Abstract
Diabetic nephropathy (DN) is the leading cause of chronic kidney disease in the United States and is a major cause of cardiovascular disease and death. DN develops insidiously over a span of years before clinical manifestations, including microalbuminuria and declining glomerular filtration rate (GFR), are evident. During the clinically silent period, structural lesions develop, including glomerular basement membrane (GBM) thickening, mesangial expansion, and glomerulosclerosis. Once microalbuminuria is clinically apparent, structural lesions are often considerably advanced, and GFR decline may then proceed rapidly toward end-stage kidney disease. Given the current lack of sensitive biomarkers for detecting early DN, a shift in focus toward examining the cellular and molecular basis for the earliest structural change in DN, i.e., GBM thickening, may be warranted. Observed within one to two years following the onset of diabetes, GBM thickening precedes clinically evident albuminuria. In the mature glomerulus, the podocyte is likely key in modifying the GBM, synthesizing and assembling matrix components, both in physiological and pathological states. Podocytes also secrete matrix metalloproteinases, crucial mediators in extracellular matrix turnover. Studies have shown that the critical podocyte-GBM interface is disrupted in the diabetic milieu. Just as healthy podocytes are essential for maintaining the normal GBM structure and function, injured podocytes likely have a fundamental role in upsetting the balance between the GBM’s synthetic and degradative pathways. This article will explore the biological significance of GBM thickening in DN by reviewing what is known about the GBM’s formation, its maintenance during health, and its disruption in DN.
Keywords: podocyte, glomerular filtration barrier, podocyte- glomerular basement membrane interface
diabetic nephropathy (DN) is a common complication of diabetes mellitus and is the leading cause of chronic kidney disease (CKD) in the United States (40). Considered a glomerulopathy, DN is characterized by extracellular matrix (ECM) accumulation in glomeruli, the highly sophisticated organelles that selectively filter circulating blood. Despite widespread use of targeted therapies to lower glucose and to antagonize the renin-angiotensin system (RAS), the prevalence of DN has remained stable (42). As demonstrated by clinical trials, multifactorial interventions can slow, but not stop, the progression of DN (56). Even mild disease is correlated with markedly increased risks of cardiovascular disease and death (5). Defining the mechanisms that underlie development and progression of DN is critical to improving outcomes of diabetic patients and to reducing the societal burden of CKD.
The natural history of DN, as defined in the 1980s based on longitudinal studies of type 1 and type 2 diabetic patients, begins with an initial long clinically silent period during which the patient remains normoalbuminuric with a normal or high glomerular filtration rate (GFR). During this clinically silent phase, structural lesions develop, including glomerular basement membrane (GBM) thickening, mesangial expansion, and glomerulosclerosis. Once microalbuminuria is clinically apparent, structural lesions are often considerably advanced, and GFR decline may then proceed rapidly toward end-stage renal disease (ESRD) (117, 118, 124). Indeed, treatment initiated after the onset of overt DN typically cannot arrest progression.
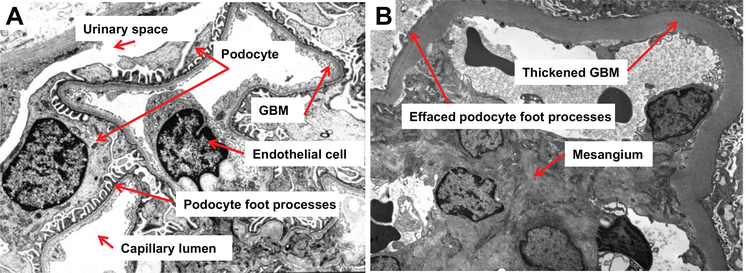
Because albuminuria and declining kidney function are insensitive biomarkers for detecting early DN (124), we have sought to examine the molecular basis for a structural lesion that occurs early in the natural history of DN, GBM thickening. GBM thickening is the earliest detectable feature of DN (132), observed within one to two years following the onset of diabetes, and precedes clinically evident albuminuria (133). Primarily due to expansion of the lamina densa, GBM thickening in diabetes is diffuse and quite uniform (Fig. 1B) (142). Thought to result from an imbalance between the synthesis and degradation of mostly normal ECM constituents, GBM thickening has been reported in patients in the preclinical stage of diabetes, so-called “prediabetes” (99). This phenomenon has also been found in experimental models: GBM thickening in the rhesus monkey is apparent before development of clinically overt diabetes, correlating this lesion with hyperinsulinemia or the prediabetic state (41).
Fig. 1.
Ultrastrnctural images of the glomerular filtration barrier (GFB), normal vs. diabetic. A: electron micrograph of the normal GFB at maturity. The micrograph shows the capillary lumen supported by the fenestrated endothelium, glomerular basement membrane (GBM) proper, and interdigitating foot processes of the visceral glomerular epithelial cells or “podocytes.” B: electron micrograph of the diseased GFB in diabetes mellitus. There is a thickened GBM due to marked widening of the lamina densa, with prominent podocyte foot process effacement. Expansion of the mesangial matrix is also present.
In an effort to identify predictors of DN risk, Caramori et al. analyzed kidney biopsies from 94 normoalbuminuric patients with long-standing type 1 diabetes. In the normoalbuminuric cohort, greater GBM width was the only renal structural parameter that independently predicted progression. GBM width in the range of the “progressors” (567.3 ± 104.1 nm compared with 459.5 ± 85.7 nm for the “nonprogressors”) increased the risk of progression from 17 to 33.3%. Despite long-standing diabetes, none of the type 1 diabetic patients with GBM width within the normal range progressed to proteinuria and/or ESRD (28).
We posit that alterations in normal GBM structure and function constitute a critical pathogenic feature of progressive DN. Expanding our understanding of the cellular and molecular events that lead to GBM thickening may aid in devising early intervention strategies to prevent the progression to irreversible DN. To that end, in this review, we will consider what is known about the GBM’s formation during development, maintenance in health, and disruption in DN. We will end with exploring the possible biological significance of GBM thickening in early DN.
THE GBM: FORMATION AND STRUCTURE
The GBM is a specialized gel-like extracellular network within the glomerulus that lies between and supports two cell types: glomerular endothelial cells (GEnCs) on the inner capillary wall and visceral glomerular epithelial cells or “podocytes” on the outer surface (Fig. 1A) (25). The typical width of the GBM (~300–350 nm) is more than two times the thickness of most other human basement membranes due to fusion of two discrete basement membranes made by GEnCs and podocytes. Ultrastructurally, when examined using conventional tissue preparative methods, the highly cross-linked network of the GBM is organized into three layers: a central electron-dense lamina densa sandwiched between two layers of lower electron density, the lamina rara interna on the endothelial side, and the lamina rara externa on the epithelial side (150). Similar to all basement membranes, the GBM is composed of glycoproteins (laminins, type IV collagen, nidogen) and proteoglycans (agrin, perlecan, type XVIII collagen) (49, 136, 179). However, functionally, the GBM is distinct in that it facilitates continuous flow across the glomerular filtration barrier (GFB) while bearing hemodynamic stresses and supporting glomerular cells (75). Any alterations in its structure are due to changes in the cells it supports, i.e., GEnCs and podocytes.
GBM Formation During Development
The GBM is built via a collective effort of the underlying GEnCs and overlying podocytes (3). Coculture studies have shown that assembly and organization of the GBM’s ECM require cross talk between GEnCs and podocytes, likely mediated by a soluble factor released by podocytes (25). In the developing glomerular capillary loop, ultrastructural studies have identified a quadrilaminar organization of the GBM, with both a subendothelial and subepithelial basement membrane, each composed of its uniquely associated lamina rara and lamina densa, separated by a space between the two basal laminae (2, 107). As development progresses, the quadrilaminar structure matures into a trilaminar basement membrane, thought to occur by fusion of closely apposed basement membrane layers (2, 107). Some studies have suggested that the observed laminae rarae may be artifacts of the fixation and rapid dehydration steps used in conventional processing of tissues for electron microscopy. In these studies, when the GBM is examined after fixation with glutaraldehyde followed by freeze substitution, the laminae rarae are not seen (31, 149). This remains an area of controversy (106), and applying newer techniques to this question may finally clarify the true in vivo substructure of the GBM.
The immature GBM consists of α1α2α1(IV)-collagen, synthesized by both podocytes and GEnCs in early comma and S-shaped stages of glomerulogenesis (113). As the GBM matures, there is protein isoform switching. Beginning at the capillary loop stage, podocytes alone appear to synthesize mature α3α4α5(IV)-collagen, replacing the α1α2α1(IV)-collagen network (4). Both cell types may be responsible for removal and replacement of the immature laminin-α1β1γ1 by the mature laminin-α5β2γ1 (160). After the mature GBM is fully assembled, the biosynthetic programs are downregulated (3).
GBM: A Complex Scaffold of Structural and Regulatory Proteins
Nine major proteins have been found in the GBM. Similar to all basement membranes, the GBM is composed of networks of laminin and type IV collagen, nidogens, and heparan sulfate proteoglycans (HSPGs) (Fig. 2). Distinct from most basement membranes, including the contiguous Bowman’s capsule and tubular basement membranes (112), there is laminin and type IV collagen isoform switching in the GBM during glomerular development and maturation and, possibly, in specific glomerular diseases (3). The GBM’s unusual composition is due, presumably, to its unique functional properties (112).
Fig. 2.
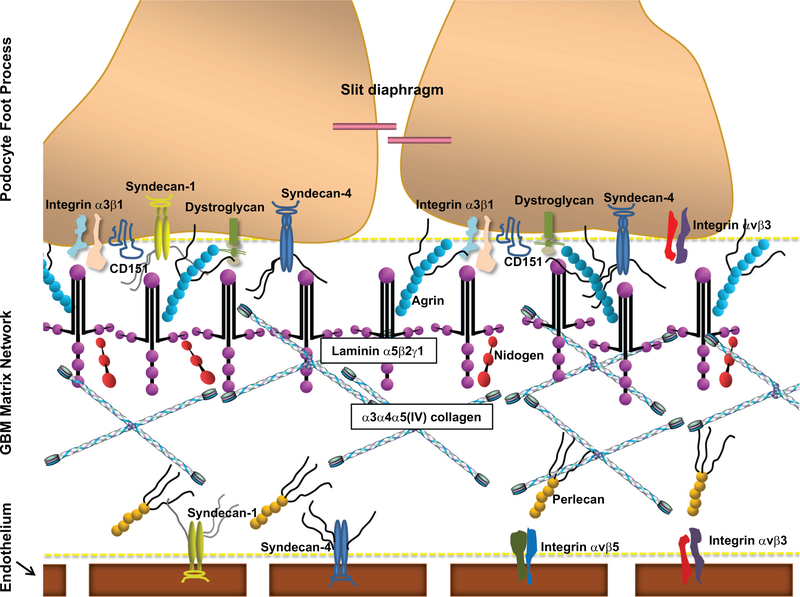
Schematic representation of the critical podocyte-GBM interface. The podocyte-GBM interface forms a signaling platform that controls many cell fate decisions. The GBM matrix is composed of networks of laminin-α5β2γ1, α3α4α5(IV)-collagen, nidogens, and heparan sulfate proteoglycans, including agrin and perlecan. The key cell-matrix adhesion receptor is integrin-α3β1, which connects laminin-α5β2γ1 in the GBM, via various adaptor proteins, to the intracellular actin cytoskeleton. Tetraspanin CD151 strongly binds to integrin-α3β1, promoting strong linkage of podocytes to the GBM. Podocytes also express other cell-matrix adhesion receptors that modulate podocyte adhesion to the GBM, including integrin-αvβ3, dystroglycan, syndecan-1, and syndecan-4. Cell-matrix adhesion receptors on the endothelial surface of the GBM include syndecan-1, syndecan-4, integrin-αvβ3, and integrin-αvβ5.
The matrix of the GBM is a highly structured composite of interacting protein networks (169), recently reviewed in detail (3, 110). Laminin and type IV collagen self-polymerize into networks that are connected to each other and/or to cell-surface receptors by HSPGs and nidogen (196). In addition to structural proteins, the GBM contains regulators (e.g., proteases, growth factors). In a recent proteomic analysis of human glomerular ECM, 144 structural and regulatory proteins were identified, with >50% of them expressed in the GBM (95). Overall, the matrix of the GBM is a unique, highly dynamic, and intricate scaffold of networking proteins (96).
Laminins
Laminin is a large (~800-kDa) heterotrimer of α-, β-, and γ-glycoprotein chains organized into a cruciform structure. Laminin-α5β2γ1 is the major isoform in the normal adult GBM, interacting with type IV collagen via nidogen (3). Mice lacking laminin-β2 exhibit massive proteinuria and die at three to five weeks. Ultrastructurally, in the absence of laminin-β2, the GBM appears disorganized (129). Patients with laminin-β2 gene mutations develop Pierson’s syndrome, characterized by congenital nephrotic syndrome with variable ocular and neurological manifestations (103, 140, 197). In mice lacking laminin-α5, the GBM disintegrates, podocytes become clustered, and GEnCs and mesangial cells fail to establish capillary loops (114). Thus, laminin is an absolute requirement for basement membrane formation.
Collagens
Type IV collagen, the most abundant protein in the GBM, is secreted as a heterotrimer composed of three coiled a-chains. In the mature GBM, the composition of type IV collagen chains is α3α4α5. Grafting experiments, in vivo, have shown that podocytes, but not GEnCs, synthesize the α3α4α5(IV) network (4). Unlike the laminin network, the type IV collagen network is dispensable for basement membrane formation. In the absence of the α3α4α5(IV) network, there is a compensatory increase in the α1α2α1(IV) network. However, the compensation is incomplete, resulting in ultrastructural abnormalities, glomerular scarring, and kidney dysfunction (96). Compared with the α1α2α1(IV) network, the α3α4α5(IV) network may be more highly cross-linked and, therefore, more resistant to proteases, producing greater strength and stability in the GBM architecture (110).
Nidogens
Nidogen-1 and −2 (also known as entactin-1 and −2) were originally considered integral basement membrane proteins that bridged laminin and type IV collagen networks. However, in mice with knockout of either isoform, basement membrane formation and organ development are normal (123, 157). The double-knockout mice appear to develop functioning kidneys, with well-defined GBMs, suggesting that nidogen is dispensable for GBM formation. However, simultaneous deletion of both nidogen-1 and −2 causes perinatal lethality due to pulmonary and cardiac failure (11). Nidogen may function to provide extra stability under situations of extreme stress (110).
Heparan Sulfate Proteoglycans
Proteoglycans consist of protein cores with covalently linked glycosaminoglycan (GAG) side chains. Frequently, these side chains are modified by sulfation, which, along with carboxyl residues (20), imparts a negative charge. Proteoglycans are thought to stabilize the basement membrane by binding laminin, type IV collagen, and nidogen (113) and to contribute to the gel-like properties of the ECM, potentially acting as “anticlogging” agents (81). The two most prominent HSPGs within the GBM are agrin and perlecan. Agrin, derived primarily from podocytes, is the most abundant HSPG at maturity (59, 60) and is present throughout the width of the adult GBM. Perlecan is exclusively present on the endothelial side of the GBM and in the mesangial matrix (61).
A third basement membrane HSPG is type XVIII collagen, a hybrid collagen-proteoglycan (158), which harbors a globular COOH-terminal noncollagenous (NC)-1 domain that contains the fragment endostatin (131). Endostatin, best known for strongly inhibiting angiogenesis and tumor growth by restricting endothelial cell proliferation (131) and migration (192), may be involved in diverse physiological processes (47). By immunogold labeling, type XVIII collagen has been localized to both the endothelial and epithelial sides of the GBM (179). A recent study suggests that type XVIII collagen has a polarized orientation in the GBM: the NH2-terminal collagen XVIII antibody shows staining on both the podocyte and endothelial edges, whereas the COOH-terminal antiendostatin antibody shows gold particle localization within the GBM (87). Indeed, the type XVIII collagen NC1 domain has been shown to bind to perlecan in vivo (115, 156). Following type XVIII collagen total knockout or knockout of the epithelial promoter 2-derived longer variant, there is podocyte foot process effacement, suggesting that type XVIII collagen may play an important role in podocyte-GBM interaction (87).
Based on their known characteristics, HSPGs would be predicted to impede filtration of macromolecules by electrostatic repulsion and steric hindrance (127). Indeed, HSPGs originally were thought to provide the basis for charge selectivity in the GFB. More recently, this concept has been challenged (108, 111). A GBM lacking HSPGS may be more susceptible to increased transcapillary filtration pressure (155). In addition to providing mechanical stability, heparan sulfate moieties sequester heparin-binding growth factors, cytokines, and chemokines, thereby controlling their release and function (181).
THE GBM AND ITS ROLE IN THE GLOMERULAR FILTRATION BARRIER
The GFB, a highly specialized unit responsible for the selective ultrafiltration of blood, is composed of three layers: a fenestrated endothelium with its associated glycocalyx, the GBM, and the filtration slits formed by the interdigitating foot processes of podocytes (Fig. 1A) (119). The GFB allows free permeability to water and small solutes, but prevents the loss of macromolecules and cells from the blood, producing a virtually protein-free primary filtrate. In humans, ~180 liters of primary urine are produced each day at capillary pressures far exceeding those of other organs. Because the interdependent layers of the GFB continuously interact with each other, both mechanically and biochemically, changes in any of them can alter glomerular permeability (172).
The GBM’s Contribution to Size Selectivity
By scanning electron microscopy, the GBM is a highly ordered labyrinth of intersecting polygonal fibrils of varying thickness (4–10 nm), most tightly packed within the core (67, 92). Although the GBM appears to be less selective than the cellular components (64), it is likely that the GBM plays an essential role in the size-selective properties of the GFB. In laminin-β2 knockout mice, the GBM’s disorganization precedes the onset of proteinuria, at a time when the podocytes ultrastructurally appear normal. Furthermore, in tracer studies, laminin-β2-deficient mice show increased GBM permeability, emphasizing that a correctly assembled GBM is vital for establishing the permselectivity of the GFB (75). Behaving more like a gel than a simple filter, the GBM’s size-selective characteristics may be determined by its diffusion properties (159), with the proteoglycan-containing layer acting as a viscous negatively charged screen in front of the lamina densa (127).
The GBM’S Contribution to Charge Selectivity
The charge selectivity of the GFB is highly controversial. For decades, the GFB had been considered to include both size- and charge-selective components (79, 80). Classic studies using tracers of comparable composition and size, but with varying degrees of positive or negative charge, led to the theory of “charge selectivity.” These studies showed that, with decreasing negative charge, the tracers penetrated into, or across, the GFB to a greater degree (22, 32). With their highly sulfated GAG side chains, HSPGs were thought to provide the negative charge that could function as the “charge-selective barrier” (121). However, recent genetic studies have challenged this long-accepted view. Podocyte-specific deletion of agrin results in a significant reduction in the GBM’s negative charge. However, alone or combined with knockout of perlecan, agrin deletion is not associated with permeability defects (57, 65). Similarly, podocyte-specific knockout of Ext1, a gene that encodes an enzyme subunit required for heparan sulfate biosynthesis, yields mild albuminuria that is not statistically different from that seen in control mice (35). Finally, in transgenic mice that widely express human heparanase, an endoglycosidase that strips heparan sulfate side chains from proteoglycans, there is a significant reduction in anionic charge in the GBM without accompanying permselectivity defects (182). These data challenge the premise that heparan sulfate plays a primary role in charge-selective filtration.
THE CRITICAL PODOCYTE-GBM INTERFACE
Podocyte-GBM adhesion is crucial for establishing and maintaining the structural and functional integrity of the GFB. The podocyte-GBM interface (Fig. 2) forms a signaling platform that greatly impacts multiple cell fate decisions, including shape, growth, differentiation, and survival. In addition, the ECM regulates cell-cell signaling by sequestering secreted growth factors and cytokines, acting as a reservoir for controlled release (96). In podocytes, the key cell-matrix adhesion receptor is integrin-α3β1, which connects laminin- α5β2γ1 in the GBM, via various adaptor proteins, to the intracellular actin cytoskeleton. Podocytes also express other cell-matrix adhesion receptors, including integrins- α2β1 and -αvβ3, dystroglycan, syndecans-1 and −4 (146, 155), and type XVII collagen (69).
Integrins
The ECM communicates with cells through transmembrane cell-surface receptors termed integrins (71). Acting as more than simple anchoring points for cell attachment, integrins are crucially involved in the cellular control of ECM deposition (62). Integrins, heterodimeric glycoproteins composed of α- and β-subunits, lack kinase activity and rely on scaffolding proteins and downstream kinases for signal transduction, all of which interact within an adhesion complex (146). Interactions with other transmembrane proteins (e.g., tetraspanins) allow further refinement. Based on cell type and context, over 200 proteins may be recruited to adhesion complexes, highlighting that adhesion signaling may possibly affect many cell fate decisions (96).
Integrin-α3β,1 shows the greatest versatility, binding to collagen, laminin, fibronectin, nidogen, and epiligrin. In human glomeruli, α3β1 has been found on podocytes, mesangial cells, and GEnCs (151). As the most highly expressed integrin on the podocyte’s cell surface (6, 89), α3β1 is found on the basolateral aspect of foot processes and is likely key in mediating the podocyte’s binding to the underlying GBM via laminin-α5β2γ1 (14, 15, 88, 162). In fact, integrin-α3β1 and its associated molecules form the podocyte’s principal adhesion complex, coupling GBM molecules to the podocyte’s actin cytoskeleton (155). This integration of the ECM and cytoskeleton provides physical reinforcement, enabling podocytes to withstand considerable mechanical stress (155).
Gene targeting studies have emphasized the importance of integrins in establishing and maintaining the glomerular capillary wall (3). Integrin-α3 knockout mice die shortly after birth with defects in kidney and lung branching morphogenesis. Glomeruli from these mice have abnormally large capillary loops, thickened and irregular GBMs, and absent podocyte foot processes (90). Similarly, podocyte-specific deletion of integrin-α3 causes severe proteinuria, massive edema, and ESRD by 6 wk of age, with ultrastructural changes including foot process effacement and GBM abnormalities (154). Likewise, children carrying deletion or homozygous missense mutations in integrin-α3 have proteinuria and atrophic glomeruli on biopsy (128). Podocyte-specific knockout of integrin-β1 results in proteinuria at birth, associated with morphological changes (GBM splitting, foot process effacement, podocyte depletion), followed by death at one to three weeks of age from eSrD (78,144). Thus, integrins appear to exert an active role in ECM assembly, regulating the GBM’s structure and permselectivity (168).
Dystroglycan
Dystroglycan is a heavily glycosylated cell receptor comprised of an extracellular a-subunit connected to a transmembrane α-subunit. Expressed at high levels, it is immunolocalized to the apical and basolateral surfaces of podocytes and their foot processes and acts as a receptor for laminin and agrin in the GBM (183). When dystroglycan is deleted from podocytes, or from the entire kidney, there are no discernible glomerular structural or functional phenotypes, and these mice do not exhibit an increased susceptibility to injury (76). These findings suggest that integrins are likely the chief laminin-binding proteins in glomeruli or that integrins can fully substitute for the loss of dystroglycan (3).
Tetraspanins
On plasma membranes, tetraspanins form multimolecular complexes, called tetraspanin-enriched microdomains, which incorporate other transmembrane proteins (e.g., integrins) (19, 66). In vivo, tetraspanin CD151 strongly binds integrin-α3β1 (195) at the base of podocyte foot processes (153, 163). Mutation of CD151 causes hereditary nephritis, characterized by focal GBM thickening and splitting (83). Global, and podocyte-specific, CD151-knockout mice develop GBM abnormalities, foot process effacement, proteinuria, and glomerulosclerosis (13, 153, 154). Likely, CD151 is involved in maintaining and/or reinforcing podocyte adhesion to laminin via integrin-α3β1. In vitro, podocytes without CD151 lose resistance to shear stress (153). Thus, the integrin-α3β1-CD151 complex promotes strong linkage of podocytes to the GBM, maintaining the GFB in high-pressure states (145).
Cell-Surface Proteoglycans
Podocytes express at least three types of cell-surface proteoglycans under physiological conditions: syndecan-1, syndecan-4, and glypican-1 (21, 30, 148). Syndecans are type 1 transmembrane HSPGs that are important in modulating podocyte adhesion to the GBM. When podocytes are unable to assemble heparan sulfate to cell-surface proteoglycan core proteins, cell-matrix interactions are compromised (34, 35). A recent study by Sugar et al. found that podocyte-specific deletion of Wdeacetylase/Wsulfotransferase 1 (NDST1), a key enzyme in the sulfation of heparan sulfate GAGs, disrupts the podocyte’s ability to adhere and properly organize on the underlying GBM. The authors conclude that the lack of N- sulfation negatively affects the lateral aggregation of syndecan-4 (167). Further supporting syndecan-4’s role in podocyte- GBM interactions, syndecan-4 knockout mice develop GBM thickening and FSGS following unilateral nephrectomy (30).
Collagen Type XVII: A Collagenous Transmembrane Protein
Type XVII collagen (also known as BP180 or BPAG2) is best characterized as a hemidesmosomal adhesion component in skin and mucosa (55) and has been identified as an autoantigen in blistering skin diseases, most notably bullous pemphigoid (180). In skin and mucosa, collagen XVII is present at sites of cell-matrix and cell-cell interactions (10, 109, 180). Bullous pemphigoid has been reported to occur in association with glomerular diseases, including anti-GBM disease and membranous nephropathy (68, 141, 152). Ultrastructurally, in normal human glomeruli, collagen XVII can be seen in podocyte foot processes and the adjacent lamina rara externa of the GBM (69). Interestingly, deletion of collagen XVII is associated with podocyte effacement and GBM splitting (69). Although the glomerular binding partners of collagen XVII are not known, its localization at the podocyte-GBM interface suggests that it indeed may also serve a function in cell-matrix interactions (69).
BALANCING GBM MATRIX SYNTHESIS AND DEGRADATION: THE ESSENTIAL ROLE OF THE PODOCYTE
During glomerulogenesis, as glomerular capillaries expand to their final dimensions, newly synthesized segments of basement membrane material emerge underneath developing podocyte foot processes and are somehow integrated in the fused GBM (1). Once fully developed, the mature GBM appears to be a strikingly stable structure. Price and Spiro demonstrated that the in vivo loss of basement membrane protein radioactivity from adult rat GBM collagen occurs at a similar rate as that from tail tendon collagen, with a half-life of >100 days (147). Nevertheless, the mature GBM’s dense network of secreted extracellular constituents requires ongoing turnover, actively remodeling during times of injury and repair (137). In the mature glomerulus, the podocyte is likely key in modifying the GBM, synthesizing and assembling basement membrane components, both in physiological and pathological states (102). By tracking silver deposits, Walker showed that the GBM is generated, slowly but continuously, on the epithelial (podocyte) surface of the glomerular capillary and migrates inward toward the endothelial surface (184). In contrast to the very slow turnover rate of GBM collagen, GBM HSPGs turn over rapidly, with a half-life of hours (17). In fact, in normal rats, the half-life of GBM perlecan is less than three hours. It is suggested that the HSPGs may require continued renewal to prevent clogging of the GFB (8). An increased understanding of the cellular and molecular events that occur during early formation of the glomerular capillary wall may provide important clues into how postdevelopment active remodeling of the GBM takes place, especially as it relates to injury responses and activation of repair pathways.
In addition to their role in the synthesis and assembly of basement membrane components, podocytes also secrete matrix metalloproteinases (MMPs), zinc endopeptidases widely recognized as crucial mediators in ECM turnover (164). Thus, the ability of the podocyte to maintain normal GBM structure and function depends on a delicate balance between synthesizing and degrading pathways. In diabetes mellitus, the podocyte is an early target of injury. Given the vital role of the podocyte in maintaining the GBM, diabetes-induced podocyte injury or “podocytopathy” has the capacity seriously to threaten the integrity of the GBM.
DIABETIC PODOCYTOPATHY: AN EARLY FEATURE OF DN
Far from being fully understood, the mechanisms leading to diabetic podocyte injury are complex and involve interplay between hemodynamic and metabolic factors, including systemic and intraglomerular hypertension, vasoactive hormones, and inflammatory and prosclerotic cytokines. Recent reviews detailing the candidate pathways in the pathogenesis of diabetic podocytopathy (46, 165, 166, 198) underscore that diabetes may activate pathways involving cellular energy production, advanced glycation end products (AGEs), reactive oxygen species, cell cycle regulatory proteins, and ECM homeostasis (54, 82).
An extensive body of work supports the role of the local intrarenal RAS in disrupting normal glomerular autoregulation. Other mediators of defective autoregulation include prostanoids, nitric oxide, atrial natriuretic factor, growth hormone, glucagon, and insulin. The impaired autoregulation leads to glomerular hyperperfusion and hyperfiltration, resulting in elevated intraglomerular pressure (82) and, ultimately, podocyte injury (198). In addition, the locally produced angiotensin II has nonhemodynamic actions, including activation of signaling pathways that lead to ECM remodeling, podocyte apoptosis, and local inflammatory response (46).
At the molecular level, the mammalian target of rapamycin (mTOR) has been recognized as an important mediator of diabetic podocyte injury. Podocyte-specific activation of mTOR complex 1, a kinase that senses nutrient availability, replicates many DN features, including podocyte injury/loss, GBM thickening, and proteinuria, in the absence of hyperglycemia (73). Similarly, podocyte-specific insulin receptor knockout produces DN-like lesions, including GBM thickening, again without systemic hyperglycemia (185). These models emphasize that diabetes is a generalized metabolic disorder, not just a state of chronic hyperglycemia, affecting multiple pathways activated by nutrient excess (172).
It is generally believed that podocytes are terminally differentiated and quiescent cells, with limited regenerative capacity in the face of injury (9, 101). Numerous studies support that podocyte injury, manifested as cell hypertrophy, foot process widening/effacement, and decreased podocyte number (i.e., podocytopenia), is an early feature of DN (133,161,165). Foot process widening/effacement is detectable early in DN, when patients are still normoalbuminuric (138). Furthermore, urinary excretion of nephrin, an important slit diaphragm protein, can be detected in ~1/3 of normoalbuminuric diabetic patients, indicating that early podocyte injury precedes the onset of microalbuminuria (135). Finally, podocytopenia contributes to the progression of DN in patients with both type 1 (161, 187) and type 2 (133) diabetes. Although the cellular and molecular mechanisms of podocytopenia have only been partially characterized (9), podocyte detachment represents one of the earliest features of DN and is a key pathogenic pathway underlying podocyte depletion and progression to glomerulosclerosis. Indeed, podocytes may detach from the GBM due to decreased expression of α3β1-integrin (33), and viable urinary podocytes have been detected in human and experimental DN (125, 126, 139). In addition to detachment, other mechanisms of podocytopenia include apoptosis, with glucose-induced oxidative stress being implicated as an important mediator (171), and hyperglycemia-induced autophagy (9).
GBM DISRUPTION IN DIABETES: THE ROLE OF THE INJURED PODOCYTE
In diabetes, an imbalance between synthesis, controlled by transcription and translation, and degradation, regulated by the interplay between MMPs and their inhibitors [tissue inhibitors of matrix metalloproteinases (TIMPs)], influences the accumulation of ECM proteins in the GBM (100). Regulating the majority of podocyte-expressed genes, the zinc fingers and homeoboxes family of transcriptional factors are likely of major importance in the pathogenesis of GBM thickening in dN (98). Furthermore, podocytes are not just passive targets but, instead, actively mediate continuing glomerular injury (36). Just as healthy podocytes are critical for maintaining the normal GBM structure and function, injured podocytes likely have a key role in upsetting the balance between the synthetic and degradative pathways of the GBM.
Increased Matrix Production in DN
Podocytes synthesize GBM matrix molecules, including type IV collagen, laminin, nidogen, and agrin, and hyperglycemia is known to stimulate the transcription of matrix genes. In cell culture, upon exposure to high glucose, injured podocytes adopt a “promatrix phenotype,” with increased synthesis of new type IV collagen chains (12, 58, 72). In addition, there is strong in vivo evidence that GBM matrix production increases early after the onset of persistent hyperglycemia (24, 37, 105). Furthermore, hyperglycemia may induce expression of transforming growth factor-β (TGF-β). This increase in TGF-β can stimulate ECM synthesis by the podocyte itself in an autocrine manner. Other pathways implicated in hyperglycemia-induced ECM production by podocytes include the mitogen-activated protein kinase pathway and the 12-lipoxygenase pathway of arachidonic acid metabolism (198).
Dysregulated Matrix Turnover in DN
The GBM is continuously remodeled through the enzymatic actions of secreted proteases (155). The balance of MMPs and TIMPs determines ECM integrity. In the kidney, the spatial expression of MMPs and TIMPs is complex and has not been completely characterized. MMP-2, −3, −9, −13, and −14 and TIMP-1 are expressed in glomeruli (29). There is accumulating evidence that hyperglycemia and/or other metabolic derangements in diabetes not only enhance the synthesis of ECM proteins but also suppress ECM degradation. A number of animal studies have demonstrated a link between aberrant MMP expression and DN progression (29). Because podocytes both produce GBM components and secrete matrix-degrading proteinases, podocyte injury resulting in abnormal MMP expression may also critically play a role in GBM thickening in DN.
Matrix Metalloproteinases
The MMPs, synthesized as inactive zymogens, are activated via proteolytic cleavage of the propeptides by trypsin, plasmin, or other MMPs. Traditionally, MMPs are classified according to their structures and/or ECM substrate specificities. For example, the gelatinases (MMP2, MMP9) cleave denatured collagen (gelatin) and type IV collagen in basement membranes (175).
As recently reviewed (29, 177, 191), the evidence supporting a role for MMPs in the pathogenesis of DN is conflicting. Initially, MMPs were thought to be globally protective through their proteolytic potential. However, growing evidence indicates that MMPs are also involved in inflammation and tissue fibrosis in kidney disease. It is increasingly clear that MMPs cleave a wide variety of substrates, ranging from cell-surface receptors and adhesion molecules (e.g., cadherins, integrins) to growth factors and cytokines (e.g., TGF-β, fibroblast growth factor receptor 1). This extensive array of substrates enables MMPs not only to regulate ECM remodeling but also many cell behaviors, including proliferation, migration, differentiation, and apoptosis (175). Indeed, MMPs can both promote and inhibit inflammation. Degrading ECM proteins may be valuable to reduce matrix accumulation; however, degraded products of the ECM may not be biologically inactive. Thus, greater ECM degradation by MMPs may not always translate into diminished fibrosis in DN (175).
Role of the gelatinases MMP2 and MMP9 in DN.
As a MMP subfamily, gelatinases MMP2 and MMP9 play an important role in ECM homeostasis and remodeling. However, conflicting results have been reported regarding their expression level and activity in DN. There are reports that MMP2 expression and activity are decreased in human and experimental DN (43, 63,170,190). Because MMP2 is responsible for degradation of type IV collagen and laminin, this reported decrease has been suggested to contribute to ECM accumulation and eventual glomerulosclerosis. However, more recently, studies indicate that the converse may be true: that MMP2 activity is higher in the serum and urine of type 1 diabetic patients, accompanied by increased expression and activity in kidneys (45, 176). MMP2 upregulation has also been reported in experimental DN (143). Whether this upregulation is protective or detrimental has been debated. A study using chronic blockade with a nonspecific MMP2 inhibitor in a diabetic rat model reported reduced proteinuria and attenuated structural changes. The authors concluded that blocking MMP2 may also block the downstream release of TGF-β from the large latent form bound to the ECM (188). However, others have proposed that MMP2 may play a protective role against progressive diabetic kidney injury. Diabetic MMP2 knockout mice exhibit increased serum blood urea nitrogen/creatinine, albuminuria, and renal structural injury, with enhanced ECM accumulation in glomeruli (173). Further studies are needed clearly to define the role of MMP2 in DN.
Despite earlier studies suggesting otherwise, there is increasing evidence that glomerular MMP9 expression and activity are enhanced in DN and that MMP9 suppression, either by genetic defect (97) or via pharmacological means (193, 194), results in attenuation of albuminuria, glomerular hyperfiltration, and structural abnormalities, including GBM thickening. Likewise, when podocytes in culture are incubated with diabetes-related cytokines (e.g., TGF-β1, tumor necrosis factor-a, vascular endothelial growth factor), MMP9 activity is upregu- lated. This increased MMP9 activity is associated with podocyte dedifferentiation and enhanced synthesis of new ECM. Finally, DN patients have higher urinary MMP9 concentrations than healthy controls, and an upregulation of plasma MMP9 is observed in diabetic patients before the onset of microalbuminuria (29). MMP9 may be key in self-propagating inflammation by producing collagen fragments that attract and stimulate neutrophils to release more MMP9. In addition, neutrophils coexpress MMP9 with neutrophil gelatinase-associated lipocalin (NGAL) (178), a factor recently hailed as a biomarker of kidney injury (116). Urinary excretion of NGAL increases in parallel with MMP9, and the interaction between NGAL and mMp9 may further prolong the activity of MMP9 (178). Thus, MMP9 has a pivotal, and likely maladaptive, role in the development of DN. Chronic MMP9 activation creates a less compact and progressively thickened GBM (97).
Interestingly, endostatin, the antiangiogenic type XVIII collagen fragment, directly inhibits the activation of pro-MMP2 and pro-MMP9 in vitro (86, 93, 130). Furthermore, the regulatory pathways between endostatin and MMPs appear to be bidirectional. In addition to the inhibitory role endostatin may play in the regulation of MMPs, MMPs may also be involved in the in vivo production of endostatin, generating endostatin fragments from proteolysis of type XVIII collagen (47, 51). Thus, it has become increasingly clear that further studies are needed to define how the actions of endostatin and MMPs may intersect at the podocyte-GBM interface in the diabetic milieu.
Tissue Inhibitors of Matrix Metalloproteinases
MMPs are regulated by a family of endogenous inhibitors known as TIMPs. The four known TIMPs (1–4) have varying specificities for different MMPs but together can inhibit all of them (175). In diabetic patients, studies have found increased expression and activity of TIMP-1, −2, and −3, with urinary TIMP1 levels correlating with increased albuminuria (29). Suggesting an early compensatory role, TIMP3, the most highly expressed TIMP in the kidney (84), is upregulated in both experimental (16) and human (48, 189) dN. In diabetic mouse models, TIMP3 knockout results in significantly increased mean glomerular area, GBM thickening, and albuminuria (16, 52). Thus, loss of TIMP3 leads to exacerbation of diabetic kidney injury.
Other Mediators That Contribute to Decreased Matrix Turnover
Hyperglycemia can trigger AGE generation. These modified proteins may accumulate because of their decreased susceptibility to enzymatic hydrolysis by MMPs (122). Furthermore, glycation of ECM proteins stiffens the GBM (113). The podocyte appears to be a specific target of AGE actions, since receptors for AGE (RAGE) are highly expressed on podocytes (186). RAGE null mice have no kidney phenotype. However, diabetic RAGE null mice have decreased proteinuria and less GBM thickening and mesangial matrix expansion compared with that seen in diabetic wild-type mice (94). These results suggest that AGE/RAGE signaling contributes to diabetic podocyte injury (120).
GBM THICKENING AS A MANIFESTATION OF DIABETIC PODOCYTE INJURY
Since 1959, when Farquhar et al. described a biopsy series of seven diabetic patients with thickening of the basement membrane proper as one of the earliest manifestations of DN (50), the significance of GBM thickening in the pathogenesis of DN has been appreciated and investigated. Several quantitative and qualitative biochemical alterations of the GBM occur in DN. The GBM’s structure may be markedly altered by an imbalance between ECM synthesis and degradation, nonenzymatic glycosylation, change in spatial distribution of some components (e.g., type IV collagen) across the GBM (18, 44, 74), and nonspecific trapping of serum proteins (7). In the early stages of DN, there are also obvious changes in the podocyte-GBM interface. In experimental and human DN, decreased podocyte expression of α3β1-integrin has been described. Experimentally, loss of α3β1-integrin occurs within 1 mo of diabetes induction and is persistent (33, 85, 151). A significant alteration of the podocyte’s most important integrin, and particularly in the domain facing the GBM, likely interferes with normal ECM deposition, leading to marked changes in GBM structure and function (151).
There are conflicting data on the alterations of HSPGs by diabetes. Some studies have described increased enzymatic degradation of heparan sulfate in diabetic kidneys from patients and experimental models (134, 174). Furthermore, high ambient glucose increases the expression of heparanase in podocytes (77). Studies have also shown decreases in the extent of sulfation in diabetic kidneys (23, 38, 39). A recent study proposes that undersulfation of heparan sulfate in diabetic kidneys may be due to changes in NDST1 level and/or activity (167). However, in other studies examining renal biopsies from patients with microalbuminuria and type 1 diabetes, glomerular staining for heparan sulfate was not different between control and diabetic specimens. Whether changes in heparan sulfate expression, structure, or sulfation play a role in the pathogenesis of DN remains unclear (198).
GBM THICKENING IN DIABETES: ADAPTIVE OR MALADAPTIVE?
Although metabolic perturbations are clear prerequisites of GBM thickening in diabetes, the pathogenesis of this process is incompletely understood (105). GBM thickening can occur in long-standing diabetes without concomitant albuminuria, suggesting that GBM structural changes are not the primary mechanism of albuminuria (26, 27, 53). In fact, in patients who develop DN, GBM thickening may precede the onset of albuminuria (133). Moreover, following pancreas transplantation in diabetic rats, the urinary excretion of albumin returns to normal.(104) This supports that the GBM, thickened by diabetes, may function normally in the animal “cured of hyperglycemia” (105) Thus, at the early stages of experimental and human DN, increased GFB permeability to protein cannot be explained by GBM thickening alone. In fact, it remains unclear what role GBM thickening plays in renal functional abnormalities. One could argue that GBM thickening in diabetes may be the injured podocyte’s response to stress, aimed at ensuring that the kidney meets its essential function of producing a relatively protein-free ultrafiltrate.
When podocytes are injured in diabetes, a primary component of the GFB, the podocyte, is structurally altered and functionally compromised. In response to the threat of filtration barrier malfunction, the podocyte may adapt by activating a series of cell signaling pathways that ultimately will increase synthesis of GBM components and lead to GBM thickening, thus preventing widespread leakiness of the filtration barrier. In the short term, the trade off for decreasing albumin leak via GBM thickening may be a loss of filtration capacity. Over time, with continued exposure to injurious stimuli, a process that began as adaptive may become maladaptive. Mechanical properties of the GBM matrix (i.e., stiffness, deformability) provide inputs into podocyte cell behavior (70). An undamaged GBM is necessary for proper adhesion and function of podocytes (119). Thus, GBM structural changes may reduce cell binding and promote podocyte detachment (91). Eventually, conditions that weaken the GBM or increase the transcapillary filtration pressure intensify the mechanical stress experienced by podocytes. Podocytes then respond by depositing additional GBM matrix in an attempt to resist the applied force and prevent further detachment. Inevitably, a vicious cycle ensues whereby the GBM becomes progressively disorganized, with subsequent barrier function failure, leading to disease progression (155).
CONCLUSION
GBM thickening is the earliest detectable morphological feature of DN and may be observed within one to two years following the onset of diabetes, preceding clinically evident albuminuria. Affixed to the underlying GBM via transmembrane cell receptors, the podocyte is likely a key culprit in GBM thickening in diabetes, responsible for both increased matrix production and dysregulated degradation/turnover. Thus, GBM thickening in diabetes may be a manifestation of subtle podocyte injury, representing the injured cell’s response to stress and appearing even before cell detachment, apoptosis, and albuminuria. A more complete understanding of the signaling events that underlie GBM thickening in diabetes will permit intervention before GBM thickening becomes maladaptive, thereby blocking progression to clinically overt DN.
ACKNOWLEDGMENTS
I thank Drs. Paul W. Sanders, Anupam Agarwal, and Sumant S. Chugh for helpful suggestions during the preparation of the manuscript and Dr. Huma Fatima for providing the electron microscopy images.
GRANTS
This work was supported by Career Development Award-2 No. 5IK2BX001942 fromthe U.S. Department ofVeterans Affairs, the Biomedical Laboratory Research and Development Program, to C. B. Marshall.
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
REFERENCES
- 1.Abrahamson DR. Development of kidney glomerular endothelial cells and their role in basement membrane assembly. Organogenesis 5: 275287, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrahamson DR. Glomerulogenesis in the developing kidney. Semin Nephrol 11: 375–389, 1991. [PubMed] [Google Scholar]
- 3.Abrahamson DR. Role of the podocyte (and glomerular endothelium) in building the GBM. Semin Nephrol 32: 342–349, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrahamson DR. Hudson BG, Stroganova L, Borza DB, St. John PL Cellular origins of type IV collagen networks in developing glomeruli. J Am Soc Nephrol 20: 1471–1479, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, Ukpds G. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63: 225–232, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Adler S Characterization of glomerular epithelial cell matrix receptors. Am J Pathol 141: 571–578, 1992. [PMC free article] [PubMed] [Google Scholar]
- 7.Adler S Structure-function relationships associated with extracellular matrix alterations in diabetic glomerulopathy. J Am Soc Nephrol 5: 1165–1172, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Akuffo EL, Hunt JR, Moss J, Woodrow D, Davies M, Mason RM. steady-state labelling approach to the measurement of proteoglycan turnover in vivo and its application to glomerular proteoglycans. Biochem J 320: 301–308, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anil Kumar P, Welsh GI, Saleem MA, Menon RK. Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front Endocrinol (Lausanne) 5: 151, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aumailley M, Has C, Tunggal L, Bruckner-Tuderman L. Molecular basis of inherited skin-blistering disorders, and therapeutic implications. Expert Rev Mol Med 8: 1–21, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Bader BL, Smyth N, Nedbal S, Miosge N, Baranowsky A, Mokkapati S, Murshed M, Nischt R. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol Cell Biol 25: 6846–6856, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai Y, Wang L, Li Y, Liu S, Li J, Wang H, Huang H. High ambient glucose levels modulates the production of MMP-9 and alpha5(IV) collagen by cultured podocytes. Cell Physiol Biochem 17: 57–68, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Baleato RM, Guthrie PL, Gubler MC, Ashman LK, Roselli S. Deletion of CD151 results in a strain-dependent glomerular disease due to severe alterations of the glomerular basement membrane. Am J Pathol 173: 927–937, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baraldi A, Furci L, Zambruno G, Rubbiani E, Annessi G, Lusvarghi E. Very late activation-3 integrin is the dominant beta 1-integrin on the glomerular capillary wall: an immunofluorescence study in nephrotic syndrome. Nephron 62: 382–388, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Baraldi A, Zambruno G, Furci L, Manca V, Vaschieri C, Lusvarghi E. Beta-1 integrins in the normal human glomerular capillary wall: an immunoelectron microscopy study. Nephron 66: 295–301, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Basu R, Lee J, Wang Z, Patel VB, Fan D, Das SK, Liu GC, John R, Scholey JW, Oudit GY, Kassiri Z. Loss of TIMP3 selectively exacerbates diabetic nephropathy. Am J Physiol Renal Physiol 303: F1341–F1352, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beavan LA, Davies M, Couchman JR, Williams MA, Mason RM. In vivo turnover of the basement membrane and other heparan sulfate proteoglycans of rat glomerulus. Arch Biochem Biophys 269: 576–585, 1989. [DOI] [PubMed] [Google Scholar]
- 18.Bendayan M Alteration in the distribution of type IV collagen in glomerular basal laminae in diabetic rats as revealed by immunocyto- chemistry and morphometrical approach. Diabetologia 28: 373–378, 1985. [DOI] [PubMed] [Google Scholar]
- 19.Berditchevski F Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci 114: 4143–4151, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Bertolatus JA, Hunsicker LG. Polycation binding to glomerular basement membrane. Effect of biochemical modification. Lab Invest 56: 170–179, 1987. [PubMed] [Google Scholar]
- 21.Bjornson Granqvist A, Ebefors K, Saleem MA, Mathieson PW, Haraldsson B, Nystrom JS. Podocyte proteoglycan synthesis is involved in the development of nephrotic syndrome. Am J Physiol Renal Physiol 291: F722–F730, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Bohrer MP, Baylis C, Humes HD, Glassock RJ, Robertson CR, Brenner BM. Permselectivity of the glomerular capillary wall. Facilitated filtration of circulating polycations. J Clin Invest 61: 72–78, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown DM, Klein DJ, Michael AF, Oegema TR. 35S-glycosamino- glycan and 35S-glycopeptide metabolism by diabetic glomeruli and aorta. Diabetes 31: 418–25, 1982. [DOI] [PubMed] [Google Scholar]
- 24.Brownlee M, Spiro RG. Glomerular basement membrane metabolism in the diabetic rat. In vivo studies. Diabetes 28: 121–125, 1979. [DOI] [PubMed] [Google Scholar]
- 25.Byron A, Randles MJ, Humphries JD, Mironov A, Hamidi H, Harris S, Mathieson PW, Saleem MA, Satchell SC, Zent R, Humphries MJ, Lennon R. Glomerular cell cross-talk influences composition and assembly of extracellular matrix. J Am Soc Nephrol 25: 953–966, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes 52: 1036–1040, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Caramori ML, Kim Y, Huang C, Fish AJ, Rich SS, Miller ME, Russell G, Mauer M. Cellular basis of diabetic nephropathy. 1. Study design and renal structural-functional relationships in patients with longstanding type 1 diabetes. Diabetes 51: 506–513, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Caramori ML, Parks A, Mauer M. Renal lesions predict progression of diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol 24: 11751181, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catania JM, Chen G, Parrish AR. Role of matrix metalloproteinases in renal pathophysiologies. Am J Physiol Renal Physiol 292: F905–F911, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Cevikbas F, Schaefer L, Uhlig P, Robenek H, Theilmeier G, Echtermeyer F, Bruckner P. Unilateral nephrectomy leads to up-regulation of syndecan-2- and TGF-beta-mediated glomerulosclerosis in syndecan-4 deficient male mice. Matrix Biol 27: 42–52, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Chan FL, Inoue S, Leblond CP. The basement membranes of cryofixed or aldehyde-fixed, freeze-substituted tissues are composed of a lamina densa and do not contain a lamina lucida. Cell Tissue Res 273: 41–52, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Chang RL, Deen WM, Robertson CR, Brenner BM. Permselectivity of the glomerular capillary wall. III. Restricted transport of polyanions. Kidney Int 8: 212–218, 1975. [DOI] [PubMed] [Google Scholar]
- 33.Chen HC, Chen CA, Guh JY, Chang JM, Shin SJ, Lai YH. Altering expression of alpha3beta1 integrin on podocytes of human and rats with diabetes. Life Sci 67: 2345–2353, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Chen S, Wassenhove-McCarthy D, Yamaguchi Y, Holzman L, van Kuppevelt TH, Orr AW, Funk S, Woods A, McCarthy K. Podocytes require the engagement of cell surface heparan sulfate proteoglycans for adhesion to extracellular matrices. Kidney Int 78: 1088–1099, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S, Wassenhove-McCarthy DJ, Yamaguchi Y, Holzman LB, van Kuppevelt TH, Jenniskens GJ, Wijnhoven TJ, Woods AC, McCarthy KJ. Loss of heparan sulfate glycosaminoglycan assembly in podo- cytes does not lead to proteinuria. Kidney Int 74: 289–299, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng H, Harris RC. The glomerulus-a view from the outside-the podocyte. Int J Biochem Cell Biol 42: 1380–1387, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen MP, Klein CV. Glomerulopathy in rats with streptozotocin diabetes. Accumulation of glomerular basement membrane analogous to human diabetic nephropathy. J Exp Med 149: 623–631, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen MP, Klepser H, Wu VY. Undersulfation of glomerular basement membrane heparan sulfate in experimental diabetes and lack of correction with aldose reductase inhibition. Diabetes 37: 1324–1327, 1988. [DOI] [PubMed] [Google Scholar]
- 39.Cohen MP, Surma ML. [(35)S]sulfate incorporation into glomerular basement membrane glycosaminoglycans is decreased in experimental diabetes. J Lab Clin Med 98: 715–722, 1981. [PubMed] [Google Scholar]
- 40.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 59: A7, e1–e420, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Cusumano AM, Bodkin NL, Hansen BC, Iotti R, Owens J, Klotman PE, Kopp JB. Glomerular hypertrophy is associated with hyperinsulin- emia and precedes overt diabetes in aging rhesus monkeys. Am J Kidney Dis 40: 1075–1085, 2002. [DOI] [PubMed] [Google Scholar]
- 42.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb Temporal trends in the prevalence of diabetic kidney disease in the United States. J Am Med Assoc 305: 2532–2539, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Del Prete D, Anglani F, Forino M, Ceol M, Fioretto P, Nosadini R, Baggio B, Gambaro G. Down-regulation of glomerular matrix metalloproteinase-2 gene in human NIDDM. Diabetologia 40: 1449–1454,1997. [DOI] [PubMed] [Google Scholar]
- 44.Desjardins M, Bendayan M. Ultrastructural distribution of glomerular basement membrane components in experimental diabetes. Diabetes Res 14: 65–73, 1990. [PubMed] [Google Scholar]
- 45.Diamant M, Hanemaaijer R, Verheijen JH, Smit JW, Radder JK, Lemkes HH. Elevated matrix metalloproteinase-2 and −9 in urine, but not in serum, are markers of type 1 diabetic nephropathy. Diabet Med 18: 423–424, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Diez-Sampedro A, Lenz O, Fornoni A. Podocytopathy in diabetes: a metabolic and endocrine disorder. Am J Kidney Dis 58: 637–646, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Digtyar AV, Pozdnyakova NV, Feldman NB, Lutsenko SV, Severin SE. Endostatin: current concepts about its biological role and mechanisms of action. Biochemistry (Mosc) 72: 235–246, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Ewens KG, George RA, Sharma K, Ziyadeh FN, Spielman RS. Assessment of 115 candidate genes for diabetic nephropathy by transmission/disequilibrium test. Diabetes 54: 3305–3318, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Farquhar MG. The glomerular basement membrane: not gone, just forgotten. J Clin Invest 116: 2090–2093, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farquhar MG, Hopper J Jr, Moon HD. Diabetic glomerulosclerosis: electron and light microscopic studies. Am J Pathol 35: 721–753, 1959. [PMC free article] [PubMed] [Google Scholar]
- 51.Ferreras M, Felbor U, Lenhard T, Olsen BR, Delaisse J. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett 486: 247–251, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Fiorentino L, Cavalera M, Menini S, Marchetti V, Mavilio M, Fabrizi M, Conserva F, Casagrande V, Menghini R, Pontrelli P, Arisi I, D’Onofrio M, Lauro D, Khokha R, Accili D, Pugliese G, Gesualdo L, Lauro R, Federici M. Loss of TIMP3 underlies diabetic nephropathy via FoxO1/STAT1 interplay. EMBO Mol Med 5: 441–455, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fioretto P, Steffes MW, Mauer M. Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes 43: 1358–1364, 1994. [DOI] [PubMed] [Google Scholar]
- 54.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev 93: 137–188, 2013. [DOI] [PubMed] [Google Scholar]
- 55.Franzke CW, Tasanen K, Schumann H, Bruckner-Tuderman L. Collagenous transmembrane proteins: collagen XVII as a prototype. Matrix Biol 22: 299–309, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358: 580–591, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Goldberg S, Harvey SJ, Cunningham J, Tryggvason K, Miner JH. Glomerular filtration is normal in the absence of both agrin and perlecan- heparan sulfate from the glomerular basement membrane. Nephrol Dial Transplant 24: 2044–2051, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greka A, Mundel P. Cell biology and pathology of podocytes. Ann Rev Physiol 74: 299–323, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Groffen AJ, Ruegg MA, Dijkman H, van de Velden TJ, Buskens CA, van den Born J, Assmann KJ, Monnens LA, Veerkamp JH, van den Heuvel LP. Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane. J Histochem Cytochem 46: 19–27, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Groffen AJ, Veerkamp JH, Monnens LA, van den Heuvel LP. Recent insights into the structure and functions of heparan sulfate proteoglycans in the human glomerular basement membrane. Nephrol Dial Transplant 14: 2119–2129, 1999. [DOI] [PubMed] [Google Scholar]
- 61.Gubler MC. Inherited diseases of the glomerular basement membrane. Nat Clin Pract Nephrol 4: 24–37, 2008. [DOI] [PubMed] [Google Scholar]
- 62.Guo L, Sanders PW, Woods A, Wu C. The distribution and regulation of integrin-linked kinase in normal and diabetic kidneys. Am J Pathol 159: 1735–1742, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han SY, Jee YH, Han KH, Kang YS, Kim HK, Han JY, Kim YS, Cha DR. An imbalance between matrix metalloproteinase-2 and tissue inhibitor of matrix metalloproteinase-2 contributes to the development of early diabetic nephropathy. Nephrol Dial Transplant 21: 2406–2416, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88: 451–487, 2008. [DOI] [PubMed] [Google Scholar]
- 65.Harvey SJ, Jarad G, Cunningham J, Rops AL, van der Vlag J, Berden JH, Moeller MJ, Holzman LB, Burgess RW, Miner JH. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol 171: 139–152, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 6: 801–811, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Hironaka K, Makino H, Yamasaki Y, Ota Z. Pores in the glomerular basement membrane revealed by ultrahigh-resolution scanning electron microscopy. Nephron 64: 647–649, 1993. [DOI] [PubMed] [Google Scholar]
- 68.Hoorn EJ, Taams NE, Hurskainen T, Salih M, Weening JJ, Jonkman MF, Pas HH, Schreurs MW. Bullous Pemphigoid With a Dual Pattern of Glomerular Immune Complex Disease. Am J Kidney Dis 67: 302–306, 2016. [DOI] [PubMed] [Google Scholar]
- 69.Hurskainen T, Moilanen J, Sormunen R, Franzke CW, Soininen R, Loeffek S, Huilaja L, Nuutinen M, Bruckner-Tuderman L, Autio-Harmainen H, Tasanen K. Transmembrane collagen XVII is a novel component of the glomerular filtration barrier. Cell Tissue Res 348: 579–588, 2012. [DOI] [PubMed] [Google Scholar]
- 70.Hynes RO. The extracellular matrix: not just pretty fibrils. Science 326: 1216–1219, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002. [DOI] [PubMed] [Google Scholar]
- 72.Iglesias-de la Cruz MC, Ziyadeh FN, Isono M, Kouahou M, Han DC, Kalluri R, Mundel P, Chen S. Effects of high glucose and TGF-beta1 on the expression of collagen IV and vascular endothelial growth factor in mouse podocytes. Kidney Int 62: 901–913, 2002. [DOI] [PubMed] [Google Scholar]
- 73.Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Ruegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 121: 2181–2196, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Inoue S, Bendayan M. High-resolution ultrastructural study of the rat glomerular basement membrane in aminonucleoside nephrosis. Ultra- struct Pathol 20: 409–416, 1996. [DOI] [PubMed] [Google Scholar]
- 75.Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities inLamb2—/— mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest 116: 2272–2279, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jarad G, Pippin JW, Shankland SJ, Kreidberg JA, Miner JH. Dystroglycan does not contribute significantly to kidney development or function, in health or after injury. Am J Physiol Renal Physiol 300: F811–F820, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int 74: 22–36, 2008. [DOI] [PubMed] [Google Scholar]
- 78.Kanasaki K, Kanda Y, Palmsten K, Tanjore H, Lee SB, Lebleu VS, Gattone VH Jr, Kalluri R. Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol 313: 584–593, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanwar YS. Continuum of historical controversies regarding the structural-functional relationship of the glomerular ultrafiltration unit. Am J Physiol Renal Physiol 308: F420–F424, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kanwar YS, Danesh FR, Chugh SS. Contribution of proteoglycans towards the integrated functions of renal glomerular capillaries: a historical perspective. Am J Pathol 171: 9–13, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanwar YS, Rosenzweig LJ. Clogging of the glomerular basement membrane. J Cell Biol 93: 489–494, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol 6: 395–423, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karamatic Crew V, Burton N, Kagan A, Green CA, Levene C, Flinter F, Brady RL, Daniels G, Anstee DJ. CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assembly of human basement membranes in kidney and skin. Blood 104: 2217–2223, 2004. [DOI] [PubMed] [Google Scholar]
- 84.Kassiri Z, Oudit GY, Kandalam V, Awad A, Wang X, Ziou X, Maeda N, Herzenberg AM, Scholey JW. Loss of TIMP3 enhances interstitial nephritis and fibrosis. J Am Soc Nephrol 20: 1223–1235, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kemeny E, Mihatsch MJ, Durmuller U, Gudat F. Podocytes loose their adhesive phenotype in focal segmental glomerulosclerosis. Clin Nephrol 43: 71–83, 1995. [PubMed] [Google Scholar]
- 86.Kim YM, Jang JW, Lee OH, Yeon J, Choi EY, Kim KW, Lee ST, Kwon YG. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res 60: 5410–5413, 2000. [PubMed] [Google Scholar]
- 87.Kinnunen AI, Sormunen R, Elamaa H, Seppinen L, Miller RT, Ninomiya Y, Janmey PA, Pihlajaniemi T. Lack of collagen XVIII long isoforms affects kidney podocytes, whereas the short form is needed in the proximal tubular basement membrane. J Biol Chem 286: 7755–7764, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Korhonen M, Ylanne J, Laitinen L, Cooper HM, Quaranta V, Virtanen I. Distribution of the alpha 1-alpha 6 integrin subunits in human developing and term placenta. Lab Invest 65: 347–356, 1991. [PubMed] [Google Scholar]
- 89.Korhonen M, Ylanne J, Laitinen L, Virtanen I. Distribution of beta 1 and beta 3 integrins in human fetal and adult kidney. Lab Invest 62: 616–625, 1990. [PubMed] [Google Scholar]
- 90.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 122: 3537–3547, 1996. [DOI] [PubMed] [Google Scholar]
- 91.Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV. response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol 304: F333–F347, 2013. [DOI] [PubMed] [Google Scholar]
- 92.Kubosawa H, Kondo Y. Ultrastructural organization of the glomerular basement membrane as revealed by a deep-etch replica method. Cell Tissue Res 242: 33–39, 1985. [DOI] [PubMed] [Google Scholar]
- 93.Lee SJ, Jang JW, Kim YM, Lee HI, Jeon JY, Kwon YG, Lee ST. Endostatin binds to the catalytic domain of matrix metalloproteinase-2. FEBS Lett 519: 147–152, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Leeuwis JW, Nguyen TQ, Dendooven A, Kok RJ, Goldschmeding R. Targeting podocyte-associated diseases. Adv Drug Deliv Rev 62: 13251336, 2010. [DOI] [PubMed] [Google Scholar]
- 95.Lennon R, Byron A, Humphries JD, Randles MJ, Carisey A, Murphy S, Knight D, Brenchley PE, Zent R, Humphries MJ. Global analysis reveals the complexity of the human glomerular extracellular matrix. J Am Soc Nephrol 25: 939–951, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lennon R, Randles MJ, Humphries MJ. The importance of podocyte adhesion for a healthy glomerulus. Front Endocrinol (Lausanne) 5: 160, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li SY, Huang PH, Yang AH, Tarng DC, Yang WC, Lin CC, Chen JW, Schmid-Schonbein G, Lin SJ. Matrix metalloproteinase-9 deficiency attenuates diabetic nephropathy by modulation of podocyte functions and dedifferentiation. Kidney Int 86: 358–369, 2014. [DOI] [PubMed] [Google Scholar]
- 98.Liu G, Clement LC, Kanwar YS, Avila-Casado C, Chugh SS. ZHX proteins regulate podocyte gene expression during the development of nephrotic syndrome. J Biol Chem 281: 39681–39692, 2006. [DOI] [PubMed] [Google Scholar]
- 99.Mac-Moune Lai F, Szeto CC, Choi PC, Ho KK, Tang NL, Chow KM, Li PK, To KF. Isolate diffuse thickening of glomerular capillary basement membrane: a renal lesion in prediabetes? Mod Pathol 17: 15061512, 2004. [DOI] [PubMed] [Google Scholar]
- 100.Mariappan MM. Signaling mechanisms in the regulation of renal matrix metabolism in diabetes. Exp Diabetes Res 2012: 749812, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marshall CB, Shankland SJ. Cell cycle regulatory proteins in podocyte health and disease. Nephron Exp Nephrol 106: e51–e59, 2007. [DOI] [PubMed] [Google Scholar]
- 102.Marshall SM. The podocyte: a potential therapeutic target in diabetic nephropathy? Curr Pharm Des 13: 2713–2720, 2007. [DOI] [PubMed] [Google Scholar]
- 103.Matejas V, Hinkes B, Alkandari F, Al-Gazali L, Annexstad E, Aytac MB, Barrow M, Blahova K, Bockenhauer D, Cheong HI, Maruniak- Chudek I, Cochat P, Dotsch J, Gajjar P, Hennekam RC, Janssen F, Kagan M, Kariminejad A, Kemper MJ, Koenig J, Kogan J, Kroes HY, Kuwertz-Broking E, Lewanda AF, Medeira A, Muscheites J, Niaudet P, Pierson M, Saggar A, Seaver L, Suri M, Tsygin A, Wuhl E, Zurowska A, Uebe S, Hildebrandt F, Antignac C, Zenker M. Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum. Hum Mutat 31: 992–1002, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mauer SM, Brown DM, Matas AJ, Steffes MW. Effects of pancreatic islet transplantation on the increased urinary albumin excretion rates in intact and uninephrectomized rats with diabetes mellitus. Diabetes 27: 959–964, 1978. [DOI] [PubMed] [Google Scholar]
- 105.Mauer SM, Steffes MW, Brown DM. The kidney in diabetes. Am J Med 70: 603–612, 1981. [DOI] [PubMed] [Google Scholar]
- 106.McAdams AJ. Glomerular capillary wall basement membrane really does have laminae lucidae: A defense. Pediatr Dev Pathol 2: 260–263, 1999. [DOI] [PubMed] [Google Scholar]
- 107.McCarthy KJ. Morphogenesis of the glomerular filter: the synchronous assembly and maturation of two distinct extracellular matrices. Microsc Res Tech 39: 233–253, 1997. [DOI] [PubMed] [Google Scholar]
- 108.McCarthy KJ, Wassenhove-McCarthy DJ. The glomerular basement membrane as a model system to study the bioactivity of heparan sulfate glycosaminoglycans. Microsc Microanal 18: 3–21, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mihai S, Sitaru C. Immunopathology and molecular diagnosis of autoimmune bullous diseases. J Cell Mol Med 11: 462–481, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miner JH. The glomerular basement membrane. Exp Cell Res 318: 973–978, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miner JH. Glomerular filtration: the charge debate charges ahead. Kidney Int 74: 259–261, 2008. [DOI] [PubMed] [Google Scholar]
- 112.Miner JH. Organogenesis of the kidney glomerulus: focus on the glomerular basement membrane. Organogenesis 7: 75–82, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miner JH. Renal basement membrane components. Kidney Int 56: 2016–2024, 1999. [DOI] [PubMed] [Google Scholar]
- 114.Miner JH, Li C. Defective glomernlogenesis in the absence of laminin alpha5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol 217: 278–289, 2000. [DOI] [PubMed] [Google Scholar]
- 115.Miosge N, Simniok T, Sprysch P, Herken R. The collagen type XVIII endostatin domain is co-localized with perlecan in basement membranes in vivo. J Histochem Cytochem 51: 285–296, 2003. [DOI] [PubMed] [Google Scholar]
- 116.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipoca- lin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003. [DOI] [PubMed] [Google Scholar]
- 117.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med 310: 356–360, 1984. [DOI] [PubMed] [Google Scholar]
- 118.Mogensen CE. Microalbuminuria, blood pressure and diabetic renal disease: origin and development of ideas. Diabetologia 42: 263–285, 1999. [DOI] [PubMed] [Google Scholar]
- 119.Moller CC, Pollak MR, Reiser J. The genetic basis of human glomerular disease. Adv Chronic Kidney Dis 13: 166–173, 2006. [DOI] [PubMed] [Google Scholar]
- 120.Mora-Fernandez C, Dominguez-Pimentel V, de Fuentes MM, Gorriz JL, Martinez-Castelao A, Navarro-Gonzalez JF. Diabetic kidney disease: from physiology to therapeutics. J Physiol 592: 3997–4012, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morita H, Yoshimura A, Kimata K. The role of heparan sulfate in the glomerular basement membrane. Kidney Int 73: 247–248, 2008. [DOI] [PubMed] [Google Scholar]
- 122.Mott JD, Khalifah RG, Nagase H, Shield CF 3rd, Hudson JK, Hudson BG. Nonenzymatic glycation of type IV collagen and matrix metalloproteinase susceptibility. Kidney Int 52: 1302–1312, 1997. [DOI] [PubMed] [Google Scholar]
- 123.Murshed M, Smyth N, Miosge N, Karolat J, Krieg T, Paulsson M, Nischt R. The absence of nidogen 1 does not affect murine basement membrane formation. Mol Cell Biol 20: 7007–7012, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Najafian B, Mauer M. Morphologic features of declining renal function in type 1 diabetes. Semin Nephrol 32: 415–422, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakamura T, Ushiyama C, Shimada N, Sekizuka K, Ebihara I, Hara M, Koide H. Effect of the antiplatelet drug dilazep dihydrochloride on urinary podocytes in patients in the early stage of diabetic nephropathy. Diabetes Care 23: 1168–1171, 2000. [DOI] [PubMed] [Google Scholar]
- 126.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Ebihara I, Koide H. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant 15: 1379–1383, 2000. [DOI] [PubMed] [Google Scholar]
- 127.Nerlich A, Schleicher E. Immunohistochemical localization of extracellular matrix components in human diabetic glomerular lesions. Am J Pathol 139: 889–899, 1991. [PMC free article] [PubMed] [Google Scholar]
- 128.Nicolaou N, Margadant C, Kevelam SH, Lilien MR, Oosterveld MJ, Kreft M, van Eerde AM, Pfundt R, Terhal PA, van der Zwaag B, Nikkels PG, Sachs N, Goldschmeding R, Knoers NV, Renkema KY, Sonnenberg A. Gain of glycosylation in integrin alpha3 causes lung disease and nephrotic syndrome. J Clin Invest 122: 4375–4387, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP. The renal glomerulus of mice lacking s-laminin/laminin beta 2: nephrosis despite molecular compensation by laminin beta 1. Nat Genet 10: 400–06, 1995. [DOI] [PubMed] [Google Scholar]
- 130.Nyberg P, Heikkila P, Sorsa T, Luostarinen J, Heljasvaara R, Stenman UH, Pihlajaniemi T, Salo T. Endostatin inhibits human tongue carcinoma cell invasion and intravasation and blocks the activation of matrix metalloprotease-2, −9, and −13. J Biol Chem 278: 2240422411, 2003. [DOI] [PubMed] [Google Scholar]
- 131.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88: 277–285, 1997. [DOI] [PubMed] [Google Scholar]
- 132.Osterby R, Gundersen HJ. Glomerular size and structure in diabetes mellitus. I. Early abnormalities. Diabetologia 11: 225–229, 1975. [DOI] [PubMed] [Google Scholar]
- 133.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Parthasarathy N, Spiro RG. Effect of diabetes on the glycosaminogly- can component of the human glomerular basement membrane. Diabetes 31: 738–741, 1982. [DOI] [PubMed] [Google Scholar]
- 135.Patari A, Forsblom C, Havana M, Taipale H, Groop PH, Holthofer H. Nephrinuria in diabetic nephropathy of type 1 diabetes. Diabetes 52: 2969–2974, 2003. [DOI] [PubMed] [Google Scholar]
- 136.Patrakka J, Tryggvason K. Molecular make-up of the glomerular filtration barrier. Biochem Biophys Res Commun 396: 164–169, 2010. [DOI] [PubMed] [Google Scholar]
- 137.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003. [DOI] [PubMed] [Google Scholar]
- 138.Perrin NE, Torbjornsdotter TB, Jaremko GA, Berg UB. The course of diabetic glomerulopathy in patients with type I diabetes: a 6-year follow-up with serial biopsies. Kidney Int 69: 699–705, 2006. [DOI] [PubMed] [Google Scholar]
- 139.Petermann AT, Pippin J, Krofft R, Blonski M, Griffin S, Durvasula R, Shankland SJ. Viable podocytes detach in experimental diabetic nephropathy: potential mechanism underlying glomerulosclerosis. Nephron Exp Nephrol 98: e114–e123, 2004. [DOI] [PubMed] [Google Scholar]
- 140.Pierson M, Cordier J, Hervouuet F, Rauber G. An unusual congenital and familial congenital malformative combination involving the eye and kidney. J Genet Hum 12: 184–213, 1963. [PubMed] [Google Scholar]
- 141.Plaisier E, Borradori L, Hellmark T, Wattiaux MJ, Flageul B, Mougenot B, Ronco P. Anti-glomerular basement membrane nephritis and bullous pemphigoid caused by distinct anti-alpha 3(IV)NC1 and anti-BP180 antibodies in a patient with Crohn’s disease. Am J Kidney Dis 40: 649–654, 2002. [DOI] [PubMed] [Google Scholar]
- 142.Ponchiardi C, Mauer M, Najafian B. Temporal profile of diabetic nephropathy pathologic changes. Curr Diab Rep 13: 592–599, 2013. [DOI] [PubMed] [Google Scholar]
- 143.Portik-Dobos V, Harris AK, Song W, Hutchinson J, Johnson MH, Imig JD, Pollock DM, Ergul A. Endothelin antagonism prevents early EGFR transactivation but not increased matrix metalloproteinase activity in diabetes. Am J Physiol Regul Integr Comp Physiol 290: R435–R441, 2006. [DOI] [PubMed] [Google Scholar]
- 144.Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, Holzman LB, Phillips CL, Fassler R, Quaggin SE, Miner JH, Zent R. Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol 316: 288–301, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pozzi A, Zent R. Hold tight or you’ll fall off: CD151 helps podocytes stick in high-pressure situations. J Clin Invest 122: 13–16, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pozzi A, Zent R. Integrins in kidney disease. J Am Soc Nephrol 24: 1034–1039, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Price RG, Spiro RG. Studies on the metabolism of the renal glomerular basement membrane. Turnover measurements in the rat with the use of radiolabeled amino acids. J Biol Chem 252: 8597–8602, 1977. [PubMed] [Google Scholar]
- 148.Pyke C, Kristensen P, Ostergaard PB, Oturai PS, Romer J. Proteoglycan expression in the normal rat kidney. Nephron 77: 461–470, 1997. [DOI] [PubMed] [Google Scholar]
- 149.Reale E, Luciano L. The laminae rarae of the glomerular basement membrane. Their manifestation depends on the histochemical and histological techniques. Contrib Nephrol 80: 32–40, 1990. [PubMed] [Google Scholar]
- 150.Reeves WH, Kanwar YS, Farquhar MG. Assembly of the glomerular filtration surface. Differentiation of anionic sites in glomerular capillaries of newborn rat kidney. J Cell Biol 85: 735–753, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Regoli M, Bendayan M. Alterations in the expression of the alpha 3 beta 1 integrin in certain membrane domains of the glomerular epithelial cells (podocytes) in diabetes mellitus. Diabetologia 40: 15–22, 1997. [DOI] [PubMed] [Google Scholar]
- 152.Ross EA, Ahmed AR. Bullous pemphigoid-associated nephropathy: report of two cases and review of the literature. Am J Kidney Dis 14: 225–229, 1989. [DOI] [PubMed] [Google Scholar]
- 153.Sachs N, Claessen N, Aten J, Kreft M, Teske GJ, Koeman A, Zuurbier CJ, Janssen H, Sonnenberg A. Blood pressure influences end-stage renal disease of Cd151 knockout mice. J Clin Invest 122: 348–358, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, Sonnenberg A Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol 175: 33–39, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sachs N, Sonnenberg A. Cell-matrix adhesion of podocytes in physiology and disease. Nat Rev Nephrol 9: 200–210, 2013. [DOI] [PubMed] [Google Scholar]
- 156.Sasaki T, Fukai N, Mann K, Gohring W, Olsen BR, Timpl R. Structure, function and tissue forms of the C-terminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin. EMBO J 17: 4249–256, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schymeinsky J, Nedbal S, Miosge N, Poschl E, Rao C, Beier DR, Skarnes WC, Timpl R, Bader BL. Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol Cell Biol 22: 6820–6830, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Seppinen L, Pihlajaniemi T. The multiple functions of collagen XVIII in development and disease. Matrix Biol 30: 83–92, 2011. [DOI] [PubMed] [Google Scholar]
- 159.Smithies O Why the kidney glomerulus does not clog: a gel permeation/ diffusion hypothesis of renal function. Proc Natl Acad Sci USA 100: 4108–4113, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.St. John PL, Abrahamson DR. Glomerular endothelial cells and podo- cytes jointly synthesize laminin-1 and −11 chains. Kidney Int 60: 10371046, 2001. [DOI] [PubMed] [Google Scholar]
- 161.Steffes MW, Schmidt D, McCrery R, Basgen JM, International Diabetic Nephropathy Study Group. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int 59: 2104–2113, 2001. [DOI] [PubMed] [Google Scholar]
- 162.Sterk LM, de Melker AA, Kramer D, Kuikman I, Chand A, Claessen N, Weening JJ, Sonnenberg A. Glomerular extracellular matrix components and integrins. Cell Adhes Commun 5: 177–192, 1998. [DOI] [PubMed] [Google Scholar]
- 163.Sterk LM, Geuijen CA, van den Berg JG, Claessen N, Weening JJ, Sonnenberg A. Association of the tetraspanin CD151 with the lamininbinding integrins alpha3beta1, alpha6beta1, alpha6beta4 and alpha7beta1 in cells in culture and in vivo. J Cell Sci 115: 1161–1173, 2002. [DOI] [PubMed] [Google Scholar]
- 164.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17: 463–516, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stieger N, Worthmann K, Schiffer M. The role of metabolic and haemodynamic factors in podocyte injury in diabetes. Diabetes Metab ResRev 27: 207–215, 2011. [DOI] [PubMed] [Google Scholar]
- 166.Stitt-Cavanagh E, MacLeod L, Kennedy C. The podocyte in diabetic kidney disease. Sci World J 9: 1127–1139, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sugar T, Wassenhove-McCarthy DJ, Orr AW, Green J, van Kup- pevelt TH, McCarthy KJ. N-sulfation of heparan sulfate is critical for syndecan-4 mediated podocyte cell-matrix interactions. Am J Physiol Renal Physiol 310: F1123–F1135, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Suh JH, Miner JH. The glomerular basement membrane as a barrier to albumin. Nat Rev Nephrol 9: 470–77, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Suleiman H, Zhang L, Roth R, Heuser JE, Miner JH, Shaw AS, Dani A. Nanoscale protein architecture of the kidney glomerular basement membrane. Elife 2: e01149, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sun SZ, Wang Y, Li Q, Tian YJ, Liu MH, Yu YH. Effects of benazepril on renal function and kidney expression of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in diabetic rats. Chin Med J (Engl) 119: 814–821, 2006. [PubMed] [Google Scholar]
- 171.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006. [PubMed] [Google Scholar]
- 172.Sward P, Rippe B. Acute and sustained actions of hyperglycaemia on endothelial and glomerular barrier permeability. Acta Physiol (Oxf) 204: 294–307, 2012. [DOI] [PubMed] [Google Scholar]
- 173.Takamiya Y, Fukami K, Yamagishi S, Kaida Y, Nakayama Y, Obara N, Iwatani R, Ando R, Koike K, Matsui T, Nishino Y, Ueda S, Cooper ME, Okuda S. Experimental diabetic nephropathy is accelerated in matrix metalloproteinase-2 knockout mice. Nephrol Dial Transplant 28: 55–62, 2013. [DOI] [PubMed] [Google Scholar]
- 174.Tamsma JT, van den Born J, Bruijn JA, Assmann KJ, Weening JJ, Berden JH, Wieslander J, Schrama E, Hermans J, Veerkamp JH. Expression of glomerular extracellular matrix components in human diabetic nephropathy: decrease of heparan sulphate in the glomerular basement membrane. Diabetologia 37: 313–320, 1994. [DOI] [PubMed] [Google Scholar]
- 175.Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol 302: F1351–F1361, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Thrailkill KM, Bunn RC, Moreau CS, Cockrell GE, Simpson PM, Coleman HN, Frindik JP, Kemp SF, Fowlkes JL. Matrix metalloproteinase-2 dysregulation in type 1 diabetes. Diabetes Care 30: 2321–2326, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Thrailkill KM, Clay Bunn R, Fowlkes JL. Matrix metalloproteinases: their potential role in the pathogenesis of diabetic nephropathy. Endocrine 35: 1–10, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tschesche H, Zolzer V, Triebel S, Bartsch S. The human neutrophil lipocalin supports the allosteric activation of matrix metalloproteinases. Eur J Biochem 268: 1918–1928, 2001. [DOI] [PubMed] [Google Scholar]
- 179.Utriainen A, Sormunen R, Kettunen M, Carvalhaes LS, Sajanti E, Eklund L, Kauppinen R, Kitten GT, Pihlajaniemi T. Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Hum Mol Genet 13: 2089–2099, 2004. [DOI] [PubMed] [Google Scholar]
- 180.Van den Bergh F, Giudice GJ. BP180 (type XVII collagen) and its role in cutaneous biology and disease. Adv Dermatol 19: 37–71, 2003. [PubMed] [Google Scholar]
- 181.van den Hoven MJ, Rops AL, Vlodavsky I, Levidiotis V, Berden JH, van der Vlag J. Heparanase in glomerular diseases. Kidney Int 72: 543–548, 2007. [DOI] [PubMed] [Google Scholar]
- 182.van den Hoven MJ, Wijnhoven TJ, Li JP, Zcharia E, Dijkman HB, Wismans RG, Rops AL, Lensen JF, van den Heuvel LP, van Kup- pevelt TH, Vlodavsky I, Berden JH, van der Vlag J. Reduction of anionic sites in the glomerular basement membrane by heparanase does not lead to proteinuria. Kidney Int 73: 278–287, 2008. [DOI] [PubMed] [Google Scholar]
- 183.Vogtlander NP, Dijkman H, Bakker MA, Campbell KP, van der Vlag J, Berden JH. Localization of alpha-dystroglycan on the podocyte: from top to toe. J Histochem Cytochem 53: 1345–1353, 2005. [DOI] [PubMed] [Google Scholar]
- 184.Walker F The origin, turnover and removal of glomerular basementmembrane. J Pathol 110: 233–244, 1973. [DOI] [PubMed] [Google Scholar]
- 185.Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstadt H, Tavare JM, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, Coward RJ. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab 12: 329–340, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, Stein G, Bierhaus A, Liliensiek B, Arnold B, Nawroth PP, Stern DM, D’Agati VD, Schmidt AM. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol 162: 1123–1137, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.White KE, Bilous RW, Marshall SM, El Nahas M, Remuzzi G, Piras G, De Cosmo S, Viberti G. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes 51: 3083–3089, 2002. [DOI] [PubMed] [Google Scholar]
- 188.Williams JM, Zhang J, North P, Lacy S, Yakes M, Dahly-Vernon A, Roman RJ. Evaluation of metalloprotease inhibitors on hypertension and diabetic nephropathy. Am J Physiol Renal Physiol 300: F983–F998, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K. Transcriptome analysis of human diabetic kidney disease. Diabetes 60: 2354–2369, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wu K, Setty S, Mauer SM, Killen P, Nagase H, Michael AF, Tsilibary EC. Altered kidney matrix gene expression in early stages of experimental diabetes. Acta Anat (Basel) 158: 155–165, 1997. [DOI] [PubMed] [Google Scholar]
- 191.Xu X, Xiao L, Xiao P, Yang S, Chen G, Liu F, Kanwar YS, Sun L. A glimpse of matrix metalloproteinases in diabetic nephropathy. Curr Med Chem 21: 3244–3260, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yamaguchi N, Anand-Apte B, Lee M, Sasaki T, Fukai N, Shapiro R, Que I, Lowik C, Timpl R, Olsen BR. Endostatin inhibits VEGF- induced endothelial cell migration and tumor growth independently of zinc binding. EMBO J 18: 4414–4423, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yao XM, Ye SD, Chen Y, Zai ZM, Li XC, Wang YX, Chen K. Rosiglitazone protects diabetic rats against kidney injury through the suppression of renal matrix metalloproteinase-9 expression. Diabetes Obes Metab 11: 519–522, 2009. [DOI] [PubMed] [Google Scholar]
- 194.Yao XM, Ye SD, Zai Z, Chen Y, Li XC, Yang GW, Wang YX, Chen K. Simvastatin protects diabetic rats against kidney injury through the suppression of renal matrix metalloproteinase-9 expression. J Endocrinol Invest 33: 292–296, 2010. [DOI] [PubMed] [Google Scholar]
- 195.Yauch RL, Berditchevski F, Harler MB, Reichner J, Hemler ME. Highly stoichiometric, stable, and specific association of integrin alpha3beta1 with CD151 provides a major link to phosphatidylinositol 4-kinase, and may regulate cell migration. Mol Biol Cell 9: 2751–2765, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yurchenco PD, O’Rear JJ. Basement membrane assembly. Methods Enzymol 245: 489–518, 1994. [DOI] [PubMed] [Google Scholar]
- 197.Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wuhl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dotsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13: 2625–2632, 2004. [DOI] [PubMed] [Google Scholar]
- 198.Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev 4: 39–45, 2008. [DOI] [PubMed] [Google Scholar]