Abstract
Catecholamines [adrenaline (A) and noradrenaline (NA)] are known to stimulate glucose metabolism at rest and in response to maximal exercise. However, training and recovery mode can alter theses hormones. Thus our study aims to examine the effects of recovery mode during High-intensity Interval Training (HIIT) on glucoregulatory hormone responses to maximal exercise in young adults. Twenty-four male enrolled in this randomized study, assigned to: control group (eg, n=6), and two HIIT groups: intermittent exercise (30 s run/30 s recovery) with active (arg, n=9) or passive (prg, n=9) recovery, arg and prg performed HIIT 3 times weekly for 7 weeks. Before and after HIIT, participants undergo a Maximal Graded Test (MGT). Plasma catecholamines, glucose, insulin, growth hormone (Gh) and cortisol were determined at rest, at the end of MGT, after 10 and 30 min of recovery. After training V02max and Maximal Aerobic Velocity (MAV) increased significantly (p<0.05) in arg. After HIIT and in response to MGT plasma glucose increase significantly (p=0.008) lesser in arg compared to prg whereas insulin concentrations were similar. The glucose/insulin ratio was significantly lower at MGT end (p=0.033) only in arg after training. After HIIT, in response to MGT, plasma A, NA, cortisol and Gh concentrations were significantly higher only in arg (p<0.05). HIIT using active recovery is beneficial for aerobic fitness, plasma glucose and glucoregulatory hormones better than HIIT with passive recovery. These findings suggest that HIIT with active recovery may improve some metabolic and hormonal parameters in young adults.
Keywords: Glucose, Insulin, Cortisol, Maximal exercise, Training recovery
Introduction
In the scientific literature related to exercise physiology, to improve aerobic fitness, it is well known that intermittent running exercise (Interval training, IT) represents an effective method [1]. Longitudinal studies have demonstrated its effectiveness in improving maximal oxygen uptake (VO2max) [2–4] as this type of training allows a high level of VO2 to be elicited by an individual [5].
It is influenced by several factors such as intensity and duration of exercise and recovery, with or without series (e.g. A number of repetitions interspersed by a relatively long recovery) and the recovery mode (passive vs. active recovery). Concerning the latter factor, recently Ben abderrahman et al. [2,3] demonstrated that an it program (30 s running at 100% of Maximal Aerobic Velocity (MAV)-30 s recovery, 3 times a week for 7 weeks) with active recovery is more efficient with respect to passive recovery to increase aerobic fitness (e.g. VO2max) and MAV. The same results were also observed by Dorado et al. [6] using another it program. Considerable information is available concerning the physiological adaptations responsible for the improvements in endurance performance observed following high- intensity it in sedentary and recreationally trained individuals [7]. In contrast, relatively little is known concerning the hormonal glucoregulation changes that occur following high- intensity it and its implication on the potential increase of performance. Since, it is well known that muscle and liver glycogen are the major endogenous energy sources for intermittent exercise and reductions of muscle glycogen reduces endurance performance and impairs high intensity exercise capacity [8].
On the other hand, it is well established that, the nervous and endocrine systems are important mediators of the body’s physiological adjustment in response to a variety of physical, environmental and behavioral stressors [9]. For example, under stress conditions, the hypothalamus controls many hormone secretions in order to adjust glucose (Glu) metabolism and energy production. To this end, Glu secretion and uptake are under the control of nervous and especially hormonal factors as catecholamines, Cortisol (C), glucagon, Growth Hormone (Gh) and Insulin (Ins) [10].
On that context, catecholamines, Adrenaline (A) and Noradrenaline (NA) are known as stress hormones and are responsible for many adaptive processes both at rest and during exercise [11]. In fact these hormones are known to affect the regulation of intermediary metabolism, affecting Glu production and muscle glycogen mobilization, all of which affect exercise metabolism and performance [9,11]. Sympatho-adrenergic activity is influenced by several factors, especially by acute exercise and chronic training. Recently, Ben abderrahman et al. [2] demonstrated that it with active recovery in comparison to passive recovery induces a significant increase of A and NA concentrations in response to maximal exercise. Hence, they observed that this type of training is also accompanied by an increase of the adrenal medulla responsiveness to the sympathetic nervous activity as shown by the significant increase of the ratio A/NA only in active recovery group. Despite the importance of the Glu energy supply for athletic performance as well as its regulation, little is known about the effect of it exercises on glucoregulatory hormone responses and especially the effect of recovery mode, active vs. passive recovery and to the best of our knowledge no study has investigated the effects of the type of recovery (active vs. passive) on glucoregulation during exercise. Consequently, the aim of this longitudinal study was to compare before and after a seven week High-Intensity It (HIIT) program, and the effects of the recovery mode (active vs. passive) on glucoregulatory hormones and physical performances. We hypothesize that Glu metabolism regulation in response to maximal exercise will be different depending on recovery mode after the HIIT program.
Materials and methods
Participants
Twenty-four male physical education students volunteered to participate in this study. Participants were assigned in randomized order to a control group (cg, n=6), and two HIIT groups: intermittent exercise (30 sie) with active (arg, n=9) or passive recovery (prg, n=9). Their age and physical characteristics measured before and after training are displayed in Table 1. All subjects were well trained, but none were specialized in middle- or long-distance running, none had ever practiced interval exercises as a training program, and none had undergone any it for 3 months before this study. They practice several sports (soccer, judo, swimming, athletic events) in their institute of physical and sports education for several hours a week (8-12 h/wk).
Table 1.
Anthropometric characteristics and maximal oxygen uptake (VO2max) of the three groups, arg, prg and cg before and after training program.
| Arg (n=9) | Prg (n=9) | Cg (n=6) | ||||
|---|---|---|---|---|---|---|
| Pre-test post-test | Pre-test | Post-test | Pre-test | Post-test | 21.1± 1 | |
| Age | 21.1± 1 | 21.1± 1 | 21± 1 | 21± 1 | 21.1± 1 | |
| (years) | 179.2±4.1 | 179±3.7 | 182.3±5 | 181.5±4.6 | 176.8±4.5 | 176.7±4.4 |
| Height | 179.2±4.1 | 179±3.7 | 182.3±5 | 181.5±4.6 | 176.8±4.5 | |
| (cm) | 76.8±10.9b | 77.3±10.4b | 74.5±10.8c | 74.1±10.3c | 66.5±6.1 | 67.2±6.5 |
| Weight | 76.8±10.9b | 77.3±10.4b | 74.5±10.8c | 74.1±10.3c | 66.5±6.1 | |
| (kg) | 10.6±3.4 | 10.8±3.5 | 11.8±4.1 | 12.5±4.6 | 10.5±1.9 | 10.9±2.2 |
| Fat mass | 10.6±3.4 | 10.8±3.5 | 11.8±4.1 | 12.5±4.6 | 10.5±1.9 | |
| (%) | 68.4±7.8 | 68.7±7.2 | 64.7±7.7 | 63.9±7.1 | 59.4±4.8 | 59.8±5.2 |
| Fat free mass | 68.4±7.8 | 68.7±7.2 | 64.7±7.7 | 63.9±7.1 | 59.4±4.8 | |
| (kg) | 16.1±1.7 | 17.3±1.2 a | 15.6±1.4 | 16.6±1.5a | 16.1±0.4 | 15.8±0.6 |
| Mav | 16.1±1.7 | 17.3±1.2a | 15.6±1.4 | 16.6±1.5a | 16.1±0.4 | |
| (km.h−1) | 4.5±0.7 | 4.8±0.7 | 4.3±0.4 | 4.4±0.5 | 4±0.4 | 4±0.4 |
| Vo2max (l.min−1) | 4.5±0.7 | 4.8±0.7 | 4.3±0.4 | 4.4±0.5 | 4±0.4 | 60±4.2 |
| Vo2max (ml.min−1.kg−1) | 59.4±9.3 | 62.9±10.3a | 58.7±5.7 | 60.4±6 | 60.4±2.7 | 199±3 |
| Vo2max | 198±6 | 197±6 | 200.1±5.2 | 198±4.7 | 200±6 | |
| (ml.min−1.kg−1ffm) | 1.1±0.03 | 1.1±0.05 | 1.1±0.06 | 1.1±0.04 | 1.1±0.1 | 1.2±0.1 |
| Hrmax (bpm) | 1.1±0.03 | 1.1±0.05 | 1.1±0.06 | 1.1±0.04 | 1.1±0.1 | 2.2±0.8 |
| Rer | 2.1±0.3 | 2.4±0.7 | 1.7±0.3 | 2.1±0.6 | 2.5±0.6 | 6.1±1.2 |
| [la]rest | 6.5±1.8 | 6.5±1.2 | 6.9±1.4 | 6.4±1.4 | 6.9±0.9 | |
| (mmol.l−1) | ||||||
Data are means (± sd). Ffm: fat free mass, hr: heart rate, rer: respiratory exchange ratio, VO2max: oxygen consumption, [la]: blood lactate concentration, mav: maximal aerobic velocity, tg: trained group, cg: control group, p1: before training, p2: after training.
A: significantly different from rest values, a p<0.05
B: significantly different between arg and cg, b p<0.01
c: significantly different between prg and cg, c p<0.01
The subjects were informed of the procedures and purposes of the study and written informed consent was obtained. Prior to testing, the participants underwent a medical examination and were fully informed about the experimental procedures. Written informed consent was obtained from all participants in accordance with the international ethical standards. The whole experimental process was conducted in accordance with the guidelines of the ethical committee of the university of rennes 2, a signed accord which had approved the experimental protocol and the procedures involved was prearranged by all participants in the study.
Experimental design
The experimental procedures were similar to that our previous studies [2, 3]. Before the training period, all subjects visited the laboratory for a familiarization session with all the material of the experiment. During this session they underwent a complete clinical and electrocardiological examination. Anthropometric measurements were performed before and after the training period by a researcher trained in anthropometric assessment.
All participants performed a maximal graded exercise test (MGT; see below) to determine their VO2max and their maximal aerobic velocity (MAV) before and after the 7-week training program. The MGT took place in the morning after a standardized breakfast (10 kcal/kg which 55% came from carbohydrates, 33% from lipids and 12% from proteins). During the mgt, respiratory gas exchange was measured breath-by-breath using a calibrated portable telemetric system (cosmed k4b2, Rome, Italy). Heart rate (HR) was continuously monitored (polar electro, kempele, Finland) with 5s interval recording. The subjects of the arg and prg performed another MGT (without expired gas measurement) at the mid of the seven weeks of high-intensity interval training program (Swhitp) to assess MAV and to update the training speeds.
Atmospheric conditions were verified before each test, making sure that all sessions were carried out under similar environmental conditions (temperature ranges from 14 to 20°c, speed of wind<2m.s−1 and humidity ranges from 50 to 70%).
Maximal graded test [12]
This MGT tests was carried out on 200 m outdoor tartan track (of which 60 m indoors) calibrated with cones. Blue cones were set at 50 m intervals along the track (inside the first line) while red cones were set 2 m behind the blue cones. The running pace was set by an examiner, equipped with a whistle and a chronometer, which made a short sound when the subject had to pass by a cone to be able to maintain a constant speed. At each sound, the subject had to be within 2 m of the blue cones. When subjects were behind a red cone three consecutive times or when the subjects stopped the exercise, judging themselves exhausted, the test ended. The initial speed was 8 km.h−1 and was increased by 1 km.h−1 every 2 min. The velocity at the last completed stage was considered as MAV. If the velocity at exhaustion was only maintained for 1 min (half of the stage duration), then MAV was considered to be equal to the velocity during the previous stage plus 0.5 km.h−1 [14]. During the test, the subjects were verbally encouraged to run for as long as possible.
Training program
Only trained groups (arg and prg) participated to an intermittent training program 3 times a week during the 7 week program (Table 2). The training sessions were exclusively composed of Intermittent Exercise (30 s/30 s ie) and were performed outside on a 400-m track. All sessions included three different periods. The session started with the warm-up including 15 min of continuous jogging, followed by 5 min of stretching exercises and 5 short bursts of accelerations. Then, the subjects executed the IE session. Subjects were divided into several groups according to their MAV values and so distances to be covered in IE. This distribution was done in order to reduce subjects’ number per IE session. During the recovery period, a loud sound was made at mi-period (15 s) to inform the subjects of the remaining time for the end of recovery. At the end of the training session subjects cooled down for about 15 min, running at low intensity and performing static stretching. At the midpoint of the training program, the trained groups performed a MGT (without respiratory-gas-exchange measurements) to assess maximal aerobic velocity to update each subject’s training speed. All training sessions were supervised by two members of our laboratory. Progressive overload was applied weekly by increasing the number and/or the intensity of IE repetition in a session (Table 2). During this period cg did not participated to any physical training program.
Table 2.
The training program for both trained groups (arg and prg).
| Week 1 | week 2 | week 3 | Week 4 | Week 5 | week 6 | Week 7 | |
|---|---|---|---|---|---|---|---|
| 2× | 2× | 2× | 2× | 2× | 2× | 2× | |
| Arg | (8×30 s ie) | (10×30 s ie) | (8×30s ie) | (10×30s ie) | (10×30s ie) | (10×30s ie) | (10×30s ie) |
| (n=9) | 100%/50% mav | 110%/50% mav | 110%/50% mav | 110%/50 mav | 110%/50% mav | 100%/50% mav | 100%/50% mav |
| R=5 min | r=5 min | r=5 min | R=5 min | R=5 min | r=5 min | R=5 min | |
| 2× | 2× | 2× | 2× | 2× | 2× | 2× | |
| Prg | (12×30s ie) | (15×30 s ie) | (12×30s ie) | (15×30 s ie) | (15×30s ie) | (15×30s ie) | (15×30s ie) |
| (n=9) | 100%/0% mav | 110%/0% mav | 110%/0% mav | 110%/0% mav | 110%/0% mav | 100%/0% mav | 100%/0% mav |
| R=5 min | r=5 min | r=5 min | R=5 min | R=5 min | r=5 min | R=5 min |
Training program for the both trained groups. Arg: active recovery group; prg: passive recovery group; MAV: maximal aerobic velocity; r: passive recovery between series. Example: [2×(8× 30sie) 100%/50% MAV. R=5 min] it means that the subject had to run 2 series of 8 times 30 s/30 s composed of 30 s running at 100% of MAV and 30 s active recovery at 50% of MAV. The subject recovers passively 5 min between each two series. Each session is repeated 3 times a week.
Blood sampling
Blood samples were obtained from an antecubital vein at rest, at the end of the MGT, after 10 and 30 min of recovery, both before and after training. Before the beginning of sampling and upon arrival, each subject lied down and a heparinized catheter (insyte-w, 1.1 mm o.d. × 30 mm) was inserted into an antecubital vein and thereafter the first blood sample (10 ml) was drawn. Standardization of the position critical, hence, participant remained in sitting position for 15 min prior to first blood collection (rest position) [15]. Collected samples using EDTA tubes and were measurement for plasma Glu, Insulin (Ins), Adrenaline (A), noradrenaline (NA), growth hormone (Gh) and cortisol (C). Plasma glucose was assayed by the glucose oxidise method (boehringer mannheim kit, meylan, France). The sensitivity of the assay was 0.12 mmol.l-1 and the coefficient of intra-assay variation was 2.4%. Plasma insulin was determined by RadioImmunoassay (RIA) using a specific kit (cis bio international, orisindustrie sa, France). The detection limit of insulin in the described method was 2μu.ml−1 and the interassay coefficient of variation was 5.5%. Plasma catecholamine concentrations were measured by high-performance liquid chromatography (chromosystems, thermofinnigan, France), following the method of Koubi et al. [16]. The detection limit of catecholamine in the described method was 0.06 nmol.l−1 and the interassay coefficient of variation was 6.4%. Plasma Gh and C were determined in duplicate in a single assay using commercially prepared ria kits (gammacoat [125i], diasorin, stillwater, mn). Lower limits of detection for Gh and C were 0.4 ng/ml and 0.2 μg/dl, respectively. The intra- assay coefficients of variation were 10.5% and 6% for Gh and C, respectively. Blood lactate concentration was determined enzymatically using a lactate analyser (microzym, cetrix, France). All the plasma concentrations were corrected taking into account the plasma volume variations [17].
Statistical analysis
Data were expressed as mean values ± Standard Deviation (SD). Sigma stat 3.10 software (SPSS, Chicago, il, USA) was used for statistical analysis. After testing for normal distribution (kolmogorov–smirnov test), differences within and between the groups were analysed using a two-way analysis of variance for repeated measurements. After confirming significant group differences over time, post-hoc Newman-keul’s tests were performed. Linear regression analyses were used to assess the independent contribution of adrenaline, noradrenaline and lactate to incident VO2max values. Relationships between parameters were assessed using a spearman’s rho correlation. A value of p<0.05 was accepted as the minimal level of statistical significance.
Results
Table 1 showed the morphological characteristics and the physiological responses to the graded maximal exercise measured before and after Swhitp. The participants of arg and prg were heavier than the cg subjects both before and after the training period (p<0.05 and 0.01, respectively, for arg and prg). However, this training program induced no significant effects on morphological characteristics. Before the Swhitp there were no significant differences between the 3 groups concerning mav and VO2max. However, our training program induced a significant increase (p<0.05) for these two parameters in only the arg. Glucose and insulin variation in response to the MGT before and after the training period plasma Glu and Ins concentrations determined at rest, at the end of the MGT, and after 10 and 30 min of recovery, before and after the Swhitp are presented in Table 3 and 4 respectively. Plasma Glu concentration increased significantly at the end of exercise and remained higher after 10 min of recovery in all groups both before and after training. No significant differences were observed between our three groups (p>0.05). However, after the training period and in response to MGT plasma Glu increase significantly (p=0.008) slightly less in arg (−0.65 ± 1.64) than prg (− 0.22 ± 1.15) as demonstrated by the delta results.
Table 3.
Plasma Glu concentrations determined before (p1) and after (p2) intermittent training program at rest, at the end of the maximal grated test, after 10 and 30 minutes recovery for the three groups, arg, prg and cg.
| Glu0 | Gluend | Glu10 | Glu30 | ||
|---|---|---|---|---|---|
| P1 | 5.50 ± 0.64 | 7.73 ± 1.09 a | 7.60 ±1.17 a | 5.76 ± 0.67 | |
| Arg (n=9) | P2 | 5.70 ± 0.53 | 7.08±1.30 ab | 7.81 ±0.91 a | 5.96 ± 0.72 |
| Delta | 0.20 ± 0.49 | −0.65 ± 1.64 | 0.21 ±0.92 | 0.20 ± 0.99 | |
| P1 | 5.20 ± 0.52 | 7.69 ± 0.50 a | 7.24 ± 0.55 a | 5.79 ± 1.22 | |
| Prg (n=9) | P2 | 5.23 ± 0.83 | 7.47 ± 0.82 | 7.23 ± 0.55 a | 5.61 ± 0.83 |
| Delta | 0.03 ± 0.45 | −0.22 ±1.15 | −0.01 ± 1.00 | −0.18 ± 1.39 | |
| P1 | 5.33 ± 0.93 | 7.31 ± 1.52 a | 7.39±1.22a | 5.55 ± 0.55 | |
| Cg (n=6) | P2 | 5.51 ± 0.54 | 7.35 ± 0.89 a | 7.33±1.29a | 5.79 ± 0.92 |
| Delta | 0.20 ± 0.74 | 0.04 ± 1.19 | −0.06 ± 0.71 | 0.24 ± 0.62 |
Data are means (±sd) plasma Glu concentration at rest (Glu0), at the end of exercise (Gluend), after 10 min of recovery (Glu10) and after 30 min of recovery (Glu30). Arg: active recovery group; prg: passive recovery group; cg; control group; p1: before training; p2: after training.
A: significant differences compared to rest values, a p<0.05.
B: significant differences between before and after training program, b p<0.05.
Table 4.
Plasma Ins (mui/ml) concentrations determined before (p1) and after (p2) intermittent training program at rest, at the end of the maximal grated test, after 10 and 30 minutes recovery for the three groups, arg, prg and cg.
| Ins0 | Insend | Ins10 | Ins30 | ||
|---|---|---|---|---|---|
| P1 | 7.66±1.81 | 15.06±3.68a | 20.09±3.70a | 14.48 ± 4.35 a | |
| Arg (n=9) | P2 | 7.77±2.15 | 16.67± 3.30 a | 29.12± 4.84 ab | 15.85 ± 5.67a |
| Delta | 0.11±1.59 | 1.61 ± 3.87 | 9.03 ± 6.48 | 1.37 ± 7.55 | |
| P1 | 7.49±1.75 | 14.87 ± 3.61a | 20.03±7.59a | 14.98 ± 4.31a | |
| Prg (n=9) | P2 | 7.93±1.37 | 14.63±2.08a | 20.92±4.17a | 15.31 ± 2.76a |
| Delta | 0.44± 1.32 | −0.24 ± 3.36 | 0.89 ± 4.62 | 0.33 ±5.19 | |
| P1 | 7.46± 1.52 | 14.43 ± 2.77a | 20.02±4.35a | 15.30 ± 3.01a | |
| Cg (n=6) | P2 | 7.45± 1.81 | 14.96 ± 5.18a | 20.37±5.06a | 15.39 ± 2.95a |
| Delta | −0.01±2.85 | 0.53 ± 4.20 | 0.35 ± 3.40 | 0.09 ± 3.60 |
Data are means (±sd) plasma Ins concentration at rest (Ins0), at the end of exercise (Insend), after 10 min of recovery (Ins10) and after 30 min of recovery (Ins30). Arg: active recovery group; prg: passive recovery group; cg; control group; p1: before training; p2: after training.
A: significant differences compared to rest values, a p<0.05.
B: significant differences between before and after training program, b p<0.05.
Plasma Ins determined at rest (Table 4) was similar between the three groups before and after Swhitp. In response to MGT and during the recovery period plasma Ins concentration increased significantly (p<0.05) both before and after the training period. Plasma Ins concentration measured 10 min after the end of MGT was more important after Swhitp with an increase of 9.03 ± 6.48 in arg and 0.89 ± 4.62 in prg.
Ratio (Glu/Ins)
Ratio (Glu/Ins) determined at rest, at the end of the maximal grated test, after 10 and 30 min recovery, before and after the Swhitp are presented in Table 5. The ratio was significantly lower at the end of exercise (p=0.033) and remained decrease at 10 min of recovery (p=0.028) only in arg after training. No significant differences were observed in either the prg or cg after the training program.
Table 5.
Ratio glucose/insulin (Glu/Ins) determined before (p1) and after (p2) intermittent training program at rest, at the end of the maximal grated test, after 10 and 30 minutes recovery for the three groups, arg, prg and cg.
| Glu0/Ins0 | Gluend/Insend | Glu10/Ins 10 | Glu30/Ins30 | |
|---|---|---|---|---|
| P1 | 0.76±0.24 | 0.54±0.14 | 0.38±0.06 | 0.45±0.20 |
| Arg (n=9) P2 | 0.80±0.27 | 0.44±0.11 b | 0.27±0.05b | 0.42±0.15 |
| Delta | 0.03±0.26 | −0.10±0.11 | −0.11±0.10 | −0.03±0.24 |
| P1 | 0.74±0.23 | 0.56±0.20 | 0.44±0.24 | 0.45±0.30 |
| Prg (n=9) P2 | 0.67±0.11 | 0.52±0.10 | 0.36 ±0.007 | 0.39±0.14 |
| Delta | −0.07±0.19 | −0.04±0.25 | −0.08±0.18 | −0.07±0.35 |
| P1 | 0.74±0.32 | 0.51±0.07 | 0.38±0.07 | 0.38±0.09 |
| Cg (n=6) P2 | 0.78±0.21 | 0.54±0.17 | 0.39±0.16 | 0.40±0.13 |
| Delta | 0.04±0.36 | 0.03±0.19 | 0.01±0.11 | 0.02±0.14 |
Data are means (±sd) ratio glucose/insulin at rest (Glu0/Ins0), at the end of exercise (Gluend/Insend), after 10 min of recovery (glu10/Ins10) and after 30 min of recovery (Glu30/Ins30). Arg: active recovery group; prg: passive recovery group; cg; control group; p1: before training; p2: after training.
B: significant differences between before and after training program, b p<0.05.
Cortisol variation in response to the MGT before and after the training period.
Plasma C concentrations determined at rest, at the end of the MGT, and after 10 and 30 min of recovery, both before and after the Swhitp and are presented in Table 6.
Table 6.
Plasma cortisol (ng.m−1) concentrations determined before (p1) and after (p2) intermittent training program at rest, at the end of the maximal grated test, after 10 and 30 min recovery for the three groups, arg, prg and cg.
| C0 | Cend | C10 | C30 | ||
|---|---|---|---|---|---|
| P1 | 176.50±102.77 | 231.50±123.06a | 275.00 ± 69.60a | 275.5 ± 79.99a | |
| Arg (n=9) | P2 | 219.50±71.97 | 252.00±62.95ab | 302.50±89.14ab | 305.50±82.91ab |
| Delta | 43.00 ± 12.13 | 20.50 ± 87.06 | 55.00 ± 86.51 | 30.00 ± 65.79 | |
| P1 | 178.00 ± 62.90 | 227.8 ± 73.40a | 261.5 ± 49.28a | 258.50 ± 50.11a | |
| Prg (n=9) | P2 | 209.00 ± 70.23 | 230.50± 6439a | 242.80±60.33a | 261.40± 48.53a |
| Delta | 31.00 ± 93.03 | 2.70 ± 34.65 | −18.70 ± 40.81 | 2.90 ± 33.81 | |
| P1 | 182.90 ± 45.10 | 235.00±77.78a | 281.10±46.63a | 269.20± 59.40a | |
| Cg (n=6) | P2 | 190.00 ± 47.17 | 225.20±43.00a | 302.20±31.94a | 316.20± 58.83a |
| Delta | 7.10 ± 46.65 | −9.80±49.83 | 21.10±66.79 | 47.0 ± 72.22 |
Data are means (±sd). Plasma c concentration at rest (C0), at the end of exercise (Cend), after 10 min of recovery (C10) and after 30 min of recovery (C30). Arg: active recovery group; prg: passive recovery group; cg; control group; p1: before training; p2: after training.
A: significant differences compared to rest values, a p<0.05.
B: significant differences between before and after training program, b p<0.05.
The MGT induced a significant increase of plasma c values determined at the end of MGT, after 10 min and 30min of recovery compared to basal values for the three groups (arg, prg and cg), both before and after the Swhitp. Before training, plasma c concentrations determined at rest, at the end of the exercise and after 10 and 30 min of recovery were similar between the 3 groups. As shown by the delta values, in response to the Swhitp, plasma c concentration was significantly higher only in arg at the end of exercise (+ 20.50 ± 87.06; p 0.046), after 10 min (+ 55.00 ± 86.51; p=0.034) and after 30 min of recovery (+ 30.00 ± 65.79; p=0.042).
Growth hormone (Gh) variation in response to the MGT before and after the training period.
Plasma Gh concentrations measured at rest, at the end of the MGT, and after 10 and 30 min of recovery, before and after the swhitp are displayed in Table 7.
Table 7.
Plasma Gh concentrations determined before (p1) and after (p2) intermittent training program at rest, at the end of the maximal grated test, after 10 and 30 minutes recovery for the three groups, arg, prg and cg.
| Gh0 | Ghend | Gh10 | Gh30 | ||
|---|---|---|---|---|---|
| P1 | 0.94±0.41 | 11.39±9.50a | 18.82±8.95a | 12.94± 10.23a | |
| Arg (n=9) | P2 | 1.27±0.57 | 16.93±4.93ab | 22.80±4.55ab | 16.18± 3.20ab |
| Delta | 0.31±0.60 | 5.54 ± 7.83 | 3.98 ±10.11 | 3.24 ± 6.77 | |
| P1 | 0.95±0.35 | 11.19±5.79a | 16.91± 5.49a | 12.69 ± 4.59a | |
| Prg (n=9) | P2 | 1.03±0.53 | 14.36 ± 3.83a | 17.27 ± 4.78a | 13.09 ± 3.24a |
| Delta | 0.08±0.55 | 3.17 ± 3.87 | 0.36 ± 4.47 | 0.40 ± 5.72 | |
| P1 | 0.92±0.23 | 11.33 ± 4.95a | 17.87 ± 6.03a | 13.94 ± 5.04a | |
| Cg (n=6) | P2 | 0.91±0.24 | 11.83 ± 4.20a | 17.48 ± 3.96a | 13.75 ± 4.72a |
| Delta | −0.01±0.23 | 0.50 ± 2.93 | −0.39 ± 2.81 | −0.19 ± 1.42 |
Data are means (±sd) plasma Gh concentration at rest (Gh0), at the end of exercise (Ghend), after 10 min of recovery (Gh10) and after 30 min of recovery (Gh30). Arg: active recovery group; prg: passive recovery group; cg; control group; p1: before training; p2: after training.
A: significant differences compared to rest values, a p<0.05.
B: significant differences between before and after training program, b p<0.05.
Plasma Gh concentrations were similar in response to the MGT between the three groups. [Gh] increased significantly at the end of the MGT and during the recovery period. As shown by the delta values, in response to the Swhitp, [Gh] measured at the end of exercise (p=0.000) and after 10 (p=0.001) and 30 min of recovery (p=0.001) was significantly higher only in arg.
Adrenaline (A) and Noradrenaline (NA) variation in response to the MGT before and after the training period
Plasma A and NA concentrations determined at rest, at the end of the MGT, and after 10 and 30 min of recovery, before and after the Swhitp are presented in Figure 1a and Figure 1b, respectively. The MGT induced a significant increase of plasma A values determined at the end of the test for the 3 groups, arg (p<0.01), prg (p<0.01) and cg (p<0.05), both before and after the training period. Before training, plasma A concentrations determined at rest, at the end of the exercise and after 10 and 30 min of recovery was similar between the 3 groups. However, after Swhitp, plasma A determined immediately at the end of the MGT were significantly higher in arg compared to prg and cg (p<0.05). Before and after the Swhitp plasma NA increased significantly (p<0.05) in all groups after the MGT. Plasma NA measured immediately at the end of the MGT, was significantly higher in arg compared to prg (p<0.05) and cg (p<0.05) only after the Swhitp.
Figure 1.
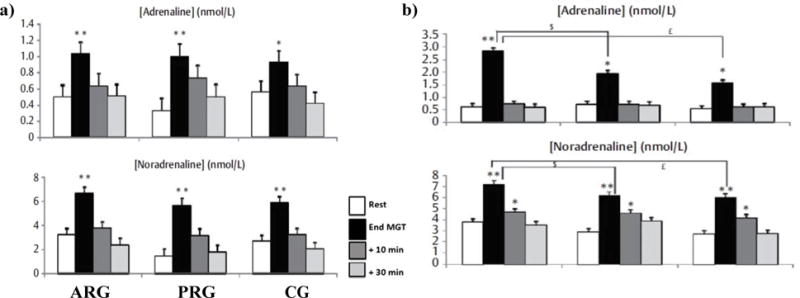
a: Plasma adrenaline and noradrenaline concentrations determined before training at rest, at the end of maximal exercise and after 10 and 30 min recovery for active recovery group (ARG). b) Plasma adrenaline and noradrenaline concentrations determined after training at rest, at the end of maximal exercise and after 10 and 30 min recovery for active recovery group (ARG),
Discussion
The main finding of this study is 7-week of HIIT with active recovery induced a more significant improvement than passive recovery in plasma Glu and glucoregulatory hormones after the MGT. This improved glucoregulation observed after the training period in arg was concomitant to an improvement of their aerobic fitness.
Effect of intermittent training on indices of aerobic fitness (MAV and VO2max)
Following 7-week HIIT, indices of aerobic performance (MAV and VO2max expressed in absolute and relative to body mass values) were significantly increased in arg, but not in prg. These findings agree with the literature [2,3,18]. These studies confirmed the effect of HIIT program on aerobic performance indices. Our results also confirmed those observed in our laboratory and in response to the same training program as previously reported [3]. Similarly, Burke et al. [4] observed in moderately trained women a significant increase of VO2max after a 7-week HIIT program (4 times per week). In fact, several studies observed improvements in both MAV and VO2max after only 4 weeks of such training [19,20].
However, this significant improvement of MAV, regardless of the recovery mode and after only 3.5 weeks of HIIT, was consistent with that of Billat et al. [19] and Denadai et al. [20] in highly trained runners and with that of Billat et al. [21] and Heubert et al. [22] in physical education students. In addition, before or after the training period, the MGT induced significant increase in [la] for all our three groups. The Swhitp did not seem to influence the [la] evolution in response to MGT. This finding might be explained by a less demand of anaerobic metabolism during endurance training mode [23] compared to resistance or sprint training.
Effect of intermittent training on catecholamine responses
Plasma A and NA concentrations increased in response to maximal exercise in all the groups before and after the Swhitp. Plasma A and NA concentrations measured in the present study in all groups at the end of the maximal exercise were higher than those measured in other studies in response to short sprint exercise [24] as well as intermittent exercise [25]. The present study showed that arg subjects exhibited significantly higher A and NA response at the end of MGT than cg and prg after Swhitp. The same results were also observed in our laboratory by Ben abderrahman et al. [2, 3] in response to the same training program. This has been explained by the fact that intermittent exercise training with active recovery (i.e., arg) is more intense and might induce more stress, which could lead to an increase of catecholamine secretion. Differences observed between arg and prg could also be explained by differences in the duration of the MGT performed after the Swhitp. However, after the training program arg performed only ~1 min longer than prg during the mgt. Effect of intermittent training on the cortisol responses before and after training, the resting plasma C concentrations for the three groups were similar. These findings agree with the available literature data [25,26], which did not showed any significant changes between the total C/free C ratio levels at rest among endurance trained and sedentary subjects. Indeed, the availability of c did not increase in endurance- trained subjects [26]. However, other studies observed an increase in basal plasma C in highly trained athletes [29]. In our study, plasma C concentrations increased significantly at the end of the maximal exercise. These findings are in accordance with literature data [30,31]. In fact, the C level was more important in response to higher intensity and duration of exercise. It was observed that plasma C level remained elevated after 30 min of recovery. Generally, in response to intense and prolonged exercise, C concentration level remained higher than basal values for at least 120 to 150 min after the exercise. In response to Swhitp, the C responses were relatively similar except in the active recovery group, the concentration of this hormone remained very elevated at 30 min of recovery.
Effect of intermittent training on the Gh responses
In the current study, in response to maximal exercise plasma Gh concentrations increased significantly in all our three groups. However, no significant differences were observed in response to the Swhitp. These results agree with several studies [26,32]. In the same context, Sasaki et al. [33] study examined the effects of a HIIT program on exercise-induced Gh responses. This study did not show any significant change in Gh secretion after this Swhitp. Moreover, many studies do not show any training effect on the total Gh secretion over 24 hours. However, Bloom et al. [34] comparing Gh responses between trained and sedentary cyclists found that sedentary subjects increased their plasma Gh concentration more than the trained subjects. These authors also observed that at the end of the exercise, plasma Gh concentration was dropped in trained cyclists while it continued to increase in sedentary subjects. In our study, the Gh levels remained elevated during recovery, which could be explained by an exercise-related Gh secretion that was prolonged during recovery phase. Nevill et al. [34] observed that upon stopping a 30s sprint the plasma Gh concentration was 10 times higher than that observed at rest. Other authors have shown that after 10 min of a race at 70% VO2max, the Gh secretion peak was observed after 30 min of recovery. After 3 sets of resistance exercises (of 6 exercises performed at 80% of the maximum repeat capacity), Gh was 31 times greater than its resting concentration at the end of exercise and stilled increasing during 60 min after exercise (at 60th min, the plasma concentration of Gh remained 2.8 times higher than its resting concentration according to Murray et al. [36].
Effect of intermittent training on the glucose and insulin responses
In our study the resting plasma Glu and Ins concentrations were similar in the trained and untrained groups. Previous studies showed that physical training reduced rest serum Ins level [37–39]. Thus our results may be explained perhaps by the training duration, which might not have been enough to reduce Glu and Ins resting concentrations. In response to MGT, there was an increase in Ins responses that occurred with a slight time lag relative to the exercise-induced plasma Glu enhance. This appears to be a classical result, allowing Ins to restore basal Glu values through stimulation of Glu movement into cells (insulin-sensitive cells such as muscles via glut4). This Glu input stimulated by Ins secretion allows the re-synthesis of the glycogen stores. In response to our training program only arg decreased their circulating glucose levels in response to maximal exercise. Such findings are possibly due to several physiological adaptations (i.e., Ins sensitivity and glucose transport) to intensive training. These results agree with others [34,37,40]. In fact, it is usually demonstrated that endurance training decreased circulating Glu both at rest and in response to exercise. However, it is important to note that this decrease was seen only in arg and not in prg in our study. The lower Glu levels measured in arg could be explained either by decreased hepatic glucose production (HGP) or increased muscle glucose uptake (MGU) following the Swhitp. However, during intense exercise, HGP is primarily controlled by A, C and Gh [41]. In response to maximal exercise, arg had higher A and C levels after the training period. Therefore, endurance interval training with active recovery is more likely to increase HGP than to decrease it. Training intensity plays a major role in stimulating hyperglycemic hormones. In fact, it has been demonstrated that intense training (30 s maximal effort exercises) increases C levels at rest and during exercise [42]. In addition, most studies have shown that endurance training did not affect C levels during moderate-intensity exercise [43–45]. The higher plasma C level during recovery we observed for this group could partly explain the differences in blood Glu concentration in the group.
Another probable mechanism resulted to endurance interval training program is the greater muscle glucose uptake (MGU). This phenomenon can be explained by an increase of the glucose transport under insulin action and an increase in insulin sensitivity. Data from the present investigation are in agreement with some studies in reporting lower or no changing systemic Ins levels at rest and in response to exercise in endurance-trained men compared with untrained ones [34,46]. Therefore, the lower Glu level observed in arg is probably the result of the high-affinity transmembrane receptor to insulin with intense training. MGU is shown to be dependent on glucose transporter (Glut4) content. During exercise, the non-insulin-dependent glut4 are the main glucose transporters involved in MGU. According to Holten et al. [47] strength training (30 minutes/day, 3 times/week) increased protein content of Glut4, insulin receptors density, and insulin action in skeletal muscles.
Moreover, it has been demonstrated that the increase of glucose transport in the absence of insulin and the Glut4 translocation are mediated by adrenaline increase [48]. Thus, the massive entry of glucose in the membrane space could be allowed by the translocation of Glut4 which is stimulated by the increase in adrenaline secretion [48,11]. This result suggests the important role that adrenaline secretion plays in glucose metabolism regulation.
Conclusion
Seven weeks of HIIT using active recovery has a beneficial efficient on aerobic fitness, as well as on plasma glucose and glucoregulatory hormones after MGT better than HIIT with passive recovery. These finding suggest that HIIT with active recovery may more effectively improve some metabolic and hormonal parameters.
Acknowledgments
The authors wish to acknowledge all the subjects for their participation in the study.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Kessler HS, Sisson SB, Short KR. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports med. 2012;42:489–509. doi: 10.2165/11630910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Ben Abderrahman A, Prioux J, Chamari K, Ben Ounis O, Tabka Z, et al. Running interval training and estimated plasma-volume variation. Int J Sports Physiol Perform. 2013a;8:358–365. doi: 10.1123/ijspp.8.4.358. [DOI] [PubMed] [Google Scholar]
- 3.Ben Abderrahman A, Zouhal H, Chamari K, Thevenet D, De Mullenheim PY, et al. Effects of recovery mode (active vs. passive) on performance during a short high- intensity interval training program: a longitudinal study. Eur J Appl Physiol. 2013b;113:1373–1383. doi: 10.1007/s00421-012-2556-9. [DOI] [PubMed] [Google Scholar]
- 4.Burke J, Thayer R, Belcamino M. Comparison of effects of two intervaltraining programmes on lactate and ventilatory thresholds. Br J Sports Med. 1994;28:18–21. doi: 10.1136/bjsm.28.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thevenet D, Tardieu M, Zouhal H, Jacob C, Ben Abderrahman A, et al. Influence of exercise intensity on time spent at high percentage of maximal oxygen uptake during an intermittent session in young endurance-trained athletes. Eur J Appl Physiol. 2007;102:19–26. doi: 10.1007/s00421-007-0540-6. [DOI] [PubMed] [Google Scholar]
- 6.Dorado C, Sanchis-Moysi J, Calbet JA. Effects of recovery mode on performance, O2 uptake, and O2 deficit during high-intensity intermittent exercise. Can J AppI Physiol. 2004;29:227–44. doi: 10.1139/h04-016. [DOI] [PubMed] [Google Scholar]
- 7.Laursen PB, Jenkins DG. The scientific basis for high-intensity interval training: optimizing training programmes and maximising performance in highly trained endurance athletes. Sports Med. 2002;32:53–73. doi: 10.2165/00007256-200232010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Hultman E. Muscle glycogen stores and prolonged exercise. In: Shephard RJ, editor. Frontiers of fitness. Springfield (II): Charles C Thomas; 1971. pp. 37–60. [Google Scholar]
- 9.Kjaer M. Hepatic glucose production during exercise. Adv Exp Med Biol. 1998;441:117–127. [PubMed] [Google Scholar]
- 10.Ciccarelli L, Connell SR, Enderle M, Mills DJ, Vonck J, et al. Structure and conformational variability of the mycobacterium tuberculosis fatty acid synthase multienzyme complex. Structure. 2013;21:1251–1257. doi: 10.1016/j.str.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Zouhal H, Jacob C, Delamarche P, Gratas-Delamarche A. Catecholamine and effects of exercise, training and gender. Sports Med. 2008;38:401–423. doi: 10.2165/00007256-200838050-00004. [DOI] [PubMed] [Google Scholar]
- 12.Léger L, Boucher R. An indirect continuous running multistage field test: the Université de Montréal track test. Can J Appl Sport Sci. 1980;5:77–84. [PubMed] [Google Scholar]
- 13.Kuipers H, Verstappen FT, Keizer HA, Geurten P, Van Kranenburg G. Variability of aerobic performance in the laboratory and its physiologic correlates. Int J Sports Med. 1985;6:197–201. doi: 10.1055/s-2008-1025839. [DOI] [PubMed] [Google Scholar]
- 14.Hackney AC, Viru A. Research Methodology: Endocrinologic Measurements in Exercise Science and Sports Medicine. J Athl Train. 2008;43:631–639. doi: 10.4085/1062-6050-43.6.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koubi HE, Desplanche D, Gabrielle C, Cottet-Emard JM, Semporer B, et al. Exercise endurance and fuel utilization: a reevaluation of the effects of fasting. J Appl Physiol. 1991;70:1337–1343. doi: 10.1152/jappl.1991.70.3.1337. [DOI] [PubMed] [Google Scholar]
- 16.Van Beaumont W, Greenleaf JE, Juhos L. Disproportional changes in hematocrit, plasma volume, and proteins during exercise and bed rest. J Appl Physiol. 1972;33:55–61. doi: 10.1152/jappl.1972.33.1.55. [DOI] [PubMed] [Google Scholar]
- 17.Ouerghi N, Ben Fradj MK, Bezrati I, Feki M, Kaabachi N, et al. Effect of High- Intensity Interval Training on Plasma Omentin-1 Concentration in Overweight/Obese and Normal-Weight Youth. Obes Facts. 2017;10:323–331. doi: 10.1159/000471882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Billat VL, Flechet B, Petit B, Muriaux G, Koralsztein JP. Interval training at V02max: effects on aerobic performance and overtraining markers. Med Sci Sports Exerc. 1999;31:156–163. doi: 10.1097/00005768-199901000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Denadai BS, Ortiz MJ, Greco CC, de Mello MT. Interval training at 95% and 100% of the velocity at VO2 max: effects on aerobic physiological indexes and running performance. Appl Physiol Nutr Metab. 2006;31:737–743. doi: 10.1139/h06-080. [DOI] [PubMed] [Google Scholar]
- 20.Billat VL, Hamard L, Koralsztein JP. The influence of exercise duration at VO2 max on the off-transient pulmonary oxygen uptake phase during high intensity running activity. Arch Physiol Biochem. 2002;110:383–392. doi: 10.1076/apab.110.5.383.11831. [DOI] [PubMed] [Google Scholar]
- 21.Heubert R, Bocquet V, Koralsztein JP, Billat V. Effect of 4 weeks of training on the limit time at VO2 max. Can J Appl Physiol. 2003;28:717–736. doi: 10.1139/h03-055. [DOI] [PubMed] [Google Scholar]
- 22.Cadefau J, Casademont J, Grau JM, Fernandez J, Balaguer A, et al. Biochemical and histochemical adaptation to sprint training in young athletes. Acta Physiol Scand. 1990;140:341–351. doi: 10.1111/j.1748-1716.1990.tb09008.x. [DOI] [PubMed] [Google Scholar]
- 23.Botcazou M, Zouhal H, Jacob C, Gratas-Delamarche A, Berthon PM, et al. Effect of training and detraining on catecholamine responses to sprint exercise in adolescentgirls. EurJ Appl Physiol. 2006;97:68–75. doi: 10.1007/s00421-006-0131-y. [DOI] [PubMed] [Google Scholar]
- 24.Eliakim A, Nemet D, Zaldivar F, McMurray RG, Culler FL, et al. Reduced exercise-associated response of the GH-IGF-I axis and catecholamines in obese children and adolescents. J Appl Physiol. 2006;100:1630–1637. doi: 10.1152/japplphysiol.01072.2005. [DOI] [PubMed] [Google Scholar]
- 25.Luger A, Deuster PA, Kyle SB, Gallucci WT, Montgomery LC, et al. Acute hypothalamic-pituitary-adrenal responses to the stress of treadmill exercise. Physiologic adaptations to physical training. N Engl J Med. 1987;316:1309–1315. doi: 10.1056/NEJM198705213162105. [DOI] [PubMed] [Google Scholar]
- 26.Jaffre C, Lac G, Benhamou CL, Courteix D. Effects of chronic intensive training on androgenic and cortisol profiles in premenarchal female gymnasts. Eur J Appl Physiol. 2002;87:85–89. doi: 10.1007/s00421-002-0605-5. [DOI] [PubMed] [Google Scholar]
- 27.Walker S, Santolamazza F, Kraemer W, Hakkinen K. Effects of prolonged hypertrophic resistance training on acute endocrine responses in young and older men. J Aging Phys Act. 2015;23:230–236. doi: 10.1123/japa.2013-0029. [DOI] [PubMed] [Google Scholar]
- 28.Kraemer WJ, French DN, Paxton NJ, Hakkinen K, Volek JS, et al. Changes in exercise performance and hormonal concentrations over a big ten soccer season in starters and nonstarters. J Strength Cond Res. 2004;18:121–128. doi: 10.1519/1533-4287(2004)018<0121:ciepah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Tanner AV, Nielsen BV, Allgrove J. Salivary and plasma cortisol and testosterone responses to interval and tempo runs and a bodyweight-only circuit session in endurance- trained men. J Sports Sci. 2014;32:680–689. doi: 10.1080/02640414.2013.850594. [DOI] [PubMed] [Google Scholar]
- 30.Alghadir AH, Gabr SA, Aly FA. The effects of four weeks aerobic training on saliva cortisol and testosterone in young healthy persons. J Phys Ther Sci. 2015;27:2029–2033. doi: 10.1589/jpts.27.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovell DI, Cuneo R, Wallace J, McLellan C. The hormonal response of older men to sub-maximum aerobic exercise: the effect of training and detraining. Steroids. 2012;77:413–418. doi: 10.1016/j.steroids.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki H, Morishima T, Hasegawa Y, Mori A, Ijichi T, et al. 4 weeks of high- intensity interval training does not alter the exercise-induced growth hormone response in sedentary men. Springer plus. 2014;3:336. doi: 10.1186/2193-1801-3-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloom SR, Johnson RH, Park DM, Rennie MJ, Sulaiman WR. Differences in the metabolic and hormonal responses to exercise between racing cyclists and untrained individuals. J Physiol. 1976;258:1–18. doi: 10.1113/jphysiol.1976.sp011403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nevill ME, Holmyard DJ, Hall GM, Allsop P, van Oosterhout A, et al. Growth hormone responses to treadmill sprinting in sprint- and endurance-trained athletes. Eur J Appl Physiol Occup Physiol. 1996;72:460–467. doi: 10.1007/BF00242276. [DOI] [PubMed] [Google Scholar]
- 35.Murray R, Bartoli WP, Eddy DE, Horn MK. Physiological and performance responses to nicotinic-acid ingestion during exercise. Med Sci Sports Exerc. 1995;27:1057–1062. doi: 10.1249/00005768-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Winder WW, Hickson RC, Hagberg JM, Ehsani AA, McLane JA. Training-induced changes in hormonal and metabolic responses to submaximal exercise. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:766–771. doi: 10.1152/jappl.1979.46.4.766. [DOI] [PubMed] [Google Scholar]
- 37.Wilmore JH, Costill DL. Physiologie du sport et de I’exercice. De Boeck Universite; Paris: Bruxelles: 1998. [Google Scholar]
- 38.Gerosa-Neto J, Antunes BM, Campos EZ, Rodrigues J, Ferrari GD, et al. Impact of long-term high-intensity interval and moderate-intensity continuous training on subclinical inflammation in overweight/obese adults. J Exerc Rehabil. 2016;12:575–580. doi: 10.12965/jer.1632770.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kjaer M, Engfred K, Fernandes A, Secher NH, Galbo H. Regulation of hepatic glucose production during exercise in humans: role of sympathoadrenergic activity. Am J Physiol. 1993;265:275–283. doi: 10.1152/ajpendo.1993.265.2.E275. [DOI] [PubMed] [Google Scholar]
- 40.Rizza R, Cryer P, Gerich J. Role of Glucagon, Catecholamines, and Growth Hormone in Human Glucose Counterregulation. J Clin Invest. 1979;64:62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreira A, Arsati F, De Oliveira Lima Arsati YB, Da Silva DA, De Araújo VC. Salivary cortisol in top-level professional soccer players. Eur J Appl Physiol. 2009;106:25–30. doi: 10.1007/s00421-009-0984-y. [DOI] [PubMed] [Google Scholar]
- 42.Mujika I, Chatard JC, Padilla S, Guezennec CY, Geyssant A. Hormonal responses to training and its tapering off in competitive swimmers: relationships with performance. Eur J Appl Physiol Occup Physiol. 1996;74:361–366. doi: 10.1007/BF02226933. [DOI] [PubMed] [Google Scholar]
- 43.Filaire E, Duché P, Lac G. Effects of amount of training on the saliva concentrations of cortisol, dehydroepiandrosterone and on the dehydroepiandrosterone: cortisol concentration ratio in women over 16 weeks of training. Eur J Appl Physiol Occup Physiol. 1998;78:466–471. doi: 10.1007/s004210050447. [DOI] [PubMed] [Google Scholar]
- 44.Filaire E, Bernain X, Sagnol M, Lac G. Preliminary results on mood state, salivary testosterone:cortisol ratio and team performance in a professional soccer team. Eur J Appl Physiol. 2001;86:179–184. doi: 10.1007/s004210100512. [DOI] [PubMed] [Google Scholar]
- 45.Poortmans J, Boisseau N. Biochemie des activités physiques. Edition De Boeck université; Bruxelles: 2003. [Google Scholar]
- 46.Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JF, et al. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes. 2004;53:294–305. doi: 10.2337/diabetes.53.2.294. [DOI] [PubMed] [Google Scholar]
- 47.Han XX, Bonen A. Epinephrine translocates GLUT-4 but inhibits insulin-stimulated glucose transport in rat muscle. Am J Physiol. 1998;274:700–707. doi: 10.1152/ajpendo.1998.274.4.E700. [DOI] [PubMed] [Google Scholar]
- 48.Minokoshi Y, Okano Y, Shimazu T. Regulatory mechanism of the ventromedial hypothalamus in enhancing glucose uptake in skeletal muscles. Brain Res. 1994;649:343–347. doi: 10.1016/0006-8993(94)91085-5. [DOI] [PubMed] [Google Scholar]


