.04* | ||
| Fragmentation | 0.21 | -0.23 | -0.07 | 0.80 | ||
| P=0.24 | P=0.20 | P=0.72 | P<0.001* | |||
| WASO (disruption) | 0.12 | -0.18 | -0.02 | |||
| P=0.53 | P=0.32 | P=0.93 | ||||
| Employed/Student | -0.33 | 0.52 | ||||
| P=0.07 | P=0.003* |
*=statistically significant (P<0.05)
VA OU= visual acuity binocular; GVF diam= Goldmann visual field log diameter; WASO=Wake After Sleep Onset (disruption);
Objective vs. Subjective Variables
Data in Table 2 indicate that the two objective measures of sleep (i.e., fragmentation and wake after sleep onset time measured by actigraphy) were highly significantly correlated (P<0.001), but each of these objective sleep measures was not statistically significantly correlated with the subjective sleep quality ratings in daily diaries or the Pittsburgh Sleep Quality Index questionnaire. The Pittsburgh Sleep Quality Index was borderline significantly related to the sleep quality ratings by diary (P=0.053). Objectively measured daytime activity levels (i.e., average total activity counts by actigraphy) were not significantly correlated with subjectively reported health-related activity limitations on the SF-36 questionnaire for the physical function subscale (P=0.52).
DISCUSSION
The findings in the present study provide evidence that people with retinitis pigmentosa who were not employed or a student, who had greater central vision loss, or who reported sleep disturbances tended to have more restrictive daytime activity levels. Decreased daytime physical activity and bouts of napping can attenuate nocturnal sleep drive, and conversely sleep disturbances may lead to fatigue and daytime sleepiness, which can create a vicious cycle. The results presented here, along with those from previous studies,23,33 highlight the importance of developing interventions for retinitis pigmentosa patients to maintain their visual functioning and thus their employability, mobility and physical activity.
An interesting finding in the present study was the lack of a significant correlation between objective sleep measures with actigraphy and subjectively reported sleep disturbances with the diaries and Pittsburgh Sleep Quality Index. Discrepanices between actigraphic and sleep diary measures have been previously reported for several other populations and are attributed to limitations of sleep diaries, which can be prone to systematic biases that lead to overestimates of sleep quality.49 Actigraphy can provide valid estimates of total sleep time, sleep percentage, and wake after sleep onset time, but its validity for assessing sleep onset latency remains suboptimal. Actigraphy is not considered a replacement for other assessment tools, such as sleep diaries or overnight polysomnography.49 The objective measures of sleep in our study focused on restlessness and time awake during the sleep period, which may be different aspects of sleep than the quality ratings in the self-reported diaries, which inquired about deep or refreshing sleep quality. Each night, participants rated their sleep quality and the sleep disruptions were measured by actigraphy, but we used their mean scores over a week for the analyses. Perhaps we would have been more likely to find a better correlation between the two variables if we had used the data for each nightly sleep period since the means do not consider the range of possible day-to-day variability. The Pittsburgh Sleep Quality Index questionnaire inquires about various aspects of sleep disturbance, including many dimensions not measured by actigraphy, such as feeling uncomfortable due to physical sensations, taking sleep medication, staying awake during the day, and enthusiasm to get things done.
The present study also found a lack of significant association between health-related activity limitations from the SF-36 questionnaire and daytime activity limitations captured objectively with actigraphy. Although researchers may be tempted to use a simple questionnaire to assess sleep or daytime activity without an additional objective measure such as actigraphy, there are likely aspects that are not fully captured by either self-reported measures or actigraphy alone, thus indicating the value of including both types of measures in future studies. Of note, the amount of time spent awake after sleep onset measured only with actigraphy was the only sleep measure that significantly correlated with reduced daytime activity levels in the present study, thus it was important to include this non-invasive, objective measure of sleep. Subjectively measured sleep quality ratings in the participants’ diaries were marginally or borderline significantly related to daytime activity, and our relatively small sample size may have not been adequately powered to detect this association. Both performance-based observational testing and self-reports are valuable measures for assessing the impact of visual dysfunction on overall lifestyle,50 as it is important to acknowledge the psychological and cognitive aspects related to patients’ mobility. Future studies should evaluate other important psychosocial factors that may be related to performance of activities in people with retinitis pigmentosa, such as fear of falling.
Another somewhat unexpected finding was that the level of visual function loss was not significantly related to severity of sleep disturbances in this cohort of v patients with some remaining vision. Very similar to a previous study,37 two-thirds of our retinitis pigmentosa patients (n=22 of 33) had a score of 5 or greater on the Pittsburgh Sleep Quality Index, indicating they were experiencing sleep disturbances. The etiology of sleep disturbances appears to be less likely due to retinal degeneration or influence of the circadian pathway for sleep-wake cycles through the intrinsically photosensitive retinal ganglion cells in the inner retina since visual function loss was not significantly associated with sleep issues. Based on our study findings in Table 2, it appears that pain is a more plausible factor that may influence sleep problems. Our study accounted for the presence of bodily pain from the SF-36, but this questionnaire does not distinguish the type of pain. However, we do not believe the participants’ pain was likely to be related to other comorbidities since the SF-36 general health subscale score was not significantly associated with our sleep variables. A landmark study published 30 years ago, in which 500 retinitis pigmentosa patients in a single clinic were systematically queried regarding their symptoms, reported that that over half (53%) had headaches.40 Future studies are needed to elucidate whether v patients’ headaches and/or mobility-related injuries are the cause of pain that appears to be associated with non-employment status and sleep disruptions. Interventions could be tailored and evaluated to attempt to reduce the cause of bodily pain once it is elucidated in future work.
The present study did not assess the uptake of interventional strategies, but the findings suggest several options that practitioners can use to help retinitis pigmentosa patients to maintain daytime activity levels. Examples include guide dogs, orientation and mobility training to adjust walking speed and increase visual scanning, flashlights for mobility at night, hats or visors to reduce glare, and route planning based on sun-shade patterns.51 Decreased sleep quality can ultimately affect processes like learning, memory, and attention, which are all important components of successful low vision rehabilitation. Therefore, it is important for providers to consider the contribution of sleep disturbances to physical functioning and offer a referral to a specialist to improve sleep hygiene for patients with daytime sleepiness. Interventions as simple as increasing the regularity of daily routines and lifestyle activities, such as exercise,52 may prove beneficial in improving sleep quality.36 Exercise stimulates beneficial effects in various physiologic pathways of the body. Based on previous research in animal models of retinitis pigmentosa, it has been proposed that regular, moderate physical activity may offer protection against the damage from increased oxidative stress in the retina,53,54 suggesting the need for clinical studies to provide evidence of long-term benefits of exercise in retinitis pigmentosa patients with regard to both sleep disturbances and visual loss.
While actigraphy provides objective measures of daytime activity levels in patients’ real-world environments and routines, the potential limitations of accelerometry are its inability to report types of activity and discriminate activity intensity.55 For future studies, it has been suggested that it is possible to modify and analyze novel intervals for accelerometer data to detect varying activity levels of real-life physical performance.56 The wrist is the preferred location for sleep measures and, while the waist has been reported as the optimal location for recording a wide range of physical activity,57 a previous study found much lower compliance rates for waist sensors compared to wrist sensors (i.e., 65% versus 99% wear time).58 This finding prompted the NHANES program to switch placement from the waist to the wrist, which did result in improved compliance rates.44 It is also convenient to obtain both physical activity and sleep measures from a single device worn on the wrist. In a previous study, the daytime total activity counts were lower for placement on the hip when compared wrist placement;59 thus the two placement locations may not be comparable. The present study did not determine whether subjects were active within or outside their homes as in a previous study using GPS devices,10 which may be a valuable additional component to add to future studies of daytime activity in retinitis pigmentosa patients.
Actigraphy may be a valuable outcome measure to use in future treatment clinical trials to evaluate for changes in daytime activity levels within v patients who may develop improvements in visual function. Actigraphy might be particularly helpful to evaluate for real-world impact of visual improvements outside of a controlled laboratory test of mobility or navigation. Similarly, studies of new navigational or low vision other rehabilitation devices for retinitis pigmentosa may find it beneficial to use actigraphy as a non-invasive, objective measure of whether there are improvements in daytime activity levels related to device usage.
ACKNOWLEDGMENTS
The authors wish to thank Erin McInrue for her assistance with the analysis of the actigraphy data.
National Institutes of Health (NIH): NIH K23 EY018356.
REFERENCES
- 1.Smith MT. Why an Actigraphy Manual Is Needed. Behav Sleep Med 2015;13(Suppl. 1):S1–3. [DOI] [PubMed] [Google Scholar]
- 2.Bussmann JB, Stam HJ. Techniques for Measurement and Assessment of Mobility in Rehabilitation: A Theoretical Approach. Clin Rehabil 1998;12:455–64. [DOI] [PubMed] [Google Scholar]
- 3.Quante M, Kaplan ER, Rueschman M, et al. Practical Considerations in Using Accelerometers to Assess Physical Activity, Sedentary Behavior, and Sleep. Sleep Health 2015;1:275–84. [DOI] [PubMed] [Google Scholar]
- 4.Zinkhan M, Berger K, Hense S, et al. Agreement of Different Methods for Assessing Sleep Characteristics: a Comparison of Two Actigraphs, Wrist and Hip Placement, and Self-report with Polysomnography. Sleep Med 2014;15:1107–14. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberger ME, Haskell WL, Albinali F, et al. Estimating Activity and Sedentary Behavior from an Accelerometer on the Hip or Wrist. Med Sci Sports Exerc 2013;45:964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Hees VT, Renström F, Wright A, et al. Estimation of Daily Energy Expenditure in Pregnant and Non-Pregnant Women Using a Wrist-Worn Tri-Axial Accelerometer. PLoS One 2011;6:e22922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, Rowlands AV, Murray P, Hurst TL. Physical Activity Classification Using the GENEA Wrist-Worn Accelerometer. Med Sci Sports Exerc 2012;44:742–8. [DOI] [PubMed] [Google Scholar]
- 8.Chen KY, Acra SA, Majchrzak K, et al. Predicting Energy Expenditure of Physical Activity Using Hip and Wrist-worn Accelerometers. Diabetes Technol Ther 2003;5:1023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swartz AM, Strath SJ, Bassett DR Jr, O’Brien WL, King GA, Ainsworth BE. Estimation of Energy Expenditure Using CSA Accelerometers at Hip and Wrist Sites. Med Sci Sports Exerc 2000;32:S450–6. [DOI] [PubMed] [Google Scholar]
- 10.Ramulu PY, Maul E, Hochberg C, et al. Real-world Assessment of Physical Activity in Glaucoma Using an Accelerometer. Ophthalmol 2012;119:1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loprinzi PD, Brodowicz GR, Sengupta S, et al. Accelerometer-Assessed Physical Activity and Diabetic Retinopathy in the United States. JAMA Ophthalmol 2014;132:1017–9. [DOI] [PubMed] [Google Scholar]
- 12.Loprinzi PD, Swenor BK, Ramulu PY. Age-Related Macular Degeneration Is Associated with Less Physical Activity among US Adults: Cross-Sectional Study. PLoS One 2015;10:e0125394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sengupta S, Nguyen AM, van Landingham SW, et al. Evaluation of Real-world Mobility in Age-related Macular Degeneration. BMC Ophthalmol 2015;15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loprinzi PD, Smit E, Lin FR, et al. Accelerometer-Assessed Physical Activity and Objectively Determined Dual Sensory Impairment in US Adults. Mayo Clin Proc 2013;88:690–6. [DOI] [PubMed] [Google Scholar]
- 15.Houwen S, Hartman E, Visscher C. Physical Activity and Motor Skills in Children with and without Visual Impairments. Med Sci Sports Exerc 2009;41:103–9. [DOI] [PubMed] [Google Scholar]
- 16.Willis JR, Jefferys JL, Vitale S, Ramulu PY. Visual Impairment, Uncorrected Refractive Error, and Accelerometer-Defined Physical Activity in the United States. Arch Ophthalmol 2012;130:329–35. [DOI] [PubMed] [Google Scholar]
- 17.Barbosa Porcellis da Silva R, Silva R, Marques AC, Reichert FF. Objectively Measured Physical Activity in Brazilians with Visual Impairment: Description and Associated Factors. Disabil Rehabil 2017;19:1–7. [DOI] [PubMed] [Google Scholar]
- 18.van Landingham SW, Willis JR, Vitale S, Ramulu PY. Visual Field Loss and Accelerometer-Measured Physical Activity in the United States. Ophthalmol 2012;119:2486–92. [DOI] [PubMed] [Google Scholar]
- 19.Leger D, Guilleminault C, Santos C, Paillard M. Sleep/wake Cycles in the Dark: Sleep Recorded by Polysomnography in 26 Totally Blind Subjects Compared to Controls. Clin Neurophysiol 2002;113:1607–14. [DOI] [PubMed] [Google Scholar]
- 20.Aubin S, Gacon C, Jennum P, et al. Altered Sleep-Wake Patterns in Blindness: A Combined Actigraphy and Psychometric Study. Sleep Med 2016;24:100–8. [DOI] [PubMed] [Google Scholar]
- 21.Rivkees SA, Fink C, Nelson M, Borchert M. Prevalence and Risk Factors for Disrupted Circadian Rhythmicity in Children with Optic Nerve Hypoplasia. Br J Ophthalmol 2010;94:1358–62. [DOI] [PubMed] [Google Scholar]
- 22.Wee R, Van Gelder RN. Sleep Disturbances in Young Subjects with Visual Dysfunction. Ophthalmol 2004;111:297–302. [DOI] [PubMed] [Google Scholar]
- 23.Latham K, Baranian M, Timmis MA, et al. Relative Difficulties of Daily Living Tasks with Retinitis Pigmentosa. Optom Vis Sci 2017;92:317–28. [DOI] [PubMed] [Google Scholar]
- 24.Geruschat DR, Turano KA, Stahl JW. Traditional Measures of Mobility Performance and Retinitis Pigmentosa. Optom Vis Sci 1998;75:525–37. [DOI] [PubMed] [Google Scholar]
- 25.Turano KA, Geruschat DR, Stahl JW, Massof RW. Perceived Visual Ability for Independent Mobility in Persons with Retinitis Pigmentosa. Invest Ophthalmol Vis Sci 1999;40:865–77. [PubMed] [Google Scholar]
- 26.Black A, Lovie-Kitchin JE, Woods RL, et al. Mobility Performance with Retinitis Pigmentosa. Clin Exp Optom 1997;1:1–12. [Google Scholar]
- 27.Haymes S, Guest D, Heyes A, Johnston A. Mobility of People with Retinitis Pigmentosa as a Function of Vision and Psychological Variables. Optom Vis Sci 1996;73:621–37. [DOI] [PubMed] [Google Scholar]
- 28.Szlyk JP, Seiple W, Fishman GA, Alexander KR, Grover S, Mahler CL. Perceived and Actual Performance of Daily Tasks: Relationship to Visual Function Tests in Individuals with Retinitis Pigmentosa. Ophthalmology 2001;108:65–75. [DOI] [PubMed] [Google Scholar]
- 29.Barbosa DG, Andrade RD, Pelegrini A, Felden ÉP. Rating of Perceived Capacity: a Proposal to Predict Adequate Levels of Physical Activity in Visually Impaired Individuals. J Sports Med Phys Fitness 2017; 10.23736/S0022-4707.17.08070-7. [DOI] [PubMed] [Google Scholar]
- 30.Schakel W, Bode C, van der Aa HPA, et al. Exploring the Patient Perspective of Fatigue in Adults with Visual Impairment: a Qualitative Study. BMJ Open 2017;7:e015023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senthil MP, Khadka J, Pesudovs K. Seeing Through their Eyes: Lived Experiences of People with Retinitis Pigmentosa. Eye 2017;31:741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.An AR, Shin DW, Kim S, et al. Health Behaviors of People with Retinitis Pigmentosa in the Republic of Korea. Ophthalmic Epidemiol 2014;21:279–86. [DOI] [PubMed] [Google Scholar]
- 33.Levinson JD, Joseph E, Ward LA, et al. Physical Activity and Quality of Life in Retinitis Pigmentosa. J Ophthalmol 2017;2017:6950642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jangra D, Ganesh A, Thackray R, et al. Psychosocial Adjustment to Visual Loss in Patients with Retinitis Pigmentosa. Ophthalmic Genet 2007;28:25–30. [DOI] [PubMed] [Google Scholar]
- 35.Myllyntausta S, Salo P, Kronholm E, et al. Changes in Sleep Difficulties During the Transition to Statutory Retirement. Sleep 2018;41: 10.1093/sleep/zsx182. [DOI] [PubMed] [Google Scholar]
- 36.Monk TH, Reynolds CF, Buysse DJ, et al. The Relationship between Lifestyle Regularity and Subjective Sleep Quality. Chronobiol Int 2003;20:97–107. [DOI] [PubMed] [Google Scholar]
- 37.Murphy C, Duponsel N, Huang XS, et al. Retinal Disorders and Sleep Disorders: Are They Genetically Related? J Vis Impair Blind 2015;109:359–70. [Google Scholar]
- 38.Gordo MA, Recio M, Sanchez-Barcelo EJ. Decreased Sleep Quality in Patients Suffering from Retinitis Pigmentosa. J Sleep Res 2001;10:159–64. [DOI] [PubMed] [Google Scholar]
- 39.Ionescu D, Driver HS, Heon E, et al. Sleep and Daytime Sleepiness in Retinitis Pigmentosa Patients. J Sleep Res 2001;10:329–35. [DOI] [PubMed] [Google Scholar]
- 40.Heckenlively JR, Yoser SL, Friedman LH, Oversier JJ. Clinical Findings and Common Symptoms in Retinitis Pigmentosa. Am J Ophthalmol 1988;105:504–11. [DOI] [PubMed] [Google Scholar]
- 41.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy Validation with Insomnia. Sleep 2006;29:232–9. [PubMed] [Google Scholar]
- 42.Ancoli-Israel S, Martin JL, Blackwell T, et al. The SBSM Guide to Actigraphy Monitoring: Clinical and Research Applications. Behav Sleep Med 2015;13(Suppl. 1):S4-S38. [DOI] [PubMed] [Google Scholar]
- 43.Korszun A, Young EA, Engleberg NC, et al. Use of Actigraphy for Monitoring Sleep and Activity Levels in Patients with Fibromyalgia and Depression. J Psychosom Res 2002;52:439–43. [DOI] [PubMed] [Google Scholar]
- 44.Freedson PS, John D. Comment on “Estimating Activity and Sedentary Behavior from an Accelerometer on the Hip and Wrist”. Med Sci Sports Exerc 2013;45:962–63. [DOI] [PubMed] [Google Scholar]
- 45.Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). I. Conceptual Framework and Item Selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 46.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 47.Beck AT, Ward CH, Mendelson M, et al. An Inventory for Measuring Depression. Arch Gen Psychiatry 1961;4:561–71. [DOI] [PubMed] [Google Scholar]
- 48.Massof RW, Dagnelie G, Benzschawel T, et al. First Order Dynamics of Visual Field Loss in Retinitis Pigmentosa. Clin Vis Sci 1990;5:1–26. [Google Scholar]
- 49.Martin JL, Hakim AD. Wrist Actigraphy. Chest 2011;139:1514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richman J, Lorenzana LL, Lankaranian D, et al. Relationships in Glaucoma Patients between Standard Vision Tests, Quality of Life, and Ability to Perform Daily Activities, Ophthalmic Epidemiol 2010;17:144–51. [DOI] [PubMed] [Google Scholar]
- 51.Geruschat DR, Turano KA. Connecting Research on Retinitis Pigmentosa to the Practice of Orientation and Mobility. J Vis Impair Blind 2002;96:69. [Google Scholar]
- 52.Yang PY, Ho KH, Chen HC, Chien MY. Exercise Training Improves Sleep Quality in Middle-aged and Older Adults with Sleep Problems: a Systematic Review. J Physiother 2012;58:157–63. [DOI] [PubMed] [Google Scholar]
- 53.Hanif AM, Lawson EC, Prunty M, et al. Neuroprotective Effects of Voluntary Exercise in an Inherited Retinal Degeneration Mouse Model. Invest Ophthalmol Vis Sci 2015;56:6839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kruk J, Kubasik-Kladna K, Aboul-Enein HY. The Role Oxidative Stress in the Pathogenesis of Eye Diseases: Current Status and a Dual Role of Physical Activity. Mini Rev Med Chem 2015;16:241–57. [DOI] [PubMed] [Google Scholar]
- 55.Verceles AC, Hager ER. Use of Accelerometry to Monitor Physical Activity in Critically Ill Subjects: A Systematic Review. Resp Care 2015;60:1330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smuck M, Tomkins-Lane C, Ith MA, et al. Physical Performance Analysis: A New Approach to Assessing Free-Living Physical Activity in Musculoskeletal Pain and Mobility-Limited Populations. PLoS One 2017;12:e0172804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cleland I, Kikhia B, Nugent C, et al. Optimal Placement of Accelerometers for the Detection of Everyday Activities. Sensors (Basel) 2013;13:9183–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Hees VT, Renstrom F, Wright A, et al. Estimation of Daily Energy Expenditure in Pregnant and Non-Pregnant Women Using a Wrist-Worn Tri-Axial Accelerometer. PLoS One 2011;6:e22922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Routen AC, Upton D, Edwards MG, Peters DM. Discrepancies in Accelerometer-Measured Physical Activity in Children Due to Cut-Point Non-Equivalence and Placement Site. J Sports Sci 2012;30:1303–10. [DOI] [PubMed] [Google Scholar]


