Abstract
Objectives
This study aimed to determine the relationship between the polymorphisms of the H1/H2 gene of platelet membrane receptor P2Y12 and cerebral infarction (CI) in a Han population in North Shandong Province, People’s Republic of China.
Patients and methods
A case–control study, which involved 168 nonstoke subjects (contrast group) and 152 CI patients (CI group), was conducted. The state of subjects in the CI group was validated by computed tomography or MRI. The clinical data were categorized into two groups. The data included age, gender, smoking, drinking, shrinkage pressure, diastolic blood pressure, blood glucose, cholesterol, triglyceride, low-density lipoprotein, high-density lipoprotein, serum uric acid, fibrinogen and homocysteine. The polymorphisms were genotyped with PCR and restriction fragment length polymorphism analysis. The distribution characteristics of nonstoke subjects and CI patients and the relationship between the polymorphisms of the H1/H2 gene of platelet membrane receptor P2Y12 and ischemic stroke were analyzed.
Results
No significant difference was found between the contrast group and CI group (P>0.05) in terms of age, gender composition, smoking, alcohol consumption, blood glucose, cholesterol, triglyceride, low-density protein, high-density lipoprotein cholesterol, uric acid and homocysteine. In contrast, significant differences were found between these two groups (P<0.01) in terms of SBP, DBP and plasma fibrinogen level. The genotyping revealed 112 carriers of the wild-type H1/H1 genotype and 40 carriers of the mutational H2 allele of P2Y12 H1/H2 in the CI group and 140 carriers of the wild-type H1/H1 genotype and 28 carriers of the mutational H2 allele of P2Y12 H1/H2 in the contrast group. Furthermore, the H1/H2 and H2/H2 gene frequencies (26.3%) were significantly higher in the CI group (χ2=4.440, P<0.05) than those in the contrast group (16.7%). Moreover, the frequencies of the H2 allele in the CI and contrast groups were 14.5% and 8.6%, respectively, and the difference was statistically significant (χ2=5.392, P<0.05). Multiple logistic regression analysis results revealed that factors associated with CI include systolic blood pressure and plasma fibrinogen level, which carry the −893T gene. After adjusting for potential confounding factors, the H2 allele carriers had a 1.928-fold increased risk for CI (OR=1.928, 95% confidence interval: 1.137–3.188; P=0.038) when compared with noncarriers.
Conclusion
The present study found that hypertension and elevated plasma fibrinogen levels are significant risk factors for ischemic stroke and confirmed that the H1/H2 and H2/H2 genes of platelet membrane glycoprotein receptor P2Y12 are risk factors of ischemic stroke.
Keywords: adenosine diphosphate, P2Y12 gene, single-nucleotide polymorphism, ischemic stroke, clopidogrel resistance
Introduction
Atherosclerosis is the main cause of ischemic stroke. In atherosclerosis, as well as in arterial thrombosis, platelet activation and aggregation are the core factors. Adenosine diphosphate (ADP) is an important medium for platelet aggregation, and P2Y12 is an ADP receptor on the platelet surface. When ADP combines with P2Y12 and G protein, it induces and stabilizes the persistent platelet aggregation effect. A study in apolipoprotein E-deficient mice revealed that P2Y12 expressed on platelets is a key factor responsible for atherosclerosis. Platelet P2Y12 deficiency suppresses platelet factor 4 secretion and P-selectin expression and critically regulates the release of platelet factor 4 through the inhibition of the cAMP/protein kinase A pathway, affecting monocyte recruitment and infiltration.1
The polymorphism of the P2Y12 receptor gene may lead to thrombosis through the influence of platelet functions.2 The present study3 confirmed that the following five sites have polymorphisms in this gene: C34T, G52T, I-C139T, i-T744C and i-ins80lA. Among these sites, C139T, i-T744C, i-ins801A and G52T are completely linked, and the resulting link is designated either as primary haploid H1 (not carrying four kinds of polymorphisms) or secondary haploid H2 (carrying all four polymorphisms).
At present, there is a common relationship between the platelet membrane glycoprotein receptor P2Y12 gene and clopidogrel.4–8 A large study in clopidogrel-treated patients suggested that carriers of the H2/H2 haplotype of the P2Y12 gene exhibited stronger aggregation and that a homozygote H2 genotype contributes to clopidogrel resistance.9 Recently, a meta-analysis revealed that C34T and G52T polymorphisms in the P2Y12 receptor gene might be risk factors for poor response to platelet in patients receiving clopidogrel therapy.10 Another study conducted by Liu et al,11 which included 191 ischemic stroke patients, revealed that the C allele in P2RY12 (rs2046934) was predicted to be a protective factor for clopidogrel resistance (CC+TC vs TT; OR: 0.407, 95% confidence interval: 0.191–0.867, P=0.018).
However, the study on the effect of the P2Y12 polymorphism of the platelet membrane glycoprotein receptor gene on ischemic stroke as an independent factor remains inconclusive. Hence, further studies are needed to determine the underlying mechanisms in ischemic stroke. The present study focuses on the correlation between the G52T polymorphism in the P2Y12 receptor gene and ischemic stroke.
Patients and methods
Study population
A total of 152 patients were assigned to the cerebral infarction (CI) group. Among these patients, 83 patients were male and 69 patients were female, and their age ranged within 36–88 years, with an average age of 63.91±11.49 years. All patients were of Han nationality residing in Shandong Province and were admitted to the neurological ward of Binzhou Medical University Hospital from July 2013 to April 2015. The criteria for enrollment conformed to the diagnostic criteria of the Fourth Session of the National Conference (People’s Republic of China) on Cerebrovascular Disease, which were confirmed by computed tomography (CT) or MRI. The exclusion criteria were as follows: patients with atrial fibrillation, history of trauma surgery, diseases of the blood system, tumors, autoimmunity and incomplete heart, lung, liver and kidney function. In addition, a total of 168 subjects were assigned to the contrast group. Among these subjects, 78 subjects were male and 90 subjects were female, and their age ranged within 38–86 years, with an average age of 61.65±11.47 years. All subjects in the contrast group were of Han nationality residing in the Shandong Province and were healthy as confirmed by CT or MRI. The following clinical data were collected: age, gender, smoking, drinking, systolic blood pressure (SBP), diastolic blood pressure (DBP), blood glucose, blood lipid (cholesterol, triglyceride, low-density lipoprotein and high-density lipoprotein), serum uric acid, plasma fibrinogen level and homocysteine level. The present study was approved by the ethics committee of Binzhou Medical University Hospital. All participants agreed to participate in this experiment and signed written informed consent.
Genotyping
DNA extraction method
Blood samples were obtained from the peripheral veins of patients and healthy subjects and were stored in 4 mL evacuated vacuum tubes containing 3.8% sodium citrate and EDTA at a ratio of 1:9. Genomic DNA was extracted from white blood cells using the centrifugal column method, according to the instructions of the blood genomic DNA extraction kit, which was purchased from TaKaRa (Dalian Treasure Biological Engineering Co., Ltd.).
Genetic screening
PCR and restriction fragment length polymorphism analysis were used to detect the G52T genotype of the P2Y12 gene. The primers used were designed according to the search results of the genebank and the reference. The sequences of the primer for the P2Y12 gene were as follows: sense primer 5′-TCACTTATCTCTGGTGAAATAATAAGATTACGTA-3′ and antisense primer 5′-GTC AGAAATGGCCTCTGTATATATCGTCATGAGTAGTCGTACG-3′. These were synthesized by Shanghai Sangon Biological Engineering Co., Ltd. The PCR for P2Y12 polymorphism typing was performed in a total volume of 50 µL containing 200 µmol/L of each dNTP, 10 pmol of each primer, 2 µg of DNA, 5 U of Taq DNA polymerase and 50 µL of liquid paraffin. The PCR parameters were as follows: initial denaturation for 4 minutes at 95°C, followed by 40 cycles of 30-second denaturation at 94°C, 30-second annealing at 56°C, 60-second extension at 72°C and a final extension for 10 minutes at 72°C. Approximately 5 µL of the PCR product was collected for electrophoresis in 2% agarose gel (ethidium bromide was added), and the 230 bp fragments confirmed under ultraviolet light was the desired product. A solution containing 1 µL of restriction enzyme RsaI, 2 µL of 10× T Buffer, 2 µL of 0.1% BSA and sterile water was added to the 5 µL PCR product to a total volume of 20 µL, and the resultant solution was stored at 30°C in water for 4 hours. Approximately 2 µL of 10× loading buffer was added to 20 µL PCR enzyme-digested products to terminate the enzyme reaction. Subsequently, 10 µL of the PCR enzyme-digested product was subjected to electrophoresis for 15 minutes in 2% agarose gel electrophoresis (ethidium bromide was added) at 110 V. The restricted DNA products were visualized by ultraviolet light and analyzed using the standard molecular weight of the DNA ladder.
Statistical analyses
All data were presented as mean±SD. The difference in measurement data between the CI group and contrast group was compared using a two sample t-test. All single-nucleotide polymorphism (SNPs) evaluated in the present study were tested for deviation from the Hardy–Weinberg equilibrium using the chi-squared test. The genotype and allele frequencies between the CI group and contrast group were compared using the chi-squared test. The relationship between the variables and CI was studied using multivariate logistic regression analysis. Statistical analysis was performed using SPSS version 19.0. A two-tailed probability value of <0.05 or 0.01 was considered as significant.
Results
Clinical characteristics
The baseline characteristics of the study groups are presented in Table 1. No significant difference (P>0.05) was found between groups with regard to age, gender, smoking, alcohol consumption, blood glucose, cholesterol, triglyceride, low-density lipoprotein, high-density lipoprotein, serum uric acid and homocysteine. However, significant differences were observed in SBP, DBP and plasma fibrinogen level between the two groups (P<0.01).
Table 1.
Comparison of demographic characteristics and risk factors of stroke between the two groups
| Category | CI group (n=152) | Contrast group (n=168) | P-value |
|---|---|---|---|
| Age, mean±SD | 63.91±11.486 | 61.65±11.486 | 0.084 |
| Gender (M/F), n | 83/69 | 78/90 | 0.148 |
| Smoking, n (%) | 19.1 (29) | 12.5 (21) | 0.124 |
| Alcohol, n (%) | 16.4 (25) | 10.7 (18) | 0.143 |
| SBP (mmHg), mean±SD | 156.82±24.015 | 140.04±24.438 | <0.001 |
| DBP (mmHg), mean±SD | 90.15±14.543 | 82.58±12.593 | <0.001 |
| Blood glucose (mmol/L), mean±SD | 6.1438±2.114 | 5.757±1.46 | 0.06 |
| Cholesterol (mmol/L), mean±SD | 4.5943±1.192 | 4.836±1.1206 | 0.063 |
| Triglyceride (mmol/L), mean±SD | 1.553±0.9221 | 1.719±1.1419 | 0.156 |
| Low-density lipoprotein (mmol/L), mean±SD | 2.9268±0.9681 | 3.006±0.9356 | 0.46 |
| High-density lipoprotein (mmol/L), mean±SD | 1.104±0.3444 | 1.1407±0.339 | 0.334 |
| Fibrinogen (g/L), mean±SD | 3.339±0.8741 | 3.091±0.6764 | 0.005 |
| Serum uric acid (µmol/L), mean±SD | 271.59±80.5959 | 278.26±94.5859 | 0.5 |
| Homocysteine levels (µmol/L), mean±SD | 16.628±12.0426 | 14.78±9.494 | 0.127 |
Abbreviations: CI, cerebral infarction; M, male; F, female; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Polymorphism analysis of the P2Y12 gene in the platelet membrane receptor
The PCR enzyme-digested products are presented in Figure 1. The length of the amplified fragment of the platelet membrane receptor P2Y12 gene G52T was 230 bp. The PCR product was digested with enzyme RsaI in the presence of the H1 allele, yielding 197 and 33 bp fragments for the H1/H1 genotype, respectively. The homozygous mutation (H2/H2 genotype) contained two RsaI enzyme cleavage sites, which yielded three fragments of 157, 40 and 33 bp for the H2/H2 genotype and four fragments of 197, 157, 40 and 33 bp for the H1/H2 genotype.
Figure 1.
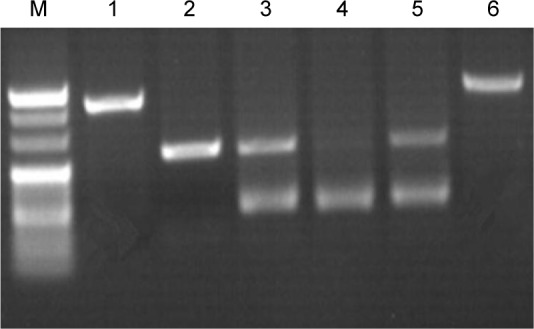
P2Y12 gene electrophoretogram.
Notes: M denotes the indicator “Mark”: the index strip from top to bottom is 250 bp, 220 bp, 200 bp, 170 bp and 150 bp. (1 and 6) The PCR product: 230 bp. (2) H1/H1: 197 bp; (3 and 5) H1/H2: 197 bp and 157 bp. (4) H2/H2: 157 bp. Magnification 1:1.
Genotype distribution
A total of 320 subjects were included in the present study. Among these subjects, 168 were healthy subjects and 152 were CI patients. In the CI group, 112 patients were H1/H1 genotype, 36 patients were H1/H2 genotype, and four patients were H2/H2 genotype. In the contrast group, 140 subjects were H1/H1 genotype, 27 subjects were H1/H2 genotype and one subject was H2/H2 genotype. The H1/H2 and H2/H2 genotype frequency (26.3%) was higher in the CI group than that in the contrast group (16.7%), and the difference was statistically significant (χ2=4.440, P<0.05). Meanwhile, the haploid H2 allele frequency (14.5%) was higher in the CI group than that in the contrast group (8.6%), and the difference was statistically significant (χ2=5.392, P<0.05). The distribution of H1/H2 gene polymorphisms of the P2Y12 gene between the CI group and contrast group is presented in Table 2.
Table 2.
Frequency comparison of the H1/H2 polymorphism of the P2Y12 gene between CI and contrast groups
| Group | n | Genotype
|
χ2 | P-value | Allele type
|
χ2 | P-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| H1/H1 (%) | H1/H2 (%) | H2/H2 (%) | H1 (%) | H2 (%) | ||||||
| CI group | 152 | 112 (73.7) | 36 (23.4) | 4 (2.9) | 4.440 | 0.035 | 260 (85.5) | 44 (14.5) | 5.392 | 0.020 |
| Contrast group | 168 | 140 (83.3) | 27 (16.1) | 1 (0.6) | 307(91.4) | 29 (8.6) | ||||
Abbreviation: CI, cerebral infarction.
Multivariate logistic regression analysis
Table 3 shows the results of the multivariate logistic regression analysis. CI was used as the dependent variable, while the following data were used as independent variables in the analysis: age, gender, smoking, alcohol consumption, SBP, DBP, blood glucose, cholesterol, triglyceride, low-density lipoprotein, high-density lipoprotein, serum uric acid, plasma fibrinogen level, homocysteine level and P2Y12 H1/H2 genotype. The results revealed that SBP, plasma fibrinogen level and -H2 gene carriers (P<0.05 or P<0.01) can affect CI. After controlling for potential confounding factors, individuals who were −8 H2 allele carriers had an ~1.928 times (OR=1.928, 95% confidence interval: 1.137–3.188, P=0.038) increase in occurrence of CI when compared with that of noncarriers. Therefore, the -H2 allele is a risk factor for CI.
Table 3.
Multivariate logistic regression analysis with CI as the dependent variable
| Independent variable | Significance | Exp (B) | 95% confidence interval of Exp (B)
|
|
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Age | 0.596 | 1.007 | 0.982 | 1.031 |
| Gender | 0.334 | 0.747 | 0.414 | 1.349 |
| Smoking | 0.585 | 1.257 | 0.553 | 2.855 |
| Drinking | 0.409 | 1.451 | 0.600 | 3.505 |
| SBP | 0.004 | 1.002 | 1.007 | 1.037 |
| DBP | 0.150 | 1.019 | 0.993 | 1.045 |
| Blood glucose | 0.150 | 1.140 | 0.954 | 1.362 |
| Cholesterol | 0.166 | 0.761 | 0.517 | 1.120 |
| Triglyceride | 0.440 | 0.887 | 0.653 | 1.203 |
| Low-density lipoprotein | 0.741 | 1.078 | 0.691 | 1.681 |
| High-density lipoprotein | 0.508 | 0.748 | 0.317 | 1.766 |
| Plasma fibrinogen level | 0.047 | 1.413 | 1.005 | 1.986 |
| Serum uric acid | 0.195 | 0.998 | 0.995 | 1.001 |
| Homocysteine level | 0.217 | 1.015 | 0.991 | 1.039 |
| Genotype | 0.038 | 1.928 | 1.137 | 3.188 |
Abbreviations: CI, cerebral infarction; SBP, systolic blood pressure; DBP, diastolic blood pressure.
The distribution of the P2Y12 genotype H1/H2 in the two groups was in line with the Hardy–Weinberg balance using the chi-squared test, indicating that the genotype distribution of the P2Y12 gene H1/H2 polymorphism in the investigated population reached a genetic equilibrium, as shown in Table 4.
Table 4.
The genotype distribution of the Hardy–Weinberg equilibrium in the two groups
| P2y12 g52t
|
|||
|---|---|---|---|
| GG | GT | TT | |
| CI group | |||
| Observed value | 112 | 36 | 4 |
| Expected value | 111.1 | 37.69 | 3.2 |
| χ2=0.289, P>0.75 | |||
| Contrast group | |||
| Observed value | 140 | 27 | 1 |
| Expected value | 140.28 | 26.4 | 1.18 |
| χ2=0.0426, P>0.75 | |||
Abbreviation: CI, cerebral infarction.
Discussion
The P2×1 receptor (ligand-gated ion channel), P2Y1 receptor and P2Y12 receptor are the three types of presently known ADP receptors on the surface of the platelet membrane. Among these receptors, the P2Y12 receptor occurs only in the platelet membrane, and its distribution is more than that of the P2Y1 receptor. It mainly combines with the Gi protein to play an important role on ADP-induced platelet aggregation. Carrying different platelet membrane receptor P2Y genotypes may interfere with P2Y receptor function and affect arterial thrombotic diseases, such as coronary heart disease and ischemic stroke, as well as the susceptibility and sensitivity of antiplatelet aggregation drugs, such as aspirin and clopidogrel.
Fontana et al3 examined ADP-induced platelet aggregation responses in 98 healthy volunteers and found that the number of H2 alleles was associated with the maximal aggregation response to ADP (P=0.007). They came to the conclusion that the haplotype H2 allele of the P2Y12 receptor is correlated to ADP-induced platelet aggregation in healthy subjects, suggesting that the P2Y12 receptor gene polymorphism may be associated with atherosclerosis. Kim et al12 also found that maximal platelet aggregation in response to ADP is associated with the P2Y12 52C>T polymorphism in a Korean population.
Zoheir et al13 studied the relationship between the T744C genetic polymorphism of the P2Y12 ADP receptor and the activation of this receptor by ADP or the response of patients to platelet inhibitors. The result revealed that the T744C polymorphism of the P2Y12 ADP receptor gene was correlated with platelet reactivity. Carrying the C allele at this position is associated with increased platelet activation response to ADP. Another study conducted by Long et al,14 which included 165 ischemic stroke cases and 152 healthy subjects, revealed that platelet ADP receptor P2Y12 gene −744TC/TT genotype frequency (43.61%) is significantly higher in the CI group (χ2=4.73, P<0.05) than in the contrast group (28.95%). Furthermore, the 744 C allele frequency was 22.03% in the CI group, which was significantly higher (χ2=5.80, P<0.05) than that in the contrast group (15.46%). After adjusting for risk factors, the carriers of the P2Y12 gene −744TC/TT genotype had an ~1.65 times (OR=1.65, 95% confidence interval: 1.03–2.75, P=0.04) increase in CI occurrence when compared with noncarriers. They concluded that the P2Y12 gene −744TC/TT genotype may be one of the risk factors of ischemic stroke.
Li et al15 investigated the correlation of clopidogrel resistance with C34T and G52T polymorphisms of ADP receptor P2Y12. All 370 patients in this study had coronary atherosclerotic heart disease. They found that the incidence of clopidogrel resistance was significantly lower in patients carrying the CC genotype when compared with that in patients carrying the CT+TT genotype (P<0.05) in the C34T locus. However, in the G52T locus, the incidence of clopidogrel resistance in patients with the GT+TT genotype was significantly higher than that in patients with the GG genotype (P<0.05). After the 1-year follow-up, patients with the CC genotype had a lower incidence of angina recurrence compared with patients with the CT+TT genotype (13.2% vs 19.6%, χ2=4.956, P<0.05), whereas patients with the GG genotype had lower incidences of emergency revascularization, angina and cardiovascular composite end point events when compared with patients with the GT+TT genotype (P<0.05). Therefore, gene mutations of P2Y12 receptors C34T and G52T may be risk factors for clopidogrel resistance and adverse cardiovascular events. The study conducted by Ziegler et al16 in peripheral artery disease patients revealed that clopidogrel response variability exists and carriers of the P2Y12 polymorphism 34T allele had a 4.02-fold increased adjusted risk for adverse neurological events (ischemic stroke and/or carotid revascularization) when compared with carriers of only 34C alleles (95% confidence interval: 1.08–14.9). However, no significant interaction between the 52G>T polymorphism and neurological events was found.
On the contrary, Bierend et al17 studied platelet aggregation in platelet-rich plasma obtained from 124 CAD patients treated with 100 mg of aspirin per day (−1). They found that low-dose aspirin inhibits platelet aggregation to the same extent as in the patients carrying or not carrying the P2Y12 H2 haplotype and 34T allele. In addition, the P2Y12 gene polymorphism did not significantly affect any of the aggregatory response. Zee et al18 carried out a prospective cohort study of 14,916 initially healthy American men to examine the possible association of P2RY12 genetic variants, particularly haplotype H2 (comprising dbSNP rs10935838, rs2046934, rs5853517 and rs6809699), among the 708 white males who subsequently developed a thromboembolic event (incident myocardial infarction [MI], ischemic stroke or deep venous thromboembolism [DVT]/pulmonary embolism [PE]) and among the equal number of age- and smoking-matched white males who remained free of reported vascular disease during the follow-up (controls). Furthermore, the haplotype H2 distribution was significantly different between the DVT/PE cases (12%) and their matched controls (21%; P=0.02). In addition, the haplotype H2 was significantly associated with a lower risk of incident DVT/PE when compared with the reference haplotype H1 (OR=0.50, 95% confidence interval=0.27–0.93, P=0.028). However, P2RY12 variants or the haplotype H2 with incident MI or ischemic stroke was found.
Some studies found that adverse clinical events may be multifactorial, but is not determined by single-gene polymorphisms. One study19 aimed to evaluate the effects of platelet receptor gene (P2Y12, P2Y1) and glycoprotein gene (GPIIIa) polymorphisms, as well as their interactions, on antiplatelet drug responsiveness and clinical outcomes in patients with acute minor ischemic stroke (MIS). This study prospectively enrolled 426 patients with acute MIS, who had been receiving combined aspirin and clopidogrel treatment for the last 3 months, and found that the responsiveness to antiplatelet drugs and the risks for adverse clinical events in this MIS cohort appear to be multifactorial, since the outcomes were not mediated by single-gene polymorphisms. The CYP2C8 rs17110453A>C, GPIIIa rs2317676A>G, and P2Y12 rs16863323C>T three-loci interaction may confer a higher risk for ischemic stroke (IS).20
The present study revealed that no significant difference (P>0.05) was present between the CI group and contrast group in terms of age, gender composition, smoking, alcohol consumption, blood glucose, cholesterol, triglyceride, low-density lipoprotein, high-density lipoprotein, cholesterol, serum uric acid and homocysteine level. Meanwhile, the two groups exhibited significant differences (P<0.01 or P<0.05) in terms of SBP, DBP and plasma fibrinogen level, indicating that high blood pressure and plasma fibrinogen levels are closely correlated to the occurrence of ischemic stroke. Other traditional stroke risk factors, such as smoking and dyslipidemia, and other differences between these two groups were not statistically significant. However, in other traditional stroke risk factors, such as smoking, the difference in dyslipidemia between the two groups was not statistically significant. This result may be because the patients selected for the present study had no first onset, and some of them had relapsed or quit smoking, and other preventive measures. In addition, they had second-level stroke prevention and long-term use of lipid-lowering drugs. These reasons caused bias in some of the present results.
In the present study, the P2Y12 gene haplotype frequency of H2 was 8.6% in the Shandong Han population, which was lower than that in Caucasians (13.8%) and Mexican Mestizos (12.8%).3,21 This result may be related to racial differences. A statistical analysis was performed on the combined H1/H2 gene and H2/H2 gene group, given the low number of the H2/H2 gene group. In the CI group, the H1/H2, H2/H2 genotype frequency was 26.3%, which was significantly higher (χ2=4.440, P<0.05) compared with the contrast group (16.7%). The haploid H2 allele frequency was 14.5%, which was significantly higher (χ2=5.392, P<0.05) than that in the contrast group (8.6%). By adjusting for age, gender, smoking, alcohol consumption, SBP, DBP, blood glucose, cholesterol, triglyceride, low-density lipoprotein, high-density lipoprotein, serum uric acid, plasma fibrinogen level and homocysteine level and using the healthy control group as a reference, the multivariate logistic regression analysis revealed that individuals who carry the haploid H2 allele had añ1.928 times (OR=1.928, 95% confidence interval: 1.137–3.188, P=0.038) increase in CI occurrence compared with non-carriers. Therefore, it could be inferred that the P2Y12 gene H1/H2 polymorphism of the platelet membrane receptor is correlated with ischemic stroke. The P2Y12 gene H1/H2 H2/H2 genotype may be one of the risk factors of stroke, and the haploid H2 allele may be the etiological factor of ischemic stroke.
Owing to differences in genetic factors among different populations, the research results may vary. Meanwhile, ischemic stroke is a multigene disease caused by the combined effects of multiple genetic factors and environmental factors. Therefore, better conclusions may be obtained by developing more selective gene loci polymorphism tests and conducting prospective studies with large sample sizes, while considering many factors and indices.
Conclusion
Hypertension and elevated plasma fibrinogen levels are significant risk factors for ischemic stroke, and the H1/H2 and H2/H2 genes of platelet membrane glycoprotein receptor P2Y12 are risk factors of ischemic stroke.
Acknowledgments
This work was supported by the Scientific Research Common Program of Bin Zhou city (No 2013ZC1713) and the Science and Technology Development Program Project of Shandong Medical and Health (No 2017WS232).
Footnotes
Author contributions
S-JL made substantial contributions to the conception of the work, analysis and interpretation of the data and drafted and revised the manuscript. X-SZ and QZ made substantial contributions to the data acquisition, analysis and interpretation of the data and also helped to draft and revise the manuscript. H-LC and Y-LG made substantial contributions to the DNA extraction and gene detection. All authors contributed toward drafting and revising the paper, and agree to be accountable for all aspects of the work.
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Li D, Wang Y, Zhang L, et al. Roles of purinergic receptor P2Y, G protein-coupled 12 in the development of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32(8):e81–e89. doi: 10.1161/ATVBAHA.111.239095. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Jung IS, Jung EJ, et al. Identification of P2Y12 single-nucleotide polymorphisms and their influences on the variation in ADP-induced platelet aggregation. Thromb Res. 2011;127(3):220–227. doi: 10.1016/j.thromres.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Fontana P, Dupont A, Gandrille S, et al. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation. 2003;108(8):989–995. doi: 10.1161/01.CIR.0000085073.69189.88. [DOI] [PubMed] [Google Scholar]
- 4.Galić E, Vrbanić L, Kapitanović S, et al. P2RY12 gene polymorphisms and effect of clopidogrel on platelet aggregation. Coll Antropol. 2013;37(2):491–498. [PubMed] [Google Scholar]
- 5.Tiu Rebrova, Muslimova EF, Afanas’ev SA, Sergienko TN, Repin AN. Resistance to clopidogrel and polymorphism of P2RY12 and GPIIIA genes in patients with chronic coronary heart disease. Klin Med. 2013;91(8):29–31. [PubMed] [Google Scholar]
- 6.Kar R, Meena A, Yadav BK, Yadav R, Kar SS, Saxena R. Clopidogrel resistance in North Indian patients of coronary artery disease and lack of its association with platelet ADP receptors P2Y1 and P2Y12 gene polymorphisms. Platelets. 2013;24(4):297–302. doi: 10.3109/09537104.2012.693992. [DOI] [PubMed] [Google Scholar]
- 7.Zhang WB, Zhang XX, Chen XY, et al. Relationship between P2Y12 gene polymorphisms and clopidogrel resistance in patients with coronary heart disease. Chin J Cardiovasc Med. 2015;20(1):18–22. [Google Scholar]
- 8.Yang HH, Chen Y, Gao CY. Associations of P2Y12R gene polymorphisms with susceptibility to coronary heart disease and clinical efficacy of antiplatelet treatment with clopidogrel. Cardiovasc Ther. 2016;34(6):460–467. doi: 10.1111/1755-5922.12223. [DOI] [PubMed] [Google Scholar]
- 9.Staritz P, Kurz K, Stoll M, Giannitsis E, Katus HA, Ivandic BT. Platelet reactivity and clopidogrel resistance are associated with the H2 haplotype of the P2Y12-ADP receptor gene. Int J Cardiol. 2009;133(3):341–345. doi: 10.1016/j.ijcard.2007.12.118. [DOI] [PubMed] [Google Scholar]
- 10.Cui G, Zhang S, Zou J, Chen Y, Chen H. P2Y12 receptor gene polymorphism and the risk of resistance to clopidogrel: A meta-analysis and review of the literature. Adv Clin Exp Med. 2017;26(2):343–349. doi: 10.17219/acem/63745. [DOI] [PubMed] [Google Scholar]
- 11.Liu R, Zhou ZY, Chen YB, et al. Associations of CYP3A4, NR1I2, CYP2C19 and P2RY12 polymorphisms with clopidogrel resistance in Chinese patients with ischemic stroke. Acta Pharmacol Sin. 2016;37(7):882–888. doi: 10.1038/aps.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KA, Song WG, Lee HM, Joo HJ, Park JY. Effect of P2Y1 and P2Y12 genetic polymorphisms on the ADP-induced platelet aggregation in a Korean population. Thromb Res. 2013;132(2):221–226. doi: 10.1016/j.thromres.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Zoheir N, Abd Elhamid S, Abulata N, El Sobky M, Khafagy D, Mostafa A. P2Y12 receptor gene polymorphism and antiplatelet effect of clopidogrel in patients with coronary artery disease after coronary stenting. Blood Coagul Fibrinolysis. 2013;24(5):525–531. doi: 10.1097/MBC.0b013e32835e98bf. [DOI] [PubMed] [Google Scholar]
- 14.Long HY, Dai W, Zhang ZP, Song YQ, Xie AM. Association of platelet membrane glycoprotein receptor P2Y12 gene polymorphism and ischemic stroke. Chin J Nerv Ment Dis. 2012;38(12):757–759. Article in Chinese. [Google Scholar]
- 15.Li XJ, Chen XM. Association between clopidogrel resistance and polymorphism of platelet adenosine diphosphate receptor in patients with coronary atherosclerotic disease. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2014;43(3):333–338. doi: 10.3785/j.issn.1008-9292.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Ziegler S, Schillinger M, Funk M, et al. Association of a functional polymorphism in the clopidogrel target receptor gene, P2Y12, and the risk for ischemic cerebrovascular events in patients with peripheral artery disease. Stroke. 2005;36(7):1394–1399. doi: 10.1161/01.STR.0000169922.79281.a5. [DOI] [PubMed] [Google Scholar]
- 17.Bierend A, Rau T, Maas R, Schwedhelm E, Böger RH. P2Y12 polymorphisms and antiplatelet effects of aspirin in patients with coronary artery disease. Br J Clin Pharmacol. 2008;65(4):540–547. doi: 10.1111/j.1365-2125.2007.03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zee RY, Michaud SE, Diehl KA, et al. Purinergic receptor P2Y, G-protein coupled, 12 gene variants and risk of incident ischemic stroke, myocardial infarction, and venous thromboembolism. Atherosclerosis. 2008;197(2):694–699. doi: 10.1016/j.atherosclerosis.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Yi X, Zhou Q, Wang C, Lin J, Liu P, Fu C. Platelet receptor Gene (P2Y12, P2Y1) and platelet glycoprotein Gene (GPIIIa) polymorphisms are associated with antiplatelet drug responsiveness and clinical outcomes after acute minor ischemic stroke. Eur J Clin Pharmacol. 2017;73(4):437–443. doi: 10.1007/s00228-017-2198-2. [DOI] [PubMed] [Google Scholar]
- 20.Yi X, Lin J, Wang Y, Zhou J, Zhou Q. Interaction among CYP2C8, GPIIIa and P2Y12 variants increase susceptibility to ischemic stroke in Chinese population. Oncotarget. 2017;8(41):70811–70820. doi: 10.18632/oncotarget.19991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vargas-Alarcón G, Ramírez-Bello J, de La Peña A, et al. Distribution of ABCB1, CYP3A5, CYP2C19, and P2RY12 gene polymorphisms in a Mexican Mestizos population. Mol Biol Rep. 2014;41(10):7023–7029. doi: 10.1007/s11033-014-3590-y. [DOI] [PubMed] [Google Scholar]


