Abstract
Background:
Health warning labels (HWLs) on cigarette packs in Australia, Canada, Mexico, and the United States include varying information about toxic cigarette smoke constituents and smoking-related health risks. HWL information changed more recently in Australia, Canada, and Mexico than in the United States.
Aims:
To investigate whether smokers’ knowledge of toxic constituents and perceived smokingrelated risks increased after adding this information to HWLs and how knowledge of toxic constituents is associated with perceptions of smoking-related risks.
Methods:
Data come from a longitudinal, online cohort of 4,621 adult smokers surveyed every four months from September 2012 (Wave 1) to January 2014 (Wave 5) in Australia, Canada, and Mexico, with the United States being surveyed from waves 2 to 5. Generalized estimating equation models estimated the association between perceived smoking-related risk at follow-up and prior wave knowledge of toxic constituents, adjusting for attention to HWLs, sociodemographics, and smoking-related characteristics.
Results:
Between 2012 and 2014, knowledge of toxic constituents increased in Australia, Canada and Mexico (p<0.001), but not in the United States. Higher levels of both attention to HWLs and knowledge of toxic constituents were associated with a higher perceived risk of smoking-related conditions at follow-up across all countries except for the United States.
Conclusions:
Our results suggest that information about toxic constituents on prominent HWLs not only increases smoker’s knowledge of toxic constituents, but that it may also reinforce the effects of HWL messages about specific, smoking-related health outcomes.
Keywords: global health, health behavior, health communications, health promotion, smoking and tobacco use, tobacco control and policy
Introduction
Cigarette smoke contains more than 9,000 different chemicals, over 60 of which are carcinogenic (Rodgman & Perfetti, 2013; US Department of Health and Human Services, 2014). The World Health Organization’s Framework Convention on Tobacco Control (FCTC) emphasizes sustained education and communication about tobacco-related health risks (World Health Organization [WHO], 2013a), including the need to effectively disclose information about the toxic constituents in tobacco products to the public, particularly if it enhances consumer understanding of smoking-related risks (WHO, 2013b).
Pictorial health warning labels (HWLs) can promote awareness of smoking-related health risks among smokers, potentially including those with low literacy (Fong, Hammond, & Hitchman, 2009; Thrasher, Carpenter, et al., 2012). Compared to text-only HWLs, pictorial HWLs are more effective in eliciting cognitive elaboration and negative affective reactions (Noar et al., 2015). By the end of 2016, more than 100 countries adopted prominent pictorial HWLs on cigarette packaging (Canadian Cancer Society, 2016).
While countries include varying amounts of and strategies for communicating information about toxic constituents on HWLs, only a few studies have investigated the impact of toxic constituent information in HWLs. Hall, Ribisl, and Brewer (2014) documented that US adults had limited knowledge about tobacco constituents and that awareness of constituents may discourage them from smoking. Studies have found differences in population levels of knowledge about toxic constituents between countries that do and do not include this information on cigarette packs (Hammond, Fong, McNeill, Borland, & Cummings, 2006; O’Connor, Kozlowski, Borland, Hammond, & McNeill, 2006; Siahpush, McNeill, Hammond, & Fong, 2006). Three studies showed that knowledge of toxic constituents increases after inclusion of this information on HWLs (R. Borland & Hill, 1997; Swayampakala et al., 2014; Thrasher, Murukutla, et al., 2012; Thrasher, Perez-Hernandez, Arillo-Santillan, & Barrientos-Gutierrez, 2012). Nevertheless, no study examined the relationship between HWL-linked knowledge of toxic constituents and perceived risks of smoking, which could inform the development of more effective HWLs.
Study Context:
Cigarette packages often display quantitative levels of toxic constituents in cigarette smoke. However, because this information has been misleading for consumers (Hammond & White, 2012), the FCTC recommends against this strategy (WHO, 2013c). For example, tar and nicotine emission numbers historically printed on cigarette packages were generated under standardized smoking machine conditions and not the conditions that human smokers experience, which may reinforce misperceptions that some cigarettes are safer than others (U.S. Department of Health and Human Services, 2014). Hence, in 2008, the United States (U.S.) Federal Trade Commission rescinded its prior approval of statements for packs about machine-produced tar and nicotine yields (Federal Trade Commission, 2008). To address these concerns, the WHO recommends qualitatively describing chemicals in tobacco products to more effectively communicate risk to consumers (Hammond & White, 2012; WHO, 2013c).
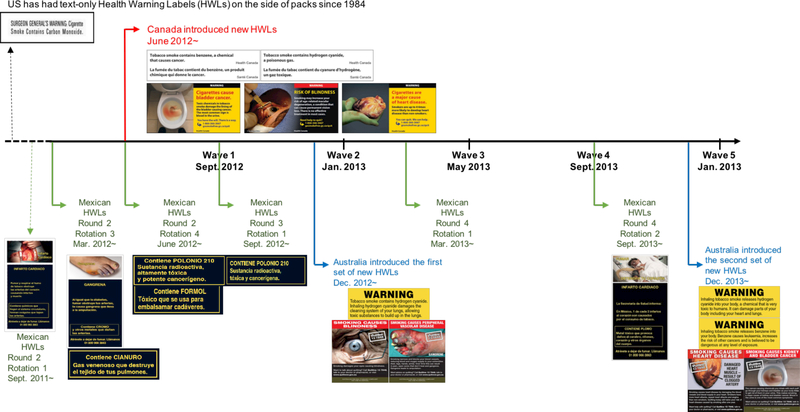
Following the WHO recommendations, Australia, Canada, and Mexico have implemented new pictorial HWLs that include descriptive information about toxic constituents (Figure 1). In December 2012, Australia was the first country to implement “plain packaging” (i.e., standardized pack sizes and prohibition of brand imagery), which included new, larger pictorial HWLs with prominently formatted (i.e., black lettering on yellow background), short statements about how toxic constituents produce disease (e.g., Inhaling hydrogen cyanide damages the cleaning system of your lungs, allowing toxic substances to build up in the lungs). That same year in June, Canada implemented new, larger pictorial HWLs, while replacing quantitative levels for different toxic constituents with short statements indicating the presence of the constituent (e.g., Tobacco smoke contains hydrogen cyanide, a poisonous gas). Since 2010, Mexico has included different, rotating statements on the back of packages that briefly describe specific toxic constituents, either linking them to a disease or to potentially familiar contexts where they may encounter the constituent (e.g., Contains Formaldehyde: a toxin that is used to embalm corpses) (Thrasher JF, 2013). The United States has not implemented pictorial HWLs and its text-only HWLs on the side of the pack include only a brief statement about carbon monoxide (Figure 1).
Figure 1. Mandated Health Warning Labels (HWLs) with Qualitative Information about Toxic Constituents and Smoking-related Risks on Cigarette Packages for Australia, Canada, Mexico, and the US in the Survey Waves*.
*Mexico changed the HWL content every six months but HWLs stay on the market for a substantial period afterwards, given that the law does not specify when HWLs are no longer allowed to be sold. Hence, information printed on packs in the period before the study began was likely to be on HWLs over the entire study period, although their presence is likely to have diminished over time.
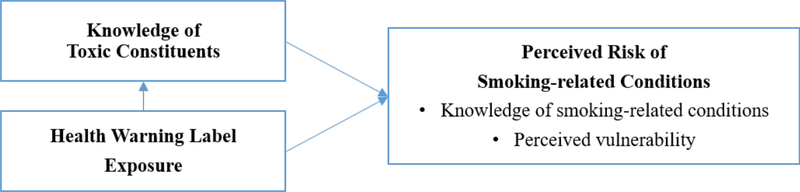
Central to our conceptual model, presented in Figure 2, is how exposure to HWLs works by influencing perceived risk, particularly the dimension of perceived susceptibility, which is drawn from the Health Belief Model (Fong et al., 2006; Yong et al., 2014) and refers to one’s subjective belief that he is likely to develop a health problem that predicts behavior change (Becker, 1974). Our conceptual model posits that HWL exposures increase knowledge of toxic constituents and perceived risk of smoking-related conditions portrayed on HWLs. Furthermore, we posit that knowledge of toxic constituents promotes risk perceptions because these constituents help explain elevated risk. In particular, we hypothesize that pictorial HWLs with qualitative information about toxic constituents promote awareness of these constituents and this awareness, in turn, enhances perceptions of smoking-related risks (Figure 2).
Figure 2. Conceptual Framework Illustrating How Knowledge of Toxic Constituents and Exposure to Health Warning Label Can Influence Perceived Risk of Smoking-related Conditions.
Methods
Sample
Our sample was recruited by e-mail invitations to Global Market Insight’s online, commercial panels (Global Market Insight, 2011), with the overall response rates ranging from 6% to 13%. Eligible panelists were between 18 and 64 years, had smoked at least once in the prior month, and had smoked more than 100 cigarettes in their lifetime. Five waves of data were collected once every four months, from September 2012 (Wave 1) to January 2014 (Wave 5) in Australia, Canada, and Mexico. As Australia introduced new HWLs in December 2012, only the four waves of data after policy implementation were analyzed. Data collection started one wave later in the United States than other countries (January 2013) since pictorial HWLs were not introduced as planned in 2012, producing four waves. Samples were replenished by recruiting new adult smokers from the same online consumer panel to maintain the sample size of approximately 1,000 smokers for each wave in Australia, Canada, and Mexico. In the United States, an oversample of 400 Latinos was surveyed at each wave to allow for comparisons with the Mexico sample (for analyses not described in this study). The analytic sample was composed of 1,036 adult smokers in Australia, 1,190 smokers in Canada, 1,166 smokers in Mexico, and 1,229 smokers in the United States. The sample of 4,621 smokers provided 9,566 baseline-to-follow-up wave pairs, with 2,136 smokers providing only one wave pair, 1,042 smokers providing two, 625 smokers providing three, 619 smokers providing four, and 199 smokers providing all five baseline-to-follow-up wave pairs. The IRB at the University of South Carolina approved the study.
Measures
Knowledge of toxic constituent index.
We showed participants a list of six toxic constituents, presented in random order, that were described on HWLs for at least one country, either in prior or newly introduced HWLs (i.e., benzene, carbon monoxide, cyanide, formaldehyde, nitrosamines, and radioactive polonium 210). Participants indicated whether each chemical is in cigarette smoke by answering “Yes” (scored 1) or “No” and “Don’t know” (scored 0). To allow for a more specific link between HWL content and consumer knowledge, country-specific indices were constructed. The indices were standardized to range from 0 to 1 by first summing responses to toxic constituents qualitatively described on HWLs for their country (Figure 1) and then dividing the sum by the number of the constituents, that is, 2 for Australia and Canada (i.e., benzene and cyanide), 3 for Mexico (i.e., cyanide, formaldehyde, and radioactive polonium 210), and 1 for the United States (i.e., carbon monoxide).
Perceived risk index.
Perceived risk of smoking-related conditions was measured by combining knowledge of specific smoking-related conditions mentioned on HWLs and perceived vulnerability to these diseases. First, knowledge of smoking-related conditions was assessed by asking participants to indicate which illnesses, if any, are caused by smoking cigarettes, followed by a list of eight diseases (i.e., emphysema, heart attacks, bladder cancer, blindness, impotence in male smokers, gangrene, hepatitis, and diseases that lead to amputation) shown in random order with three response options (“Yes,” “No,” “Don’t know”). For four of the listed diseases (i.e., bladder cancer, blindness, gangrene, and heart attacks), perceived vulnerability was assessed by asking participants to compare their own chance of getting the disease in the future to the chance of a non-smoker if they continue to smoke the amount that they currently do, with response options from 1 (Just as likely) to 4 (Much more likely), as well as a “Don´t know” option which was counted as 1 (just as likely) (Costello, Logel, Fong, Zanna, & McDonald, 2012). Based on responses to the knowledge and vulnerability questions, participants were scored from 0 to 2 for each disease outcome that was portrayed on their country’s HWLs (Australia = 0 to 8; Canada = 0 to 6; Mexico = 0 to 4; the United States = 0 to 2; See Figure 1). Participant responses were scored 0 if they did not indicate that the disease is caused by smoking cigarettes, 1 if they indicated that the disease is caused by smoking but answered that their own chance of getting the disease is just as likely as that of non-smokers, and 2 if they indicated that the disease is caused by smoking and their chance of getting the disease is a greater than that of non-smokers. Responses were averaged to create standardized, country-specific indices of perceived risk (range = 0 to 2). In other words, scores for each disease outcome described on HWLs for their country (Figure 1) were summed and divided by the number of the diseases queried that were on HWLs (i.e., 4 diseases for Australia = bladder cancer, blindness, gangrene, heart disease); 3 for Canada = bladder cancer, blindness, heart disease; 2 for Mexico = gangrene and heart disease; 1 for the United States = heart disease).
Attention to HWLs.
The level of attention to HWLs was assessed by asking participants how often they had read or looked closely at the HWLs on cigarette packages in the last month, with five response options for each question ranging from 1 (“never”) to 5 (“very often”). The question was asked only to those who indicated that they had noticed the HWLs in the last month. Those who answered that they “never” noticed the HWLs were categorized as 1 (“never”) for attention to HWLs.
Adjustment variables.
Sociodemographic variables included age (18–24; 25–34; 35–44; 45–54; 54–64), sex, educational attainment (high school or less; some college or university; university or more), and household income (i.e., $29,999 or less, $30,000-$59,999, and $60,000 or more for annual household income in Australia, Canada, and the US; $5,000 or less, $5,001-$10,000, and $10,001 or more pesos per month in Mexico). Smoking intensity was assessed with the Heaviness of Smoking Index (HSI) (Heatherton, Kozlowski, Frecker, Rickert, & Robinson, 1989), which combined information about both the number of cigarettes per day (0: 1–10 or non-daily smokers, 1: 11–20, 2: 21–30, 3: 31+) and the time to first cigarette (0: ≤ 5 or non-daily smokers, 1: 6–30, 2: 31–60, 3: 61+ min) and ranged from 0 to 6. Because of the substantial percentage of non-daily smokers, particularly in Mexico, an indicator for daily vs non-daily smoking was also included. Furthermore, a “time-in-sample” indicator was derived to adjust for possible instrumentation bias, indicating the number of prior surveys the participant had completed at the time of the survey (range = 0–5).
Statistical analysis
All analyses were conducted using STATA, v 13. Generalized estimating equation (GEE) models were estimated to assess within-country changes in knowledge of toxic constituent index and perceived smoking-related risks by regressing these indices on the survey wave, since GEE models can use incomplete data from those who failed to participate in every wave, while adjusting for repeated observations. For each country, bivariate and adjusted GEE models regressed risk perceptions at the subsequent wave (i.e., t + 1) on prior wave assessments (i.e., time t) of attention to HWLs, knowledge of toxic constituents, perceived risk index, all adjustment variables, survey wave, and the number of surveys participated.
Sensitivity analyses were conducted to assess potential issues with selection bias and attrition. First, we assessed changes in knowledge of toxic constituent index and perceived risk index over time among all smokers, including those lost to follow-up (Supplementary Figures 1 and 2). All models were also re-estimated using weights developed based on age, gender, and educational profile of smokers in the general population for each country. Furthermore, propensity scores (i.e., predicted probabilities of completing survey waves) were derived using baseline measures of variables that are potentially associated with participation and included as adjustment variables. Finally, all models were rerun to adjust for potential biases from yeah-saying, by including an adjustment variable, indication of knowledge that nitrosamines is in cigarette smoke—a tobacco constituent that did not appear on HWLs in any of the countries. The pattern of results from these additional analyses was consistent in direction, magnitude, and, with minor exceptions, statistical significance of effects. Because reporting these results would not meaningfully change the results or their interpretation, these results are not provided (other than the supplementary figures 1 and 2). The data shown are from unweighted models.
Results
Sample characteristics
Table 1 presents the sample characteristics for each country. The mean scores for knowledge of toxic constituent index, standardized based on the HWL content for each country (range: 0 to 1), were 0.4 (Standard deviation [SD] = 0.4) in Australia, 0.6 (SD = 0.4) in Canada, 0.3 (SD = 0.3) in Mexico, and 0.7 (SD = 0.4) in the United States. (Note: these scores are not comparable between countries.) A majority of smokers in all countries were aware that carbon monoxide is in cigarette smoke (ranging from 66% in Australia to 79% in Canada), while less than 30% of the smokers were aware that nitrosamines or radioactive polonium is in cigarette smoke. The proportion of smokers who acknowledged the presence of benzene, cyanide, and radioactive polonium in cigarette smoke was larger if their countries included the information on HWLs, compared to that of smokers from countries without such information; the same did not apply to carbon monoxide and formaldehyde. For instance, about 44% of Australian smokers and 61% of Canadian smokers were aware that cigarette smoke contains benzene, compared to 35% of both Mexican and U.S. smokers.
Table 1.
Analytic Sample Characteristics of Adult Smokers (n=4,621) by Country, % or mean (SD)
| Australia | Canada | Mexico | United States | |
|---|---|---|---|---|
| Age* | ||||
| 18–24 | 4% | 7% | 16% | 11% |
| 25–34 | 20% | 20% | 31% | 27% |
| 35–44 | 24% | 22% | 22% | 21% |
| 45–54 | 26% | 25% | 16% | 21% |
| 54–64 | 26% | 26% | 15% | 20% |
| Female* | 51% | 51% | 44% | 47% |
| Education* | ||||
| High school or less | 33% | 28% | 29% | 28% |
| Some college or university | 42% | 44% | 19% | 39% |
| University or more | 26% | 28% | 52% | 32% |
| Income* | ||||
| Low | 23% | 24% | 34% | 25% |
| Middle | 26% | 30% | 36% | 36% |
| High | 51% | 45% | 30% | 39% |
| Daily smoking* | 89% | 84% | 50% | 82% |
| Heaviness of smoking index* | 2.8 (1.6) | 2.4 (1.5) | 0.8 (1.2) | 2.4 (1.5) |
| Quit Intention* | 38% | 42% | 47% | 41% |
| Previous Quit attempt* | 33% | 38% | 53% | 37% |
| Knowledge of toxic constituent index | 0.4 (0.4) | 0.6 (0.4) | 0.3 (0.3) | 0.7 (0.4) |
| Benzene* | 44%a | 61%a,b | 35%c | 35%c |
| Carbon Monoxide* | 66%b | 79%b | 75%b | 74%a,b |
| Cyanide* | 42%a | 65%a,b | 52%a | 38%c |
| Formaldehyde* | 35%c | 63%b | 28%a | 47%c |
| Nitrosamines* | 24%b | 26%b | 26%c | 22%c |
| Radioactive Polonium* | 16%c | 13%c | 29%a | 14%c |
| Perceived risk index | 1.1 (0.6) | 1.1 (0.6) | 1.2 (0.6) | 1.4 (0.8) |
| Bladder cancer* | ||||
| No knowledge of disease | 55%a | 46%a | 71%c | 69%c |
| Only knowledge of disease | 12%a | 13%a | 6%c | 6%c |
| Knowledge of disease and perceived risk | 33%a | 41%a | 24%c | 25%c |
| Blindness* | ||||
| No knowledge of disease | 28%a,b | 51%a | 75%c | 79%c |
| Only knowledge of disease | 22%a,b | 14%a | 5%c | 5%c |
| Knowledge of disease and perceived risk | 50%a,b | 34%a | 20%c | 16%c |
| Gangrene* | ||||
| No knowledge of disease | 24%a,b | 73%c | 46%a,b | 76%c |
| Only knowledge of disease | 23%a,b | 9%c | 13%a,b | 7%c |
| Knowledge of disease and perceived risk | 53%a,b | 18%c | 41%a,b | 17%c |
| Heart Disease* | ||||
| No knowledge of disease | 20%a,b | 14%a,b | 15%a,b | 22%a,b |
| Only knowledge of disease | 17%a,b | 14%a,b | 12%a,b | 12%a,b |
| Knowledge of disease and perceived risk | 63%a,b | 72%a,b | 74%a.b | 66%a,b |
| Nobs | 2,292 | 2,554 | 2,246 | 2,474 |
| Nsmokers | 1,036 | 1,190 | 1,166 | 1,229 |
Note. HWL = health warning label.
The information was included in newly introduced HWLs.
The information was included in the past HWLs.
The information was never included in the HWLs.
significant difference across countries at p < .01 according to chi-square and f tests. We have not compared the difference in country-specific indices across countries.
The mean scores for perceived risk indices, standardized to represent each country’s HWL content (range: 0 to 2) and thus not comparable between countries, were 1.1 (SD=0.6) in Australia, 1.1 (SD = 0.6) in Canada, 1.2 (SD = 0.6) in Mexico, and 1.4 (SD = 0.8) in the United States. The proportion of smokers who acknowledged that smoking causes bladder cancer, blindness, and gangrene and believed that they were at a higher risk to develop the conditions compared to non-smokers was also larger if their countries included the information on HWLs. For instance, about 33% of Australian smokers and 41% of Canadian smokers reported that cigarette smoking causes bladder cancer and their risk of getting bladder cancer is higher than non-smokers, compared to 24% of Mexican and 25% of U.S. smokers.
Changes over Time in Knowledge of Toxic Constituents and Perceived Risk
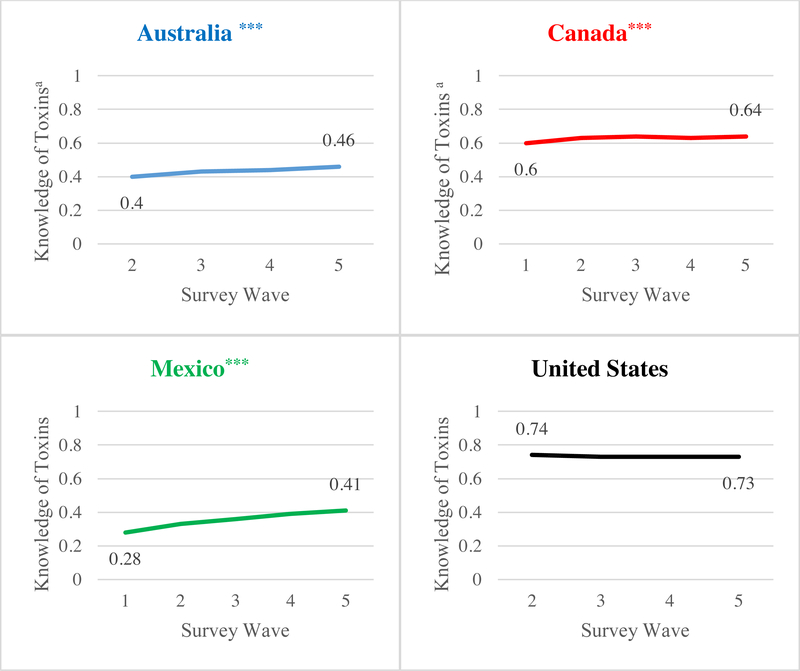
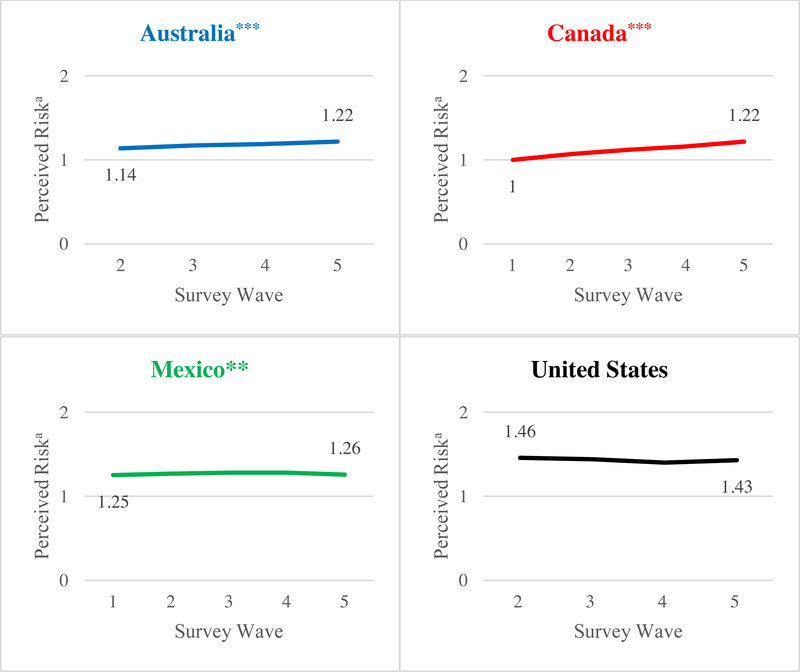
Figure 3 presents a significant linear trend towards greater levels of knowledge of toxic constituent index over time in Australia, Canada, and Mexico (p < .001). Figure 4 also shows a significant increase in mean perceived risk index over time in Australia (p < .001), Canada (p < .001), and Mexico (p < .01), although the index did not increase over time in Mexico when those lost to follow-up were included in the analysis (Supplementary Figure 2). Neither knowledge of toxic constituent index nor perceived risk index changed significantly over time in the United States.
Figure 3. Changes in knowledge of toxic constituent index over time.
aMeans adjusted for age, sex, education, income, daily smoking, wave, and time-in-sample. Indices were standardized by averaging the responses to the number of toxic constituents qualitatively described on each country’s HWLs, that is, 2 for Australia and Canada, 3 for Mexico, and 1 for the United States. Numbers of observations were 2,292 in Australia, 2,554 in Canada, 2,246 in Mexico, and 2,474 in the United States, and the numbers of smokers were 1,036 in Australia, 1,190 in Canada, 1,166 in Mexico, and 1,229 in the United States. Data were collected once every four months from September 2012 (Wave 1) to January 2014 (Wave 5) in Australia, Canada, and Mexico. For Australia, only the four waves of data after policy implementation were used for analysis, since new HWLs were introduced 3 months after data collection started. Data collection started one wave later in the United States due to parent project aims, producing four waves of data. ***p < .001 for linear trend
Figure 4. Changes in perceived risk of smoking-related conditions index over time.
a Means adjusted for age, sex, education, income, daily smoking, wave, and time-in-sample; Indices were standardized (range: 0 to 2) by averaging the responses to the number of smoking-related conditions included in each country’s HWL content, that is, 4 for Australia, 3 for Canada, 2 for Mexico, and 1 for the United States; Numbers of observations were 2,292 in Australia, 2,554 in Canada, 2,246 in Mexico, and 2,474 in the United States, and the numbers of smokers were 1,036 in Australia, 1,190 in Canada, 1,166 in Mexico, and 1,229 in the United States; Data were collected once every four months from September 2012 (Wave 1) to January 2014 (Wave 5) in Australia, Canada, and Mexico. For Australia, only the four waves of data after policy implementation were used for analysis, since new HWLs were introduced 3 months after data collection started. Data collection started one wave later in the United States due to parent project aims, producing four waves of data.
***P < .001, **p < .01 for linear trend
Perceived Risk at Follow-up
Table 2 shows that attention to HWLs, knowledge of toxic constituent index, and prior wave perceived risk index were all significantly and independently associated with stronger perceived risk index at the follow-up wave in Australia (p < .01 for attention to HWLs and knowledge of toxic constituent index, p < .001 for prior wave perceived risk), Canada (p < .001), and Mexico (p < .001). In the United States, only knowledge of toxic constituent index and prior wave perceived risk index were independently associated with perceived risk at follow-up (p < .001).
Table 2.
Associations of perceived risk at follow-up with prior wave attention to warnings, knowledge of toxic constituent, and perceived risk
| Australia | Canada | Mexico | United States | |||||
|---|---|---|---|---|---|---|---|---|
| ba | bb | ba | bb | ba | Bb | ba | ba | |
| Age | ||||||||
| 25–34 | 0.11 | 0.09 | 0.04 | −0.03 | −0.02 | −0.05 | 0.14* | 0.1 |
| 35–44 | 0.18* | 0.15* | 0.02 | 0.05 | 0 | −0.03 | 0.32*** | 0.22*** |
| 45–54 | 0.13 | 0.13* | −0.06 | −0.09 | −0.01 | −0.05 | 0.46*** | 0.33*** |
| 55–64 | 0.04 | 0.1 | −0.07 | −0.09 | −0.06 | −0.08 | 0.40*** | 0.29*** |
| Female | 0.17*** | 0.05** | 0.13*** | 0.10*** | 0.10** | 0.07** | 0.01 | 0.01 |
| Education | ||||||||
| Some college or university | 0.01 | −0.01 | 0.06 | 0.05 | −0.04 | −0.04 | 0.02 | 0 |
| University or more | 0.03 | 0 | 0.11* | 0.06 | 0.01 | −0.03 | 0.04 | 0.04 |
| Income | ||||||||
| Middle | 0.08 | 0.01 | 0.05 | 0.05 | 0.10** | 0.08* | −0.03 | 0.01 |
| High | 0.12** | 0.04 | 0.18*** | 0.15*** | 0.12** | 0.08* | 0 | 0.05 |
| Daily smoking | 0.01 | 0.02 | 0.10** | 0.10* | 0.10*** | 0.07* | 0.09 | 0.02 |
| Heaviness of Smoking Index | −0.01 | 0 | 0.02 | 0 | 0.03* | 0 | 0.04** | 0.01 |
| Quit Intention | 0.03 | 0 | 0.02 | 0.02 | 0.07* | 0.07* | 0 | −0.03 |
| Previous quit attempt | 0.06* | 0 | 0 | −0.01 | 0.03 | −0.01 | 0.03 | 0.07 |
| Attention to warnings | 0.06*** | 0.03** | 0.06*** | 0.04*** | 0.06*** | 0.04*** | −0.02 | 0.00 |
| Knowledge of toxic constituent indexc | 0.21*** | 0.07** | 0.20*** | 0.15*** | 0.34*** | 0.20*** | 0.35*** | 0.27*** |
| Perceived risk (prior wave)cd | 0.76*** | 2.71*** | 0.43*** | 0.36*** | 0.30*** | 0.23*** | 0.38*** | 0.33*** |
| Nobs | 2,292 | 2,554 | 2,246 | 2,474 | ||||
| Nsmokers | 1,036 | 1,190 | 1,166 | 1,229 | ||||
Bivariate Models.
Adjusted models which included all covariates (socio-demographic characteristics, daily smoking, heaviness of smoking index, quit intentions, quit attempt, wave, time-in-sample & perceived risk at time ‘t’) in one model
Indices were standardized (range: 0 to 1) by averaging the responses to the number of toxic constituents qualitatively described on each country’s HWLs, i.e., 2 for Australia and Canada, 3 for Mexico, and 1 for the United States.
Indices were standardized (range: 0 to 2) by averaging the responses to the number of smoking-related conditions included in each country’s HWL content, i.e., 4 for Australia, 3 for Canada, 2 for Mexico, and 1 for the United States.
p < .05,
p < .01,
p < .001
Discussion
Our study suggests that qualitative information about toxic constituents on HWLs can promote awareness of toxic constituents, which, in turn, may promote understanding of smoking-related risks. As in previous studies (R. Borland & Hill, 1997; Swayampakala et al., 2014), smokers’ knowledge of toxic cigarette smoke constituents increased over the period after information about toxic constituents was added to HWLs in Australia, Canada, and Mexico. Furthermore, awareness of toxic constituents was generally higher in countries with information in newly introduced HWLs, with the only exceptions being carbon monoxide and, to a lesser extent, formaldehyde. More than 65% of participants in Australia, Canada, and Mexico knew that cigarette smoke contains carbon monoxide, which is unsurprising because Australian and Canadian HWLs had long included carbon monoxide. Mexico has also long been displaying quantitative levels of carbon monoxide emissions on the side of cigarette packs. Canadian HWLs had described formaldehyde for more than 10 years, while the newly introduced Mexican HWLs included the information only for a year. In general, participants had limited knowledge about other toxic constituents, such as nitrosamines and radioactive polonium. Novel constituents may be worth studying for inclusion in HWL messaging because smokers desire to learn about tobacco constituents and the associated health harms (Moracco et al., 2016) and engage more in novel HWL information (Borland, 1997), while some suggested that familiar constituents can better discourage smoking (Hall et al., 2014).
Perceived smoking-related risks significantly increased over time in Australia and Canada, where new, prominent HWLs covered 75% of the front and back of packs included diseases that had not been described on prior HWLs, such as bladder cancer in Australia and blindness and bladder cancer in Canada. In Mexico, where pictures were changed but the diseases they described did not, smokers’ perceived risks also increased. On the other hand, the United States did not change its HWLs and perceived risks—or knowledge of toxic constituents, in this case carbon monoxide—did not change over time among U.S. smokers. These findings confirm previous studies that prominent pictorial HWLs with rotating content are effective in increasing perception of smoking-related risks (Hammond et al., 2006; Swayampakala et al., 2014), as the FCTC and U.S. legislation mandates.
As predicted, both attention to HWLs and knowledge of toxic constituents were independently associated with subsequent perceptions of smoking-related risks in Australia, Canada, and Mexico, even after adjusting for perceived risk at the prior wave. In the United States, attention to HWLs was unassociated with risk perceptions. Additional analysis showed that attention to HWLs was also unassociated with baseline knowledge of toxic constituents in the United States (data not shown). The findings agree with a previous study, which found that salient HWLs promote negative perceptions of cigarette products (Yong et al., 2014). Since perceived risk promotes behavioral intention and change (Costello et al., 2012; Jacobson, Catley, Lee, Harrar, & Harris, 2014; Janz & Becker, 1984; Rogers, 1975; Romer & Jamieson, 2001), our findings suggest that, given its link with perceived risk of smoking-related conditions, knowledge of toxic constituents could further promote cessation behaviors.
This study has several limitations. First, we could not definitively determine whether and how the presentation of toxic constituent information influences risk perceptions and subsequent cessation behaviors, independent of HWL information about smoking-related risks. The effect of constituent knowledge on risk perception could be due to yeah-saying, although we found the same pattern of results when analyses controlled for awareness of a toxicant that was not on HWLs. Nevertheless, future studies may be needed to more directly assess whether communication of information about specific chemicals increases generalized concern about smoking-related harms independent of HWL messages about those harms. Second, our observational study could not fully control for other policies that may have influenced the study results. For instance, during the study period, Australia increased tobacco taxes, and this may have influenced results. However, neither Canada nor Mexico adopted any federal-level tobacco control policy besides changing HWLs, and the results were generally consistent with Australia. Furthermore, our models tested psychosocial pathways that were specific to HWL content, thereby providing additional assurance around the validity of our findings. Third, differential attrition may have biased our results; for instance, perceived risk of smoking-related conditions did not increase over time in Mexico when those lost to follow-up were included in the analysis. However, our analyses controlled for time-in-sample and we produced results consistent with those reported when including propensity scores to adjust for factors associated with attrition. Fourth, we did not evaluate the effect of varying representations of HWL content, such as how constituent information is displayed (e.g., prominence, formatting) or described. Studies of this kind could help develop more effective HWLs, as, for example, some emerging research suggests that short descriptions of how constituents produce disease are more effective in motivating people to not smoke than short casual statements that merely link constituents to disease (Salloum et al., 2017). Future studies may also benefit from considering perceived vulnerability for diseases other than the four diseases assessed in the current study to fully capture perceived risk of smoking-related conditions. Last, the substances mentioned on HWLs varied by country. Since only carbon monoxide was described on HWLs in the United States and was already widely known, the knowledge measure may not be comparable with the more complex, rotating HWL information about constituents in the other countries. That said, the increases in the other countries are notable. Future research on how messages about tobacco constituents influence risk perceptions should consider assessing the relative importance of both cognitive and affective pathways, as well as the potentially bi-directional and reinforcing influence of constituent and health risk information.
Despite these limitations, our findings provide the first evidence that HWLs with qualitative information about toxic constituents can help promote perceptions of smoking-related risks. Future research should determine the specific characteristics of toxic constituent messages on cigarette HWLs that maximize consumer understanding of product harm and increase personal risk perceptions. Such research is critical for governments to better communicate about toxic constituents, which is a fundamental obligation under the WHO’s FCTC.
Supplementary Material
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Data collection and analyses were supported by a grant from the U.S. National Cancer Institute (R01 CA167067). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Footnotes
Declaration of Conflicting Interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Cummings has received payment as an expert witness in litigation filed against the tobacco industry where he testifies about matters of addiction, consumer knowledge, advertising and marketing, cigarette design, industry documents and concealment, and the impact of product warnings.
References
- Becker MH (1974). The health belief model and personal health behavior (Vol. 2): Slack. [Google Scholar]
- Borland R (1997). Tobacco health warnings and smoking-related cognitions and behaviours. Addiction, 92(11), 1427–1435. doi: 10.1111/j.1360-0443.1997.tb02864.x [DOI] [PubMed] [Google Scholar]
- Borland R, & Hill D (1997). Initial impact of the new Australian tobacco health warnings on knowledge and beliefs. Tob Control, 6(4), 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello MJ, Logel C, Fong GT, Zanna MP, & McDonald PW (2012). Perceived risk and quitting behaviors: results from the ITC 4-country survey. Am J Health Behav, 36(5), 681–692. doi: 10.5993/ajhb.36.5.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Trade Commission. (2008). FTC rescinds guidance from 1966 on statements concerning tar and nicotine yields [Press release]. Retrieved from https://www.ftc.gov/newsevents/press-releases/2008/11/ftc-rescindsguidance-1966-statements-concerning-tarnicotine
- Fong GT, Cummings KM, Borland R, Hastings G, Hyland A, Giovino GA, . . . Thompson ME (2006). The conceptual framework of the International Tobacco Control (ITC) Policy Evaluation Project. Tobacco Control, 15(suppl 3), iii3–iii11. doi: 10.1136/tc.2005.015438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong GT, Hammond D, & Hitchman SC (2009). The impact of pictures on the effectiveness of tobacco warnings. Bull World Health Organ, 87(8), 640–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GMI. (2011). GMI Global Panel Book. Bellevue, WA: Global Market Insight, Inc. [Google Scholar]
- Hall MG, Ribisl KM, & Brewer NT (2014). Smokers and Nonsmokers Beliefs About Harmful Tobacco Constituents: Implications for FDA Communication Efforts. Nicotine & Tobacco Research, 16(3), 343–350. doi: 10.1093/ntr/ntt158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D, Fong GT, McNeill A, Borland R, & Cummings KM (2006). Effectiveness of cigarette warning labels in informing smokers about the risks of smoking: findings from the International Tobacco Control (ITC) Four Country Survey. Tob Control, 15 Suppl 3, iii19–25. doi: 10.1136/tc.2005.012294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D, & White CM (2012). Improper disclosure: Tobacco packaging and emission labelling regulations. Public Health, 126(7). doi: 10.1016/j.puhe.2012.03.012 [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, & Robinson J (1989). Measuring the Heaviness of Smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction, 84(7), 791–800. doi: 10.1111/j.1360-0443.1989.tb03059.x [DOI] [PubMed] [Google Scholar]
- Jacobson JD, Catley D, Lee HS, Harrar SW, & Harris KJ (2014). Health risk perceptions predict smoking-related outcomes in Greek college students. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors, 28(3), 743–751. doi: 10.1037/a0037444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz NK, & Becker MH (1984). The Health Belief Model: A Decade Later. Health Education & Behavior, 11(1), 1–47. doi: 10.1177/109019818401100101 [DOI] [PubMed] [Google Scholar]
- Moracco KE, Morgan JC, Mendel J, Teal R, Noar SM, Ribisl KM, . . . Brewer NT (2016). “My First Thought was Croutons”: Perceptions of Cigarettes and Cigarette Smoke Constituents Among Adult Smokers and Nonsmokers. Nicotine & Tobacco Research, 18(7), 1566–1574. doi: 10.1093/ntr/ntv281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noar SM, Hall MG, Francis DB, Ribisl KM, Pepper JK, & Brewer NT (2015). Pictorial cigarette pack warnings: a metaanalysis of experimental studies. Tob Control. doi: 10.1136/tobaccocontrol-2014-051978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RJ, Kozlowski LT, Borland R, Hammond D, & McNeill A (2006). Relationship between constituent labelling and reporting of tar yields among smokers in four countries. J Public Health (Oxf), 28(4), 324–329. doi: 10.1093/pubmed/fdl056 [DOI] [PubMed] [Google Scholar]
- Rodgman A, & Perfetti TA (2013). The chemical components of tobacco and tobacco smoke. Boca Raton, FL: CRC press. [Google Scholar]
- Rogers RW (1975). A Protection Motivation Theory of Fear Appeals and Attitude Change. The Journal of Psychology, 91(1), 93–114. doi: 10.1080/00223980.1975.9915803 [DOI] [PubMed] [Google Scholar]
- Romer DJP. (2001). The role of perceived risk in starting and stopping smoking (Vol. 59). Thousand Oaks, CA, US: Sage Publications, Inc. [Google Scholar]
- Salloum R, Lourviere J, Getz K, Islam F, Anshari D, Cho YJ, . . . Thrasher J (2017). Evaluation of strategies to communicate harmful and potentially harmful constituents (HPHC) information through cigarette package inserts: A discrete choice experiment. Tobacco control, tobacco-control2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siahpush M, McNeill A, Hammond D, & Fong GT (2006). Socioeconomic and country variations in knowledge of health risks of tobacco smoking and toxic constituents of smoke: results from the 2002 International Tobacco Control (ITC) Four Country Survey. Tob Control, 15 Suppl 3, iii65–70. doi: 10.1136/tc.2005.013276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayampakala K, Thrasher JF, Hammond D, Yong HH, Bansal-Travers M, Krugman D, . . . Hardin J (2014). Pictorial health warning label content and smokers’ understanding of smoking-related risks-a cross-country comparison. Health Education Research, 30(1), 35–45. doi: 10.1093/her/cyu022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher JF, Carpenter MJ, Andrews JO, Gray KM, Alberg AJ, Navarro A, . . . Cummings KM (2012). Cigarette warning label policy alternatives and smoking-related health disparities. Am J Prev Med, 43(6), 590–600. doi: 10.1016/j.amepre.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher JF, Murukutla N, Pérez-Hernández R, Alday J, Arillo-Santillán E, Cedillo C, & Gutierrez JP (2012). Linking mass media campaigns to pictorial warning labels on cigarette packages: a cross-sectional study to evaluate effects among Mexican smokers. Tobacco Control. doi: 10.1136/tobaccocontrol-2011-050282 [DOI] [PubMed] [Google Scholar]
- Thrasher JF, Perez-Hernandez R, Arillo-Santillan E, & Barrientos-Gutierrez I (2012). Towards informed tobacco consumption in Mexico: effect of pictorial warning labels in smokers. Salud Publica Mex, 54(3), 242–253. [PMC free article] [PubMed] [Google Scholar]
- Thrasher JF, R.-S. L, Lazcano-Ponce E, Hernández-Ávila M, Editors. (2013). Reacción de fumadores y no fumadores hacia las advertencias sanitarias más impactantes en el Distrito Federal, Guadalajara y Monterrey [Public health & tobacco, Volume 2: Warning labels in Latin America and the Caribbean; ] (pp. 162–173). [Google Scholar]
- US Department of Health and Human Services. (2014). Reports of the Surgeon General The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US). [Google Scholar]
- World Health Organization. (2013a). Guidelines for implementation of Article 11 of the WHO FCTC. Retrieved from http://www.who.int/fctc/guidelines/adopted/ar ticle_11/en/
- World Health Organization. (2013b). Guidelines for implementation of Article 12 of the WHO FCTC. Retrieved from http://www.who.int/fctc/guidelines/Decision.p df?ua=1
- World Health Organization. (2013c). Guidelines for implementation of Articles 9 and 10 of the WHO FCTC. Retrieved from http://www.who.int/fctc/guidelines/Guidelines s_Articles_9_10_rev_240613.pdf?ua=1
- Yong HH, Borland R, Thrasher JF, Thompson ME, Nagelhout GE, Fong GT, . . . Cummings KM (2014). Mediational Pathways of the Impact of Cigarette Warning Labels on Quit Attempts. Health Psychology, 33(11), 1410–1420. doi: 10.1037/hea0000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.