Abstract
Introduction
Most pregnant smokers report abruptly reducing their cigarettes per day (CPD) by ~50% after learning of pregnancy and making further smaller reductions over the remainder of their pregnancy. Laboratory and naturalistic studies with non-pregnant smokers have found that these types of reductions often lead to changes in smoking topography (i.e., changes in smoking intensity to maintain a desired blood-nicotine level). If pregnant women smoke more intensely, they may expose themselves and their offspring to similar levels of toxicants despite reporting reductions in CPD.
Methods
Pregnant and non-pregnant female smokers (n = 20 and 89, respectively) participated. At the experimental session, after biochemical confirmation of acute abstinence, participants smoked one usual brand cigarette ad lib through a Borgwaldt CReSS Desktop Smoking Topography device. Carbon monoxide (CO) and measures of nicotine withdrawal, craving, and reinforcement derived from smoking were also collected.
Results
The two groups did not differ on demographic or smoking characteristics at screening, except nicotine metabolism rate, which as expected, was faster in pregnant smokers. Analyses suggest that none of the smoking topography parameters differed between pregnant and non-pregnant smokers, although pregnant smokers had a significantly smaller CO boost. Both groups reported similar levels of relief of withdrawal and craving after smoking, but other subjective effects suggest that pregnant smokers find smoking less reinforcing than non-pregnant smokers.
Conclusions
Pregnant smokers do not smoke cigarettes differently than non-pregnant women, but appear to find smoking comparatively less pleasurable.
Implications
This is the first study to assess smoking topography in pregnant women. Pregnant women appear to be at increased risk for smoking cigarettes with more intensity because of (1) their tendency to make significant abrupt reductions in the number of cigarettes they smoke each day after learning of pregnancy and (2) an increase in nicotine metabolism induced by pregnancy. Despite these changes, the present results suggest that pregnant women do not smoke cigarettes more intensely or in a way that causes more toxicant exposure, perhaps due to a reportedly less pleasurable smoking experience.
Introduction
Maternal cigarette smoking is the leading preventable cause of poor pregnancy outcomes.1 Nevertheless, approximately 15% of pregnant women are regular cigarette smokers. Fifty percent reductions in cigarettes per day (CPD) in early pregnancy have been reliably reported across many studies.2–6 In one study, pregnant women self-reported that the bulk of this change in smoking rate takes place within the first few days after learning of their pregnancy.7 Surprisingly, this reduction in CPD occurs despite a pregnancy-induced increase in the metabolism of nicotine.8,9 In non-pregnant populations of smokers, higher rates of nicotine metabolism are associated with smoking more CPD.10
While pregnant smokers report making reductions in CPD to reduce harm to their offspring,11 previous research suggests that self-reported reductions may not correspond with decreased toxicant exposure. For example, self-reported reductions in CPD among pregnant smokers enrolled in clinical trials for smoking cessation are not reliably accompanied by corresponding reductions in biochemical markers of smoke exposure.12 Indeed, despite reporting a one-third reduction in CPD between 10 and 14 weeks gestation, urine cotinine levels among pregnant smokers in the Heil et al. study decreased by only 10% and carbon monoxide (CO) levels remained unchanged. Correlations between CPD and biochemical markers tend to vary widely among pregnant smokers, with a range across reports of 0.32–0.74 and a median of 0.44.13–17
One commonly cited potential explanation for these variations in the relationship between nicotine exposure and CPD is that pregnant smokers change their smoking topography (e.g., increase the number of puffs per cigarette, the duration of each puff, the volume of each puff, etc.) in an effort to maintain the same blood-nicotine level despite smoking fewer CPD.13–18 To our knowledge, there are no prior reports examining smoking topography among pregnant smokers. In laboratory and naturalistic studies with non-pregnant smokers involving similar discrepancies between reductions in CPD and corresponding reductions in smoking biomarkers, discrepancies have been attributed to increases in smoking intensity.19,20 If pregnant women smoke cigarettes more intensely, they may inadvertently continue to expose themselves and their offspring to relatively high levels of toxicants despite making reductions in CPD.
The present study compared the smoking topography of usual brand cigarettes in pregnant and non-pregnant female smokers currently smoking approximately the same number of CPD. If pregnant smokers were trying to sustain pre-pregnancy nicotine levels, they would be expected to evidence a pattern of more intensive smoking (e.g., larger puff volumes) compared to non-pregnant women who smoke at a comparable daily rate. Women of lower socioeconomic status (SES) were of particular interest in the present study because socioeconomically disadvantaged women are at increased risk for (1) smoking, (2) nicotine dependence, (3) smoking more CPD, (4) smoking higher nicotine yield cigarettes and (5) continuing to smoke after becoming pregnant and thus would be expected to be at relatively high risk for increased smoking intensity.21,22 Measures of withdrawal, craving and reinforcement were also collected to examine mechanisms that may influence smoking topography in these two groups.
Methods
Participants and Inclusion/Exclusion Criteria
Pregnant and non-pregnant smokers were recruited via ads on Facebook, Craigslist, and in local newspapers and with flyers posted on community bulletin boards between March 2015 and November 2016. Pregnant participants were also recruited from OB/GYN clinics. All potential participants completed a brief phone screen and those who appeared eligible were invited to attend an in-person screening session to determine final eligibility. After providing informed consent, participants submitted breath samples (Micro+ Smokerlyzer; coVita/Bedfont, Haddonfield, NJ) and urine samples (NicAlert cotinine test strip; Nymox, Hasbrouck Heights, NJ) to verify smoking status. Urine was tested to determine pregnancy status, to test for illicit drug use, and to quantify cotinine levels via enzyme immunoassay technique (MGC240; Microgenics, Fremont, CA). Additionally, participants provided saliva samples which were analyzed for cotinine and trans-3’-hydroxycotinine (3-HC), the major metabolite of cotinine. 3-HC was divided by cotinine to calculate a nicotine metabolite ratio (NMR), which is strongly correlated with nicotine clearance.23 Saliva was analyzed by liquid chromatography mass spectrometry.
Next, potential participants completed sociodemographic (e.g., age, race/ethnicity, education, marital status, etc.) and medical history questionnaires developed in our laboratory and filled out a series of standardized questionnaires, including the Fagerstrӧm Test for Nicotine Dependence and the Mini International Neuropsychiatric Interview (MINI).24–27
Eligible non-pregnant participants had to self-report smoking at least 5 CPD for the past year and have an intake breath CO sample >8 ppm. There was no minimum CPD or breath CO level for the pregnant participants. Rather, smoking status was confirmed among pregnant participants with a urine cotinine value >100 ng/ml (>2 on NicAlert strip). Educational attainment served as a proxy for SES. As such, all participants had to have less than an Associate’s degree. Individuals were excluded if they reported exclusively rolling their own cigarettes, using other tobacco or nicotine products more than 9 days in the last 30, intentions to quit in the next 7 days if pregnant and 30 days if non-pregnant, or any smoking cessation product use in the last 30 days. All participants were without a current serious mental disorder and could not test positive for illicit drug use, except for THC. Opioid-dependent pregnant and non-pregnant women who were stable in opioid agonist maintenance treatment were eligible. All potential participants were compensated $50 for completing the screening session.
Procedures
If deemed eligible, participants were invited back for an experimental session. The session took place in a private room located in a suite specifically built for indoor smoking. Participants were instructed to abstain from smoking prior to the session for at least 6 hours and had to meet at least a 50% reduction in their screening breath CO level in order to begin the experimental session; this criterion is widely used as a marker of acute abstinence in smoking research.28–30 If CO values were >50% of screening CO values, the session was rescheduled and they were instructed to abstain for a longer period of time before their next scheduled session. After abstinence was confirmed, all participants took two puffs from their usual brand cigarette to standardize the time since each participant last smoked.31,32 After taking these two puffs but before smoking a full cigarette, participants completed the Minnesota Nicotine Withdrawal Scale (MNWS) and the Questionnaire of Smoking Urges - Brief (QSU-Brief). Thirty minutes after taking two puffs, participants smoked one usual brand cigarette through a CReSS Desktop smoking topography device (Borgwaldt, Richmond, VA) with no instruction (i.e., ad libitum puffing). The device measured and recorded a number of smoking topography parameters, namely: (1) number of puffs per cigarette, (2) puff duration, (3) inter-puff interval, (4) puff volume and (5) maximum puff velocity. The CReSS smoking topography device has been shown to have good reliability and validity even in single trials.33,34
Immediately after smoking the cigarette, participants completed the modified Cigarette Evaluation Questionnaire (mCEQ). The mCEQ consists of 12 items which query how smoking the cigarette made the participant feel (e.g., “Did the cigarette taste good?”, “Did the cigarette help you concentrate?”).35 Designated items are averaged to generate five subscale scores, namely (1) Satisfaction, (2) Psychological Reward, (3) Aversion, (4) Enjoyment of Respiratory Tract Sensations and (5) Craving Reduction. This measure has demonstrated good reliability and validity.36
CO was collected in 15-minute increments in the hour following smoking to assess CO boost, another measure of smoke exposure and intensity of smoking.37,38 To measure CO boost, pre-cigarette CO was subtracted from each CO value measured after smoking the cigarette. Withdrawal and craving were also measured in 15-minute increments during that hour using the MNWS and QSU-Brief. The MNWS measured eight nicotine withdrawal symptoms (e.g., craving, irritability, anxiety).39–41 Mean withdrawal is derived as the average of seven of the eight symptoms, with the item “Desire or Craving to Smoke” analyzed separately.40 The MNWS has good reliability and validity.38–40 The QSU-Brief is comprised of 10 statements indicating current cravings to smoke (e.g., “A cigarette would taste good right now.”, “I could control things better right now if I could smoke.”).42,43 The instrument is scored such that two factors are derived, with Factor 1 often described as a measure of the anticipation of positive reinforcing effects of smoking and Factor 2 a measure of the anticipation of negative reinforcing effects of smoking. The QSU-Brief is a reliable and valid measure of smoking urges.44
Participants were compensated $135 for their time. Pregnant participants ended their participation after this session. For non-pregnant women, this session was their first in a larger 14-visit study designed to test the acute effects of cigarettes with varying nicotine levels described elsewhere.30
Statistical Method
Independent t-tests and Fisher’s exact tests were used to compare demographics, smoking characteristics and baseline biochemical measures between the two groups. Nicotine metabolite ratio was log-transformed prior to statistical comparison. In comparisons where variances were not equal, corrected Satterthwaite approximations were used.
Given unequal sample sizes, non-uniform allocation was employed and the two groups were also frequency matched on all demographic characteristics (i.e., age, race, education and opioid dependence). All topography measures were log-transformed to meet normal distribution requirements so that independent t-tests could be used to compare the two groups; effect sizes (Cohen’s ds) were also calculated. A parallel set of analyses including all demographic and smoking variables as covariates was conducted with smoking topography data. Because the results of these analyses were the same as analyses that did not include covariates, the simpler set of results is presented. To explore whether topography changed as a function of increasing gestational age, a Pearson product-moment correlation coefficient was also computed to assess whether estimated gestational age (EGA) and any of the smoking topography parameters were related.
The five mCEQ subscales were compared using independent t-tests. CO boost, mean total MNWS score, MNWS item “Desire or Craving to Smoke”, QSU-Brief Factor 1 and QSU-Brief Factor 2 were compared between the two groups and across time points using repeated measures ANOVAs, with time as the within-subjects factor and pregnancy status as between-subject factor.
CO boost was also characterized using area under the curve. To do so, trapezoids were constructed with the x- and y-axis coordinates for each data point and the combined area of the three trapezoids summed. Significance for all tests was set at p < .05.
Power Analysis
Sample size calculations were based on smoking topography data collected from non-pregnant women participating in the pilot phase of a study testing the acute effects of cigarettes with differing levels of nicotine.30 Assuming a Type I error rate of 5% and power of 80%, it was determined that a sample size of 20 pregnant women would provide sufficient power to detect 20–30% differences in smoking topography measures. Differences of this magnitude are commonly observed in studies comparing smoking topography between groups.29,45,46
Results
Participant Characteristics
Twenty pregnant and 89 non-pregnant female smokers completed the experimental session. On average, participants were 30 years old, Caucasian, and had a high school education or less (Table 1). One-third of participants in both groups were opioid-maintained. Pregnant smokers averaged 22 weeks EGA at screening and reported cutting down from smoking 22 CPD prior to pregnancy to 13 CPD at screening (a 41% reduction; range: 15–75% reduction). Women in both groups tended to smoke approximately 14 high nicotine yield, non-menthol cigarettes per day, had moderate levels of nicotine dependence, started smoking around 15 years of age and had average urine cotinine levels of 870 ng/ml. There was no difference in past 30 day other nicotine/tobacco use between the two groups. As expected due to physiological changes induced by pregnancy, NMR was significantly higher among pregnant smokers as compared to non-pregnant smokers (t(107) = 2.81, p < .01).
Table 1.
Demographics and Smoking Characteristics
| Pregnant (n = 20) |
Non-Pregnant (n = 89) |
p value | |
|---|---|---|---|
| Demographics | |||
| Age | 30.3 ± 4.9 | 29.7 ± 6.4 | .72 |
| % White | 90.0 | 96.6 | .23 |
| % High school graduate or less | 55.0 | 50.0 | .63 |
| % Opioid-dependent | 35.0 | 30.9 | .79 |
| Estimated weeks gestational age | 22.2 ± 9.9 | N/A | |
| Smoking Characteristics | |||
| Pre-pregnancy cigarettes per day | 22.1 ± 7.8 | N/A | |
| Cigarettes per day at screening | 12.6 ± 5.4 | 15.3 ± 6.1 | .07 |
| Nicotine yield for usual brand cigarette | 1.1 ± 0.2 | 1.1 ± 0.1 | .62 |
| % Menthol | 25.0 | 27.0 | .98 |
| Fagerström Test for Nicotine Dependence | 4.4 ± 2.2 | 4.6 ± 2.2 | .69 |
| Age first started smoking | 14.7 ± 2.9 | 15.5 ± 2.6 | .24 |
| Biochemical Measures | |||
| Urine cotinine (ng/ml) | 821.6 ± 517.6 | 913.1 ± 462.4 | .44 |
| Nicotine metabolite ratio | 0.65 ± 0.30 | 0.44 ± 0.27 | .003 |
Values in the table are means ± standard deviations unless otherwise noted. Nicotine yield values come from the Federal Trade Commission’s Tar, Nicotine and Carbon Monoxide Report from 1999 to 2005.
Smoking Topography
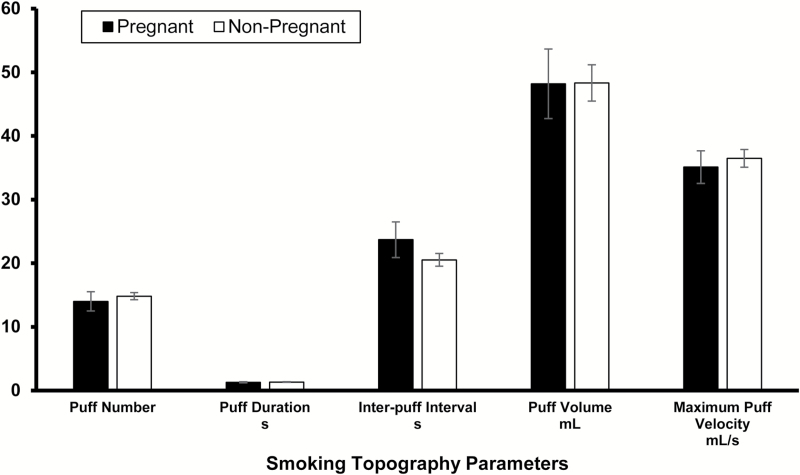
Comparing pregnant and non-pregnant women, there were no significant differences in mean (± SEM) puff number (14.00 ± 1.39 vs. 14.82 ± 0.58), puff duration (1.30 ± 0.09 vs. 1.34 ± 0.05), inter-puff interval (23.70 ± 2.58 vs. 20.52 ± 1.00), puff volume (48.21 ± 5.04 vs. 48.34 ± 2.86), and maximum puff velocity (35.11 ± 2.37 vs. 36.47 ± 1.39) (all ps > .25, ds range: −0.3 to 0.3). Differences between groups averaged less than 5% across parameters (Figure 1). Within the pregnant smoker sample, there were no significant correlations between EGA and any smoking topography parameter.
Figure 1.
Mean ± SEM for smoking topography parameters for pregnant and non-pregnant smokers as measured by the CReSS Desktop Smoking Topography device. There were no significant differences between groups on any parameter.
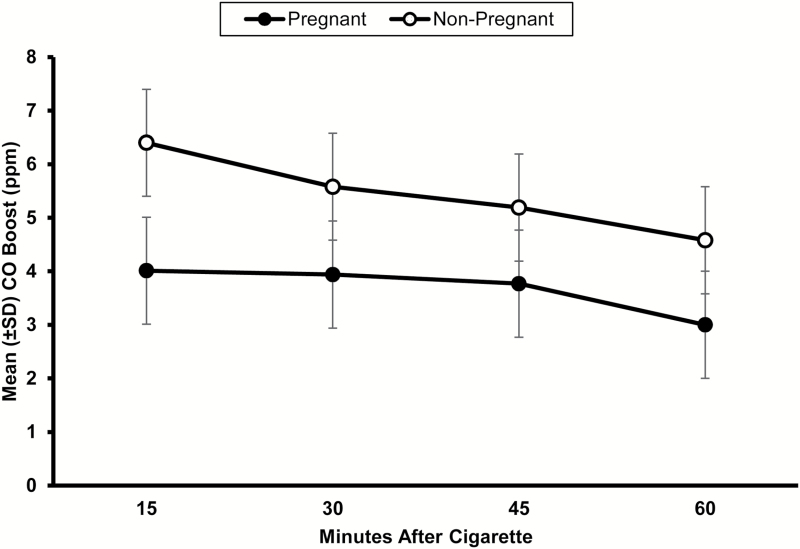
CO boost AUC was 39% greater in non-pregnant as compared to pregnant smokers, (F(1, 107) = 19.02, p< .01). CO boost decreased in a parallel fashion in both groups over time (F(3, 321) = 2.25, p < .05; Figure 2).
Figure 2.
Mean ± SD carbon monoxide (CO) boost 15, 30, 45 and 60 min after pregnant and non-pregnant smokers smoked one usual brand cigarette. There were significant effects of group and time (ps < .05), but no interaction on CO boost area under the curve.
Subjective Effects
mCEQ
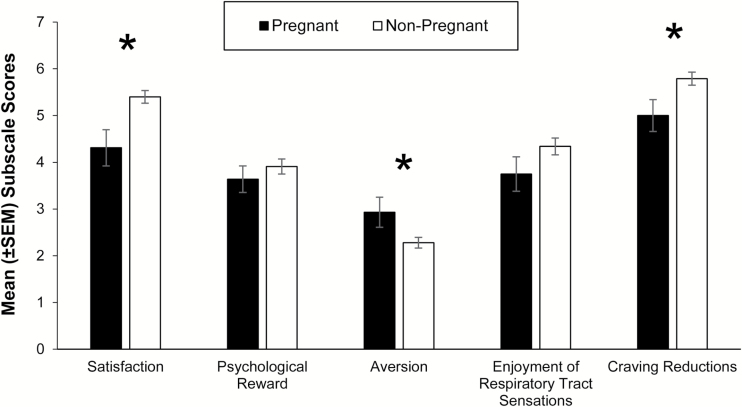
Compared to non-pregnant smokers, pregnant smokers reported significantly lower Satisfaction and Craving Reduction scores and higher Aversion mCEQ scores (t(107) = 10.32, p < .01, t(107) = 5.54, p = .02, t(107) = 5.23, p= .02; Figure 3).
Figure 3.
Mean ± SEM Modified Cigarette Evaluation Questionnaire subscale scores immediately after pregnant and non-pregnant smokers smoked one usual brand cigarette. An asterisk (*) indicates a significant effect of group (p < .05).
MNWS
There were no significant differences between groups on mean MNWS scores or on the MNWS item “Desire or Craving to Smoke”. Scores decreased in both groups 15 min after smoking the cigarette followed by increasing scores across subsequent time points (F(4, 427) = 8.53, p < .01, F(4, 427) = 20.78, p < .01; see online Supplementary Materials).
QSU-Brief
There were significant differences between groups on both QSU-Brief Factor 1 and QSU-Brief Factor 2 scores. While scores on both factors were comparable in pregnant and non-pregnant women prior to smoking, pregnant women reported significantly lower positive and negative anticipation of reinforcing effects from smoking compared to non-pregnant smokers (F(1, 107) = 17.27, p < .01, F(1, 107) = 4.22, p < .05). Scores in both groups then increased in a parallel fashion across time points on both factors (F(4, 428) = 22.02, p < .01, F(4, 427) = 13.83, p < .01; see online Supplementary Materials).
Discussion
To our knowledge, this is the first study comparing smoking topography between pregnant and non-pregnant smokers. Despite reporting decreases in their CPD and experiencing increases in nicotine metabolism rate, smoking topography of pregnant women did not differ significantly from non-pregnant smokers who reported comparable levels of CPD. While it is possible that there were no differences between groups because the non-pregnant smokers were also smoking more intensely, this seems unlikely given the large number of participants in this group.
Although no differences were discernible on any topography parameters, CO boost was less among pregnant smokers, an effect opposite of what would be expected with compensatory smoking. It is well known that CO can cross the placenta. As such, this smaller CO boost may be a function of some of the CO inhaled by the mother entering the placental-fetal compartment. Hormonal and anatomical changes in the respiratory system during pregnancy that increase overall tidal volume (the total amount of air in one inhale and one exhale combined) may also contribute. With a larger tidal volume, perhaps more CO can be exhaled with every breath, leading to a smaller CO boost. Whatever the mechanisms, a smaller CO boost is consistent with previous studies suggesting there are differences between pregnant and non-pregnant women with regards to CO levels.47,48
Across self-report questionnaires in the present study, two themes emerged. The first was that both groups experienced similar levels of relief from withdrawal after smoking, with consistent results across mean MNWS and mCEQ Psychological Reward. Two self-report measures also suggested that pregnant smokers do not find smoking as pleasurable or reinforcing as non-pregnant smokers. QSU-Brief Factor 1 scale scores, a measure of the anticipation of positive reinforcing effects from smoking, were lower among pregnant smokers, as was mCEQ Satisfaction subscale scores. Also according to the mCEQ, pregnant smokers indicated that smoking was more aversive and was not as effective at reducing cravings immediately after smoking a cigarette. This composite of lower positive subjective effects and greater aversive effects is consistent with a lower relative reinforcing effect of smoking.49 It is possible that decreases in the overall enjoyment of cigarette smoking facilitates the substantial reductions most female smokers report during pregnancy and may also explain why they do not engage in compensatory smoking following such substantial reductions. However, this study was not specifically designed to investigate this question and this conclusion should be cautiously considered.
It was surprising that baseline cotinine levels did not differ between pregnant and non-pregnant smokers. While both groups reported smoking about the same number of CPD, NMR was 30% faster among pregnant smokers, suggesting that their cotinine levels should have been lower if they were smoking approximately the same number of CPD and given no differences in the smoking topography parameters measured. It is possible that pregnant and non-pregnant smokers differ on other topography parameters, such as how much air was mixed with the puff during inhalation and how long the puff was held in the lungs, that offset faster metabolism among pregnant smokers. It is also possible that there may still be some social pressure on pregnant smokers to underreport their level of smoking.
These findings should be considered in light of some limitations. First, the pregnant sample in this study is relatively small. However, previous studies with similar sample sizes have found differences in smoking topography.29,46 Additionally, the control group was closely matched on a number of sociodemographic and smoking characteristics which are known to influence smoking topography, which eliminated variability that may have made it more difficult to detect differences between the groups. Furthermore, the differences in topography measures between non-pregnant and pregnant smokers were relatively small (<5% on average), which does not suggest that the study was underpowered. Likely the best way to answer questions about changes in smoking upon learning of pregnancy would be to conduct a large prospective longitudinal observational study of non-pregnant female smokers of reproductive age to see, for example, how smoking topography and subjective measures change in the subset of women who become pregnant, but it does not seem likely that such a study will be conducted given the time, effort, and costs such a study would require.
This study has several notable strengths. To our knowledge, it is the first study to capture a variety of variables during a single cigarette smoking bout among pregnant smokers. More specifically, this study characterized (1) smoking topography of usual brand cigarettes, (2) changes in CO in the hour that followed smoking, and (3) subjective effects of this smoking experience. Most noteworthy among these is smoking topography. For the past 20 years, researchers have speculated about whether pregnant smokers engage in compensatory smoking. This was the first study to directly address this question. In addition, a large sample of non-pregnant female smokers who did not differ from the pregnant smokers on important sociodemographic or smoking characteristics was included for comparison.
There are a number of future directions that could be explored. First, in regards to studying smoking topography during pregnancy, future studies should replicate this study in different contexts. For example, smoking topography can be measured using a portable version of the CReSS device used in the present study that can be sent home with participants to record data across multiple smoking bouts in the participant’s usual smoking environment. These studies would help validate the findings reported in this paper and may help overcome changes in smoking topography due to social pressure. Additional studies are also needed to more firmly establish the relationship between CPD and biochemical markers of smoking during pregnancy. A recent study by Denlinger and colleagues (2016) assessed non-pregnant smokers in a controlled, but not entirely artificial, environment (i.e., a hotel that permitted smoking) for 5 days.50 This study allowed researchers to precisely quantify how many CPD participants smoked and the levels of cotinine and other biomarkers that resulted. A similar study with pregnant women could generate population estimates that could be used for research and clinical purposes.
In summary, results of the present study suggest that the smoking topography of pregnant smokers does not differ from that of non-pregnant female smokers and that pregnant smokers find smoking less reinforcing. These changes in reinforcement may help pregnant smokers make the substantial reductions in CPD typically reported during pregnancy and may also protect them from engaging in compensatory smoking.
Supplementary Material
Supplementary data are available at Nicotine and Tobacco Research online.
Funding
This project was supported by a Tobacco Centers of Regulatory Science (TCORS) award (P50DA036114) from the National Institute on Drug Abuse and Food and Drug Administration.
Declaration of Interests
None declared.
Supplementary Material
References
- 1. U.S. Department of Health and Human Services. The health consequences of smoking: 50 years of progress. A report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2. Coleman T, Cooper S, Thornton JG et al. . A randomized trial of nicotine-replacement therapy patches in pregnancy. N Engl J Med. 2012;366(9):808–818. [DOI] [PubMed] [Google Scholar]
- 3. Heil SH, Higgins ST, Bernstein IM et al. . Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction. 2008;103(6):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Higgins ST, Heil SH, Solomon LJ et al. . A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res. 2004;6(6):1015–1020. [DOI] [PubMed] [Google Scholar]
- 5. Rigotti NA, Park ER, Regan S et al. . Efficacy of telephone counseling for pregnant smokers: a randomized controlled trial. Obstet Gynecol. 2006;108(1):83–92. [DOI] [PubMed] [Google Scholar]
- 6. Ussher M, Lewis S, Aveyard P et al. . The London Exercise and Pregnant smokers (LEAP) trial: A randomised controlled trial of physical activity for smoking cessation in pregnancy with an economic evaluation. Health Technol Assess. 2015;19(84):1–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heil SH, Herrmann ES, Badger GJ et al. . Examining the timing of changes in cigarette smoking upon learning of pregnancy. Prev Med. 2014;68:58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bowker K, Lewis S, Coleman T, Cooper S. Changes in the rate of nicotine metabolism across pregnancy: a longitudinal study. Addiction. 2015;110(11):1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dempsey D, Jacob P, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;310(2):594–598. [DOI] [PubMed] [Google Scholar]
- 10. Pianezza ML, Sellers EM, Tyndale RF. Nicotine metabolism defect reduces smoking. Nature. 1999;393:750. [DOI] [PubMed] [Google Scholar]
- 11. Graham H, Flemming K, Fox D, Heirs M, Sowden A. Cutting down: insights from qualitative studies of smoking in pregnancy. Health Soc Care Community. 2014;2(3):259–267. [DOI] [PubMed] [Google Scholar]
- 12. Heil SH, Solomon LJ, Skelly JM, Bernstein IM, Higgins ST.. Correspondence between self-reported and biochemical measures of cigarette smoking in pregnant women. Phoenix, AZ: Oral presentation at the 77th meeting of the College on Problems of Drug Dependence; 2015. [Google Scholar]
- 13. Boyd NR, Windsor RA, Perkins LL, Lowe JB. Quality of measurement of smoking status by self-report and saliva cotinine among pregnant women. Matern Child Health J. 1998;2(2):77–83. [DOI] [PubMed] [Google Scholar]
- 14. Ellard GA, Johnstone FD, Prescott RJ, Ji-Xian W, Jian-Hua M. Smoking during pregnancy: the dose dependence of birthweight deficits. Br J Obstet Gynaecol. 1996;103(8):806–813. [DOI] [PubMed] [Google Scholar]
- 15. Klebanoff MA, Levine RJ, Clemens JD, DerSimonian R, Wilkins DG. Serum cotinine concentration and self-reported smoking during pregnancy. Am J Epidemiol. 1998;148(3):259–262. [DOI] [PubMed] [Google Scholar]
- 16. Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatr Perinat Epidemiol. 2005;19(5):368–376. [DOI] [PubMed] [Google Scholar]
- 17. Dukic VM, Niessner M, Benowitz N, Hans S, Wakschlag L. Modelling the relationship of cotinine and self-reported measures of maternal smoking during pregnancy: a deterministic approach. Nicotine Tob Res. 2007; 9(4):453–465. [DOI] [PubMed] [Google Scholar]
- 18. Lindqvist R, Lendahls L, Tollbom O, Aberg H, Hakansson A. Smoking during pregnancy: Comparison of self-report and cotinine levels in 496 women. Acta Obstet Gynecol Scand. 2002;81(3):240–244. [DOI] [PubMed] [Google Scholar]
- 19. Benowitz NL, Jacob P, Kozlowski LT, Yu L. Influence of smoking fewer cigarettes on exposure to tar, nicotine, and carbon monoxide. N Engl J Med. 1986;315(21):1310–131. [DOI] [PubMed] [Google Scholar]
- 20. Jarvis MJ, Giovino GA, O’Connor RJ, Kozlowski LT, Bernert JT. Variation in nicotine intake among U.S. cigarette smokers during the past 25 years: Evidence from NHANES surveys. Nicotine Tob Res. 2014;16(12):1620–1628. [DOI] [PubMed] [Google Scholar]
- 21. Chilcoat HD. An overview of the emergence of disparities in smoking prevalence, cessation, and adverse consequences among women. Drug Alcohol Depend. 2009;104: S17–S23. [DOI] [PubMed] [Google Scholar]
- 22. Higgins ST, Heil SH, Badger GJ et al. . Educational disadvantage and cigarette smoking during pregnancy. Drug Alcohol Depend. 2009;104(suppl 1):S100–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dempsey D, Tutka P, Jacob P et al. . Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76(1):64–72. [DOI] [PubMed] [Google Scholar]
- 24. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991. 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- 25. Pomerleau CS, Majchrzak MJ, Pomerleau OF. Nicotine dependence and the Fagerström Tolerance Questionnaire: A brief review. J Subst Abuse. 1989;1(4):471–477. [PubMed] [Google Scholar]
- 26. Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the Fagerstrӧm Tolerance Questionnaire and the Fagerstrӧm Test for Nicotine Dependence. Addict Behav. 1994;19(1):33–39. [DOI] [PubMed] [Google Scholar]
- 27. Sheehan DV, Lecrubier Y, Sheehan KH et al. . The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998; 59(suppl 20): 22–33. [PubMed] [Google Scholar]
- 28. Johnson MW, Bickel WK, Kirshenbaum AP. Substitutes for tobacco smoking: a behavioral economic analysis of nicotine gum, denicotinized cigarettes, and nicotine containing cigarettes. Drug Alcohol Depend. 2004;74:253–264. [DOI] [PubMed] [Google Scholar]
- 29. Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug Alcohol Depend. 2005;20:259–265. [DOI] [PubMed] [Google Scholar]
- 30. Higgins ST, Heil SH, Sigmon SC. Response to varying the nicotine content of cigarettes in vulnerable populations: an initial experimental examination of acute effects. Psychopharmacology. 2016;234(1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Henningfield JE, Griffiths RR. Cigarette smoking and subjective response: Effects of d-amphetamine. Clin Pharmacol Ther. 1981;30(4):497–505. [DOI] [PubMed] [Google Scholar]
- 32. Shahan TA, Bickel WK, Madden GJ, Badger GJ. Comparing the reinforcing efficacy of nicotine containing and de-nicotinized cigarettes: a behavioral economic analysis. Psychopharmacology. 1999;147(2):210–216. [DOI] [PubMed] [Google Scholar]
- 33. Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009;11(7): 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB. Smoking topography: Reliability and validity in dependent smokers. Nicotine Tob Res. 2003;5(5):673–679. [DOI] [PubMed] [Google Scholar]
- 35. Westman E, Levin E, Rose J. Smoking while wearing the nicotine patch: Is smoking satisfying or harmful?Clin Res. 1992;40:871–877. [Google Scholar]
- 36. Cappeleri JC, Bushmakin AG, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32(5):912–923. [DOI] [PubMed] [Google Scholar]
- 37. Strasser AA, Ashare RL, Kozlowski LT, Pickworth WB. The effect of filter blocking and smoking topography on carbon monoxide levels in smokers. Pharmacol Biochem Behav. 2005;82(2):320–329. [DOI] [PubMed] [Google Scholar]
- 38. Zacny JP, Stitzer ML, Brown FJ, Yingling JE, Griffiths RR. Human cigarette smoking: effects of puff and inhalation parameters on smoke exposure. J Pharmacol Exp Ther. 1987;240(2):554–564. [PubMed] [Google Scholar]
- 39. Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. [DOI] [PubMed] [Google Scholar]
- 40. Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry. 1991;48(1):52–59. [DOI] [PubMed] [Google Scholar]
- 41. Hughes J, Hatsukami DK. Errors in using tobacco withdrawal scale. Tob Control. 1998;7:92–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-Brief) in a laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–17. [DOI] [PubMed] [Google Scholar]
- 43. Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. [DOI] [PubMed] [Google Scholar]
- 44. Toll BA, Katalua NA, McKee SA. Investigating the factor structure of the Questionnaire on Smoking Urges – Brief (QSU-Brief). Addict Behav. 2006;31(7):1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eissenberg T, Adams C, Riggins EC, Likness M. Smokers’ sex and the effects of tobacco cigarettes: subject-rated and physiological measures. Nicotine Tob Res. 1999;1(4):317–324. [DOI] [PubMed] [Google Scholar]
- 46. Strasser AA, Malaiyandi V, Hoffmann E, Tyndale RF, Lerman C. An association of CYP2A6 genotype and smoking topography. Nicotine Tob Res. 2007;9(4):511–518. [DOI] [PubMed] [Google Scholar]
- 47. Higgins ST, Heil SH, Badger GJ et al. . Biochemical verification of smoking status in pregnant and recently postpartum women. Exp Clin Psychopharmacol. 2007; 15(1):58–66. [DOI] [PubMed] [Google Scholar]
- 48. Bailey BA. Using expired air carbon monoxide to determine smoking status during pregnancy: preliminary identification of an appropriately sensitive and specific cut-point. Addict Behav. 2013; 38(10):2547–2550. [DOI] [PubMed] [Google Scholar]
- 49. Arger CA, Heil SH, Sigmon SC et al. . Preliminary validity of the modified cigarette evaluation questionnaire in predicting the reinforcing effects of cigarettes that vary in nicotine content. Exp Clin Psychopharmacol. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Denlinger RL, Smith TT, Murphy S et al. . Characterizing biomarkers of nicotine exposure when smoking very low nicotine content cigarettes in a controlled access environment. Tob Reg Science. 2016;2:186–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.