Abstract
Recent studies suggest that environmentally induced effects on sperm phenotype can influence offspring phenotype beyond the classic Mendelian inheritance mechanism. However, establishing whether such effects are conveyed purely through ejaculates, independently of maternal environmental effects, remains a significant challenge. Here, we assess whether environmentally induced effects on sperm phenotype affects male reproductive success and offspring fitness. We experimentally manipulated the duration of sperm storage by males, and thus sperm age, in the internally fertilizing fish Poecilia reticulata. We first confirm that sperm ageing influences sperm quality and consequently males reproductive success. Specifically, we show that aged sperm exhibit impaired velocity and are competitively inferior to fresh sperm when ejaculates compete to fertilize eggs. We then used homospermic (noncompetitive) artificial insemination to inseminate females with old or fresh sperm and found that male offspring arising from fertilizations by experimentally aged sperm suffered consistently impaired sperm quality when just sexually mature (four months old) and subsequently as adults (13 months old). Although we have yet to determine whether these effects have a genetic or epigenetic basis, our analyses provide evidence that environmentally induced variation in sperm phenotype constitutes an important source of variation in male reproductive fitness that has far reaching implications for offspring fitness.
Keywords: Epigenetics, male sperm storage, paternal effects, sperm ageing, sperm competition, sperm velocity
Impact Summary.
Prolonged sperm storage is associated with a reduction in sperm quality in many species, including humans. Such effects have potentially important implications for a male's reproductive fitness because males that store sperm for prolonged periods (e.g., because they become isolated from females, or fail to secure mates) may suffer compromised fertility, or reduced fertilization success when their sperm compete with rival (fresher) sperm during sperm competition. However, in addition to such direct costs associated with male sperm storage, recent studies have suggested a link between environmentally induced changes in sperm quality and offspring traits. Sperm ageing therefore constitutes a potentially widespread source of nongenetic (i.e., not linked to genes) variance in offspring fitness. Here, using the live‐bearing guppy (Poecilia reticulata), we provide experimental support for these ideas, showing that sperm storage has far reaching implications for male reproductive fitness. First, we show that males whose sperm were held longer inside their reproductive organs fertilize relatively fewer eggs when in competition with those from males who produced fresher sperm. Second, we provide empirical evidence that the reduction in sperm quality caused by long‐term sperm storage has effects that transcend generations by influencing the reproductive fitness of adult offspring; offspring sired by males with aged sperm themselves suffer impaired sperm quality when they reached adulthood. We conclude, therefore, that prolonged sperm storage has profound negative consequences for males by compromising not only their own reproductive performance but also that of their adult male offspring.
Introduction
Environmental sources of variance may affect sperm phenotype both before and after the release of sperm (Marshall 2015). The prerelease environment coincides with the paternal environment, where sperm are produced and subsequently stored. Evidence for prerelease environmental effects on sperm phenotype and fertilization rates comes primarily from studies that manipulate male condition (e.g., through diet or immunity challenges) or extrinsic factors such as temperature, salinity, mating rate, and social experience (Kilgallon and Simmons 2005; Adriaenssens et al. 2012; Gasparini et al. 2013; Jensen et al. 2014). The postrelease environment refers to the conditions experienced by sperm after they are released (e.g., water in external fertilizers and the female reproductive tract in internal fertilizers) (for a recent review see Reinhardt et al. 2015). In both cases, environmentally induced changes in sperm phenotype can have important implications for male reproductive fitness, influencing both male fertility and fertilization success when ejaculates from two or more males compete to fertilize eggs (sperm competition, Parker 1970) (Almbro et al. 2011; Rahman et al. 2014a; Vasudeva et al. 2014).
There is increasing recognition that environmentally induced changes in sperm phenotype can also have implications for offspring fitness (Bonduriansky and Head 2007; Bonduriansky and Day 2009; Crean et al. 2013; Crean and Bonduriansky 2014; Zajitschek et al. 2014). These findings have potentially profound implications for evolutionary biologists because they challenge the widely held assumption that any variance in offspring fitness that is transmitted solely via sperm (e.g., inferred from quantitative genetic breeding designs) will be attributable to additive genetic variation (i.e., sire genetic variance). However, providing evidence that environmentally induced changes in sperm phenotype translate into changes in offspring phenotype is far from straightforward, especially for the paternal (prerelease) environment. This is because experimental changes in male condition may influence both the male's ability to mate (e.g., the amount of sperm transferred) and patterns of female reproductive investment (e.g., differential maternal allocation; Sheldon 2000), which can then manifest as environment‐dependent paternal effects if not experimentally controlled. Studies using artificial fertilization techniques (e.g., in vitro fertilization and artificial insemination) have great potential to circumvent this problem because they experimentally control for potentially confounding factors when evaluating paternal environmental effects on offspring fitness (Evans et al. 2004). Accordingly, recent studies employing in vitro fertilization in external fertilizers have provided evidence that paternal environmental effects, transmitted exclusively through ejaculates as a consequence of environmentally moderated changes to sperm phenotype, can influence the fitness of resulting embryos. For example, in the zebrafish Danio rerio (Zajitschek et al. 2014) and the solitary ascidian Styela plicata (Crean et al. 2013), experimentally moderated changes in sperm phenotype in response to changes in social environment influenced early offspring development and survival.
The length of time that sperm are retained in the male testes (or storage organs) prior to ejaculation represents a widespread source of paternal (prerelease) environmental variance that influences sperm phenotype. During storage, sperm inevitably undergo ageing (postmeiotic sperm ageing sensu Pizzari et al. 2008) and evidence that the duration of sperm storage by males, and hence sperm ageing, alters sperm phenotype has been reported in humans (e.g., see Tarin et al. 2000 for a review) and other animals (El Jack and Lake 1966; Froman and Bernier 1987; Reinhardt 2007; Gasparini et al. 2014). Given the ubiquity of sperm storage by males in animals (i.e., sperm production is inevitably temporally separated from sperm release/transfer), and the fact that sperm storage will likely vary among individuals according to ecological conditions, mate availability, and female choice (Reinhardt 2007), sperm ageing associated with male sperm storage constitutes a potentially widespread source of environmentally induced variation in sperm phenotype in many taxa. Changes in sperm phenotype associated with sperm age may therefore offer an obvious but often overlooked explanation for the lack of repeatability reported in many studies looking at ejaculate traits within the same male (Siva‐Jothy 2000; Reinhardt 2007; Pizzari et al. 2008; Reinhardt et al. 2015). Nevertheless, providing unequivocal evidence that sperm age affects offspring fitness is logistically challenging, not least because other potential sources of variance in offspring fitness (e.g., male mating history, male age, and differential maternal effects) need to be controlled experimentally.
In this study, we determine whether the experimental manipulation of the length of sperm storage by males influences direct components of male and female reproductive fitness and components of offspring fitness using the guppy Poecilia reticulata. Guppies are live‐bearing fish that are ideal subjects for addressing this question; the duration of sperm storage can be readily manipulated experimentally (see below) to control sperm age (postmeiotic prerelease), as males cannot dump or reabsorb sperm during storage and sperm accumulate for up to 60 days in the testicular ducts (Billard and Puissant 1969). Sperm ageing due to sperm storage within males is known to influence sperm quality; previous work has shown that stored sperm exhibit slower swimming speed compared to fresh sperm produced by the same male (Gasparini et al. 2014). Importantly, environmentally induced variation in sperm phenotype associated with sperm age is ecologically and physiologically relevant in guppies. In natural populations, males are often found in male‐only or male‐biased pools during the dry season creating the opportunities for long periods of sexual abstinence or low mating rate (Houde 1997). From a practical perspective, the development of artificial insemination techniques in this system (Evans et al. 2003) means that sperm of different ages can be delivered to females in a way that controls for variation in male mating history, ejaculate size, and possible differential maternal effects mediated by male–female interactions.
Our experiment had two broad aims. First, we explored the direct fitness implications of environmentally induced changes in sperm phenotype associated with sperm age for both males and females using a split‐ejaculate design (see Methods and Fig. 1). In the case of males, we used a portion of the ejaculate to conduct a series of heterospermic (mixed ejaculate) artificial inseminations involving sperm obtained after short or long storage to determine whether the duration of sperm storage affects the success of ejaculates when they compete to fertilize a female's eggs (i.e., sperm competition; Parker 1970). In the case of females, we used a subsample of the same ejaculate used for the heterospermic inseminations to perform a series of homospermic (single ejaculate) inseminations to determine whether sperm age influences female fecundity. Second, we tested whether there are trans‐generational consequences of sperm ageing for offspring fitness by assessing early (juvenile) and late (adult) components of fitness in male and female offspring that arose from the homospermic inseminations. Specifically, we contrasted the survival and size of juvenile offspring arising from aged‐ and fresh‐sperm treatments, and subsequently evaluated components of reproductive fitness of adult offspring (ejaculate traits of males and body size as a proxy of fecundity in females). Our results reveal that sperm ageing has important reproductive consequences for males; sperm ageing affects sperm velocity and compromises sperm competitive ability and these effects carryover to offspring, whereby males fathered by males with aged sperm exhibit compromised sperm velocity when tested at two stages during adulthood (four and 13 months).
Figure 1.
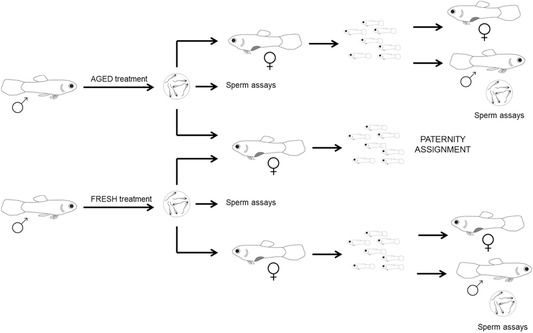
Schematic view of the experimental design. The length of sperm storage was manipulated in two groups of adult males to obtain fresh and aged sperm (see Methods for further details). Ejaculates were then collected and used in (i) sperm assays and (ii) artificial inseminations in both competitive (heterospermic) and noncompetitive (homospermic) fertilization trials.
Methods
FISH MAINTENANCE
The fish used in the experiment were reared from the descendants of fish captured in 2006 from a natural population in Queensland (Alligator creek). Virgin females were used to standardize mating history, age, and social experience, and to avoid the possibility that fertilizations were attributable to sperm stored from prior matings. Virgin females (six months old) were reared in single sex tanks until required for the experiment, while experimental males of the same age were reared in mixed‐sex aquaria from birth. All tanks were maintained at 26 ± 1°C and illuminated on a 12:12 light/dark cycle. All fish were fed five days per week on a mixed diet of Artemia nauplii and commercial dry food. This research was approved by the University of Western Australia's Animal Ethics Committee (approval number: RA/3/100/1050).
MANIPULATION OF SPERM STORAGE LENGTH
Sixty adult males were used in the experiment. These males were assigned haphazardly to one of two experimental treatments (hereafter “aged” and “fresh,” obtained from long and short storage). Males in both groups were exactly the same age (six months old ± 2 days) to avoid confounding sperm age with male age (Pizzari et al. 2008). Each male was placed individually in 2 L plastic tanks equipped with gravel and an airstone and were maintained under the same conditions as the stock population. Males were kept in these tanks for one week before commencing the experiment to standardize their recent social and mating history.
After the seven‐day isolation period, the males were stripped of all available sperm (strip 0) to have males entering into the treatment phase with no previously stored sperm and also to provide baseline data for sperm production and sperm velocity. To achieve this, each male was anaesthetized and placed on a glass slide under a dissecting microscope with its gonopodium (intromittent organ) swung forward; sperm were collected by applying gentle pressure to the abdomen to release the ejaculate onto a drop of saline solution (0.9% NaCl). All sperm assays were performed blind of treatment. In guppies, sperm are packaged in bundles (termed spermatozeugmata), each containing approximately 21,000 sperm (Boschetto et al. 2011). Sperm production (in millions) could therefore be calculated from the number of sperm bundles released by each male. Sperm velocity (measured as sperm curvilinear velocity, VCL, μm/s), which is positively associated with competitive fertilization success in this species (Boschetto et al. 2011), was assessed using the CEROS sperm tracker (Hamilton‐Thorne Research, Beverly, MA, USA) as previously described (Gasparini et al. 2014). Males assigned to the “fresh” treatment were stripped of all available sperm each week for the following three weeks (i.e. until week four of the experiment). In the context of our study, individuals assigned to the fresh treatment would represent males that successfully copulated on a weekly basis (note that males can easily deplete almost all of their available sperm within a mating; Pilastro and Bisazza 1999). Males assigned to the aged treatment were subjected to a “sham” stripping to control for any potential effect of anesthesia and fish handling. Sham strips involved the same procedure but without the release of sperm (i.e., pressure was applied to a slightly different position of the male's abdomen that does not cause sperm release, see Gasparini et al. 2014). The aged treatment therefore simulates a situation in which males are precluded from mating, for example due to temporal isolation from females. At the end of the four‐week treatment period, all males were stripped again and ejaculates were collected and split for sperm assays (sperm number and sperm velocity as above) and artificial inseminations (see below) (Fig. 1).
ARTIFICIAL INSEMINATIONS
For each sperm competition trial, an equal number of sperm from two males (one from the aged treatment and one from the fresh treatment) was used to artificially inseminate a virgin female. For these heterospermic artificial inseminations, 10 sperm bundles were collected from each of the two male ejaculates (same ejaculate obtained for sperm assays, see Fig. 1) and mixed gently in an eppendorf tube (note that the number of sperm per bundle has been shown to be constant across individual sperm bundles and among males; Evans et al. 2003; Gasparini et al. 2010 and we confirm here that sperm numbers per bundle do not change with the length of storage; see Supplementary Material). The order in which males were stripped was randomized between treatments. Each female was then anaesthetized and placed under a dissecting microscope with her genital pore exposed. We used a 3 μL micropipette to inseminate each female with the mixed (fresh and aged) ejaculates. At this stage, female body size (in mm) was recorded to account for possible differences in female fecundity attributable to variation in body size across our sample. We formed 30 pairs of competitor males (60 males in total, same individuals as above), which were used to inseminate up to three virgin females per pair (total of n = 87 females). Where more than one of the females per replicate produced broods, we selected the largest brood for our subsequent molecular paternity analysis (see below).
We used the same artificial insemination procedure to inseminate a separate sample of virgin females for the noncompetitive (homospermic) fertilization trials, except that in these cases we used 20 sperm bundles from a single male (either aged or fresh treatment, same individuals as above; see Fig. 1) for each insemination. The sperm from each male were used to inseminate 2–3 females (to maximize the chances of obtaining offspring) for a total of n = 132 females. Offspring from these homospermic inseminations were used subsequently to compare female fecundity, offspring fitness, and brood sex ratios between treatments (see below). Females were placed in small (2 L) tanks until they produced their first brood. Measures of female fecundity, offspring fitness, and sex ratio were made blind to experimental treatment. In cases where more than one female produced a brood in each replicate, we selected the largest brood for the fecundity, fitness, and sex ratio measures.
PATERNITY ANALYSIS FOLLOWING HETEROSPERMIC ARTIFICIAL INSEMINATIONS
The whole bodies of newborn offspring along with caudal fin clips from adults were preserved in absolute ethanol until required. DNA was extracted using the EDNA Hispex Tissue Kit (Fisher Biotec). Up to four microsatellites were used to assign paternity according to the sharing of unique alleles between offspring and the putative sires (for details see Supplementary Material). We assigned paternity for offspring that matched the genotype of only one of the two potential sires.
FEMALE FECUNDITY, BROOD PRODUCTION TIME, EARLY OFFSPRING FITNESS, AND SEX RATIO
When females from the noncompetitive fertilization trials gave birth, newborn offspring were counted, and placed in small plastic tanks (2 L). At this stage, the time (in days) elapsed from artificial insemination to birth was recorded (“brood production time”). Note that being livebearers, fertilization is internal in guppies and once fertilized the eggs develop inside the female for roughly one month until they are fully mature and females give birth. The tanks were equipped as described above for the males. We attempted to standardize fish numbers in each tank (maximum of four fish per tank) to avoid density‐dependent effects on growth rate/offspring size. However, because some tanks inevitably contained fewer than four fish, we also included fish density as a covariate in our statistical models (see statistical analyses). Digital photos of the offspring were taken when fish were seven days old. Body size (the distance in mm between the snout and the tip of the caudal peduncle; standard length, in mm) was measured from the photographs using ImageJ software v 1.4 (http://rsb.info.nih.gov/ij/). Sex ratio was recorded when fish approached sexual maturity (within three months) and thus were easily distinguishable as either male or female (see Houde 1997).
OFFSPRING TRAITS
When offspring arising from homospermic artificial insemination trials reached sexual maturity (three months of age) we counted the number of surviving fish and photographed the left side of each fish. Male and female standard length was then measured using ImageJ. In female guppies, body size can be used as a proxy for fecundity (number of eggs produced, see Evans and Gasparini 2013). After photography, up to three male offspring were selected haphazardly from each family and housed individually in 2 L plastic tanks for a further month (under the conditions described above). A total of 96 male offspring were isolated from 38 families (n = 19 from each treatment). When the males were four months old (112 days ± 3.4 SD), ejaculates were collected and sperm number and velocity were measured as described above. One male from each family was then selected (again haphazardly) and maintained under the same conditions until 13 months old (403 days ± 8.47 SD), at which point sperm number and velocity were again assessed.
STATISTICAL ANALYSES
All analyses were performed in R v. 3.1.2 (R Development Core Team 2014). Model types depended on the underlying distributions and properties of the data. In all cases, treatment (fresh or aged) was included as a fixed effect. Proportional data (paternity, survival, sex ratio) were analyzed using generalized linear mixed‐effects models in which we specified a binomial distribution and a logit link function (glmer function of “lme4” package). The Χ 2 statistics and P‐values for the fixed effects for these models were obtained from the univariate “Anova” function (from “Car” package). The model used to analyze paternity share included the offspring sired by each male in the pair as the response variable and treatment (fresh or aged) as the fixed effect. The model takes into account the total number of offspring in each replicate (i.e., brood size), and included family identity (mother ID) as a random factor (because multiple offspring came from the same mother) and an observation‐level random effect to account for overdispersion. In all analyses, diagnostic plots were examined to inspect the distribution of the residuals and thus confirm normality of errors. Continuous variables were analyzed using either linear models, or linear mixed‐effects models when female or male identity had to be included to account for the nonindependence of traits collected from offspring sharing the same mother/father (e.g., body size or sperm traits). Female (maternal) body size was included as a covariate in our analysis of female fecundity (i.e., brood size). When analyzing offspring body size at maturity, the number of fish in the rearing tank was included as a covariate and the identity of the rearing tank was entered as a random factor.
Results
THE EFFECT OF SPERM‐STORAGE TREATMENT ON SPERM PHENOTYPE
Prior to the start of the experiment (i.e. at “strip 0,” see Materials and Methods) males (n = 60) from both treatment groups did not differ in sperm swimming velocity (mean ± SE, fresh: 105.4 μm/s ± 2.05, aged: 104.8 μm/s ± 2.13, F1,58 = 0.0354, P = 0.852) or sperm production (mean ± SE, fresh: 2.97 × 106 ± 0.26, aged: 3.05 × 106 ± 0.24, F1,58 = 0.0542, P = 0.817). However, after the treatment period (i.e., four weeks after strip 0) we found that males assigned to the fresh treatment produced significantly faster swimming sperm (mean ± SE, fresh: 114.7 μm/s ± 2.15, aged: 106.4 μm/s ± 2.92, F1,57 = 5.2125, P = 0.026, see Fig. 2A) and smaller ejaculates (mean ± SE, fresh: 2.55 × 106 sperm ± 0.24, aged: 5.00 × 106 ± 0.34, F1,58 = 34.834, P < 0.001, see Fig. S1) than their aged‐sperm counterparts.
Figure 2.
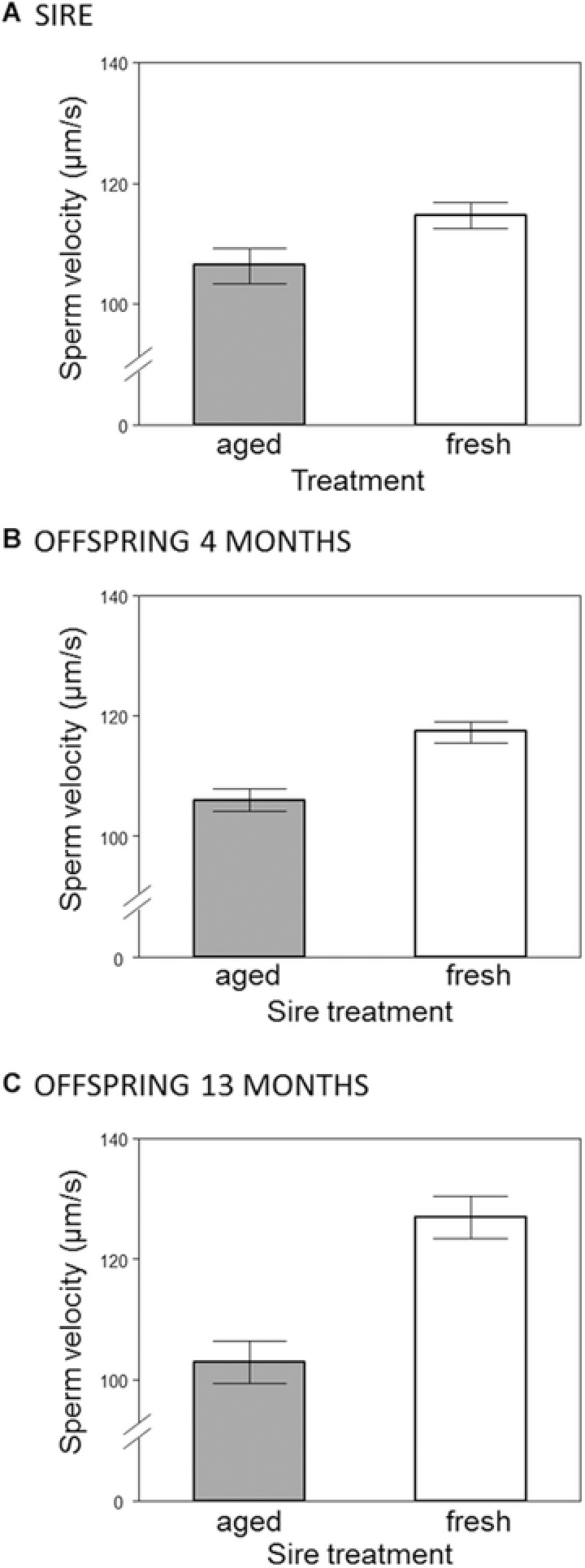
(A) In vitro sperm swimming velocity of males according to their treatment (“fresh” or “aged”). (B–C) Sperm swimming velocity measured from male offspring according to the treatment experienced by their fathers, measured at the onset of sexual maturity (four months of age, panel B), and at 13 months of age (panel C). Bars represent mean ± SE.
OUTCOME OF SPERM COMPETITION TRIALS
Of the 87 females that were artificially inseminated with mixed ejaculates from two rival males, 69 gave birth (79%, brood size, mean ± SE: 7.25 ± 0.49, min–max: 1–17). To obtain reliable estimates of paternity success we selected the largest brood for each pair of males (30 females in total, one for each of the 30 pairs of males). We analyzed 303 offspring arising from these 30 females for the paternity analysis (brood size, mean ± SE: 10.1 ± 0.64, min–max: 4–17). From these offspring, we were able to unequivocally assign parentage to 261 individuals (∼86%). Males assigned to the fresh‐sperm treatment sired a significantly higher proportion of offspring than their rivals assigned to the aged treatment (proportion of offspring sired, mean ± SE: 0.6 ± 0.06; Χ 2 = 7.2015, P = 0.007; Fig. 3). Our results from the homospermic insemination treatments revealed no significant difference in brood size between aged‐ and fresh‐sperm treatments (see below). We conclude, therefore, that the differences in paternity success seen in our heterospecific insemination trials were likely to be a result of differential fertilization success and not differential embryo survival.
Figure 3.
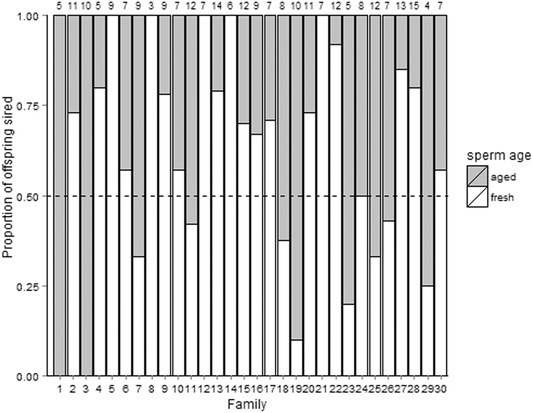
Proportion of offspring sired by males from aged‐ or fresh‐sperm group in each of the (n = 30) families. Numbers at top indicate the number of offspring per brood.
FEMALE FECUNDITY, BROOD PRODUCTION TIME, OFFSPRING SURVIVAL, AND SEX RATIO
A total of 132 females were inseminated in the noncompetitive (i.e., homospermic) artificial insemination trials. Of these, 101 (∼76%) gave birth, with no differences in the number of females giving birth between aged and fresh sperm treatments (no. females giving birth/total females inseminated: 53/67 fresh, 48/65 aged; Fisher exact test: P = 0.54). From these, we selected the largest brood for each male and obtained a sample size of n = 54 independent broods for our analyses (26 aged and 28 fresh, comprising 434 offspring). Note that the results for female fecundity, brood production time (i.e., time in days from insemination to parturition) and offspring body size at birth did not change when including all females in the analysis (see Supplementary Material). We found no effect of sperm‐age treatment on female fecundity (number of offspring produced mean ± SE, fresh group: 7.96 ± 0.93, n = 28, aged group: 8.12 ± 0.7, n = 26; treatment: F1,51 = 0.413, P = 0.52, covariate female SL: F1,51 = 10.30, P = 0.002) or brood production time (mean ± SE, fresh group: 33.64 ± 1.32 days, range 21–44, n = 28; aged group: 33.92 ± 1.41 days, range 21–46, n = 26; t‐test: t 52 = 0.146, P = 0.89). Similarly, mean offspring body size did not differ between treatments (mean ± SE, fresh group: 3.35 ± 0.02, n = 220; aged group: 3.37 ± 0.02, n = 206; treat: Χ 2 = 0.0007, P = 0.98), and the inclusion of brood size as a covariate did not alter this finding (data not shown). A total of 402 offspring survived to three months of age (92%, n = 53 families); treatment had no significant effect on either offspring survival (Χ 2 = 0.026, P = 0.871) or sex ratio (Χ 2 = 1.37, P = 0.24).
REPRODUCTIVE TRAITS OF ADULT OFFSPRING
Female offspring
As expected, the number of fish in each tank affected body size at maturity. The higher the number of fish in the tank (up to four, see Materials and Methods) the smaller the fish (no. fish: Χ 2 = 49.84, P < 0.001), and the effect was more pronounced in females than in males (no. fish × sex interaction: Χ 2 = 10.16, P = 0.001). Females were larger than males of the same age (mean ± SE, females: 13.67 ± 0.09 mm, males: 13.38 ± 0.05 mm) but there was no significant difference in body size for either sex between treatments (treatment: Χ 2 = 0.035, P = 0.85, sex: Χ 2 = 16.60, P < 0.001, treatment × sex interaction not significant). This indicates that treatment did not affect daughters’ body size, used here as a proxy for fecundity (Evans and Gasparini 2013).
Male offspring
A total of n = 96 adult male offspring were tested for sperm production and quality. Of these, three males did not produce ejaculates so our final sample comprised n = 93 males (n = 48 fresh‐sperm treatment, n = 45 aged‐sperm treatment). In the initial assays performed on four‐month old male offspring we found that males whose fathers were assigned to the aged treatment (who themselves produced slower sperm, see above) produced significantly slower swimming sperm than offspring sired by males in the fresh treatment (mean ± SE, fresh: 117.4 μm/s ± 1.70, aged: 106.1 μm/s ± 1.87; Χ 2 = 11.428, P = 0.001, see Fig. 2B). However, we found no significant difference in sperm production between these groups (mean ± SE, fresh: 2.81 × 106 ± 0.24, aged: 2.60 × 106 ± 0.27; Χ 2 = 0.44, P = 0.51). We found the same pattern in the subsequent assays performed on males aged 13 months (n = 37 males; 19 fresh, 18 aged); sons sired by males assigned to the aged treatment produced significantly slower swimming sperm than those sired by males in the fresh treatment (mean ± SE, fresh: 127.0 μm/s ± 3.50 SE, aged: 103.2 μm/s ± 3.62 SE; F1,35 = 22.32, P < 0.001, see Fig. 2C) and there was no significant difference in sperm production between treatments (mean ± SE, fresh: 3.83 × 106 ± 0.36, aged: 3.20 × 106 ± 0.34; F1,35 = 1.61, P = 0.21). Within individual males, sperm velocity did not change over time (paired t‐test: t 36 = 0.69, P = 0.50) while sperm production increased with age (paired t‐test: t 36 = 2.73, P = 0.009). Finally, we found no evidence that the strength of the treatment effect on sire sperm velocity (i.e., the difference between the initial sperm velocity measures and those taken after the four week treatment period) was correlated with the mean offspring sperm velocity in each family (Pearson correlation, r = –0.017, P = 0.918, n = 38).
Discussion
Our study reveals important fitness consequences of environmentally induced variance in sperm phenotype. The length of sperm storage (and hence sperm age), independent of male age, mating history, and potentially confounding maternal effects, has effects on the adult male's sperm quality and sperm competitive ability. When looking at cross‐generational effects we found that the length of sperm storage affects the reproductive traits of male offspring but there was no effect on offspring survival or growth. We therefore provide evidence that the observed effects on sperm phenotype in fathers are associated with decreased sperm quality in sons. Moreover, this effect was consistently expressed at two temporally separated time points during adulthood. As such, our study presents a rare example that links environmentally induced changes in sperm phenotype to offspring reproductive fitness. Moreover, we also show that such effects have long‐term consequences for offspring fitness that extend well beyond the existing evidence linking environmental effects on sperm to embryonic and juvenile stages of development (Crean et al. 2013; Immler et al. 2014; Zajitschek et al. 2014).
The length of sperm storage as a source of variance in offspring fitness has been well studied in females (female sperm storage, see Holt and Lloyd 2010; Orr and Brennan 2015). For example, sperm storage inside females has been associated with hatching failure and chick condition at hatching in a monogamous seabird species, Rissa tridactyla (White et al. 2008). Far less is known about the fitness consequences of sperm storage in males. Tan et al. (2013) showed an effect of sperm age on egg‐to‐adult viability in Drosophila melanogaster, but in that case the effect of sperm ageing could not be disentangled from that attributable to egg ageing. The paucity of studies focusing on variation in sperm storage by males is surprising, given that this is likely to represent a ubiquitous source of variance influencing sperm phenotype (i.e., not simply confined to internal fertilizers, as for female sperm storage). Fluctuations in female availability and low mating encounter rate are likely to exacerbate the effects of sperm storage, as in natural populations of guppies, where males and females are often isolated or in same‐sex pools for extended periods during the dry season (Houde 1997).
The mechanisms that link sperm age with offspring traits have yet to be determined, but may include various genetic and/or epigenetic factors that are transferred through sperm and/or components of the seminal fluid. Sperm storage is associated with thermodynamic and oxidative stress, which in turn may affect sperm cell membrane structure or components in the seminal fluid (Siva‐Jothy 2000; Reinhardt 2007; Pizzari et al. 2008). Oxidative stress can interfere with the regulation of gene expression, cause histone modifications, and induce changes in DNA methylation patterns (e.g., Franco et al. 2008); the transfer of these modifications through the sperm can influence offspring phenotype via epigenetic factors (Bonduriansky and Day 2009; Curley et al. 2011). Other possible mechanisms include differential sperm survival (filtering of specific sperm phenotype) and reactive oxygen species (ROS)‐induced DNA alterations to the Y chromosome (e.g., de novo mutations or epigenetic effects) (Aitken and Krausz 2001). Also, there is no a priori reason to expect a causal relationship between the effects of sperm ageing on sperm quality in fathers and those that generate the paternal environmental effects. Importantly, although we refer to “sperm ageing” in our article, our results may be attributable to the environmentally induced effects on other (nonsperm) components of the ejaculate (e.g., proteins, enzymes, sugars etc. contained within the seminal fluid Poiani 2006; Perry et al. 2013). Clearly, as with other studies that have revealed associations between environmentally induced changes in sperm phenotype and offspring traits (e.g., Crean et al. 2013; Immler et al. 2014; Zajitschek et al. 2014), the next step is to provide a mechanistic understanding of the pathways that account for such covariance. As noted in recent commentaries (Crean et al. 2013; Crean and Bonduriansky 2014; Marshall 2015), the list of potential mechanisms is large and growing, and identifying these remains a major challenge.
Our findings have potentially important implications for studies that use quantitative genetic designs to partition genetic from environmental sources of variance, particularly those based on the phenotypic (co)variance among paternal half siblings. In such designs, the resemblance (covariance) among paternal half‐siblings is assumed to be caused primarily by additive genetic variation in cases where males contribute nothing but sperm (which deliver the sire genetic component) at mating (Falconer and Mackay 1996; Lynch and Walsh 1998). The occurrence of paternal environmental effects mediated exclusively through the ejaculate complicates this assumption by revealing that sperm can be important conduits for nongenetic sources of variance. Given the accumulating (and increasingly widespread) evidence for phenotypic plasticity in a range of ejaculate traits (e.g., due to condition dependence, changes in social environment, etc.; see for example Simmons et al. 2007; Crean and Marshall 2008; Gasparini et al. 2009; Immler et al. 2010; Simmons and Fitzpatrick 2012; Rahman et al. 2014b), the possibility that estimates of additive genetic variance contain a substantial environmental component cannot be ignored (Evans et al. 2015). We see enormous potential for future experiments designed to quantify the impact of such effects, for example by determining the extent to which “additive genetic” variance and covariance is influenced by experimental changes in sperm phenotype.
To our knowledge, our study is the first to draw an explicit link between the length of sperm storage by males and sperm competitive ability, despite prior evidence that sperm storage can compromise in vitro measures of sperm performance (Gasparini et al. 2014; but see Firman et al. 2015). We interpret this finding as a fertilization bias, rather than a bias attributable to variance in embryo viability, as we found no significant effect of sperm age on offspring production (i.e., our fecundity measure) in the noncompetitive fertilization trials. This suggests that sperm ageing has no (or a negligible) effect on embryo viability (similar to that reported in the hide beetle Dermestes maculatus, see Jones and Elgar 2004). These findings implicate sperm competition as a factor generating paternity biases in favor of males delivering fresh sperm. In the context of our current study one can argue that during natural matings the negative effects of sperm storage on sperm velocity may be offset by sperm quantity, as we found that males with long‐sperm storage have also larger sperm reserves (i.e., sperm accumulate in the testes with time, see also below). Although this may be the case in some species, the same is unlikely to apply in guppies, where sexual selection has been shown to favor males with relatively low sperm reserves (Head et al. 2008). Moreover, in guppies, the number of sperm transferred during a mating is known to be under female control (via copula duration, Pilastro et al. 2007) and is not affected by the size of the male's sperm reserves (Pilastro et al. 2002). Thus, the detrimental effect of prolonged sperm storage on sperm velocity is likely to impose an important reproductive fitness cost on males that is unlikely to be offset by the accumulation of higher numbers of sperm.
Given the costs of sperm ageing incurred by males, one might expect them to discard aged sperm periodically, as seen in some mammals, birds, insects, and crustaceans (for a review see Reinhardt 2007). By contrast, the results from the present study showing that males in the aged sperm treatment produced significantly larger ejaculates than those assigned to the fresh sperm treatment suggests that males were not able to discharge old sperm during the four‐week treatment period. Consistent with this idea, previous work on guppies has shown that once mature, sperm are stored in the testicular duct (sperm storage site) for up to 60 days (Billard and Puissant 1969). This suggests that male guppies lack an effective mechanism for discharging aged sperm, at least over the time period chosen for our study. Nevertheless, we cannot exclude the possibility that under natural conditions, males exploit other behavioral strategies (e.g., the repeated use of forced copulations) to expel aged sperm.
We found no significant trans‐generational effects of sperm storage time on female reproductive traits. However, unlike for males, where we were able to use a straightforward assay as a proxy for male reproductive fitness (sperm quality), our assay of reproductive “fitness” for female offspring was limited to body size, which provides a reliable proxy for fecundity in guppies (Evans and Gasparini 2013). We acknowledge that other (unmeasured) traits in female offspring, for example egg quality, may have been affected by the duration of sperm storage. We hope that the present experiment will stimulate the development of assays to reliably assess female reproductive traits in guppies to test for additional trans‐generational fitness consequences of sperm ageing.
In conclusion, our findings indicate that environmental sources of variance influencing sperm phenotype can have important within‐ and trans‐generational fitness consequences. Our results contribute toward an emerging body of literature revealing the deleterious effects of sperm age on sperm phenotype, but go beyond this by revealing direct fitness implications in terms of competitive fertilization success and trans‐generational consequences in terms of offspring fitness. Our results also add to recent studies revealing paternal effects attributable entirely to environmental effects on sperm phenotype (Crean et al. 2013; Zajitschek et al. 2014). Finally, given the increasing awareness that ejaculates exhibit considerable levels of phenotypic plasticity, our findings support the recent assertion that environmentally induced paternal effects may be more general and widespread than anticipated in species where males contribute nothing but ejaculates at reproduction (Crean and Bonduriansky 2014).
C.G. conceived the experiment and analyzed the data with input from J.P.E. C.G. and R.D. conducted the experiment. C.G. and J.P.E. wrote the article. All authors approved the final version of the manuscript for submission.
Supporting information
Figure S1. The number of sperm (mean ± SE) produced by males in the two groups at the end of the experimental treatment.
ACKNOWLEDGMENTS
We thank Cameron Duggin for his help with fish husbandry. This research was funded by a Marie Curie International Outgoing Fellowship within the 7th European Community Framework Programme to C.G. (grant no. 272613).
The authors declare no conflict of interest.
DATA ARCHIVING
Data are available from Figshare (DOI: https://doi.org/10.6084/m9.figshare.4649113).
LITERATURE CITED
- Adriaenssens, B. , van Damme R., Seebacher F., and Wilson R. S.. 2012. Sex cells in changing environments: can organisms adjust the physiological function of gametes to different temperatures? Glob. Change Biol. 18:1797–1803. [Google Scholar]
- Aitken, R. J. , and Krausz C.. 2001. Oxidative stress, DNA damage and the Y chromosome. Reproduction 122:497–506. [DOI] [PubMed] [Google Scholar]
- Almbro, M. , Dowling D. K., and Simmons L. W.. 2011. Effects of vitamin E and beta‐carotene on sperm competitiveness. Ecol. Lett. 14:891–895. [DOI] [PubMed] [Google Scholar]
- Billard, R. , and Puissant C.. 1969. La spermatogenèse de Poecilia reticulata. II.—La production spermatogénétique. Annales de biologie animale, biochimie, biophysique 9:307–313. [Google Scholar]
- Bonduriansky, R. , and Day T.. 2009. Nongenetic inheritance and its evolutionary implications. Annu. Rev. Ecol. Evol. Syst. 40:103–125. [Google Scholar]
- Bonduriansky, R. , and Head M.. 2007. Maternal and paternal condition effects on offspring phenotype in Telostylinus angusticollis (Diptera: Neriidae). J. Evol. Biol. 20:2379–2388. [DOI] [PubMed] [Google Scholar]
- Boschetto, C. , Gasparini C., and Pilastro A.. 2011. Sperm number and velocity affect sperm competition success in the guppy (Poecilia reticulata). Behav. Ecol. Sociobiol. 65:813–821. [Google Scholar]
- Crean, A. J. , and Bonduriansky R.. 2014. What is a paternal effect? Trends Ecol. Evol. 29:554–559. [DOI] [PubMed] [Google Scholar]
- Crean, A. J. , Dwyer J. M., and Marshall D. J.. 2013. Adaptive paternal effects? Experimental evidence that the paternal environment affects offspring performance. Ecology 94:2575–2582. [DOI] [PubMed] [Google Scholar]
- Crean, A. J. , and Marshall D. J.. 2008. Gamete plasticity in a broadcast spawning marine invertebrate. Proc. Natl. Acad. Sci. USA 105:13508–13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley, J. P. , Mashoodh R., and Champagne F. A.. 2011. Epigenetics and the origins of paternal effects. Horm. Behav. 59:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Jack, M. H. , and Lake P. E.. 1966. The effect of resting roosters from ejaculation on the quality of spermatozoa in semen. J. Reprod. Fertil. 11:489–491. [DOI] [PubMed] [Google Scholar]
- Evans, J. P. , and Gasparini C.. 2013. The genetic basis of female multiple mating in a polyandrous livebearing fish. Ecol. Evol. 3:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, J. P. , Kelley J. L., Bisazza A., Finazzo E., and Pilastro A.. 2004. Sire attractiveness influences offspring performance in guppies. Proc. R. Soc. B 271:2035–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, J. P. , Rahman M. M., and Gasparini C.. 2015. Genotype‐by‐environment interactions underlie the expression of pre‐ and post‐copulatory sexually selected traits in guppies. J. Evol. Biol. 28:959–972. [DOI] [PubMed] [Google Scholar]
- Evans, J. P. , Zane L., Francescato S., and Pilastro A.. 2003. Directional postcopulatory sexual selection revealed by artificial insemination. Nature 421:360–363. [DOI] [PubMed] [Google Scholar]
- Falconer, D. S. , and Mackay T. F. C.. 1996. Introduction to quantitative genetics. Longman Group Ltd, London. [Google Scholar]
- Firman, R. C. , Young F. J., Rowe D. C., Duong H. T., and Gasparini C.. 2015. Sexual rest and post‐meiotic sperm ageing in house mice. J. Evol. Biol. 28:1373–1382. [DOI] [PubMed] [Google Scholar]
- Franco, R. , Schoneveld O., Georgakilas A. G., and Panayiotidis M. I.. 2008. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 266:6–11. [DOI] [PubMed] [Google Scholar]
- Froman, D. P. , and Bernier P. E.. 1987. Identification of heritable spermatozoal degeneration within the ductus deferens of the chicken (Gallus domesticus). Biol. Reprod. 37:969–977. [DOI] [PubMed] [Google Scholar]
- Gasparini, C. , Devigili A., Dosselli R., and Pilastro A.. 2013. Pattern of inbreeding depression, condition dependence, and additive genetic variance in Trinidadian guppy ejaculate traits. Ecol. Evol. 3:4940–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini, C. , Kelley J. L., and Evans J. P.. 2014. Male sperm storage compromises sperm motility in guppies. Biol. Lett. 10:20140681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini, C. , Marino I. A. M., Boschetto C., and Pilastro A.. 2010. Effect of male age on sperm traits and sperm competition success in the guppy (Poecilia reticulata). J. Evol. Biol. 23:124–135. [DOI] [PubMed] [Google Scholar]
- Gasparini, C. , Peretti A. V., and Pilastro A.. 2009. Female presence influences sperm velocity in the guppy. Biol. Lett. 5:792–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head, M. L. , Lindholm A. K., and Brooks R.. 2008. Operational sex ratio and density do not affect directional selection on male sexual ornaments and behavior. Evolution 62:135–144. [DOI] [PubMed] [Google Scholar]
- Holt, W. V. , and Lloyd R. E.. 2010. Sperm storage in the vertebrate female reproductive tract: how does it work so well? Theriogenology 73:713–722. [DOI] [PubMed] [Google Scholar]
- Houde, A. E. 1997. Sex, color and mate choice in guppies. Princeton Univ. Press, Princeton. [Google Scholar]
- Immler, S. , Hotzy C., Alavioon G., Petersson E., and Arnqvist G.. 2014. Sperm variation within a single ejaculate affects offspring development in Atlantic salmon. Biol. Lett. 10:20131040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immler, S. , Pryke S. R., Birkhead T. R., and Griffith S. C.. 2010. Pronounced within‐individual plasticity in sperm morphometry across social environments. Evolution 64:1634–1643. [DOI] [PubMed] [Google Scholar]
- Jensen, N. , Allen R. M., and Marshall D. J.. 2014. Adaptive maternal and paternal effects: gamete plasticity in response to parental stress. Funct. Ecol. 28:724–733. [Google Scholar]
- Jones, T. M. , and Elgar M. A.. 2004. The role of male age, sperm age and mating history on fecundity and fertilization success in the hide beetle. Proc. R. Soc. B 271:1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgallon, S. J. , and Simmons L. W.. 2005. Image content influences men's semen quality. Biol. Lett. 1:253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, M. , and Walsh B.. 1998. Genetics and analysis of quantitative traits. Sinauer Associates, Inc, Sunderland. [Google Scholar]
- Marshall, D. J. 2015. Environmentally induced (co) variance in sperm and offspring phenotypes as a source of epigenetic effects. J. Exp. Biol. 218:107–113. [DOI] [PubMed] [Google Scholar]
- Orr, T. J. , and Brennan P. L. R.. 2015. Sperm storage: distinguishing selective processes and evaluating criteria. Trends Ecol. Evol. 30:261–272. [DOI] [PubMed] [Google Scholar]
- Parker, G. A. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45:525–567. [Google Scholar]
- Perry, J. C. , Sirot L., and Wigby S.. 2013. The seminal symphony: how to compose an ejaculate. Trends Ecol. Evol. 28:414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilastro, A. , and Bisazza A.. 1999. Insemination efficiency of two alternative male mating tactics in the guppy (Poecilia reticulata). Proc. R. Soc. B 266:1887–1891. [Google Scholar]
- Pilastro, A. , Evans J. P., Sartorelli S., and Bisazza A.. 2002. Male phenotype predicts insemination success in guppies. Proc. R. Soc. B 269:1325–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilastro, A. , Mandelli M., Gasparini C., Dadda M., and Bisazza A.. 2007. Copulation duration, insemination efficiency and male attractiveness in guppies. Anim. Behav. 74:321–328. [Google Scholar]
- Pizzari, T. , Dean R., Pacey A., Moore H., and Bonsall M. B.. 2008. The evolutionary ecology of pre‐ and post‐meiotic sperm senescence. Trends Ecol. Evol. 23:131–140. [DOI] [PubMed] [Google Scholar]
- Poiani, A. 2006. Complexity of seminal fluid: a review. Behav. Ecol. Sociobiol. 60:289–310. [Google Scholar]
- R Development Core Team . 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Rahman, M. M. , Gasparini C., Turchini G. M., and Evans J. P.. 2014a. Experimental reduction in dietary omega‐3 polyunsaturated fatty acids depresses sperm competitiveness. Biol. Lett. 10:20140623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, M. M. , Turchini G. M., Gasparini C., Norambuena F., and Evans J. P.. 2014b. The expression of pre‐ and postcopulatory sexually selected traits reflects levels of dietary stress in guppies. PLoS One 9:e105856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt, K. 2007. Evolutionary consequences of sperm cell aging. Q. Rev. Biol. 82:375–393. [DOI] [PubMed] [Google Scholar]
- Reinhardt, K. , Dobler R., and Abbott J.. 2015. An ecology of sperm: sperm diversification by natural selection. Annu. Rev. Ecol. Evol. Syst. 46:435–459. [Google Scholar]
- Sheldon, B. C. 2000. Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 15:397–402. [DOI] [PubMed] [Google Scholar]
- Simmons, L. W. , Denholm A., Jackson C., Levy E., and Madon E.. 2007. Male crickets adjust ejaculate quality with both risk and intensity of sperm competition. Biol. Lett. 3:520–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons, L. W. , and Fitzpatrick J. L.. 2012. Sperm wars and the evolution of male fertility. Reproduction 144:519–534. [DOI] [PubMed] [Google Scholar]
- Siva‐Jothy, M. T. 2000. The young sperm gambit. Ecol. Lett. 3:172–174. [Google Scholar]
- Tan, C. K. W. , Pizzari T., and Wigby S.. 2013. Parental age, gametic age, and inbreeding interact to modulate offspring viability in Drosophila melanogaster . Evolution 67:3043–3051. [DOI] [PubMed] [Google Scholar]
- Tarin, J. J. , Perez‐Albala S., and Cano A.. 2000. Consequences on offspring of abnormal function in ageing gametes. Hum. Reprod. Update 6:532–549. [DOI] [PubMed] [Google Scholar]
- Vasudeva, R. , Deeming D. C., and Eady P. E.. 2014. Developmental temperature affects the expression of ejaculatory traits and the outcome of sperm competition in Callosobruchus maculatus . J. Evol. Biol. 27:1811–1818. [DOI] [PubMed] [Google Scholar]
- White, J. , Wagner R. H., Helfenstein F., Hatch S. A., Mulard H., Naves L. C., and Danchin E.. 2008. Multiple deleterious effects of experimentally aged sperm in a monogamous bird. Proc. Natl. Acad. Sci. USA 105:13947–13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajitschek, S. , Hotzy C., Zajitschek F., and Immler S.. 2014. Short‐term variation in sperm competition causes sperm‐mediated epigenetic effects on early offspring performance in the zebrafish. Proc. R. Soc. B 281:20140422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The number of sperm (mean ± SE) produced by males in the two groups at the end of the experimental treatment.


