Abstract
Objective:
Febrile neutropenia (FN) is one of the most serious clinical problems in patients with hematologic malignancies and patients receiving chemotherapy. The present study was implemented to determine precisely how FN is managed in most referral hospitals in Isfahan (Iran) and what are the characteristics of FN patients as well as risk factors associated with FN development.
Methods:
This study was a cross-sectional study performed over a period of 6 months on patients hospitalized in the Hematology-Oncology Center of Omid Hospital, Isfahan, Iran. The information was collected by filling the designed data abstraction form.
Findings:
A total of 115 oncology patients were admitted with or encountered to FN. This equates to a cumulative incidence of 1.26% of FN cases per 1000 oncology admissions. The average age was 49.5 ± 18.02 years (range 18–85 years), with 42.6% of patients being female. The most frequently prescribed antibiotic agents were meropenem (91.3%) and vancomycin (47.82%) alone or in combination. Empiric antifungal agents initiated in 20.86% of cases, and we could not find any patients who needed to receive antiviral treatment. From all positive cultures, Gram-positive microorganisms were the most found pathogen. Among them, female sex (42.6%) and lymphopenia (26.5%) were the most noted predictors. Neutropenia (81.7%) was the most reported risk factors for serious complications.
Conclusion:
Although our center is university-affiliated, there are still several points, and pitfalls must be considered and revised in the management of FN patients. Obtaining and assessing the samples microbiologically and antibiotic therapy accordingly were the most troublesome complications.
KEYWORDS: Cancer, febrile neutropenia, incidence, outcome
INTRODUCTION
Febrile neutropenia (FN) is one of the most common and serious clinical problems in patients with hematologic malignancies and/or patients receiving chemotherapy. Approximately, 50% of patients with FN will develop infection, of which 20% of patients with profound neutropenia will find documented bacteremia.[1]
Recently, mortality due to FN has been remarkably reduced because of advances in diagnostic methods and development in broad-spectrum new antibiotics. It has been estimated that 30-day mortality ranges from 6% to 10% among patients who were suffering from FN.[2]
Cancer patients receiving antineoplastic therapy are susceptible to be adversely affected by chemotherapy-induced side effects such as myelosuppression or mucositis, which make them at risk for bacterial and fungal infections. Since inflammatory response is muted in neutropenic patients, a fever may be the earliest and the only sign of infection. It is, therefore, critical to recognize fever early in neutropenic patients and to initiate antibacterial therapy promptly to avoid progression to sepsis syndrome and possibly death.[3]
Several factors can affect clinical outcome of patients suffering from FN. These include the patient's underlying disease, age, patients' clinical condition, number of infectious foci, duration of the neutropenia, onset of antibiotic or antifungal therapy, geographical location, and local profile of antimicrobial resistance.[4]
In reality, despite availability of different international guidelines such as the Infectious Diseases Society of America (IDSA)[1] and the 2013 American Society of Clinical Oncology (ASCO) guidelines,[5] we are still facing many challenges in the management of patients with FN.
However, to the best of our knowledge, there is no specific comprehensive report on FN incidence rate, complication, and its routine management in our oncological centers in Iran. Furthermore, the present study was designed to determine precisely how FN is managed in most referral hospitals in Isfahan, Iran, and what the characteristics of FN patients have and also risk factors associated with FN development.
METHODS
This is an observational, cross-sectional study that was performed over a period of 6 months (from the beginning of April to the end of September 2016) on patients hospitalized in the Hematology-Oncology Center of the Omid Hospital affiliated by the Isfahan University of Medical Sciences. The hospital is a referral and well-equipped 200-bed center in Iran specialized in the treatment of oncology and hematology patients.
Over a 6-month period, we assessed all adult patients (18 years of age or above) admitted to the hospital due to primarily FN diagnosis or encountered to FN after receiving chemotherapy during the hospitalization. We included both patients with solid tumors and those suffering from hematologic malignancies.
We defined fever and neutropenia according to the ASCO guidelines[5] that fever defines in neutropenic patients as a single oral temperature of >38.3°C (101°F) or a temperature of >38.0°C (100.4°F) sustained for >1 h. Although the definition of neutropenia differs from institution to institution, we defined neutropenia as an absolute neutrophil count (ANC) <500 cells/μL or an ANC that is expected to decrease to <500 cells/μL during the next 48 h.
We considered all patients who fulfilled one of the below criteria as FN.[1]
Microbiologically documented infection: FN with a microbial focus of infection and an associated pathogen
Clinically documented infection: FN with a clinical focus (e.g., cellulitis and pneumonia) but without the isolation of an associated pathogen
Unexplained fever: FN without a microbial or clinical focus.
The medical ethics committee of the hospital approved the study, and written consent was obtained from all included patients. The required information was collected by an educated pharmacy student via filling the designed data collection sheet including:
Baseline characteristics of the study population (sociodemographic data, clinical data, and patients' drug and medical history)
Methods used to investigate the patients diagnosed by FN (physical examinations, imaging, and microbiological assays)
Management of the included patients according to antibiotic and antifungal therapy
Assessment of risk factors related to patient's outcome.
Our data collection sheet included data around demographic characteristic on age, gender, underlying cancer, disease burden, comorbidities (including diabetes, chronic obstructive pulmonary disease [COPD], and heart, liver, and kidney disease), recent chemotherapy regimen, antibiotic treatment, history of prior invasive fungal or microbial infection, granulocyte colony-stimulating factor (G-CSF) treatment, length of hospital stay, and outcome of treatment recorded by the investigators. The data from positive blood cultures were collected from the reports of microbiology laboratory.
Chemotherapy regimens preceding the FN episode were recorded from each patient and categorized into regimens associated with low (<10%), medium (10%–20%), or high (>20%) risk of inducing FN according to the European Organisation for Research and Treatment of Cancer guidelines.[6] If patients had received any chemotherapy regimen before inducing FN episode, data were collected.
We also assessed the risk factors for FN by considering the predictors which were related to patients such as age, gender, poor nutritional status, defined as serum albumin levels <3.5 g/dL, poor performance status, defined as Eastern Cooperative Oncology Group (ECOG) performance scale <2, and high body surface area (BSA), defined as BSA ≥2 m2; disease-related predictors such as elevated lactate dehydrogenase (LDH), having myelophthisis or lymphopenia, and advanced stage of the underlying malignancy; and anticancer treatment-related predictors such as receiving high-dose chemotherapy regimens or failure to administer prophylactic hematopoietic growth factor support to patients receiving high-risk regimens.
The other risk factors such as risk factors for serious complications were defined according to the ASCO guidelines,[1] including neutropenia (ANC <500 cells/μL) anticipated to last >7 days, presence of any comorbid medical problems, alemtuzumab use within the past 2 months, inpatient status at the time of development of fever, and uncontrolled or progressive cancer.
In addition, patients were classified based on the risk of acute problems into two categories of low-risk and high-risk patients. This classification can play an important role in the basic approach to therapy, including the need for inpatient admission, intravenous (IV) antibiotics, and length of hospitalization. As an instance, low-risk patients are defined as those expected to be neutropenic (ANC <500 cells/μL) for ≤7 days and those with no comorbidities or hepatic or renal dysfunction. Most patients receiving chemotherapy for solid tumors are considered to be low risk for serious complications. Conversely, high-risk patients are defined as those who are expected to be neutropenic (ANC <500 cells/μL) for >7 days. Patients with hepatic or renal dysfunction are also considered to be high risk, regardless of the duration of neutropenia.[1] In this study, we considered severity of FN based on ANC count, defining as a severe FN (ANC: <100/μl) and slight-moderate FN (ANC: 100–500/μl or <1000 cell/μl with tendency toward reduction).
The Multinational Association for Supportive Care in Cancer (MASCC) risk index was calculated as previously defined and related to the risk of complicated FN.[7] This point-based risk score (maximum of 26) was established according to the patients' characteristics including burden of illness (mild = 5; moderate = 3), absence of hypotension = 5, absence of COPD = 4, no previous fungal infection = 4, absence of dehydration = 3, outpatient status at onset of fever = 3, and age <60 years = 2. The score was aimed to classify patients into low FN risk (>21 points) or high FN risk (<21 points) patients. We calculated the MASCC score for all included patients.
Microbiological information was recorded from the hospital's microbiology laboratory. We recorded all data around clinical specimens for microbial investigation (blood culture, urine, sputum, feces, catheter, and wound swabs) ordered by the attending physician at the time of admission to the emergency room or following FN diagnosis. All isolated microorganisms were processed and identified from the registry information system at the hospital. Interpretations were made according to the Clinical and Laboratory Standards Institute criteria.[8]
After recording the primal evaluation of FN patients, the way of empiric antibiotic administration according to drug regimen as well as the time in which antibiotic has been administrated was noted. We also recorded modification of treatment after releasing the causative microorganism from sent cultures.
One other important factor checked by the investigator was the probable indication for adding vancomycin into the antibiotic combination. Vancomycin (or other agents that target Gram-positive cocci) is not recommended as a standard part of the initial regimen in the management of FN, unless patients have one of following findings such as hemodynamic instability or other signs of severe sepsis, pneumonia, positive blood cultures for Gram-positive bacteria, suspected central venous catheter-related infection, skin or soft-tissue infection, and severe mucositis in patients who were receiving prophylaxis with fluoroquinolone.[1]
In addition, in the case of uncontrolled fever or fungal infection, the whole data around antifungal therapy were assessed. The most administrated antifungal agent and duration of therapy were precisely recorded. The outcome was measured as crude mortality (total number of all deaths) or attributed mortality, where the cause of death was primarily due to infection judged on clinical and laboratory parameters.
We used the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) version 20 for the data analysis. Student's t-test for parametric tests and Mann–Whitney U-test for nonparametric tests were used to compare two-sample means. The Pearson's chi-squared test of association and Fisher's exact test were used for analyzing the frequency of discrete variables and also relationship between patients' risk factors and their outcome. A two-tailed P < 0.05 was considered statistically significant.
RESULTS
During the 6-month study period, a total of 115 oncology patients were admitted with or encountered to FN during hospital stay. This equates to an incidence of 1.26% (12.6 patients of FN cases per 1000 oncology admissions). Their mean age was 49.5 ± 18.02 years (range 18–85 years), with 42.6% of patients being female [Table 1]. Among patients, 25.2% were at least 65 years old or over [Table 2]. The mean ECOG performance status in patients was 1.67 ± 1.0.
Table 1.
Demographic and clinical characteristics of febrile neutropenia patients (n=115)
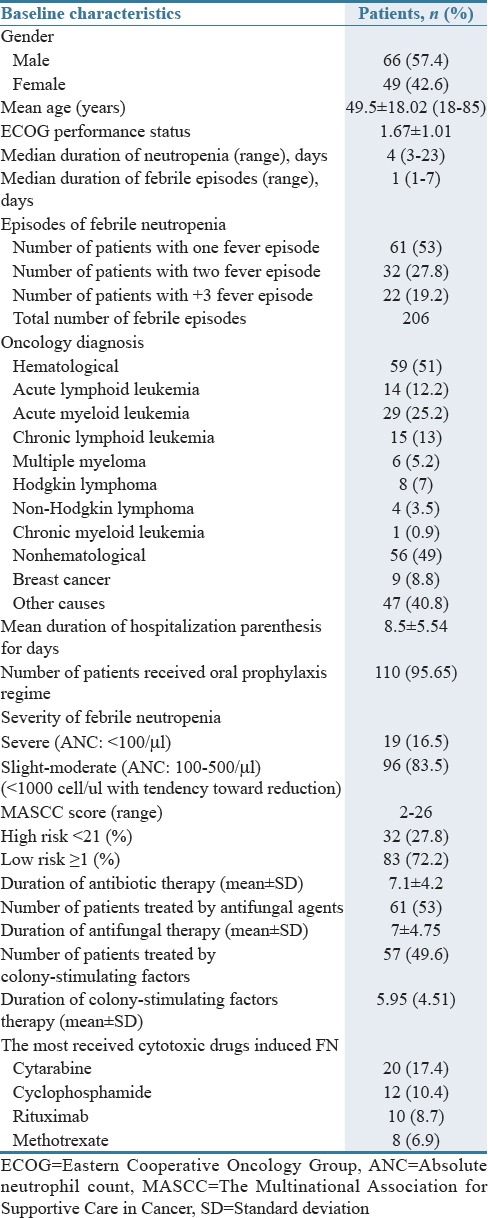
Table 2.
Risk assessment of febrile neutropenia patients (n=115)

The majority of patients suffered from severe neutropenia (ANC <500 cells/μL) with a mean neutrophil count of 0.30 ± 0.11 × 109/L.
The mean hospital length of stay was 8.5 ± 5.54 days, with a range from 2 to 33 days. The most common underlying cancer types were hematological cancer (including acute myeloid leukemia [AML], chronic myeloid leukemia, acute lymphocytic leukemia], chronic lymphocytic leukemia, and lymphoma) in 51% and the rest (49%) were with solid tumor (including breast, colorectal, lung, ovary, gastric, esophagus, pancreas, and prostate) [Table 1].
According to the MASCC score, 27.8% and 72.2% of patients were in high-risk and low-risk categories, respectively.
Median duration of neutropenia (defined as an ANC <500 cells/μL) was 4 days, and the median duration of febrile episodes was 1 day.
As shown in Table 1, the number of patients with one fever episode, two fever episodes, and three or more was 53%, 27.8%, and 19.2%, respectively. More than 95.65% of patients received oral prophylaxis regimens including antifungal agent (mostly fluconazole), antiviral agent (mostly acyclovir), and to some extent fluoroquinolone (mostly ciprofloxacin) alone or in combination.
General physical examination has been performed for all patients by emphasizing on sites most likely to be infected, including the skin, catheter sites, teeth, oropharynx and gingival surfaces, sinuses, lungs, abdomen, genitals, and perianal area. Frequent routine laboratory tests such as complete blood count with differential and creatinine, liver function tests, and electrolytes were ordered for all patients.
Based on clinical, microbiological, and radiological examination, 31.17% of all patients presented with signs and symptoms of a specific focal infection. These included respiratory tract infections (6.95%), gastrointestinal illnesses such as diarrhea or peritonitis (19%), catheter-associated sepsis (0.87%), and cellulitis (4.35%). In the other 68.83% of cases, patients presented with rigors and fever only without specific signs or symptoms of a defined focal infection.
The time when empiric antibiotics were initiated was divided into three categories, initiation time between 0–30, 30–60, and >60 min. The percentage of patients in each of categories was 37.4%, 49.6%, and 13%, respectively. All patients were treated by parenteral antibiotics on the day of admission in the emergency room or as soon as the infection was suspected.
The most prescribed antibiotics in the emergency room after diagnosing FN was meropenem (91.3%) by the dose of 1 g every 8 h and in second place, vancomycin in combination with meropenem therapy (47.82%). In most cases, the main reason for addition of vancomycin was hemodynamic instability (22%). In addition, 97.4% of administrated antipseudomonal antibiotics including carbapenem or extended-spectrum penicillins were in accordance with the IDSA guideline[1] for antibiotic indication recommendation; further, 95% of doses for prescribed antibiotics were in accordance with the guideline[1] as well. Prompt antibiotic therapy under 60 min of FN recognition occurred for 87% of cases accordingly.
Apart from meropenem and vancomycin, the most frequently prescribed antibiotic agents other were ciprofloxacin (18.26%), teicoplanin (Targocid®) (6.95%), ceftriaxone (4.37%), imipenem (2.6%), and piperacillin/tazobactam (Tazobactam®) (2.6%).
Mean duration of parenteral therapy (alone or in combination with oral treatment) was 7.15 days (minimum and maximum days of treatment were 1–33 days). In 100% of patients receiving parenteral therapy, treatment was continued with oral agents (mostly ciprofloxacin or levofloxacin) after discharge for a median duration of 5.6 days. According to the IDSA guideline,[1] 47.8% of our cases could complete the duration of antibiotic therapy at least for 7 days, the least recommended duration for FN treatment.
In 34.8% of patients, the initial antibiotic regimen was changed mainly due to lack of clinical response and in 6.95% of patients due to mismatch by antibiogram results.
During follow-up, we discovered that only 27.8% of patients had indication for vancomycin empiric therapy according to the IDSA guideline.[1] Empiric antifungal agents initiated in 20.86% of cases (caspofungin [50%], conventional amphotericin b [33.33%], and liposomal amphotericin b [16.66%]), when fever was not subsided by antimicrobial agents after 4 days. Antifungal regimen was parentally administrated for all patients. The mean duration of antifungal therapy, in our patients, was 8.66 ± 6 days (range from 1 to 20). The mean time of initiation of the antifungal agents from detecting the infectious complication according to the recommendations was 5.47 ± 3.55 days.
In 35.4% of our cases, the initiation of antifungal agents was under 4 days of fever's appearance; hence, it was not in accordance with the IDSA guideline.[1] Regarding the prescribed doses, 91.66% were in accordance with the IDSA guideline.[1]
For treatment of 67.82% of patients, at least two antimicrobial combinations, except for prophylaxis agents, were used in which the most combination regimen was meropenem and vancomycin.
None of our patients even in low-risk category have been treated by oral antibiotic agents or in outpatients setting. About half (49.6%) of all cases received G-CSF during FN for adjuvant treatment by antibiotics or antifungal agents by duration time of 5.94 ± 4.51 days (range 1–28 days). Most of them (82.45%) received G-CSF by the dose of 300 mcg/day subcutaneously.
During follow-up, we could not find any patients who needed to receive antiviral treatment. However, all of our patients received acyclovir by dose of 400 every 12 h for herpes simplex virus's prophylaxis. Only in 39.13% of patients, the positive inflammatory phase reactants such as C-reactive protein and erythrocyte sedimentation rate were checked.
Blood cultures (two sets: one peripheral and one from central venous catheter) and antimicrobial susceptibility testing were sent for microbiological evaluation for 61% of patients. Of them, 17.4% were positive. For 5.2% of patients, urine culture was ordered while it was negative after microbial evaluation. Checking serial fungal markers from the serum such as the Aspergillus galactomannan antigen was carried out in 2.6% of patients suspected of fungal infection. The ratio of microbiological samples per patients in our investigation was about 0.6, and the ratio of number of bacteria per positive culture was about 0.3. From all positive cultures, Gram-positive microorganisms were the most identified pathogen. Among them, Staphylococcus epidermidis was most prevalent (30%) followed by Staphylococcus aureus (15%) [Table 3].
Table 3.
Microbiological isolates from blood cultures of patient with febrile neutropenia
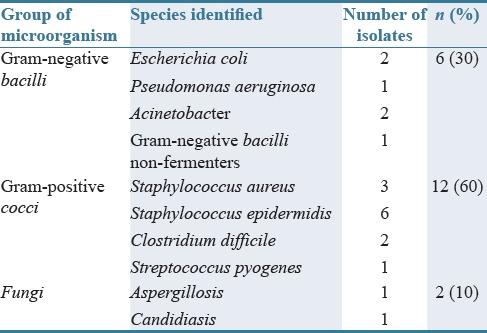
All Gram-positive Staphylococcus cultures were resistant to methicillin. On the other hand, we have reported two cultures of Acinetobacter baumannii; in one of them, isolated microorganism was only sensitive to colistimethate sodium (Colistin®).
During follow-up, 70% of patients have undergone imaging procedure including plain radiographs, computed tomography (CT) scans from suspected sites, echocardiography, and magnetic resonance imaging (MRI).
As shown in Table 2, risk factors of FN were categorized into three classes including patients, disease, and anticancer treatment risk factors. Among them, female sex (42.6%) and lymphopenia (26.5%) were the most noted predictors. Neutropenia (42.6%) was the most reported risk factors for serious complications.
The outcome of patients was assessed according to the mentioned risk factors [Table 2]. We found that poor performance status based on preexisting active cardiovascular, renal, endocrine, or pulmonary comorbidities, advanced stage of the underlying malignancy, neurologic or mental status changes of new onset, uncontrolled or progressive cancer, and new pulmonary infiltrate or hypoxemia had significant associations with patients' mortality. Totally, the attributed mortality for FN patients and crude mortality in our center during investigation were 7% and 14.9%, respectively. Among dead cases, 66.66% had the progressive and uncontrolled AML.
DISCUSSION
FN is a frequently reported complication of chemotherapy, especially among patients with hematologic malignancies. A predominant number of FN episodes are associated with infection which is one of the most causes of morbidity and mortality in cancer patients.[2]
Our study has shown that an overall FN incidence led to hospitalization in our center was about 1.26% (12.6 patients per 1000 oncology admissions) with attributed mortality of 7% among cancer patients admitted to the hospital. A mortality rate in FN patients varies 2.6%–50.6%, depending on the presence of comorbidity and underlying cancer.[9] Solid tumor patients have lower rate of mortality compared with hematological malignancies, which the latter also associates higher rate of bacteremia and opportunistic infections.[9] In an epidemiological survey by the UK group in 2011, the annual incidence of FN among solid tumor patients was estimated about 2/1000 oncology admissions, while the attributed mortality was 12.5%.[10] Furthermore, it appears the mortality rate and infectious condition has a decreasing trend during recent years. Advancements in prompt using of empiric antimicrobial therapy and prophylaxis have led to improving outcomes.[9]
One of the purposes of the present study was to find the existence pitfalls in FN management in spite of several guideline recommendations[5,6] in a university-affiliated oncology center in Isfahan, Iran. Treatment of FN episodes in accordance with existing guidelines showed a reduction in mortality.[10] Furthermore, deviation from clinical practice guidelines is associated with inappropriate prescribing of antibiotics and increased health-care costs.[10]
Historically, guidelines for the management of FN have focused on antimicrobial administration. Fever in chemotherapy-received cancer patients requires prompt attention by healthcare professionals due to potential for serious impact on mortality and overall health-care costs. In a multicenter trial between 1995 and 2000, Kuderer et al.[11] reported an average length of hospital stay of FN patients around 11 days. In a similar manner, the mean length of hospitalization was 8.5 ± 5.54 days in our survey.
Early identification of fever and sepsis and timely administration of antibiotic are highly recommended in the management of FN, and concurrent overviewing of system should be integrated by health-care facilities. Antibiotic administration should be initiated as soon as possible (<60 min of fever detection).[1] Existing data support improved outcomes with rapid therapy.[12] In our study, antibiotic therapy was initiated in <60 min in about 88% of FN patients. Although not completely compatible with guidelines recommendation, it was an acceptable range.
First-line antibiotic therapy varies based on local practice but typically includes a broad-spectrum cephalosporin with antipseudomonal activity, carbapenem, or extended-spectrum penicillin.[10] As noted, meropenem, one of the carbapenem families, was the most prescribed antibiotic in the emergency room. The choice of first-line antibiotics and the prescribed doses of antibiotics in our center were according to the guidelines' recommendations. In a retrospective study performed in one of the referral hematopoietic stem cell transplantation centers in Iran, carbapenems (imipenem and meropenem) were the first prescribed antibiotics (78.8% of all) for the treatment of FN patients.[13]
According to the guideline recommendation, addition of Gram-positive antibiotic coverage to the initial empiric antibiotic regimen has not been associated with significant clinical benefit.[1,14,15,16] A meta-analysis of 14 randomized trials found that addition of Gram-positive antibiotic coverage to standard empiric therapy did not reduce all-cause mortality in patients with cancer and neutropenic fever.[14] However, in our study, vancomycin was added to near 50% of initial empiric antibiotic regimen because most of our practitioners had fear of upcoming hemodynamic instability.
Although hemodynamic instability or other signs of severe sepsis were recommended as a standard part of the initial regimen,[14] we believe that in our center, vancomycin has been overused by a physician to lower the risk of any further complication. The overuse of special antibiotics creates the possibility of resistant microorganism, about vancomycin; we are worry of appearing vancomycin-resistant enterococci. Moreover, the appropriate use of antibiotics has been proved to minimize mortality from life-threatening infection during FN.[17]
It is noted that bacteremia is only detectable in 10%–25% of FN episodes, and clinically documented infections are found in 20%–30% of FN patients.[1,7] Before recent advances in the use of efficient prophylactic antibiotics, Gram-negative organisms were the most documented infections. Today, trend is changing to Gram-positive organisms in FN era. In line with this, S. epidermidis and S. aureus constituted the most common group of pathogens found among all the isolated cultures in our study. This is in contrast to other studies where Gram-negative bacilli were found to cause a majority of infections in FN episodes.[18]
In another retrospective multicenter study took place in four hospitals in Tehran and Ahwaz, Iran, 89 patients suffering from leukemia were assessed microbiologically and it was noted that 85.4% of total culture had Gram-negative bacteria with a dominance of Escherichia coli colonies.[19]
One of the main reasons for not sending immediate culture by our clinicians was previous experience of finding any responsible pathogen during microbiology investigation. Furthermore, only about 60% of our patients had the order of sending blood culture before administrating any antibiotic therapy, while it is recommended as an early approach to management of all patients with FN. However, the percent of documented infections in our study was same as previous report.[1]
Colony-stimulating factors are not recommended for routine use in patients with established fever and neutropenia.[1] However, about 50% of our patients received G-CSF during FN episode. The overuse of this agent has been observed in our center. Irrational overuse of G-CSF has been confirmed in another observational study of teaching hospital in Isfahan, Iran. The study demonstrated that about one-third of administrated G-CSF was not in accordance with the ASCO guideline.[20] According to the guideline, antibiotic therapy in a case of unidentified source must be continued after resolution of fever and clear evidence of bone marrow recovery. It is also recommended that afebrile patients for at least 2 days with ANC >500 cells/μL do not need further antibiotic therapy.[1]
The duration of antibiotic therapy was 7.1 ± 4.2 days. Withholding of antibiotics in our center was in conservative manner and in accordance with the guideline,[1] after complete resolving of clinical symptom with simultaneous recovery of bone marrow.
On the other hand, guideline[1] recommendation for addition of an empiric antifungal agent is 4–7 days after persistent or recurrent fever or in high-risk neutropenic patients who are expected to have a total duration of neutropenia >7 days in whom reassessment does not yield a cause. Our center approaches for initiation of broad-spectrum antifungal agents was in accordance with the guideline (5.47 ± 3.55 days). In our center, patients who have not been receiving antifungal prophylaxis treated by caspofungin in the first step; however, in patients receiving fluconazole prophylaxis, due to possible risk of fluconazole-resistant Candida spp. and invasive mold infection, amphotericin b (conventional or liposomal) was administrated. Furthermore, only half of our patients received amphotericin b for empiric antifungal therapy.
Risk factors for mortality in FN patients are multifactorial, including patient, disease, and treatment-related factors. Several risks of failure to respond to the initial empiric antibacterial therapy are defined and must be considered by the clinician. The most determining and prominent risk factors are documented infections, clinical or microbiologic (rather than unexplained neutropenic fevers), high-risk patients than low-risk patients, for example, patients with hematologic malignancies in comparison with solid tumor, delay in the initiation of appropriate and effective antibacterial therapy, poor baseline performance status of patients, and finally failure to administer guideline-driven initial empiric antibacterial therapy.[21,22,23,24] Identifying reversible factors that are amenable to change is an important and necessary process to mitigate risk and optimize outcomes for patients with FN. In our institute, poor performance status of patients, advanced stage of the underlying malignancy, neurologic or mental status changes of new onset, uncontrolled or progressive cancer, and new pulmonary infiltrate or hypoxemia had significant associations with mortality of FN patients.
The efficacy of the FN treatment by prompt initiation of empiric coverage has improved enormously since 1970 as demonstrated by a progressive decline in mortality rate.[24] Before routine use of empiric therapy, prior the 1960s, Gram-negative bacilli were responsible for documented mortality rates of 90% in neutropenic patients.[25] Sepsis due to Pseudomonas aeruginosa or E. coli induced prompt mortality within 48 h after the first blood culture had been drawn in approximately one-half of patients.[26] On the contrary, mortality rate has improved recently, and in a study of 41,779 adults with cancer who were hospitalized with FN in the United States between 1995 and 2000, the in-hospital mortality rate was 9.5%.[27] Similarly, the attributed mortality rate for FN patients in our center was around 7%.
Our study had sample size limitation; checking several items during the investigation and following up of the patients for approximately long duration had not allowed continuing the study for an expanded time with more number of patients. This study was an observational one; we tried to find the way of approaching and managing FN patients and find the facing challenges in our center by the aim of error reductions and giving feedback to the responsible healthcare professions.
Since our center is specialized cancer center with highly professional clinician, we had expected to encounter the guideline-matched behavior in our center; however, more attention should be considered for deescalating the antibiotics according to culture results, repeatedly sending the culture to find the responsible microorganism, and educating the clinician to not overuse of the antibacterial and other agents (e.g., colony-stimulating factors).
AUTHORS' CONTRIBUTION
A.M and F.A equally contributed to the conception and design of the research; F.K and A.D contributed to data gathering from mentioned hospital; A.M, F.K, and F.A contributed to the acquisition and analysis of the data; and A.M and F.K drafted the manuscript. All authors critically revised the manuscript, agreed to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Financial support and sponsorship
This study was part of an Iranian Pharm. D thesis that has been supported by Isfahan University of Medical Sciences (Grant number: 395860).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank the oncologists, nursing staff, and patients of all wards involved in this study.
REFERENCES
- 1.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2011;52:e56–93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 2.Cortés JA, Cuervo S, Gómez CA, Bermúdez D, Martínez T, Arroyo P, et al. Febrile neutropenia in the tropics: A description of clinical and microbiological findings and their impact on inappropriate therapy currently used at an oncological reference center in Colombia. Biomedica. 2013;33:70–7. doi: 10.1590/S0120-41572013000100009. [DOI] [PubMed] [Google Scholar]
- 3.Oberoi S, Suthar R, Bansal D, Marwaha RK. Febrile neutropenia: Outline of management. Indian J Pediatr. 2013;80:138–43. doi: 10.1007/s12098-012-0901-y. [DOI] [PubMed] [Google Scholar]
- 4.Rolston KV. Challenges in the treatment of infections caused by gram-positive and gram-negative bacteria in patients with cancer and neutropenia. Clin Infect Dis. 2005;40(Suppl 4):S246–52. doi: 10.1086/427331. [DOI] [PubMed] [Google Scholar]
- 5.Flowers CR, Seidenfeld J, Bow EJ, Karten C, Gleason C, Hawley DK, et al. Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2013;31:794–810. doi: 10.1200/JCO.2012.45.8661. [DOI] [PubMed] [Google Scholar]
- 6.Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Klastersky J, Paesmans M, Rubenstein EB, Boyer M, Elting L, Feld R, et al. The multinational association for supportive care in cancer risk index: A multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol. 2000;18:3038–51. doi: 10.1200/JCO.2000.18.16.3038. [DOI] [PubMed] [Google Scholar]
- 8.Clinical Laboratory and Standards Institute. [Last accessed on 2017 Jun 08]. Available from: http://www.clsi.org/
- 9.White L, Ybarra M. Neutropenic fever. Hematol Oncol Clin North Am. 2017;31:981–93. doi: 10.1016/j.hoc.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Schelenz S, Giles D, Abdallah S. Epidemiology, management and economic impact of febrile neutropenia in oncology patients receiving routine care at a regional UK cancer centre. Ann Oncol. 2012;23:1889–93. doi: 10.1093/annonc/mdr520. [DOI] [PubMed] [Google Scholar]
- 11.Kuderer N, Cosler L, Crawford J, Dale D, Lyman G. Cost and mortality associated with febrile neutropenia in adult cancer patients. Proc Am Soc Clin Oncol. 2002;15:2258–66. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 12.Zuckermann J, Moreira LB, Stoll P, Moreira LM, Kuchenbecker RS, Polanczyk CA, et al. Compliance with a critical pathway for the management of febrile neutropenia and impact on clinical outcomes. Ann Hematol. 2008;87:139–45. doi: 10.1007/s00277-007-0390-7. [DOI] [PubMed] [Google Scholar]
- 13.Amini S, Hadjibabaie M, Jahangard-Rafsanjani Z, Ashuri A, Torkamandi H, Ghavamzadeh A, et al. Evaluation of febrile neutropenia in patients undergoing hematopoietic stem cell transplantation. Acta Med Iran. 2014;52:38–42. [PubMed] [Google Scholar]
- 14.Beyar-Katz O, Dickstein Y, Borok S, Vidal L, Leibovici L, Paul M, et al. Empirical antibiotics targeting gram-positive bacteria for the treatment of febrile neutropenic patients with cancer. Cochrane Database Syst Rev. 2017;6:CD003914. doi: 10.1002/14651858.CD003914.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pizzo PA. Management of fever in patients with cancer and treatment-induced neutropenia. N Engl J Med. 1993;328:1323–32. doi: 10.1056/NEJM199305063281808. [DOI] [PubMed] [Google Scholar]
- 16.Vardakas KZ, Samonis G, Chrysanthopoulou SA, Bliziotis IA, Falagas ME. Role of glycopeptides as part of initial empirical treatment of febrile neutropenic patients: A meta-analysis of randomised controlled trials. Lancet Infect Dis. 2005;5:431–9. doi: 10.1016/S1473-3099(05)70164-X. [DOI] [PubMed] [Google Scholar]
- 17.McCabe WR, Jackson GG. Gram-negative bacteremia: II. Clinical, laboratory, and therapeutic observations. Arch Intern Med. 1962;110:856–64. [Google Scholar]
- 18.Bodey GP. Infection in cancer patients. A continuing association. Am J Med. 1986;81:11–26. doi: 10.1016/0002-9343(86)90510-3. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadzadeh A, Varnasseri M, Jalili MH, Maniavi F, Valizadeh A, Mahmoodian M, et al. Infection pattern of neutropenic patients in post-chemotherapy phase of acute leukemia treatment. Hematol Rep. 2013;5:e15. doi: 10.4081/hr.2013.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mousavi S, Dadpoor M, Ashrafi F. Granulocyte colony-stimulating factor use in a large Iranian hospital: Comparison with American society of clinical oncology (ASCO) clinical practice guideline. Int J Hematol Oncol Stem Cell Res. 2016;10:85–91. [PMC free article] [PubMed] [Google Scholar]
- 21.Freifeld A, Marchigiani D, Walsh T, Chanock S, Lewis L, Hiemenz J, et al. A double-blind comparison of empirical oral and intravenous antibiotic therapy for low-risk febrile patients with neutropenia during cancer chemotherapy. N Engl J Med. 1999;341:305–11. doi: 10.1056/NEJM199907293410501. [DOI] [PubMed] [Google Scholar]
- 22.Park CM, Koh Y, Jeon K, Na S, Lim CM, Choi WI, et al. Impact of eastern cooperative oncology group performance status on hospital mortality in critically ill patients. J Crit Care. 2014;29:409–13. doi: 10.1016/j.jcrc.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Wright JD, Neugut AI, Ananth CV, Lewin SN, Wilde ET, Lu YS, et al. Deviations from guideline-based therapy for febrile neutropenia in cancer patients and their effect on outcomes. JAMA Intern Med. 2013;173:559–68. doi: 10.1001/jamainternmed.2013.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viscoli C, Varnier O, Machetti M. Infections in patients with febrile neutropenia: Epidemiology, microbiology, and risk stratification. Clin Infect Dis. 2005;40(Suppl 4):S240–5. doi: 10.1086/427329. [DOI] [PubMed] [Google Scholar]
- 25.McCabe W, Jackson G. Gram-negative bacteremia. 2. Clinical, laboratory, and therapeutic observation. Arch Intern Med. 1962;110:856. [Google Scholar]
- 26.Bodey GP, Jadeja L, Elting L. Pseudomonas bacteremia. Retrospective analysis of 410 episodes. Arch Intern Med. 1985;145:1621–9. doi: 10.1001/archinte.145.9.1621. [DOI] [PubMed] [Google Scholar]
- 27.Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106:2258–66. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]


