Abstract
Purpose
Osteoporosis is one of the major health concerns among the elderly population, especially in postmenopausal women. Many menopausal women over 50 years of age lose their bone density and suffer bone fractures. In addition, many mortality and morbidity cases among the elderly are related to hip fracture. This study aims to investigate the effect of Lactobacillus helveticus (L. helveticus) on bone health status among ovariectomized (OVX) bone loss-induced rats.
Methods
The rats were either OVX or sham OVX (sham), then were randomly assigned into three groups, G1: sham, G2: OVX and G3: OVX+L. helveticus (1 mL of 108–109 colony forming units). The supplementation was force-fed to the rats once a day for 16 weeks while control groups were force-fed with demineralized water.
Results
L. helveticus upregulated the expression of Runx2 and Bmp2, increased serum osteocalcin, bone volume/total volume and trabecular thickness, and decreased serum C-terminal telopeptide and total porosity percentage. It also altered bone microstructure, as a result increasing bone mineral density and bone strength.
Conclusion
Our results indicate that L. helveticus attenuates bone remodeling and consequently improves bone health in OVX rats by increasing bone formation along with bone resorption reduction. This study suggests a potential therapeutic effect of L. helveticus (ATCC 27558) on postmenopausal osteoporosis.
Keywords: Lactobacillus helveticus, bone mineral density, bone loss, osteoporosis, ovariectomized rats
Introduction
Osteoporosis is a prevalent skeletal disease which is mainly related to aging and menopause. The risk of osteoporosis due to estrogen deficiency in menopausal women is high1 and closely related to the imbalance of bone metabolism. This is accompanied by greater bone resorption rather than bone formation which increases fragility and fracture risk.2
One of the important risks for those with osteoporosis is fracture risk since 51% of total disability worldwide was reported as a result of fracture.3 In this regard, many menopausal women over 50 years of age suffer from osteoporosis due to the reduction of bone density and bone micro-architecture.4 Since one-third of a woman’s life span is spent in the menopausal condition,5 reduction of the adverse effect of menopause in women’s health is vital.
One of the most common therapies for osteoporosis is bisphosphonates such as alendronate,6,7 risedronate8 and zoledronic acid.9,10 However, this therapy causes some side effects including atypical subtrochanteric fractures11 and osteonecrosis of the jaw.12 The other therapy for osteoporosis is hormone replacement therapy (HRT).13 Some studies show a strong effect of HRT in reducing fracture risks among postmenopausal women;14 however, no effect of HRT to reduce fracture risk among postmenopausal women also has been reported.15 It seems HRT is mostly useful for treating low bone density rather than treating postmenopausal women with a stabilized osteoporosis.16 HRT, like bisphosphonates, also has some side effects and can cause different types of cancer including breast17 and ovarian cancers.18 Besides that, long-term usage of HRT has been shown as one of the causes of stroke.19 Therefore, prolonged HRT for osteoporosis treatment is not recommended. In addition to this, reduction of bone density occurs more rapidly if HRT is stopped compared to normal reduction of bone density in menopausal women suffering from osteoporosis.20 Thus, due to the several adverse effects of HRT, women prefer to have nonhormonal treatments to manage their postmenopausal osteoporosis.21
In this regard, the effect of beneficial microbes on different organs and in reducing the risk of getting different diseases is of growing interest.22,23 Due to this, probiotics are considered as an alternative osteoporosis treatment and have shown inhibitory effects on osteoclastic bone resorption and/or properties of osteoblastic bone formation.24 However, most osteoporosis therapies prevent bone loss by inhibiting bone resorption,22 while increasing the bone formation and bone volume (BV) is also required for osteoporosis treatment. Probiotics, in this regard, have been shown to change both osteoclastic and osteoblastic activities.
Probiotics are live microorganisms which confer a health benefit on a host when consumed in adequate amounts.25 Probiotics, after consumption, normally lead to changes in host gut microbiota (GM).26 Probiotics perform their function by manipulating intestinal microbiota and stimulating proliferation and differentiation of epithelial cells, leading to an improved immune system.27 T cells have a critical role in immune response and can also affect bone remodeling.28 T cell deficiency in mice has been shown to increase osteoclast (Oc) number and reduce bone mass.29 Indeed, activation of T cells involves the autoimmune system, and in consequence reducing inflammation, bone loss, and osteoporosis.30 Beneficial microbes in this regard reduce the expression of pro-inflammatory cytokines in the jejunum and ileum and activate the immune system, finally inhibiting reduction of bone mass.23
Bone is persistently remodeled by osteoblasts (Obs), the bone formation component, and Ocs, the bone resorption component.31 Regulation of bone metabolism is managed by complex mechanisms.32 In this regard,33 osteoblast regulators Osterix,34 Bmp2,35 Runx236 and Oc regulator RANKL37 have shown some regulatory effects on bone metabolism. Therefore, our aim was to investigate the effects of Lactobacillus helveticus on Runx2 and Bmp2 expression and bone metabolism, and to evaluate bone mineral density (BMD) in bone loss-induced rats resulting from ovariectomy.
Materials and methods
Animal design and their intake
Twenty-four mature Sprague–Dawley rats, aged 10 weeks, with a body weight range of 280–290 g were enrolled in this research. The body weight-matched rats were randomly divided into three groups: G1: sham, G2: ovariectomized (OVX) and G3: OVX+L. helveticus, with eight rats in each group. Each rat was placed in a separate normal plastic cage in a controlled environment animal facility at 22°C±2°C with a 12-hour light/dark cycle and allowed to acclimatize for 2 weeks. The animals were fed with a standard normal chow pellet (Ridley Agri Products, Sydney, NSW, Australia) and ad libitum water access. The composition of rat chow pellet is shown in Table 1. The study was approved by the Animal Care and Use Committee (ACUC) of the Faculty of Medicine and Health Sciences, University Putra Malaysia (UPM), with approval number of UPM/FPSK/PADS/BR-UUH/00483. All the research was performed in the animal house of the Faculty of Medicine and Health Sciences, UPM, and guidelines for the use of animals were strictly followed (Universiti Putra Malaysia code of practice for the care and use of animals for scientific purposes).
Table 1.
Nutrients composition of rat chow diet
| Standard rat chow diet ingredient | |
|---|---|
| Crude protein | 21.0% |
| Crude fiber | 7.0% |
| Crude fat | 3.0% |
| Moisture | 13.0% |
| Ash | 8.0% |
| Calcium | 1.0% |
| Phosphorus | 0.5% |
| Nitrogen-free extract | 46.5% |
Surgical procedure
Surgical OVX was performed for G2 and G3 and sham ovariectomized for G1. The rats were anesthetized with an intraperitoneal injection using a combination of xylazine and ketamine with a dosage of 12/80 mg per kg rat body weight.38
Following anesthesia, the rats were shaved on left and right laterals with an electric razor, and then hair was removed from part of the skin. The area was then washed and disinfected with povidoneiodine and ethanol 70% using gauze bandage. Two incisions 1 cm long were made on each of the caudal parts of the dorsolateral area of the rats using a sharp scalpel after lifting the skin with a tomforceps. Muscles were separated along their fibers and ovaries were located, double-ligated and removed. After the removal of ovaries, the skin was closed in two layers with absorbable sutures. Then, the animals were covered with paper and located separately in a clean normal cage (one animal/cage) and left for 2 weeks to recover. After two weeks, the animal was transferred to their cages separately for the entire study.
L. helveticus cultivation
L. helveticus (ATCC 27558) was commercially purchased as the lyophilized form from the American Type Culture Collection (ATCC). Then, based on the protocol of the company, the bacteria were revived three continuous times in de Man, Rogosa and Sharpe (MRS) broth. After that, the bacteria were subcultured in MRS agar several times with the aim of refreshing. Then, the bacteria from MRS agar were inoculated again to MRS broth to get ready for force-feeding. The bacterial concentration in colony forming units (CFU) was selected based on a previous study.39 The rats in G3 were then force-fed with 1 mL of 108–109 CFU of L. helveticus in phosphate buffer saline daily via oral gavage for 16 weeks; sham and OVX groups were force-fed with 1 mL of demineralized water.
Animal euthanasia and sample collection
One day before culling, the animals were transferred to a metabolic cage individually and left overnight, and then fresh fecal samples were collected the next day in order to enumerate lactobacillus colonies. Then, the rats were subsequently anesthetized with a combination of xylazine and ketamine, and blood was collected via cardiac puncture. After that the rats were immediately euthanized and sacrificed, and then their femurs were removed gently. The left femurs were preserved in 10% buffered formalin for 24 hours, and then the solution is changed to 70% ethanol, and right femurs were snap-frozen and stored at −80°C until the assessment day. The micro-CT (μ-CT) analysis was done on the left femur of the animal; after that, a breaking force was applied to measure the stress of the bone on the same femurs; the proximal left femur was used for histology analysis and the distal left femur was used to measure Ca, Mg and Zn content of the femurs. The right femur was used for RNA isolation.
Blood biochemical analysis
The collected blood was transferred into plain tubes and centrifuged at 3,000 rpm for 10 minutes at 4°C to isolate serum for biochemical assay. Calcium and magnesium in the serum were analyzed using a Roche Cobas® C-311 Japan analyzer (Hitachi Ltd., Tokyo, Japan). Osteocalcin (OC) and C-terminal telopeptide (CTX) were analyzed using standard commercial ELISA kits; OC was analyzed using Rat-MID™ OC EIA (IDS, Fareham, UK) and CTX was analyzed using RatLaps™ EIA (IDS).
Micro-CT analysis
Images of the left femurs were obtained using μ-CT (SkyScan, 1176; Bruker microCT, Kontich, Belgium) at a resolution of 35 μm, filter 0.5, exposure 100, voltage 40, and current 100 A to measure BMD (g/cm3) and various bone structures. To get the images, the left femurs were wrapped in a moistened tissue paper and placed in the μ-CT scan device. The images were then reconstructed using NRecon software version 1.6.3.3 and analyzed with SkyScan CT analyzer software version 1.9.1. Then, BMD (g/cm3) of the femurs was calculated by the formula which was created by the analyzer. In addition to this, BV/total volume (TV) percentage, trabecular thickness (Tb.Th; mm), trabecular number (Tb.N; mm−1), trabecular separation (Tb.Sp; mm) and total porosity percentage (pro.tot) of the femurs were measured with the software. All values are reported as mean of cortical and trabecular bones. The correction of beam hardening was conducted using a metal filter during the scanning and reconstructing to improve the quality of the μ-CT images. Calibration of the μ-CT analyzer was carried out by phantoms with known density of calcium hydroxyapatite, size 4 mm in pairs. A concentration of 0.25 g/cm3 was regarded as lower density and 0.75 g/cm3 as higher density.
Stress of the femur
The stress of the left femur was measured using a three-point bending method from the center of the femur using a universal testing machine (8874; Instron Ltd., High Wycombe, UK) equipped with 5 kN load transducer. The procedure was based on the work of Goda et al.40 Briefly, the midpoint of the femur was marked, and then a breaking force was applied. After that, inside and outside radii were measured to calculate the area moment of inertia. Medullary canal diameter was calculated by subtracting the thickness of the medial and lateral walls from the diameter of the diaphysis. Moment was calculated by applied force of the machine multiplied by support points, and then divided by 4. Finally, the stress of the femur was calculated by multiplying the moment to the farthest point in the cross section to the neutral axis, and then divided by the moment of inertia.41
Bone histomorphometric measurements
Bone histomorphometric measurements were done as recommended by the American Society of Bone Mineral Research Histomorphometry Nomenclature Committee (ASBMR).42 The secondary spongy area of the left femurs, 1 mm from the lateral cortex and 3–7 mm from the lowest point of the growth plate, was used for histomorphometric purposes.42 As a brief explanation for the sample preparation, the left femurs were decalcified in 5% nitric acid for 24 hours. Then, the decalcified samples were transferred to an automated vacuum tissue processor (Leica ASP 300; Leica, Wetzlar, Germany). After that, the samples were embedded in paraffin histology wax and then sectioned at 6 μm thickness using a microtome (Leica). Finally, the samples were stained with hematoxylin and eosin (H&E) and observed under a light microscope (Olympus BX51TRF-CCD Microscope, Tokyo, Japan). After that, osteoclast surface/bone surface (OcS/BS), osteoblast surface/bone surface (ObS/BS), eroded surface/bone surface (ES/BS), osteoid surface/bone surface (OS/BS) and osteoid volume (OV)/BV ratios were measured using Weibel’s technique.43
Calcium, magnesium and zinc content of the femur
The calcium, magnesium and zinc content of the left femurs was determined by atomic absorption spectrophotometry (novAA® 400; Analytik Jena, Jena, Germany) according to the procedure of Harrison et al44 with slight modification. Distal left femurs were dried at 105°C for 24 hours. Then, they were placed in a muffle furnace at 550°C for another 24 hours to get the ash samples. The ashes were then crushed and hydrolyzed with 6 M HCl. Finally, the solution was used to determine the mineral content of the femurs. Calcium was determined at a wavelength of 422.7 nm and lamp current 4.0 mA, magnesium was determined at a wavelength of 285.2 nm and lamp current 2.0 mA and zinc was determined at a wavelength of 213.9 nm and lamp current 2.0 mA.
RNA isolation and quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the right frozen femurs using a HiYield® Total RNA Mini Kit (Real Biotech, Taipei, Taiwan) using the manufacturer’s protocol. Extracted RNA was only used if the absorbance ratio at A260/230 and A260/280 was between 1.8 and 2.0 with specific bands on gel electrophoresis.45 Then, cDNA was synthesized from 1 μg of RNA from each sample in a total 20 μL reactions using an i-script cDNA synthesis kit (Bio-Rad). PCR reagent was prepared from cDNA in a reaction mixture containing 3 μL of cDNA in a total volume of 25 μL with 2× SYBR® Green I Hot-Start. Amplifications were then placed in a thermal cycler (Eppendorf, Hamburg, Germany) with initial denaturation at 95°C for 15 minutes, followed by 40 cycles at 95°C for 15 seconds, 58.6°C for 40 seconds and 68°C for 20 seconds. The data were then calculated as fold changes compared to the control group, relative to the housekeeping gene. The primers were designed based on sequences obtained from the ENA and GenBank (http://www.ncbi.nlm.nih.gov/tools/primer-blast) and supplied by Helix Biotech Ltd. (Richmond, BC, Canada) (Table 2) and were diluted to a final concentration of 100 μL with nuclease-free water.
Table 2.
List of genes and primer sequences
| Genes | Accession number | Primer sequences (5→3) |
|---|---|---|
| Runx2 | NM_053470 | F: GCGTCCTATCAGTTCCCAAT |
| R: ATCAGCGTCAACACCATCAT | ||
| Bmp2 | NM_017178 | F: CAGGTCTTTGCACCAAGATG |
| R: GCTGGACTTAAGACGCTTCC | ||
| GAPDH (housekeeping) | NM_017008 | F: TCAAGAAGGTGGTGAAGCAG |
| R: AGGTGGAAGAATGGGAGTTG |
Abbreviations: Bmp2, bone morphometric protein; Runx2, runt-related transcription factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Enumeration of Lactobacillus in the feces
Fresh rat feces were diluted and cultivated in MRS agar, then incubated for 48 hours at 37°C. Then, lactobacilli were enumerated using a colony counter (Stuart Scientific, Staffordshire, UK) and reported as log10 CFU/g.
Statistical analysis
The univariate procedure of SAS software (version 8.2, SAS Institute Inc., Cary, NC, USA) was used for the normality test. Then, data were analyzed by one-way analysis of variance (ANOVA) with PROC MIXED in SAS. The results were then reported as mean±SEM and differences between the groups were analyzed using a post hoc Duncan’s test. Microbial data were converted to log10 and reported on a wet weight basis. Significance was considered at P<0.05.
Results
Effect of L. helveticus on serum biomarkers
Table 3 shows the analysis of biochemical assays on the serum. The OVX group showed a significantly lower level of OC and a higher level of CTX in the serum (P=0.0001) compared to other groups. However, the group of rats supplemented with L. helveticus showed a significantly increased level of serum OC (P=0.0001) and a decreased level of serum CTX (P=0.0001). These results indicate that the OVX+L. helveticus group had more bone formation and less bone resorption activity than the OVX group. L. helveticus had no significant effects on serum Ca and Mg levels.
Table 3.
Effect of L. helveticus on serum biomarkers
| Serum parameters | Sham | OVX | OVX+ L. helveticus | P-value |
|---|---|---|---|---|
| OC (ng/mL) | 144±2.2a | 77±6.3b | 118±6.4c,* | 0.0001 |
| CTX (ng/mL) | 174±14.7a | 391±22.6b | 195±7.7c,* | 0.0001 |
| Ca (mmol/L) | 2.30±0.13 | 2.24±0.10 | 2.23±0.07 | 0.96 |
| Mg (mmol/L) | 0.98±0.07 | 0.90±0.10 | 0.93±0.04 | 0.73 |
Notes: Ten-week-old female Sprague–Dawley rats were supplemented with 1 mL L. helveticus (108–109 CFU) once a day during 16 weeks, starting 2 weeks after surgery, while sham and OVX groups received 1 mL of demineralized water. At the end of the study, serum of the rats was analyzed for OC, CTX, Ca, and Mg. Values are presented as mean±SEM (n=8 in each group).
Values with different letters are significantly different at *P<0.05 based on one-way ANOVA, followed by Duncan post hoc test. a: different from b; c, b: different from a; and c, c: different from a and b.
Abbreviations: OVX, ovariectomized; OC, osteocalcin; CTX, C-terminal telopeptide; CFU, colony forming units; SEM, standard error from mean; L. helveticus, Lactobacillus helveticus.
Effect of L. helveticus on BMD and trabecular structure changes
Table 4 presents BMD and trabecular structure changes and Figure 1 shows reconstructed cross-sectional CT scan images, 1 cm above the lateral condyles of the femur. The OVX group showed a significantly lower BMD of the femur (P<0.05) than the other groups; however, the OVX+L. helveticus group showed a higher BMD of the femur which was nearest to the sham group (P<0.05). Tb.Th showed a higher value in sham and a lower value in OVX groups (P<0.05), while the L. helveticus-fed group showed a significant increase of Tb.Th compared to the OVX group (P<0.05). L. helveticus also affected the total porosity percentage and BV/TV of the femur. In this regard, L. helveticus significantly increased BV/TV and decreased total porosity percentage of the femur compared to the OVX group (P<0.05). Tb.N and Tb.Sp were not affected by L. helveticus.
Table 4.
Effect of L. helveticus on micro-CT scan analysis of the femur
| Parameters | Sham | OVX | OVX+ L. helveticus | P-value |
|---|---|---|---|---|
| BV/TV (%) | 76±6.30a | 49±5.7b,* | 57±6.6 | 0.05 |
| Porosity (%) | 25±5.3a | 53±4.6b,* | 42±6.6 | 0.02 |
| Tb.Th (mm) | 7.6±0.5a | 5.3±0.4b,* | 5.8±0.5 | 0.03 |
| Tb.Sp (mm) | 5.2±0.4 | 6.4±0.6 | 6.1±0.4 | 0.69 |
| Tb.N (mm−1) | 0.10±0.00 | 0.09±0.01 | 0.09±0.00 | 0.95 |
| BMD (g.cm−3) | 1.07±0.02a | 0.76±0.06b,* | 0.91±0.05a | 0.003 |
Notes: Ten-week-old female Sprague–Dawley rats were supplemented with 1 mL L. helveticus (108–109 CFU) once a day during 16 weeks, starting 2 weeks after surgery, while sham and OVX groups received 1 mL of demineralized water. At the end of the study, dissected femurs were analyzed with micro-CT scan analyzer. Values are presented as mean±SEM analysis of cortical and trabecular femur (n=8 in each group).
Values with different letters are significantly different at *P<0.05 based on one-way ANOVA, followed by Duncan post hoc test. a: different from b.
Abbreviations: OVX, ovariectomized; BV/TV, bone volume/total volume; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; Tb.N, trabecular number; BMD, bone mineral density; CFU, colony forming units; SEM, standard error from mean; L. helveticus, Lactobacillus helveticus.
Figure 1.
Reconstructed cross-sectional CT-scan images of the femur. (A) Sham, (B) OVX, (C) OVX+L. helveticus.
Notes: Ten-week-old female Sprague–Dawley rats were supplemented with 1 mL L. helveticus (108–109 CFU) once a day during 16 weeks, starting 2 weeks after surgery, while sham and OVX groups received 1 mL of demineralized water. At the end of the study, the cross-sectional images of the femur were performed using micro-CT and reconstructed using NRecon software version 1.6.3.3. The pictures display the reconstructed images from 1 cm above the lateral condyles of the femur.
Abbreviations: CFU, colony forming units; OVX, ovariectomized; L. helveticus, Lactobacillus helveticus.
Effect of L. helveticus on stress of the femur
Femur breaking forces are shown in Figure 2. The significantly highest and lowest stress of femurs were reported in the sham and OVX groups, respectively (P<0.05). L. helveticus group showed increasing the stress of the femur compared to the OVX group (P<0.05).
Figure 2.
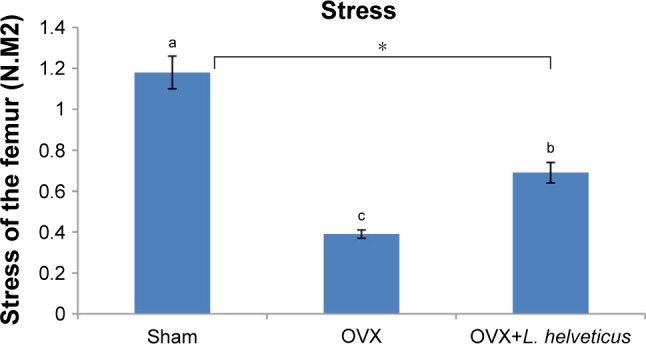
Effect of L. helveticus on stress of the femur.
Notes: Ten-week-old female Sprague–Dawley rats were supplemented with 1 mL L. helveticus (108–109 CFU) once a day during 16 weeks, starting 2 weeks after surgery, while sham and OVX groups received 1 mL of demineralized water. At the study, the stress of the femur was tested using the three-point bending method in the center of the femur with a universal testing machine. Values are presented as mean of each group in column graph with standard error bar from mean (n=8 in each group). a,bValues with different letters are significantly different at *P<0.05 based on one-way ANOVA, followed by Duncan post hoc test. a: different from b and c; b: different from a and c; c: different from a and b.
Abbreviations: CFU, colony forming units; OVX, ovariectomized; L. helveticus, Lactobacillus helveticus.
Effect of L. helveticus on Ob, Oc, OS, ES and OV
ObS/BS, OS/BS and OV/BV were significantly lower and ES/BS and OcS/BS were significantly higher in the OVX group (P=0.0001) compared to the other groups. However, OV+L. helveticus-fed group had higher ObS/BS, OS/BS and OV/BV and lower OcS/BS and ES/BS compared to the OVX group (P=0.0001), as shown in Table 5.
Table 5.
Effect of L. helveticus on histomorphometric measurements of the femur
| Parameters (%) | Sham | OVX | OVX+ L. helveticus | P-value |
|---|---|---|---|---|
| ObS/BS | 53±0.4a | 37±0.4b,* | 46±0.5 | 0.0001 |
| OcS/BS | 23±0.5a | 28±0.4b,* | 25±0.4 | 0.0001 |
| OS/BS | 24±0.2a | 18±0.2b,* | 23±0.3 | 0.0001 |
| OV/BV | 13±0.3a | 7±0.2b,* | 10±0.2 | 0.0001 |
| ES/BS | 29±0.1a | 38±0.3b,* | 34±0.1 | 0.0001 |
Notes: Ten-week-old female Sprague–Dawley rats were supplemented with 1 mL L. helveticus (108–109 CFU) once a day during 16 weeks, starting 2 weeks after surgery, while sham and OVX groups received 1 mL of demineralized water. At the end of the study, percentage of ObS/BS, OcS/BS, ES/BS, OS/BS and OV/BV were measured from histology slide (H&E) method of dissected femur. Values are presented as mean±SEM (n=8 in each group). a,bValues with different letters are significantly different at *P<0.05 based on one-way ANOVA, followed by Duncan post hoc test. a: different from b.
Abbreviations: OVX, ovariectomized; ObS/BS, osteoblast surface/bone surface; OcS/BS, osteoclast surface/bone surface; ES/BS, eroded surface/bone surface; OS/BS, osteoid surface/bone surface; OV/BV, osteoid volume/bone volume; CFU, colony forming units; SEM, standard error from mean; L. helveticus, Lactobacillus helveticus; H&E, hematoxylin and eosin.
Effect of L. helveticus on Ca, Mg and Zn content of the femur
L. helveticus in this study failed to have any significant effects on bone mineral content including Ca, Mg and Zn, as shown in Table 6.
Table 6.
Effect of L. helveticus on Ca, Mg and Zn content of the femur
| Ca, Mg and Zn content of the femur (mg/L) | Sham | OVX | OVX+ L. helveticus | P-value |
|---|---|---|---|---|
| Ca | 492±2.3 | 484±1.3 | 487±2.0 | 0.06 |
| Mg | 33±0.4 | 32±0.2 | 33±0.2 | 0.09 |
| Zn | 17±1.4 | 14±0.6 | 14±0.8 | 0.12 |
Notes: Ten-week-old female Sprague–Dawley rats were supplemented with 1 mL L. helveticus (108–109 CFU) once a day during 16 weeks, starting 2 weeks after surgery, while sham and OVX groups received 1 mL of demineralized water. At the end of the study, Ca, Mg and Zn content of the femur were analyzed. Values are presented as mean±SEM (n=8 in each group).
Abbreviations: OVX, ovariectomized; CFU, colony forming units; SEM, standard error from mean; L. helveticus, Lactobacillus helveticus.
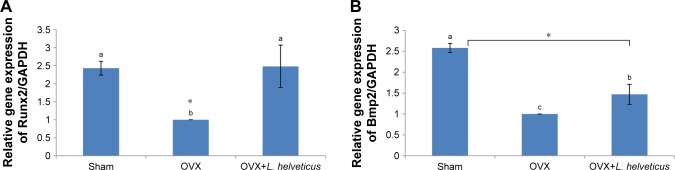
Effect of L. helveticus on expression of Runx2 and Bmp2
The mRNA expression of Runx2 and Bmp2 was lower in the OVX group compared to the sham group (P<0.05). However, OVX+L. helveticus-fed group had increased mRNA expression of Runx2 and Bmp2 and was nearest to the sham group (P<0.05; Figure 3A and B).
Figure 3.
Effect of L. helveticus on mRNA expression of (A) Runx2, (B) Bmp2.
Notes: Ten-week-old female Sprague–Dawley rats were supplemented with 1 mL L. helveticus (108–109 CFU) once a day during 16 weeks, starting 2 weeks after surgery, while sham and OVX groups received 1 mL of demineralized water. At the end of the study, the mRNA expression of Runx2 and Bmp2 was estimated by qRT-PCR analysis. Values are presented as mean of each group in column graph with standard error bar from mean (n=8 in each group). a,bValues with different letters are significantly different at *P<0.05 based on one-way ANOVA, followed by Duncan post hoc test. a: different from b and c; b: different from a and c; c: different from a and b.
Abbreviations: CFU, colony forming units; OVX, ovariectomized; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; Runx2, runt related transcription factor 2; Bmp2, Bone morphometric protein 2; L. helveticus, Lactobacillus helveticus; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Lactobacillus enumeration in the feces
The total lactobacillus count from animal feces is presented in Figure 4. At the end of the experiment, the number of lactobacillus colonies increased significantly in the feces of rats fed with L. helveticus compared to the sham and OVX groups (P<0.05).
Figure 4.
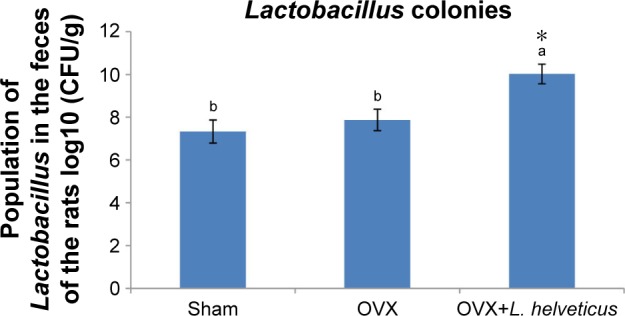
Total Lactobacillus colonies in the fecal samples.
Notes: Ten-week-old female Sprague–Dawley rats were supplemented with 1 mL L. helveticus (108–109 CFU) once a day during 16 weeks, starting 2 weeks after surgery, while sham and OVX groups received 1 mL of demineralized water. At the end of the study, the amount of Lactobacillus was quantified in the feces. Values are presented as mean of each group in column graph with standard error bar from mean (n=8 in each group). a,bValues with different letters are significantly different at *P<0.05 based on one-way ANOVA, followed by Duncan post hoc test. a: different from b.
Abbreviations: CFU, colony forming units; OVX, ovariectomized; L. helveticus, Lactobacillus helveticus.
Discussion
Beneficial bacteria supplementation affects the GM such as by increasing the population of bacteria in the host, which in turn affect the host’s health.28 Similarly, in the current study, we showed that the amount of lactobacillus in rat feces increased via feeding of L. helveticus. This means the bacteria remain alive after eating and passage from the gastrointestinal (GI) tract. Considering this aspect helps justify the better function of lactobacillus on the host. Generally, probiotics modulate GM, influence different target organs and alter the host’s functions.46 Previously, it has been shown that a lack of GM leads to elevated bone mass; however, colonization of mice with normal GI microbes normalized bone mass.47 In this regard, beneficial microbes stimulate endocrine cells which are located in the gut and improve the immune system.46 Since stem cells in the bone marrow generate T cell precursors, the immune system interacts with these pathways.48
Estrogen deficiency leads to changes in immune status via increases of inflammatory factors and, therefore, decreases the osteoprotegerin level.49 Indeed, probiotics have the capacity to increase immune system function by attenuating the elevation of inflammatory cytokines, which is shown in OVX rats,50 or via the production of bioactive peptides and consequent increase of absorption of minerals such as calcium,23 which leads to calcium accumulation in bones and thereby preventing deterioration of bone mass. However, this study failed to detect a significant increase of minerals in the serum and bone accumulation, but the changes of Ob and Oc activity as well as the modulation of bone remodeling were unblemished.
OVX caused elevation of CTX in the serum of the rats; however, those OVX rats fed with L. helveticus did not show elevated CTX in the serum. This means that L. helveticus caused a reduction in the level of CTX in the serum, and since CTX is a bone resorption indicator, it could be the consequence of bone resorption activity reduction occurring in the L. helveticus-fed group. In contrast, OC in the serum was increased with L. helveticus, suggesting that bone formation activity was increased via this bacterium. A possible improvement of bone formation activity is supported by upregulation of the two osteogenic genes measured in this study, Runx2 and Bmp2. These two genes augment Ob functions; in fact, Ob differentiation is regulated by cytokines, a range of hormones and some transcription factors.51 Bmp is one of the important keys to regulate the maintenance of postnatal bone and has an essential role in endochondral bone development.52 Bmp2, a member of Bmps, has a key role in cell growth, embryogenesis and bone formation progress as well as repairing bone fractures,53 and massively increases OC expression.54 Bmp2 performs its osteogenic action by activation of some signals such as Smad1/5/8 and transcript regulation of some other osteogenic genes such as Runx2.55 Runx2 regulates the expression of other osteoblastic genes such as collagen type 1.56 Moreover, Runx2 is a critical transcription factor for Ob differentiation.57 This is well understood in Runx2-deficient rats which show a complete lack of mature Obs and bone formation.58,59
Collectively, the present results indicate that the effectiveness of L. helveticus on bone might be due to the attenuation of bone remodeling, specifically Ob proliferation and differentiation and increasing bone formation activity. This aspect which showed the protective effect of probiotics on bone via alteration of bone formation and resorption is confirmed by the Oc and Ob numbers on the bone surface. In the current study, ObS/BS, OS/BS and OV/BV were found to be increased; in contrast, OcS/BS and ES/BS were decreased by L. helveticus in OVX rats. As a result of increasing the number of Obs and osteoids, bone formation activity is increased and, in consequence, serum OC also increases. However, if the amount of Oc and eroded surface is decreased, it can be suggested that bone resorption activity is decreased and, following that, serum CTX is also decreased.60
As a result of increased bone formation and decreased bone resorption activity, the percentage of BV will be increased; in contrast, the percentage of total porosity will be decreased. Since BMD and bone strength are associated with an increase of BV and a reduction of porosity percentage, our results regarding improvement of BMD and bone strength along with a reduction of total porosity percentage which occurred in the L. helveticus-fed group can be confirmed. Thereby, L. helveticus treatment has the capacity to reduce fracture risk and/or prevent the femur from easily fracturing.61
Conclusion
L. helveticus increases the expression of some osteoblastic genes and thereby bone formation activity. Therefore, by the attenuation of bone remodeling which is obtained via L. helveticus consumption, OVX rats can be protected from bone loss and fracture risk. These data suggest a potential therapeutic effect of L. helveticus on postmenopausal osteoporosis.
Acknowledgments
This study was fully funded by University Putra Malaysia. The authors would like to thank the staff of the Nutrition and Biochemistry Laboratories from the Faculty of Medicine and Health Sciences, University Putra Malaysia (UPM) and the Tissue Engineering Center, National University of Malaysia (UKM) for their kind help and expert assistance. The authors would also like to thank Dr Mehdi Ebrahimi for excellent help in analyzing the data.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jochems C, Islander U, Erlandsson M, Verdrengh M, Ohlsson C, Carlsten H. Osteoporosis in experimental postmenopausal polyarthritis: the relative contributions of estrogen deficiency and inflammation. Arthritis Res Ther. 2005;7(4):R837. doi: 10.1186/ar1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JG, Lee E, Kim SH, Whang KY, Oh S, Imm J-Y. Effects of a Lactobacillus casei 393 fermented milk product on bone metabolism in ovariectomised rats. Int Dairy J. 2009;19(11):690–695. [Google Scholar]
- 3.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 4.Abdul-Majeed S, Mohamed N, Soelaiman IN. Effects of tocotrienol and lovastatin combination on osteoblast and osteoclast activity in estrogen-deficient osteoporosis. Evid Based Complement Alternat Med. 2012;2012:1–9. doi: 10.1155/2012/960742. Article ID 960742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38(3):425–440. doi: 10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwamoto J, Uzawa M, Sato Y, Takeda T, Matsumoto H. Effect of alendronate on bone mineral density and bone turnover markers in post-gastrectomy osteoporotic patients. J Bone Miner Metab. 2010;28(2):202–208. doi: 10.1007/s00774-009-0116-0. [DOI] [PubMed] [Google Scholar]
- 7.Zein CO, Jorgensen RA, Clarke B, et al. Alendronate improves bone mineral density in primary biliary cirrhosis: a randomized placebo-controlled trial. Hepatology. 2005;42(4):762–771. doi: 10.1002/hep.20866. [DOI] [PubMed] [Google Scholar]
- 8.Delmas PD, Benhamou CL, Man Z, et al. Monthly dosing of 75 mg risedronate on 2 consecutive days a month: efficacy and safety results. Osteoporos Int. 2008;19(7):1039–1045. doi: 10.1007/s00198-007-0531-9. [DOI] [PubMed] [Google Scholar]
- 9.Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT) J Bone Miner Res. 2015;30(5):934–944. doi: 10.1002/jbmr.2442. [DOI] [PubMed] [Google Scholar]
- 10.Lyles KW, Colón-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kharwadkar N, Mayne B, Lawrence JE, Khanduja V. Bisphosphonates and atypical subtrochanteric fractures of the femur. Bone Joint Res. 2017;6(3):144–153. doi: 10.1302/2046-3758.63.BJR-2016-0125.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filleul O, Crompot E, Saussez S. Bisphosphonate-induced osteonecrosis of the jaw: a review of 2,400 patient cases. J Cancer Res Clin Oncol. 2010;136(8):1117–1124. doi: 10.1007/s00432-010-0907-7. [DOI] [PubMed] [Google Scholar]
- 13.Gambacciani M, Vacca F. Postmenopausal osteoporosis and hormone replacement therapy. Minerva Med. 2004;95(6):507–520. [PubMed] [Google Scholar]
- 14.Cranney A, Tugwell P, Adachi J, et al. Meta-analyses of therapies for postmenopausal osteoporosis. III. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev. 2002;23(1):517–523. doi: 10.1210/er.2001-3002. [DOI] [PubMed] [Google Scholar]
- 15.Ensrud KE, Stock JL, Barrett-Connor E, et al. Effects of raloxifene on fracture risk in postmenopausal women: the raloxifene use for the heart trial. J Bone Miner Res. 2008;23(1):112–120. doi: 10.1359/jbmr.070904. [DOI] [PubMed] [Google Scholar]
- 16.Qaseem A, Forciea MA, McLean RM, Denberg TD, Clinical Guidelines Committee of the American College of Physicians Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(11):818–839. doi: 10.7326/M15-1361. [DOI] [PubMed] [Google Scholar]
- 17.Zbuk K, Anand SS. Declining incidence of breast cancer after decreased use of hormone-replacement therapy: magnitude and time lags in different countries. J Epidemiol Community Health. 2012;66(1):1–7. doi: 10.1136/jech.2008.083774. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez E, Gallus S, Bosetti C, Franceschi S, Negri E, La Vecchia C. Hormone replacement therapy and cancer risk: a systematic analysis from a network of case–control studies. Int J Cancer. 2003;105(3):408–412. doi: 10.1002/ijc.11083. [DOI] [PubMed] [Google Scholar]
- 19.Hawkes N. HRT increases risk of blood clots and stroke, finds new analysis. BMJ. 2015;350:h1336. doi: 10.1136/bmj.h1336. [DOI] [PubMed] [Google Scholar]
- 20.Simon JA, Wehren LE, Ascott-Evans BH, Omizo MK, Silfen SL, Lombardi A. Skeletal consequences of hormone therapy discontinuance: a systematic review. Obstet Gynecol Surv. 2006;61(2):115–124. doi: 10.1097/01.ogx.0000189152.95070.f8. [DOI] [PubMed] [Google Scholar]
- 21.Davison S, Davis SR. Hormone replacement therapy: current controversies. Clin Endocrinol. 2003;58(3):249–261. doi: 10.1046/j.1365-2265.2003.01774.x. [DOI] [PubMed] [Google Scholar]
- 22.O’Connell MB. Prescription drug therapies for prevention and treatment of postmenopausal osteoporosis. J Manag Care Pharm. 2006;12(6 Suppl A):S10–S19. doi: 10.18553/jmcp.2006.12.S6-A.S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parvaneh K, Jamaluddin R, Karimi G, Erfani R. Effect of probiotics supplementation on bone mineral content and bone mass density. ScientificWorldJournal. 2014;2014:595962. doi: 10.1155/2014/595962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parvaneh K, Ebrahimi M, Sabran MR, et al. Probiotics (Bifidobacterium longum) increase bone mass density and upregulate Sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int. 2015;2015:897639. doi: 10.1155/2015/897639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howarth GS, Wang H. Role of endogenous microbiota, probiotics and their biological products in human health. Nutrients. 2013;5(1):58–81. doi: 10.3390/nu5010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bron PA, van Baarlen P, Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol. 2012;10(1):66–78. doi: 10.1038/nrmicro2690. [DOI] [PubMed] [Google Scholar]
- 27.Thomas CM, Versalovic J. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes. 2010;1(3):148–163. doi: 10.4161/gmic.1.3.11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6(1):39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Toraldo G, Li A, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109(9):3839–3848. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116(5):1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.New SA, Robins SP, Campbell MK, et al. Dietary influences on bone mass and bone metabolism: further evidence of a positive link between fruit and vegetable consumption and bone health? Am J Clin Nutr. 2000;71(1):142–151. doi: 10.1093/ajcn/71.1.142. [DOI] [PubMed] [Google Scholar]
- 33.Johnsson M, Jonsson KB, Andersson L, Jensen P, Wright D. Genetic regulation of bone metabolism in the chicken: similarities and differences to Mammalian systems. PLoS Genet. 2015;11(5):e1005250. doi: 10.1371/journal.pgen.1005250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha KM, Zhou X. Genetic and molecular control of osterix in skeletal formation. J Cell Biochem. 2013;114(5):975–984. doi: 10.1002/jcb.24439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Styrkarsdottir U, Cazier JB, Kong A, et al. Linkage of osteoporosis to chromosome 20p12 and association to BMP2. PLoS Biol. 2003;1(3):E69. doi: 10.1371/journal.pbio.0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010;339(1):189–195. doi: 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- 37.Paternoster L, Lorentzon M, Vandenput L, et al. Genome-wide association meta-analysis of cortical bone mineral density unravels allelic heterogeneity at the RANKL locus and potential pleiotropic effects on bone. PLoS Genet. 2010;6(11):e1001217. doi: 10.1371/journal.pgen.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muhammad SI, Ismail M, Mahmud RB, Salisu AM, Zakaria ZA. Germinated brown rice and its bioactives modulate the activity of uterine cells in oophorectomised rats as evidenced by gross cytohistological and immunohistochemical changes. BMC Complement Altern Med. 2013;13(1):198. doi: 10.1186/1472-6882-13-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiang SS, Pan TM. Antiosteoporotic effects of Lactobacillus – fermented soy skim milk on bone mineral density and the microstructure of femoral bone in ovariectomized mice. J Agric Food Chem. 2011;59(14):7734–7742. doi: 10.1021/jf2013716. [DOI] [PubMed] [Google Scholar]
- 40.Goda T, Kishi K, Ezawa I, Takase S. The maltitol-induced increase in intestinal calcium transport increases the calcium content and breaking force of femoral bone in weanling rats. J Nutr. 1998;128(11):2028–2031. doi: 10.1093/jn/128.11.2028. [DOI] [PubMed] [Google Scholar]
- 41.An YH, Draughn RA. Mechanical Testing of Bone and the Bone– Implant Interface. Boca Raton, London, New York, Washington, DC: CRC Press; 1999. pp. 207–217. [Google Scholar]
- 42.Baldock PA, Morris HA, Need AG, Moore RJ, Durbridge TC. Variation in the short-term changes in bone cell activity in three regions of the distal femur immediately following ovariectomy. J Bone Miner Res. 1998;13(9):1451–1457. doi: 10.1359/jbmr.1998.13.9.1451. [DOI] [PubMed] [Google Scholar]
- 43.Hfreere RH, Weibel ER. Stereologic techniques in microscopy. J R Microsc Soc. 1967;87(1):25–34. doi: 10.1111/j.1365-2818.1967.tb04489.x. [DOI] [PubMed] [Google Scholar]
- 44.Harrison E, Adjei A, Ameho C, Yamamoto S, Kono S. The effect of soybean protein on bone loss in a rat model of postmenopausal osteoporosis. J Nutr Sci Vitaminol. 1998;44(2):257–268. doi: 10.3177/jnsv.44.257. [DOI] [PubMed] [Google Scholar]
- 45.Wilfinger WW, Mackey K, Chomczynski P. Effect of pH and ionic strength on the spectrophotometric assessment of nucleic acid purity. Biotechniques. 1997;22(3):474–476. doi: 10.2144/97223st01. [DOI] [PubMed] [Google Scholar]
- 46.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6(5):306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sjögren K, Engdahl C, Henning P, et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27(6):1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005;26(7):360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev. 2005;208(1):207–227. doi: 10.1111/j.0105-2896.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 50.Ohlsson C, Engdahl C, Fåk F, et al. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014;9(3):e92368. doi: 10.1371/journal.pone.0092368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jang WG, Kim EJ, Kim DK, et al. BMP2 protein regulates osteocalcin expression via Runx2-mediated Atf6 gene transcription. J Biol Chem. 2012;287(2):905–915. doi: 10.1074/jbc.M111.253187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell. 2002;3(3):439–449. doi: 10.1016/s1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 53.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24(2):218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 54.Wu M, Chen G, Li YP. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee MH, Kim YJ, Kim HJ, et al. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem. 2003;278(36):34387–34394. doi: 10.1074/jbc.M211386200. [DOI] [PubMed] [Google Scholar]
- 56.Jensen ED, Gopalakrishnan R, Westendorf JJ. Regulation of gene expression in osteoblasts. Biofactors. 2011;36(1):25–32. doi: 10.1002/biof.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilbert L, He X, Farmer P, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem. 2002;277(4):2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 58.Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 59.Tu Q, Zhang J, Paz J, Wade K, Yang P, Chen J. Haploinsufficiency of Runx2 results in bone formation decrease and different BSP expression pattern changes in two transgenic mouse models. J Cell Physiol. 2008;217(1):40–47. doi: 10.1002/jcp.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 61.Britton RA, Irwin R, Quach D, et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014;229(11):1822–1830. doi: 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]