Abstract
Background
Pulmonary dysfunction predicts incident cardiovascular disease (CVD).
Objectives
To evaluate whether longitudinal decline in lung function is associated with incident heart failure (HF), coronary disease (CHD), and stroke.
Methods
Among 10,351 participants in the Atherosclerosis Risk in Communities Study free of CVD, rapid lung function decline was defined as the greatest quartile (n=2,585) of decline in either forced expiratory volume in 1 second (FEV1; >1.9% decline/year) or forced vital capacity (FVC; >2.1% decline/year) over 2.9±0.2 years. The relationship between rapid decline in FEV1 or FVC and subsequent incident HF, CHD, stroke, or a composite of these was assessed using multivariable Cox regression adjusting for the baseline spirometry value, demographics, height, body mass index, heart rate, diabetes, hypertension, LDL, use of lipid lowering medication, NT-proBNP and smoking.
Results
The mean age was 54 ± 6 years, 56% were women, and 81% were white. At 17±6 years of follow-up, HF occurred in 14%, CHD 11%, stroke 6%, and the composite 24%. Rapid decline in FEV1 and in FVC were both associated with a heightened risk of incident HF (HR=1.17, 95% CI 1.04–1.33; p=0.010 and HR=1.27, 95% CI 1.12–1.44; p<0.001, respectively), with rapid decline in FEV1 most prognostic in the first year of follow-up (HR=4.22, 95% CI 1.34–13.26; p=0.01). Rapid decline in FEV1 was also associated with incident stroke (HR=1.25, 95% CI 1.04–1.50; p=0.015).
Conclusion
Rapid decline in lung function, assessed by serial spirometry, is associated with a higher incidence of subsequent CVD, particularly incident HF.
Keywords: lung function, FEV1, FVC, cardiovascular disease, heart failure
Introduction
Cardiovascular disease is the leading cause of death in the United States, while chronic pulmonary disease is the third leading cause of mortality (1). Interactions between cardiac and pulmonary function in relation to clinical outcomes are well recognized (2). Impaired lung function is associated with nearly two-fold higher risk of cardiovascular mortality (3). Conversely, coronary heart disease (CHD) and heart failure (HF) are more common among people with pulmonary disease, even after accounting for common cardiovascular risk factors, including smoking.(4,5) Asthma and chronic obstructive pulmonary disease (COPD), the most prevalent respiratory diseases worldwide,(6) are associated with a systemic inflammatory response(7) and with systemic endothelial dysfunction and impaired vascular reactivity,(8) which also promote cardiovascular disease(9) and represent potential mechanisms underlying these cardiopulmonary interactions.
Age-related decline in lung function starts at approximately 25 years of age, with an average loss of 20 ml per year in the forced expiratory volume in one second (FEV1).(10) Forced vital capacity (FVC) also declines with age to approximately 75% of the best value.(10) Environmental and behavioral factors, most notably cigarette smoking, in addition to genetics, interact to determine the speed of the decline in the lung function.(11) Rapid decline in lung function is a predictor of incident COPD.(12, 13) However, while rate of decline in lung function is associated with coronary disease mortality,(14,15) the association with incident cardiovascular events is not known. Furthermore, incipient or early heart failure may cause rapid deterioration in spirometric measures, and FEV1 in particular, due to interstitial and alveolar edema, and consequent airway compression.(16) However, rapid lung function decline secondary to early and undiagnosed HF (reserve causality) would be expected to predict incident HF during short-term – as opposed to long-term – follow-up. We hypothesize that greater decline in pulmonary function will be associated with a heightened risk of incident HF, stroke, coronary disease, and death over 20 year follow-up among middle-aged persons from a community-based sample free of prevalent cardiovascular disease.
Methods
Study population
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective cohort study of 15,792 persons, aged 45 and 64 years old at the time of recruitment.(17) Participants were recruited using a probability sampling approach in four different centers in United States: Forsyth County, North Carolina; Jackson, Mississippi; the northwest suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Since study inception (1987–1989, Visit 1), four subsequent study visits were performed. Spirometry was performed at Visit 1 and at Visit 2 (1990–1992) in all participants attending each visit. The study was approved by institutional review boards from each site and all participants gave written informed consent.
For this analysis, we included participants who underwent spirometry as part of Visit 1(1987–1989) and Visit 2 (1990–1992). We excluded subjects with prevalent HF (n=700), CHD (n=661), and stroke (n=177) at Visit 2, those without information on HF (n=234) and death (n=1), and participants without spirometry data at Visits 1 or 2 (n=312; Supplemental Figure 1). We additionally excluded subjects with poor performance on spirometry as indicated by tests lasting less than 6 seconds or with irregular tracing; or with spirograms considered not reproducible (n=1912) following the previously published American Thoracic Society guidelines (18). A total of 10,351 participants were included in this analysis.
Assessment of lung function and rapid decline in lung function
Spirometry was performed following the American Thoracic Society guidelines at Visits 1 and 2.(18) Spirometry was conducted using a water-sealed Collins Survey II volume displacement spirometer (Collins Medical, Inc) and Pulmo-Screen II software (PDS Healthcare Products, 496 Inc). Three or more acceptable spirograms were obtained from at least 5 forced expirations. The best single spirogram was identified by a computer and confirmed by a technician (18). FEV1 was assessed as the volume of gas exhaled in the first second of expiration. FVC was assessed as the volume of gas forcefully exhaled after maximal inspiratory effort. Equations for predicting FEV1 and FVC were developed to obtain the percentage of predicted FEV1 and FVC adjusted for age, sex, race, and height in ARIC Study.(19) The primary exposure variable for this analysis was the change in percent predicted FEV1 between Visits 1 and 2 and the change in percent predicted FVC between Visits 1 and 2 (Visit 1 value –Visit 2 value for both). We primarily used FEV1 and FVC as percentage of predicted instead of absolute values because the percentage predicted is a more reliable measurement of changes in spirometry over a time period of less than 5 years.(20) Supplemental analyses were performed with the FEV1/FVC ratio as the primary predictor.
Ascertainment of incident cardiovascular events
Incident HF, CHD, stroke and death were ascertained through 2012 from ongoing surveillance methods as previously described.(21,22) Incident HF was defined as the first occurrence of a hospitalization with a HF diagnosis according to the International Classification of Diseases-9th Revision (ICD-9) code 428 (428.0 to 428.9) in any position ascertained by ARIC Study retrospective surveillance of hospital discharges, or a death certificate with death from HF in any position or with an ICD-9 code of 428 or with an ICD-10 code of I50 among any of the listed diagnoses or underlying causes of death.(23) CHD was defined as a hospitalized acute myocardial infarction or CHD mortality. CHD was ascertained from yearly follow-up phone calls, study visits, hospital discharge information on fatal and non-fatal myocardial infarction, next-of-kin interviews, physician-completed questionnaires, and death certification, and was adjudicated by the ARIC Mortality and Morbidity Classification Committee as previously described.(21) Stroke was defined as rapid onset of a focal neurological deficit lasting >24 hours or until death in the absence of a non-stroke cause. A stroke event was identified from annual phone calls, visits, or surveillance of ARIC community hospitals. A hospitalization was considered for validation of stroke event if the discharge diagnosis contained a cerebrovascular disease diagnosis code (ICD-9 codes 430 – 438), if the discharge summary included a key word related to cerebrovascular procedure or disease, or if there was imaging evidence of cerebrovascular disease. Following the National Survey of Stroke criteria for stroke definition,(24) a computerized algorithm and a physician reviewer independently classified strokes as definitive or probable.(22) Death was ascertained through linkage with the National Death Index.
Covariates
We used covariates measured at both Visit 1 and Visit 2. Race was self-reported. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or use of antihypertensive medication. Body mass index (BMI) was calculated by dividing measured weight (in kilograms) by height (in meters) squared and was defined as previously described in ARIC.(25) Diabetes mellitus was defined as a baseline fasting glucose level ≥126 mg/dl, baseline non-fasting glucose level ≥200 mg/dl, self-reported physician diagnosis of diabetes or the presence of medication for diabetes. Smoking was assessed by an interviewer-administrated questionnaire. Smoking history was ascertained by an interviewer-administered questionnaire, and categorized as never smokers, former smokers, and current smokers. Pack-years were obtained from the multiplication of average number of cigarette per day and number of years of smoking reported until visit 2 as previously described.(26,27) The N-terminal fragment of prohormone for BNP (NT-proBNP) and the high-sensitivity C reactive protein (hs-CRP) were measured from visit 2 serum samples that had previously been stored at −70°C. NT-proBNP measurements were performed with a sandwich immunoassay method on the Roche Elecsys 2010 Analyzer (Roche Diagnostics Corp, Indianapolis, IN). The assay coefficients of variation were 5.4% at an NT-proBNP value of 133 pg/mL and 4.3% at a value of 4516 pg/mL. High sensitivity CRP was measured using an immunoturbidimetric assay on the Roche Modular P chemistry analyzer (Roche Diagnostics, Indianapolis, IN). The coefficient of variation was 7.0%.(28)
Analytical approach
For each spirometry measure, initial quartile analysis suggested non-linear associations of change between Visits 1 and 2 and cardiovascular outcomes, with risk primarily associated with the greatest quartile of decline (Online Table 1). Therefore, change in each spirometric measure was dichotomized, with rapid decline defined as the greatest quartile of change between Visits 1 and 2. Non-rapid decliners were defined as those participants in the remaining three quartiles and served as the reference group. This approach is concordant with several prior publications assessing rapid decline in lung function.(14,29) Participant characteristics were described among rapid and non-rapid decliners. Categorical data were reported as percent frequencies and compared by chi-squared test. Continuous normally distributed data were displayed as mean and standard deviation, and continuous non-normally distributed data were displayed as median and 25th percentile and 75th percentile. Comparisons of continuous variables between rapid decliners and non-rapid decliners were performed using t-test or Wilcoxon rank-sum test, accordingly.
Rates of incident HF, CHD, stroke, death, and the composite of these are presented as events per 1000 person-years at risk. The risk associated with rapid decline was assessed using multivariable Cox proportional hazards regression models with the non-rapid decliner group as the reference population. Three models were constructed adjusting for covariates selected based on prior literature. Model 1 was unadjusted; Model 2 adjusted for the baseline spirometry value, age, sex, race, and height; Model 3 additionally adjusted for ARIC field center, BMI, heart rate, low-density-lipoprotein cholesterol, use of lipid lowering medications, NT-proBNP, diabetes, hypertension, and smoking (smoking status and pack-years). Outcomes included HF, CHD, and stroke individually, the composite of these (HF, CHD, and stroke), and the composite of these events in addition to death (HF, CHD, and stroke, and death). Effect modification of age, race, and baseline spirometry on the relationship between rapid decline in lung function and cardiovascular outcomes was assessed using multiplicative interaction terms. The proportional hazards assumption was tested for all models. In analyses where a violation of the proportional hazards assumption was detected with the plots of log (−log (survival function)) vs. log (time) for the association between rapid decline in FEV1 and HF, we also assessed the relationship over more restricted time intervals, partitioning the follow-up time as less than or more than one year, and as less than or more than ten years. These analyses were performed by placing landmarks at one and ten years respectively, and removing all patients that had a previous event or that had died. Second events were not taken into account. We assessed for competing risk of death for cardiovascular events using the Fine and Gray competing risks regression model to estimate the corresponding sub-distribution hazard ratio.(30) All statistical analyses were performed with STATA version 13.1 (Stata Corp., College Station, TX, USA). P-values <0.05 were considered statistically significant.
Results
Population characteristics
The mean age of the cohort was 53.7±5.7 years, 56% were women, and 81% were white (Table 1, Table 2). By definition, 25% of participants were classified as rapid decliners by the FEV1 criteria of a >1.9%/year decrease in FEV1, and 25% by the FVC criteria of a >2.1%/year decrease in FVC. 16% of participants met the definition of rapid decline by both FEV1 and FVC criteria. Compared to ARIC participants included in this analysis, those not included due to poor quality or missing spirometry at Visit 2 tended to be older, more frequently black, with higher BMI and prevalence of hypertension, diabetes, and smoking (Online Table 2).
Table 1.
Baseline characteristics according to the decline in percentage of predicted FEV1
| Characteristics | Visit 1 | Visit 2 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Rapid decliners n= 2,585 | Non-rapid decliners n= 7,766 | p | Rapid decliners n= 2,585 | Non-rapid decliners n= 7,766 | p | |
| Demographics | ||||||
| Age –years | 54.4 ± 5.7 | 53.5 ± 5.6 | <0.001 | 57.7 ± 5.7 | 56.9 ± 5.6 | <0.001 |
| Male gender (%) | 1236 (48) | 3307 (43) | <0.001 | - | - | - |
| Race – White (%) | 2097 (81) | 6234 (80) | 0.31 | - | - | - |
| Center | <0.001 | - | - | - | ||
| Forsyth (%) | 727 (28) | 1990 (26) | - | - | - | |
| Jackson (%) | 418 (16) | 1337 (17) | - | - | - | |
| Minneapolis (%) | 881 (34) | 2286 (29) | - | - | - | |
| Washington (%) | 559 (22) | 2153 (28) | - | - | - | |
| Medical History | ||||||
| Hypertension (%) | 744 (29) | 2176 (28) | 0.45 | 881 (34) | 2290 (30) | <0.001 |
| Anti-hypertensive medications (%) | 618 (24) | 1793 (23) | 0.38 | 591 (23) | 1705 (22) | 0.30 |
| Diabetes (%) | 211 (8) | 634 (8) | 0.99 | 319 (12) | 906 (12) | 0.34 |
| LDL cholesterol | 137 ± 38 | 137 ± 38 | 0.86 | 134 ± 37 | 133 ± 36 | 0.20 |
| Lipid lowering medications (%) | 68 (3) | 187 (2) | 0.53 | 122 (5) | 441 (6) | 0.06 |
| Smoking Status | <0.001 | <0.001 | ||||
| Current (%) | 762 (30) | 1656 (21) | 731 (28) | 1500 (19) | ||
| Former (%) | 851 (33) | 2578 (33) | 961 (37) | 2981 (38) | ||
| Never (%) | 964 (37) | 3530 (46) | 892 (35) | 3283 (42) | ||
| NT-proBNP (pg/mL) | - | - | - | 54 [28 – 100] | 48 [26 – 85] | <0.001 |
| hs-CRP (mg/L) | - | - | - | 2.4 [1.2 – 5.0] | 2.0 [0.9 – 4.1] | <0.001 |
| Physical Exam | ||||||
| Height - cm | 168.9 ± 9.2 | 168.3 ± 9.3 | 0.001 | - | - | - |
| BMI(kg/m2) | 27.3 ± 5.0 | 27.2 ± 4.9 | 0.48 | 28.3 ± 5.4 | 27.4 ± 5.0 | <0.001 |
| Heart rate - bpm | 66.2 ± 9.9 | 66.1 ± 9.7 | 0.59 | 65.7 ± 10.3 | 65.3 ± 9.6 | 0.073 |
| Systolic BP (mmHg) | 119.8 ± 17.3 | 119.3 ± 17.2 | 0.21 | 123.1 ± 18.6 | 119.5 ± 17.4 | <0.001 |
| Diastolic BP (mmHg) | 72.9 ± 10.8 | 73.0 ± 10.5 | 0.57 | 72.8 ± 10.4 | 71.7 ± 9.8 | <0.001 |
| Spirometry | ||||||
| FEV1 (liters) | 2.96 ± 0.77 | 2.91 ± 0.74 | 0.009 | 2.60 ± 0.74 | 2.84 ± 0.72 | <0.001 |
| Δ FEV1 (liters/yr) | - | - | - | 0.12 ± 0.06 | 0.02 ± 0.05 | <0.001 |
| Δ % predicted FEV1 (%/yr) | - | - | - | 3.27 ± 1.79 | 0.02 ± 1.75 | - |
| FVC (liters) | 4.00 ± 0.99 | 3.89 ± 0.97 | <0.001 | 3.60 ± 0.97 | 3.78 ± 0.94 | <0.001 |
| Δ FVC (liters/yr) | - | - | - | 0.14 ± 0.11 | 0.04 ± 0.07 | <0.001 |
| Δ % predicted FVC (%/yr) | - | - | - | 3.09 ± 2.63 | 0.37 ± 1.81 | <0.001 |
FEV1: forced expiratory volume in 1 second; BMI: body mass index; FVC: forced vital capacity; Δ: delta; Δ % predicted FEV1 per year: delta in the percentage of predicted FEV1; Δ % predicted FVC: delta in the percentage of predicted FVC per year. BP: blood pressure; LDL: low-density cholesterol.
Table 2.
Baseline characteristics according to the decline in percentage of predicted FVC
| Characteristics | Visit 1 | Visit 2 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Rapid decliners n=2,585 | Non-rapid decliners 7,766 | p | Rapid decliners n=2,585 | Non-rapid decliners 7,766 | p | |
| Demographics | ||||||
| Age –years | 54.6 ± 5.7 | 53.4 ± 5.6 | <0.001 | 58.0 ± 5.7 | 56.8 ± 5.6 | <0.001 |
| Male gender (%) | 1113 (43) | 3430 (44) | 0.32 | - | - | - |
| Race – White (%) | 2029 (79) | 6302 (81) | 0.003 | - | - | - |
| Center | <0.001 | - | - | - | ||
| Forsyth (%) | 898 (35) | 1819 (23) | - | - | - | |
| Jackson (%) | 467 (18) | 1288 (17) | - | - | - | |
| Minneapolis (%) | 667 (26) | 2500 (32) | - | - | - | |
| Washington (%) | 553 (21) | 2159 (28) | - | - | - | |
| Medical History | ||||||
| Hypertension (%) | 805 (31) | 2115 (27) | <0.001 | 904 (35) | 2267 (29) | <0.001 |
| Anti-hypertensive medications (%) | 673 (26) | 1738 (22) | <0.001 | 639 (25) | 1657 (21) | <0.001 |
| Diabetes (%) | 214 (8) | 631 (8) | 0.81 | 334 (13) | 891 (12) | 0.046 |
| LDL cholesterol | 136.7 ± 37.4 | 137.0 ± 38.8 | 0.7385 | 134.2 ± 36.8 | 132.7 ± 36.1 | 0.077 |
| Lipid lowering medications (%) | 78 (3) | 177 (2) | 0.036 | 118 (5) | 445 (6) | 0.024 |
| Smoking status | <0.001 | <0.001 | ||||
| Current (%) | 730 (28) | 1688 (22) | 693 (27) | 1538 (20) | ||
| Former (%) | 848 (33) | 2581 (33) | 954 (37) | 2988 (39) | ||
| Never (%) | 1000 (39) | 3494 (45) | 937 (36) | 3238 (42) | ||
| NT-proBNP (pg/mL) | - | - | - | 54 [28 – 99] | 48 [26 – 85] | <0.001 |
| hs-CRP (mg/L) | - | - | - | 2.6 [1.2 – 5.4] | 1.9 [0.9 – 4.0] | <0.001 |
| Physical Exam | ||||||
| Height - cm | 168.3 ± 9.4 | 168.6 ± 9.3 | 0.17 | - | - | - |
| BMI(kg/m2) | 27.5 ± 5.0 | 27.2 ± 5.0 | 0.002 | 28.6 ± 5.4 | 27.3 ± 5.0 | <0.001 |
| Heart rate - bpm | 66.5 ± 9.8 | 66.1 ± 9.8 | 0.047 | 66.0 ± 10.2 | 65.2 ± 9.6 | <0.001 |
| Systolic BP (mmHg) | 120.1 ± 17.6 | 119.2 ± 17.1 | 0.024 | 123.2 ± 18.5 | 119.5 ± 17.5 | <0.001 |
| Diastolic BP (mmHg) | 72.8 ± 10.7 | 73.1 ± 10.5 | 0.15 | 72.7 ± 10.4 | 71.7 ± 9.8 | <0.001 |
| Spirometry | ||||||
| FEV1(liters) | 3.95 ± 0.99 | 3.95 ± 0.99 | 0.065 | 2.56 ± 0.73 | 2.85 ± 0.72 | <0.001 |
| Δ FEV1 (liters/yr) | - | - | - | 0.10 ± 0.07 | 0.03 ± 0.05 | <0.001 |
| Δ % predicted FEV1 (%/yr) | - | - | - | 2.69 ± 2.18 | 0.17 ± 1.91 | <0.001 |
| FVC (liters) | 2.86 ± 0.76 | 2.94 ± 0.74 | <0.001 | 3.49 ± 0.94 | 3.82 ± 0.94 | <0.001 |
| Δ FVC (liters/yr) | - | - | - | 0.16 ± 0.10 | 0.03 ± 0.06 | <0.001 |
| Δ % predicted FVC (%/yr) | - | - | - | 3.70 ± 2.31 | 0.17 ± 1.60 | - |
FVC: forced vital capacity; BMI: body mass index; FEV1: forced expiratory volume in 1 s; Δ: delta; Δ % predicted FEV1 per year: delta in the percentage of predicted FEV1; Δ % predicted FVC: delta in the percentage of predicted FVC per year; BP: blood pressure; LDL: low-density cholesterol.
Rapid decline in FEV1 and incident cardiovascular events
The mean annual rate of decline in percent predicted FEV1 was 3.27 ± 1.79 %/year for FEV1 rapid decliners and 0.02 ± 1.75 %/year for non-rapid decliners. Rapid decliners started with a higher FEV1 in Visit 1 but ended with a lower FEV1 at Visit 2 (Table 1). FEV1 rapid decliners also demonstrated a greater annual decline in percent predicted FVC compared to non-rapid decliners (3.09 ± 2.63 %/year versus 0.37 ± 1.81 %/year, respectively).
Compared to non-rapid decliners, FEV1 rapid decliners were older, more likely to be male, slightly taller, and had a higher prevalence of current smoking (Table 1). The prevalence of diabetes and hypertension were similar between groups, as were cholesterol levels. At Visit 1, BMI and blood pressure were not different among rapid and non-rapid decliners. However, by Visit 2, rapid decliners had higher BMI and blood pressure levels. High-sensitivity CRP, a marker of systemic inflammation, was significantly higher among rapid decliners (2.4 [1.2, 5.0] mg/L versus 2.0 [0.9, 4.1] mg/L, p <0.001). NT-proBNP assessed at Visit 2 was also higher among rapid decliners (53.8 [27.9, 99.7] pg/mL versus 47.8 [25.5, 84.7] pg/mL, p<0.001)
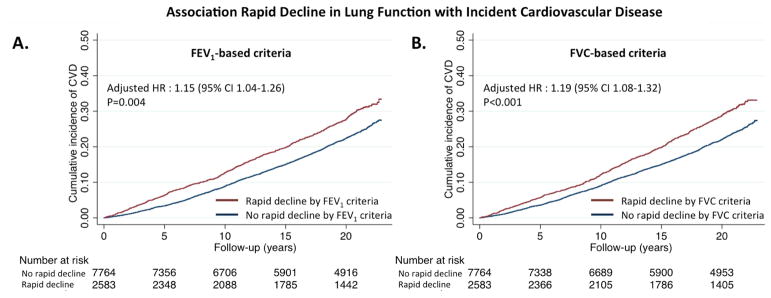
At a mean follow-up of 17±6 years, cardiovascular disease occurred in 2490 (24%) participants. HF occurred in 1473 (14%), CHD in 1093 (11%), stroke in 664 (6%) and death in 2852 (28%) (Table 3). These incidence rates are similar to those reported in other general U.S. population studies.(31–34) FEV1 rapid decliners showed a higher rate of incident cardiovascular disease compared to non-rapid decliners (composite of HF, CHD, and stroke; 16.7 (15.5–18.0)/1000 and 12.9 (12.3–13.5)/1000 person-years, respectively; Figure 1) After adjusting for baseline FEV1, age, sex, race, height, ARIC center, body mass index, heart rate, low-density-lipoprotein cholesterol, NT-proBNP, diabetes, hypertension, and smoking, rapid FEV1 decline remained associated with a higher risk of the composite endpoint (HR 1.15, 95% CI 1.04–1.26; p=0.004; Table 3 and Central Illustration). Rapid decliners also had a higher risk of death (Table 3). After accounting for competing risk of non-cardiovascular death, the risk of incident cardiovascular disease associated with rapid decline in FEV1 remained similar to that observed in the primary analysis (Table 3).
Table 3.
Risk of cardiovascular disease according to decline in percentage of predicted FEV1
| Outcomes | n | Events | Incidence rate/1000p-y | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | ||||
|
|
|
|
|
||||||
| Heart failure | |||||||||
| Non-rapid decliners | 7766 | 1043 | 7.2 (6.8–7.7) | Ref | Ref | Ref | |||
| Rapid decliners | 2585 | 430 | 9.5 (8.6–10.4) | 1.35 (1.20–1.51) | <0.001 | 1.31(1.17–1.46) | <0.001 | 1.17(1.04–1.33) | 0.010 |
| Coronary disease | |||||||||
| Non-rapid decliners | 7766 | 786 | 5.5 (5.1–5.9) | Ref | Ref | Ref | |||
| Rapid decliners | 2585 | 307 | 6.6 (5.9–7.4) | 1.24 (1.08–1.41) | 0.002 | 1.16(1.02–1.33) | 0.028 | 1.05(0.91–1.22) | 0.50 |
| Stroke | |||||||||
| Non-rapid decliners | 7766 | 467 | 3.1 (2.9–3.5) | Ref | Ref | Ref | |||
| Rapid decliners | 2585 | 196 | 4.3 (3.7–4.9) | 1.35 (1.15–1.60) | <0.001 | 1.29(1.09–1.52) | 0.003 | 1.25(1.04–1.50) | 0.015 |
| Death | |||||||||
| Non-rapid decliners | 7766 | 1989 | 13.1 (12.5–13.7) | Ref | Ref | Ref | |||
| Rapid decliners | 2585 | 863 | 17.9 (16.8–19.2) | 1.41 (1.30–1.52) | <0.001 | 1.32 (1.22–1.43) | <0.001 | 1.18 (1.08–1.28) | <0.001 |
| Composite endpoint without death | |||||||||
| Non-rapid decliners | 7766 | 1759 | 12.9 (12.3–13.5) | Ref | Ref | Ref | |||
| Rapid decliners | 2585 | 706 | 16.7 (15.5–18.0) | 1.31 (1.20–1.43) | <0.001 | 1.25 (1.15–1.36) | <0.001 | 1.15 (1.04–1.26) | 0.004 |
| Composite endpoint including death | |||||||||
| Non-rapid decliners | 7766 | 2849 | 20.5 (19.9–21.3) | Ref | Ref | Ref | |||
| Rapid decliners | 2585 | 1160 | 26.9 (25.4–28.5) | 1.34 (1.25–1.43) | <0.001 | 1.26 (1.18–1.35) | <0.001 | 1.14 (1.06–1.23) | <0.001 |
Model 1: unadjusted; Model 2: adjusted for baseline FEV1, age, sex, race, and height; Model 3: additionally adjusted for ARIC center, body mass index, heart rate, low-density-lipoprotein cholesterol, lipid-lowering medication, NT-proBNP, diabetes, hypertension, and smoking. Composite endpoint includes heart failure, stroke, and coronary disease. FEV1: forced expiratory volume in 1 s; HR (95%CI): hazard ratio and 95% confidence interval.
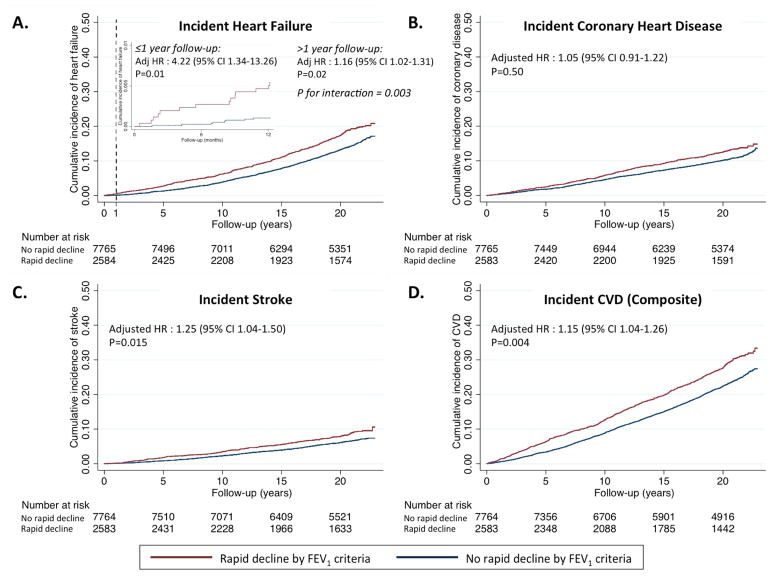
Figure 1. Association of rapid decline in FEV1 with incident cardiovascular disease.
Rapid decline is defined as greatest quartile of decline in FEV1 over a mean of 2.9 years. Kaplan-Meier curves demonstrate the rates of (A) incident heart failure, (B) incident coronary heart disease, (C) incident stroke, and (D) the composite of these among the rapid decliner and non-rapid decliner groups. Hazard ratios (HR) and associated 95% confidence intervals are calculated from Cox regression models adjusted for age, sex, race, ARIC center, height, heart rate, body mass index, LDL-cholesterol, NT-proBNP, diabetes, hypertension, and smoking. For heart failure, a violation of the proportional hazards assumption was noted for this endpoint, indicated by the significant p for interaction for decline in percentage of predicted FEV1 and time with respect to incident heart failure. The Panel A inset shows the Kaplan-Meier curve restricted to the 1st year of follow-up. HRs are provided separately for pre-1 year and post-1year follow-up (indicated by dashed line), with associated P for interaction.
Central Illustration. Association of Rapid Decline in Lung Function With Subsequent Cardiovascular Disease.
Rapid decline is defined as the greatest quartile of decline in either FEV1 (Panel A) or FVC (Panel B) over a 2.9 year period. Kaplan-Meier curves demonstrate rates of subsequent cardiovascular disease (CVD) among the rapid decliner and non-rapid decliner groups. CVD is defined as a composite of heart failure, coronary disease, and stroke. Hazard ratios (HR) and associated 95% confidence intervals are calculated from Cox regression models adjusted for age, sex, race, ARIC center, height, heart rate, body mass index, LDL-cholesterol, NT-proBNP, diabetes, hypertension, and smoking.
Rapid decliners had a higher risk of incident HF (HR 1.17, 95%CI 1.04–1.33; p=0.010), which persisted after excluding the 1093 subjects with incident CHD (HR 1.21, 95% CI 1.03–1.39; p=0.013). A violation of the proportional hazard assumption (p = 0.003) was observed for the relationship between decline in FEV1 and incident HF, such that the risk associated with FEV1 rapid decline was greater during the early follow-up period and attenuated during late follow-up. For example, FEV1 rapid decline was associated with a higher risk of HF in the first 1 year of follow-up (Table 4 and Figure 1; Supplemental Figure 2). Similarly, rapid decline was associated with a higher risk of HF during the first 10 years of follow-up but not after this point (Table 4). Rapid decline in FEV1 was also associated with a heightened risk of stroke (HR 1.25, 95% CI 1.04–1.50; p=0.015; Table 3), with no evidence of a change in the magnitude of risk over the follow-up period. Rapid decline in FEV1 was not associated with incident coronary disease (Table 3). In supplemental analyses using rapid decline in the FEV1/FVC ratio, rapid decline in the ratio was similarly associated with incident HF, and the composite of HF, CHD, and stroke (Online Table 3).
Table 4.
Risk of heart failure associated with rapid decline in lung function defined based on percentage of predicted FEV1 stratified by follow-up time (≤ or > 1 year or 10 years)
| ≤1 year | p | > 1 year | p | ≤10 year | p | > 10 year | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||
| Events | HR (95%CI) | Events | HR (95%CI) | Events | HR (95%CI) | Events | HR (95%CI) | ||||
| 22 | 4.22 (1.34–13.26) | 0.014 | 1451 | 1.16 (1.02–1.31) | 0.023 | 445 | 1.47 (1.18–1.83) | 0.001 | 1028 | 1.07 (0.92–1.24) | 0.40 |
FEV1: forced expiratory volume in 1 s; HR (95%CI): hazard ratio and 95% confidence interval. HRs are from a fully adjusted Model 3: baseline FEV1, age, sex, race, height, ARIC center, body mass index, heart rate, low-density-lipoprotein cholesterol, lipid-lowering medication, NT-proBNP, diabetes, hypertension, and smoking.
Rapid decline in FVC and incident cardiovascular events
FVC rapid decliners had a lower FVC at Visit 1 compared to non-rapid decliners (Table 2), and demonstrated an annual decline of 3.70 ± 2.31 %/year compared to 0.17 ± 1.60 %/year for non-rapid decliners. FEV1 was similar between these groups at baseline but demonstrated greater decline in the FVC rapid compared to non-rapid decline groups (2.69 ± 2.18 versus 0.17 ± 1.91%/year respectively; Table 2). FVC rapid decliners were older and more likely to be black. At Visit 1, they had higher BMI, heart rate, prevalence of hypertension, and active smoking (Table 2). These patterns persisted at Visit 2, but the magnitude of the difference in BMI, heart rate and blood pressure between groups was more prominent. Both hs-CRP and NT-proBNP were higher among rapid decliners (2.6 [1.2, 5.4] vs 1.9 [0.9, 4.0] mg/L, p <0.001; 53.4 [27.6, 98.9] vs 47.7 [25.7, 85.3] pg/mL, p<0.001 respectively).
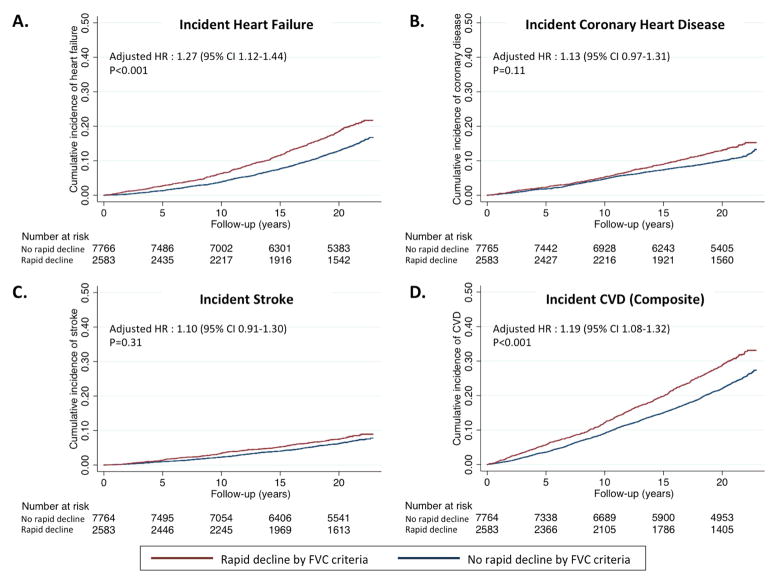
FVC rapid decline was associated with a higher rate of the composite of incident HF, CHD, and stroke (incidence rate 16.6 (15.5–18.0)/1000 in rapid decliners and 12.6 (12.0–13.2)/1000 person-years in non-rapid decliners; Table 5, Figure 2). After full adjustment, rapid FVC decline was associated with a 20% higher risk of composite endpoint (HR 1.19, 95% CI 1.08–1.32, p<0.001; Table 5). FVC rapid decline was also associated with a heightened risk of death (Table 5). In competing risk models, death was not a significant competing risk for cardiovascular disease. For components of this composite, FVC rapid decline was associated with an increased risk of incident HF (HR 1.27, 95% CI 1.12–1.44; p<0.001), which persisted after excluding participants with incident CHD (HR 1.29, 95% CI 1.11–1.50; p=0.001). Unlike FEV1 rapid decline, no significant variation in the risk associated with FVC rapid decline was noted through the follow-up period. No association was noted between FVC rapid decline and incident stroke or CHD (Table 4, Figure 2).
Table 5.
Risk of cardiovascular disease according to decline in percentage of predicted FVC
| Outcomes | n | Events | Incidence rate/1000p-y | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | ||||
|
|
|
|
|
||||||
| Heart failure | |||||||||
| Non-rapid decliners | 7766 | 1021 | 7.1 (6.6–7.5) | Ref | Ref | Ref | |||
| Rapid decliners | 2585 | 452 | 10.0 (9.1–11.0) | 1.46 (1.31–1.64) | <0.001 | 1.49 (1.33–1.67) | <0.001 | 1.27 (1.12–1.44) | <0.001 |
| Coronary disease | |||||||||
| Non-rapid decliners | 7766 | 779 | 5.4 (5.0–5.8) | Ref | Ref | Ref | |||
| Rapid decliners | 2585 | 314 | 7.3 (6.6–8.2) | 1.31 (1.15–1.49) | <0.001 | 1.31 (1.14–1.49) | <0.001 | 1.13 (0.97–1.31) | 0.11 |
| Stroke | |||||||||
| Non-rapid decliners | 7766 | 481 | 3.3 (3.0–3.6) | Ref | Ref | Ref | |||
| Rapid decliners | 2585 | 182 | 4.0 (3.4–4.6) | 1.23 (1.03–1.46) | 0.019 | 1.18 (0.99–1.41) | 0.061 | 1.10 (0.91–1.30) | 0.31 |
| Death | |||||||||
| Non-rapid decliners | 7766 | 1975 | 12.9 (12.4–13.6) | Ref | Ref | Ref | |||
| Rapid decliners | 2585 | 877 | 18.3 (17.1–19.6) | 1.46 (1.35–1.58) | <0.001 | 1.43 (1.32–1.55) | <0.001 | 1.27 (1.16–1.39) | <0.001 |
| Composite endpoint without death | |||||||||
| Non-rapid decliners | 7766 | 1748 | 12.6 (12.0–13.2) | Ref | Ref | Ref | |||
| Rapid decliners | 2585 | 717 | 16.6 (15.5–18.0) | 1.35 (1.24–1.47) | <0.001 | 1.36 (1.25–1.49) | <0.001 | 1.19 (1.08–1.32) | <0.001 |
| Composite endpoint including death | |||||||||
| Non-rapid decliners | 7766 | 2835 | 20.4 (19.7–21.2) | Ref | Ref | Ref | |||
| Rapid decliners | 2585 | 1174 | 27.3 (25.8–28.9) | 1.37 (1.28–1.47) | <0.001 | 1.36 (1.27–1.46) | <0.001 | 1.21 (1.12–1.30) | <0.001 |
Model 1: unadjusted; Model 2: adjusted age, sex, race, and height; Model 3: additionally adjusted for ARIC center, body mass index, heart rate, low-density-lipoprotein cholesterol, lipid-lowering medication, NT-proBNP, diabetes, hypertension, and smoking. Composite endpoint includes heart failure, stroke, and coronary disease.
FVC: forced vital capacity; HR (95%CI): hazard ratio and 95% confidence interval.
Figure 2. Association of rapid decline in FVC with incident cardiovascular disease.
Rapid decline is defined as greatest quartile of decline in FVC over a mean of 2.9 years. Kaplan-Meier curves demonstrate the rates of (A) incident heart failure, (B) incident coronary heart disease, (C) incident stroke, and (D) the composite of these among the rapid decliner and non-rapid decliner groups. Hazard ratios (HR) and associated 95% confidence intervals are calculated from Cox regression models adjusted for age, sex, race, ARIC center, height, heart rate, body mass index, LDL-cholesterol, NT-proBNP, diabetes, hypertension, and smoking.
No heterogeneity of effect by race or sex was detected for the association between either rapid decline in FEV1 or in FVC with heart failure, CHD, or stroke (all p for interaction >0.05). Baseline FEV1 did significantly modify the relationship between rapid decline in FEV1 and incident HF (p for interaction 0.004), such that the association of rapid decline in FEV1 was only observed among participants with a percent predicted FEV1<80% at baseline (Online Table 4). Baseline FEV1 did not significantly modify the relationship between rapid decline in FEV1 and the other outcomes assessed. Similarly, no interaction was noted between baseline FVC and the relationship between rapid decline in FVC and any of the studied outcomes. Similar associations with outcomes were noted when change in both percent predicted FEV1 and percent predicted FVC were modeled as a continuous variables instead of categorical variables (Online Table 5). In a sensitivity analysis stratified by smoking status (current and former smokers versus never smokers), similar findings were noted as in the primary analysis (Online Tables 6 and 7). No heterogeneity of effect was noted, except for the relationship between rapid decline in FEV1 and incident CHD (p for interaction by smoking status =0.04) such that rapid decline in FEV1 predicted incident CHD among never smokers but not smokers. Similar findings as in the primary analysis were also noted after exclusion of participants with probable COPD at Visit 1 (2.5% of participants; Online Table 8).
Discussion
In a bi-racial community-based sample of 10,351 participants followed for approximately 17 years, rapid decline in lung function is associated with a higher risk of cardiovascular disease, independent of established cardiovascular risk factors (Central Illustration). Rapid decline in FEV1 is associated with a higher incidence of HF, stroke and death, while rapid decline in FVC is associated with a higher incidence of HF and death. Notably, decline in FEV1 was predictive of incident HF mainly in the first year of follow-up. In contrast, a consistent risk throughout the follow-up period was noted for the association of decline in FVC with incident HF, and for the association of both exposure measures with the other study endpoints. Neither sex nor race significantly modified these associations. These findings demonstrate that deterioration in lung function is a predictor of incident cardiovascular disease, independent of smoking status and baseline lung function.
Decline in lung function starts in young adulthood. An annual fall of 25–30 ml in FEV1 is considered normal (13,35). Accelerated decline in pulmonary function results from a combination of genetic determinants and environmental exposure, and increases the risk of pulmonary diseases, mainly COPD.(36) Previous studies have shown an association between accelerated decline in lung function with systemic inflammatory activation (37), decline in arterial elasticity (38), and hypertension (39). Importantly, these same pathways have also been implicated in the development of atherosclerotic CHD,(40,41) ischemic stroke,(42) and HF.(43) Recent attention has focused on the role of co-morbidity driven systemic inflammation in the development of LV diastolic dysfunction and HFpEF in particular,(44,45) highlighting the potential parallel effects of inflammation on cardiac and pulmonary function. As a result of these shared pathophysiologic pathways, we therefore hypothesized that accelerated decline in pulmonary function would predict incident cardiovascular events. Indeed, participants with rapid decline in lung function in our analysis demonstrated higher concentrations of hsCRP – an established marker of systemic inflammation – compared to those without rapid decline. In the current study, FEV1 rapid decline in lung function was defined as the greatest quartile of decline, with FEV1 rapid decliners demonstrating an average decline of 120 ml/year, and FVC rapid decliners demonstrating an average 160 ml/year decline. These values are consistent with prior studies of rapid decline in pulmonary function.(29) Both FEV1 rapid decline and FVC rapid decline were predictive of incident cardiovascular disease independent of established cardiovascular risk factors and after accounting for the competing risk of non-cardiovascular death.
Rapid decline in both FEV1 and FVC was associated with a higher risk of incident HF, even after adjusting for potentially confounding cardiovascular risk factors including smoking status and accounting for pack-years, and NT-proBNP. In addition, the association persisted after excluding participants with incident CHD, showing that incident CHD or myocardial infarction does not explain this association. We are not aware of other studies demonstrating a relationship between changes in lung function and HF risk. Prior studies have shown a relationship between low baseline lung function and incident HF (4,5,46) and have suggested that higher levels of inflammatory markers identify persons with poor lung function who are particularly high risk for HF (29). While the HF phenotype in these studies (HF with preserved [HFpEF] versus reduced [HFrEF] ejection fraction) is not clear, impaired lung function appears differentially predictive of HFpEF compared to HFrEF(47) and the potential role for systemic inflammation in HFpEF has recently generated considerable interest.(44,45) Activation of inflammatory cascade may therefore be one mechanism linking lung function and HF, although further study is needed to support this hypothesis.(46) Previous studies showed an association between inflammation and impaired pulmonary function.(7) Indeed, the current analysis, hs-CRP - a marker of systemic inflammation - was significantly higher among FEV1 and FVC rapid decliners than non-rapid decliners.
Rapid decline in FEV1 was associated with heightened risk of subsequent HF hospitalization predominantly during the early follow-up period, with an attenuated magnitude of association later in the follow-up period, suggesting that reverse causality may be present such that FEV1 decline in some cases may be an early manifestation of HF. In contrast, rapid decline in FVC was associated with HF risk consistently throughout follow-up. One explanation for these findings is that rapid decline in FEV1 specifically may be largely an early manifestation of subclinical HF, with pulmonary congestion leading to interstitial and alveolar edema, consequent compression of the airways, and a rapid fall in FEV1.(16) Indeed, rapid decline in FEV1 secondary to early and undiagnosed HF (reserve causality) is expected to predict incident HF primarily during short-term follow-up, as observed in our analysis. In contrast, the consistent HF risk associated with rapid decline in FVC suggests a primary alteration in lung function predicting incident HF. Progressive deterioration in lung function may result in pulmonary hypertension and associated right heart failure when severe, although this is uncommon on a population level.(48) An alternative, or additional, explanation is shared pathophysiologic alterations including loss of elasticity in lung and vasculature,(38) and possibly the myocardium, with resulting coupled impairments in cardiovascular and pulmonary reserve. For any degree of cardiovascular dysfunction, impaired pulmonary function may then predispose to manifestation of HF signs and symptoms. Future studies with serial echocardiography and spirometry will be necessary to interrogate this hypothesis.
We noted differential independent associations of rapid decline in FEV1 with the atherosclerotic endpoints of stroke. The association between decline in FEV1 and coronary disease mortality was previously demonstrated in the Copenhagen City Heart Study,(14) and Baltimore Longitudinal Study of Aging.(15) We also noted an association of rapid decline in FEV1 with incident CHD in unadjusted models and after adjustment for participant demographics. However, this association did not persist after further accounting for established cardiovascular risk factors. The reasons for these between study differences are unclear but may relate to differences in the populations studied and in the adjustment variables between studies. To the extent that inflammation is one mechanism mediating the relationship between decline in pulmonary function and incident cardiovascular disease, one potential explanation for this lack of association with CHD independent of established cardiovascular risk factors is that – for this endpoint at least – the additional contribution of pulmonary dysfunction to inflammation beyond established risk factors is modest. Similarly, in unadjusted analysis, both FEV1 rapid decline and FVC rapid decline were associated with higher risk of stroke. However, only rapid decline in FEV1 remained independently associated with incident stroke in fully adjusted models. Although reduced FEV1 has been demonstrated as related to incident stroke,(49) to our knowledge, this is the first study demonstrating an association between rapid decline in FEV1 and stroke. Inflammation-associated pro-atherosclerotic mechanisms may help explain this association. In addition, decline in lung function is associated with hypertension,(39) which is a major stroke risk factor and potential mediator.(42) In our analysis, the association of rapid decline in FEV1 and incident stroke persisted after accounting for hypertension and blood pressure. Low pulmonary function has been associated with a higher risk of atrial fibrillation.(50) Although the relationship between changes in lung function and incident atrial fibrillation is not known, a higher incidence of atrial fibrillation among the rapid decline group is also a possible explanation. Finally, rapid decline in lung function is associated with a higher incidence of COPD,(13,36) which may in turn lead to higher levels of haematocrit and hemoglobin, and could predispose to stroke through elevation in the blood viscosity.(51) However, we did not find any indication that hemoglobin is a mediator in the relationship between decline in FEV1 and stroke in our analysis (data not shown).
Current guidelines recommend serial spirometry for individuals at higher risk of developing pulmonary disease, such as heavy smokers and workers with an occupational exposure.(20) Our results provide additional evidence that, in addition to providing information regarding risk of pulmonary disease, serial changes in spirometry also provides the clinician information regarding the risk of cardiovascular disease. Our findings also demonstrate that rapid decline in FEV1 in particular predicts increase risk of incident HF. This risk was particularly high, 4-fold increased, within 12 months, suggesting that clinicians should carefully consider incipient HF in patients with rapid changes in FEV1. Further studies are necessary to determine whether strategies to reduce the rate of decline in FEV1 and FVC reduce the incidence of cardiovascular diseases.
Some limitations in our analyses should be noted. The 3-year period between the two spirometry tests is relatively short and test-retest variability may limit our ability to identify participants with actual accelerated decline in lung function impairment. However, spirometry is a reliable and reproducible test, with a coefficient of variation within subjects of about 5%,(52) making variability between the two tests unlikely to bias our findings. Only pre-bronchodilator spirometric measurements are available in this study, although post-bronchodilator measures are also recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD).(11) Finally, unmeasured and residual confounding of the relationship between change in lung function and cardiovascular outcomes could not be completely addressed by multivariate modeling.
Conclusions
Rapid decline in lung function over a 3-year period is associated with a heightened risk of incident cardiovascular disease, including HF and stroke, independent of established cardiovascular risk factors and smoking. Rapid decline in FVC is associated with heighten risk of HF throughout the approximately 17 year follow-up period, while rapid decline in FEV1 specifically predicts a 4-fold increase in the risk of incident HF at 1 year, and could be an early manifestation of HF.
Supplementary Material
CLINICAL PERSPECTIVES.
Competencies in Patient Care
In addition to predicting pulmonary disease, a rapid decline in spirometric measures of lung function, FEV1 and FVC is associated a higher risk of cardiovascular disease. Specifically, rapid decline in FEV1 is associated with a 4-fold increased risk of incident heart failure during the first 12 months of follow-up, suggesting that clinicians should be alert to manifestation of heart failure when patients exhibit rapid deterioration of FEV1.
Translational Outlook
Further studies are necessary to determine whether worsening of these indices of pulmonary function reflect incipient heart failure and whether strategies to retard the decline in lung function can prevent or delay the onset of cardiovascular disease.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The work for this manuscript was also supported by NHLBI grants K08HL116792 and R01HL135008 (A.M.S.), AHA grant 14CRP20380422 (A.M.S.), the Brigham and Women’s Hospital Heart and Vascular Center Watkins Discovery Award (A.M.S.), Brazilian National Council for Scientific and Technological Development Grant 249481/2013-8 (W.N.J.), and the J.P. Lemann Foundation Jorge Paulo Lemann Harvard Medical School Cardiovascular Fellowship (O.M.S.). Dr. London is supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (ZO1 ES043012).
ABREVIATIONS LIST
- CHD
coronary disease
- CVD
cardiovascular disease
- COPD
chronic obstructive pulmonary disease
- BMI
body mass index
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- HF
heart failure
Footnotes
Conflict of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Global regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roversi S, Fabbri LM, Sin DD, Hawkins NM, Agusti A. Chronic Obstructive. Pulmonary Disease and Cardiac Diseases. An Urgent Need for Integrated Care. Am J Respir Crit Care Med. 2016;194:1319–36. doi: 10.1164/rccm.201604-0690SO. [DOI] [PubMed] [Google Scholar]
- 3.Sin DD, Wu L, Man SFP. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 4.Engström G, Melander O, Hedblad B. Population-based study of lung function and incidence of heart failure hospitalisations. Thorax. 2010;65:633–638. doi: 10.1136/thx.2010.135392. [DOI] [PubMed] [Google Scholar]
- 5.Georgiopoulou VV, Kalogeropoulos AP, Psaty BM, et al. Lung function and risk for heart failure among older adults: the Health ABC Study. Am J Med. 2011;124:334–341. doi: 10.1016/j.amjmed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. [Accessed November 14, 2016];Chronic Respiratory Disease.pdf. Available at: http://www.who.int/gard/publications/chronic_respiratory_diseases.pdf.
- 7.Gan WQ, Man SFP, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eickhoff P, Valipour A, Kiss D, et al. Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:1211–1218. doi: 10.1164/rccm.200709-1412OC. [DOI] [PubMed] [Google Scholar]
- 9.Bonetti PO, Lerman LO, Lerman A. Endothelial Dysfunction A Marker of Atherosclerotic Risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 10.Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13:197–205. doi: 10.1034/j.1399-3003.1999.13a36.x. [DOI] [PubMed] [Google Scholar]
- 11. [Accessed September 1, 2015];GOLD_Report_2015.pdf. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015.pdf.
- 12.Petersen H, Sood A, Meek PM, et al. Rapid lung function decline in smokers is a risk factor for COPD and is attenuated by angiotensin-converting enzyme inhibitor use. Chest. 2014;145:695–703. doi: 10.1378/chest.13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange P, Celli B, Agusti A, et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med. 2015;373(2):111–22. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 14.Baughman P, Marott JL, Lange P, et al. Combined effect of lung function level and decline increases morbidity and mortality risks. Eur J Epidemiol. 2012;27:933–943. doi: 10.1007/s10654-012-9750-2. [DOI] [PubMed] [Google Scholar]
- 15.Tockman MS, Pearson JD, Fleg JL, et al. Rapid decline in FEV1. A new risk factor for coronary heart disease mortality. Am J Respir Crit Care Med. 1995;151:390–398. doi: 10.1164/ajrccm.151.2.7842197. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins NM, Petrie MC, Jhund PS, Chalmers GW, Dunn FG, mcmurray JJV. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail. 2009;11:130–139. doi: 10.1093/eurjhf/hfn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 18.Standardization of spirometry--1987 update. Statement of the American Thoracic Society. Am Rev Respir Dis. 1987;136:1285–1298. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 19.Atherosclerosis Risk in Communities Study Manual 4: Pulmonary Function. Chapel Hill, NC: Chapel Hill NNH, Lung, and Blood Institute of the National Institutes of Health, Collaborative Studies Coordinating Center, University of North Carolina; 1987. [Google Scholar]
- 20.Redlich CA, Tarlo SM, Hankinson JL, et al. Official American Thoracic Society technical standards: spirometry in the occupational setting. Am J Respir Crit Care Med. 2014;189:983–993. doi: 10.1164/rccm.201402-0337ST. [DOI] [PubMed] [Google Scholar]
- 21.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 22.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 23.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 24.The National Survey of Stroke. National Institute of Neurological and Communicative Disorders and Stroke. Stroke. 1981;12:I1–91. [PubMed] [Google Scholar]
- 25.Folsom AR, Yamagishi K, Hozawa A, Chambless LE Atherosclerosis Risk in Communities Study Investigators. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howard G, Wagenknecht LE, Cai J, Cooper L, Kraut MA, Toole JF. Cigarette smoking and other risk factors for silent cerebral infarction in the general population. Stroke. 1998;29:913–917. doi: 10.1161/01.str.29.5.913. [DOI] [PubMed] [Google Scholar]
- 27.Nadruz W, Gonçalves A, Claggett B, et al. Influence of cigarette smoking on cardiac biomarkers: the Atherosclerosis Risk in Communities (ARIC) Study. Eur J Heart Failure. 2016;18:629–637. doi: 10.1002/ejhf.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michos ED, Selvin E, Misialek JR, et al. 25-Hydroxyvitamin D Levels and Markers of Subclinical Myocardial Damage and Wall Stress: The Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2016;5:e003575. doi: 10.1161/JAHA.116.003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mannino DM, Davis KJ. Lung function decline and outcomes in an elderly population. Thorax. 2006;61:472–477. doi: 10.1136/thx.2005.052449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 31.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 33.Safford MM, Brown TM, Muntner PM, et al. Association of Race and Sex With Risk of Incident Acute Coronary Heart Disease Events. JAMA. 2012;308(17):1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carandang R, Seshadri S, Beiser A, et al. Trends in Incidence, Lifetime Risk, Severity, and 30-Day Mortality of Stroke Over the Past 50 Years. JAMA. 2006;296(24):2939–2946. doi: 10.1001/jama.296.24.2939. [DOI] [PubMed] [Google Scholar]
- 35.Vestbo J, Lange P. Natural history of COPD: Focusing on change in FEV1. Respirology. 2016;21:34–43. doi: 10.1111/resp.12589. [DOI] [PubMed] [Google Scholar]
- 36.Tang W, Kowgier M, Loth DW, et al. Large-scale genome-wide association studies and meta-analyses of longitudinal change in adult lung function. Plos ONE. 2014;9:e100776. doi: 10.1371/journal.pone.0100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hancox RJ, Poulton R, Greene JM, et al. Systemic inflammation and lung function in young adults. Thorax. 2007;62:1064–1068. doi: 10.1136/thx.2006.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duprez DA, Hearst MO, Lutsey PL, et al. Associations among lung function, arterial elasticity, and circulating endothelial and inflammation markers: the multiethnic study of atherosclerosis. Hypertension. 2013;61:542–548. doi: 10.1161/HYPERTENSIONAHA.111.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobs DR, Yatsuya H, Hearst MO, et al. Rate of decline of forced vital capacity predicts future arterial hypertension: the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2012;59:219–225. doi: 10.1161/HYPERTENSIONAHA.111.184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 41.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 42.Kannel WB, Wolf PA, mcgee DL, Dawber TR, mcnamara P, Castelli WP. Systolic Blood Pressure, Arterial Rigidity, and Risk of strokethe Framingham Study. JAMA. 1981;245(12):1225–1229. [PubMed] [Google Scholar]
- 43.Vasan RS, Sullivan LM, Roubenoff R, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–91. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 44.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 45.Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agarwal SK, Heiss G, Barr RG, et al. Airflow obstruction, lung function, and risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) study. Eur J Heart Fail. 2012;14:414–422. doi: 10.1093/eurjhf/hfs016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lam CSP, Lyass A, Kraigher-Krainer E, et al. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30. doi: 10.1161/CIRCULATIONAHA.110.979203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seeger W, Adir Y, Barbera JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62:D109–D116. doi: 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 49.Truelsen T, Prescott E, Lange P, Schnohr P, Boysen G. Lung function and risk of fatal and non-fatal stroke. The Copenhagen City Heart Study. Int J Epidemiol. 2001;30:145–151. doi: 10.1093/ije/30.1.145. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Agarwal SK, Alonso A, et al. Airflow Obstruction, Lung Function, and Incidence of Atrial Fibrillation. Circulation. 2014;129:971–980. doi: 10.1161/CIRCULATIONAHA.113.004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wannamethee G, Perry IJ, Shaper AG. Haematocrit, hypertension and risk of stroke. J Intern Med. 1994;235:163–168. doi: 10.1111/j.1365-2796.1994.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 52.Laszlo G. Standardisation of lung function testing: helpful guidance from the ATS/ERS Task Force. Thorax. 2006;61:744–746. doi: 10.1136/thx.2006.061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.