Abstract
Introduction
Men and women may be differentially sensitive to the acute perceptual responses to smoking cigarettes that vary in nicotine content (“dose”) but are matched on non-nicotine constituents.
Methods
Dependent adult smokers (43 M, 31 F) took four controlled puffs from Spectrum research cigarettes that were moderate (16–17 mg/g) or very low (0.4 mg/g) in nicotine content, and matched on “tar.” To ensure reliable responses, each cigarette was administered singly five times in random order under blind conditions, with one or the other provided every 15 minutes over a 2.5-hour session following overnight abstinence. Subjective perceptions (eg, “satisfying”, “how much nicotine”) were rated after each cigarette.
Results
Subjective ratings differed due to cigarette nicotine content, as expected, and did so differentially between men and women. The interaction of nicotine content by sex was significant for most rated subjective perceptions of the cigarette, as multivariate analyses showed that differences due to nicotine content were highly significant for men (p < .001) but only marginal for women (p = .08).
Conclusions
Relative to men, women’s subjective responses to acute smoking are less sensitive to differences in cigarette nicotine content. To our knowledge, this is the first comparison of sex differences in response to very carefully controlled doses of smoked nicotine per se. Further research should examine possible sex differences in nicotine dosing administered by other smoked and nonsmoked methods, as well as the developmental pattern of these differences during onset and during cessation of dependent smoking.
Implications
Subjective perceptions of smoking cigarettes varying in nicotine contents differ between men and women. These results with research cigarettes are similar to other studies with carefully dosed nicotine administration by other means, supporting the notion that women, relative to men, are less sensitive to pharmacological factors and more sensitive to nonpharmacological factors in acute cigarette smoking. Future studies are warranted to examine sex differences in other responses to controlled nicotine intake via smoking, and via other smoked and nonsmoked methods of administering nicotine doses.
Introduction
Pleasurable subjective responses to acute nicotine intake contribute substantially to the positively reinforcing effects of cigarette smoking behavior,1,2 separate from its relief of abstinence-induced withdrawal or craving (ie, negative reinforcement).3 Yet, smokers differ in the magnitude, and perhaps even the pattern, of pleasurable responses to acute nicotine.4,5 For example, men and women differ in sensitivity of some responses to acute nicotine dosing.6,7 Because cigarette smoking stimuli involve more than just intake of nicotine,8,9 the relative contributions of pharmacological versus nonpharmacological factors to sex differences in acute responses to smoking need to be taken into consideration.
Regarding pharmacological factors, men and women generally do not appear to differ in nicotine pharmacokinetics (ie, rate of uptake and clearance of nicotine),10 unless women are taking oral contraceptives.11 However, sex differences in pharmacodynamic responses to controlled nicotine dosing may be likely for some acute effects.12–14 Even less explored are sex differences in responses to the nonpharmacological factors involved in acute smoking, which requires careful control over nicotine dosing (ie, pharmacological factors). We have long observed that acute smoking behavior in women, relative to that of men, is influenced less by intake of nicotine per se and more by non-nicotine factors,15–18 perhaps similar to some findings in preclinical research.19 Acute smoking studies manipulating the amount of nicotine exposure (via FTC-determined “yield” of commercial cigarettes) have found that women, compared to men, are generally less sensitive to nicotine per se on subjective ratings of “reward”20,21 and more sensitive to non-nicotine factors (eg, cues, olfactory smoke stimuli).16,18,22 “Reward” is not precisely defined but often refers to the pleasurable hedonic value of a substance, usually assessed with self-report measures of “liking”, “satisfying”, “good drug effects”, etc.23
On the other hand, even these sex differences in subjective responding to nicotine via smoking may be uncertain. As noted, this prior research used commercial brands varying in nicotine yield (eg, “full flavor” vs. “light” brands), in which nicotine delivery is engineered by manipulating the filter ventilation holes, rather than by the actual nicotine content of the tobacco. Yet, smokers can override effects of this ventilation, by intensifying their puff topography or by covering over the holes.24,25 For this reason, a commercial cigarette’s labeled yield is limited as a measure to assess delivery of its nicotine dose,26 raising the possibility that variations between cigarettes other than precise differences in intake of nicotine may have influenced the differential reward responses between men and women. This may include variable contents of other constituents in commercial cigarettes that could affect responses, beyond their nicotine yield.27
Research cigarettes varying in documented nicotine content of tobacco (ie, “dose”), while matched on non-nicotine constituents, are now available (called Spectrum, through the National Institute on Drug Abuse). These research cigarettes are engineered to provide specific nicotine contents, from very low to moderate (relative to commercial brands), to aid study of effects due to known amounts of nicotine delivery.28,29 Unlike with commercial cigarettes, smokers cannot readily obtain greater nicotine from a research cigarette with specific contents, allowing much better control over dosing and, therefore, testing of acute responding to differences in nicotine doses via smoking. Also advantageous is the fact that these cigarettes are available in menthol or nonmenthol versions, allowing for testing with smokers preferring one or the other brand of cigarettes. Inclusion of menthol smokers broadens the generalizability of results due to nicotine contents, as menthol brands are preferred by about one-third of U.S. smokers30 and more often among women and among African American smokers.31
Given the recency of their availability, controlled research is limited on acute subjective responses to Spectrum cigarettes. One study assessed subjective effects of an exposure to each of several Spectrum cigarettes varying in lower nicotine contents after a smoking-satiated baseline condition, finding generally dose-dependent effects.29 We also recently found differences in similar subjective responses between Spectrum cigarettes that were barely discriminable, relative to comparison Spectrum cigarettes that were not discriminable,32,33 further supporting the relevance of acute subjective effects of smoking to behavioral responses.
The current within-subjects study assessed acute subjective perceptions of Spectrum research cigarettes with moderate (16–17 mg/g) or very low (0.4 mg/g) nicotine contents, those differing more widely in nicotine than in the recent studies. Participants were male and female dependent smokers abstinent at baseline. Also, five exposures to each cigarette helped establish reliable responding. We hypothesized that differences due to cigarette nicotine content would be less in women compared to men. These differences were expected owing to generally lesser sensitivity of women to nicotine intake per se and greater sensitivity to the non-nicotine aspects of smoking, which here were closely matched between cigarettes.
Methods
Participants
Eligible subjects were required to be aged 18–65 years, smoke for ≥ 1 year, nicotine-dependent, fluent in English, and not currently diagnosed with serious medical or psychological problems (eg, cancer, heart disease, psychosis, major depression). All were those not interested in quitting smoking soon who responded to online or posted study ads (briefly describing the study and offering payment for participation). Enrolled participants were dependent smokers who either preferred nonmenthol (n = 29; 21 M, 8 F) or menthol (n = 45; 22 M, 23 F) cigarette brands. Nicotine dependence was confirmed by presence of DSM-V criteria,34 using a structured interview updated from Breslau et al.35 All also completed the Fagerstrom Test of Nicotine Dependence (FTND).36 Mean (SD) characteristics for the 43 men and 31 women are shown in Table 1. No differences due to sex were significant for most characteristics, but women were more likely to prefer menthol, χ2(1, N = 74) = 4.0, p < .05, as commonly reported.31 For this reason, menthol preference was a blocking variable in the primary comparisons of subjective reward in response to the cigarette’s nicotine content due to subject sex (see Analysis section).
Table 1.
Mean (SD) Characteristics for Male and Female Participants
| Characteristic | Men (n = 43) | Women (n = 31) |
|---|---|---|
| Age | 32.8 (9.6) | 34.7 (12.4) |
| Cigarettes per day | 16.9 (7.0) | 15.6 (3.7) |
| Nicotine yield of preferred brand | 1.2 (0.2) | 1.1 (0.2) |
| FTND | 5.1 (1.9) | 5.2 (1.5) |
| Menthol smokers* | 51.2% | 74.2% |
| Ethnicity | ||
| Caucasian | 74.4% | 64.5% |
| African American | 18.6% | 32.3% |
| More than one race | 7.0% | 3.2% |
*p < .05 for main effect of sex; menthol preference used as blocking variable in analyses.
Research Cigarettes
Menthol and nonmenthol versions of the Spectrum investigational research cigarettes, manufactured by 22nd Century Group (Clarence NY; http://www.xxiicentury.com/), were obtained from NIDA’s Drug Supply Program. Selected for the current study were the two most widely differing in nicotine contents but similar on “tar” yield (to isolate only their differences in nicotine per se). By design, the menthol and nonmenthol versions of these Spectrum cigarettes were closely matched on nicotine contents. Contents for menthol were approximately 16 mg and 0.4 mg of nicotine per gram of tobacco (ie, mg/g), while the corresponding contents for nonmenthol were 17 mg/g and 0.4 mg/g (combining from two Spectrum batches). All also had about 9–10 mg “tar”. (To compare with commercial brands, both of these Spectrum menthol and nonmenthol research cigarettes correspond to approximately 0.8, and 0.03 mg nicotine “yields” by FTC method, as reported in http://grants.nih.gov/grants/guide/notice-files/NOT-DA-14-004.html. Commercial brands typically yield about 0.9 mg nicotine, and 10 mg “tar.”27) Note that we matched menthol versus nonmenthol Spectrum cigarettes with smokers who preferred menthol or nonmenthol commercial brands, respectively, to ensure their responses would reflect how smokers in the natural environment would likely respond to cigarettes differing in nicotine content. Doing so avoided the unknown relevance of reward responses by smokers administered cigarettes with nonpreferred flavorings.37
Control of Exposure to Each Cigarette
The portable Clinical Research Support System (CReSS; Borgwaldt KC, Inc., Richmond VA) was used to standardize smoke intake from all cigarettes at four puffs per trial of exposure to one or the other cigarette (see Procedures), similar to prior studies testing subjective responses to cigarettes differing in nicotine.20 One puff, with a 2-second “hold” duration, was taken every 30 seconds, according to computer-displayed instructions standardizing smoke intake at approximately 60 mL per puff to simulate that from typical ad lib puffing.38,39 Intertrial intervals of 15 minutes minimized smoking satiation or toxicity. Smoking four puffs per cigarette was also that chosen by Hatsukami et al.29 and is the typical exposure by a smoker when forming expectations about a cigarette.40 In short, four puffs per trial delivered a “dose” of nicotine sufficient to elicit the cigarette’s subjective rewarding effects but to also prevent smoke satiation or toxicity.32
Procedures
Participants were abstinent from smoking overnight prior to the session, confirmed by CO ≤ 10 ppm41 assessed by BreathCO CO monitor (Vitalograph, Lenexa, KS). The two cigarettes were presented individually in random order across the four-puff trials (five per cigarette type), one every 15 minutes. In brief, the first two trials involved one exposure to one and then one exposure to the other of the two cigarettes, again randomly determined, but subsequent trials could involve exposures to the same cigarette on two consecutive trials (but not more than two). In other words, each cigarette had a 50% probability of being administered on a given trial, and trial orders were equal for men and women. After the last puff in each trial, subjects completed a brief self-report measure on their subjective perceptions of that cigarette. This measure included six items expected to be sensitive to nicotine content,21,22 asking how much “nicotine”, “flavor”, and “liking” they experienced, and how “satisfying”, “strong”, and “similar to own brand” the cigarette was. Each was rated on a 0–100 visual analog scale (VAS), anchored by “not at all” to “very much.”39 Thus, this measure focused on the participants’ ratings of the hedonic value of the cigarette, and not on changes in their emotional state that can be assessed independently of smoking behavior. This study, approved by the University of Pittsburgh Institutional Review Board, assessed responses from all who were enrolled in a project that involved a variable number of subsequent sessions conducted with a smaller subset of participants.33
Data Analyses
Subjective perceptions of the cigarettes were compared using repeated measures multivariate analysis of variance (RM MANOVA), with nicotine content as a within-subjects factor, sex as a between-subjects factor, and menthol as a between-subjects blocking variable. These same analyses were used to test puff topography from the two cigarettes, to confirm similar smoking exposure. As a test of assumptions for using menthol as a blocking variable, interactions between menthol, nicotine content, and/or sex were examined for both sets of outcomes. Multivariate analyses for the cigarette perceptions found no effects of menthol by nicotine, F(6,65) = 0.35, p = .91, sex by menthol, F(6,65) = 1.33, p = .26, or sex by nicotine by menthol, F(6,65) = 0.93, p = .48. For puff topography, there were no effects of menthol by nicotine, F(1,70) = 0.15, p = .70, sex by menthol, F(1,70) = 0.07, p = .79, or sex by nicotine by menthol, F(1,70) = 0.82, p = .37. Thus, the assumption of no interactions between the blocking variable (menthol) and the other independent variables was met. Follow-up univariate RM ANOVAs were performed for each individual VAS response, using the same within- and between-subjects factors. Partial eta square (η2p) was calculated as a measure of effect size.
Results
As intended, no differences in smoking topography were found for the main effect of nicotine content, F(1,70) = 1.9 ns, with means (SEM) of 63.0 (2.2) versus 61.7 (1.9) mL for the 16–17 mg/g versus 0.4 mg/g nicotine cigarettes, respectively, or for the interaction of sex with nicotine content, F(1,70) = 0.2 ns. However, consistent with other studies,42,43 there was a main effect of sex on puff volume, F(1,70) = 17.80, p < .001, η2p = 0.2, as men had higher mean puff volumes compared to women, 70.7 (2.2) versus 53.4 (2.6) mL, respectively. Yet, because of the within-subjects design of this study, these preliminary analyses document that smoking exposure was equal between the two cigarettes differing in nicotine content, for men and for women.
Multivariate analyses revealed a significant main effect of nicotine content across the linear combination of the subjective responses, F(6,65) = 12.71, p < .001, η2p = 0.5. As expected, univariate follow-ups showed significantly higher ratings for the 16–17 mg/g versus 0.4 mg/g cigarettes, respectively, for (mean [SEM]) liking (55.2 [2.5] vs. 32.8 [2.7]), satisfying (55.9 [2.4] vs. 32.3 [2.6]), how much nicotine (58.1 [2.2] vs. 34.8 [2.1]), strong (52.2 [2.0] vs. 32.6 [2.3]), flavor (53.2 [2.2] vs. 37.0 [2.5]), and similar to own brand (40.3 [2.9] vs. 19.2 [2.2]), F(1,70)’s > 36, p’s < .001. Moreover, there was a significant multivariate main effect of sex on the subjective responses F(6,65) = 3.2, p < .05, η2p = 0.2. Yet, follow-up analyses showed a significant main effect of sex only for flavor, F(1,70) = 4.8, p < .05, η2p = 0.1, with women (49.3 [3.0]) rating the cigarettes higher than men (41.0 [2.3]), overall.
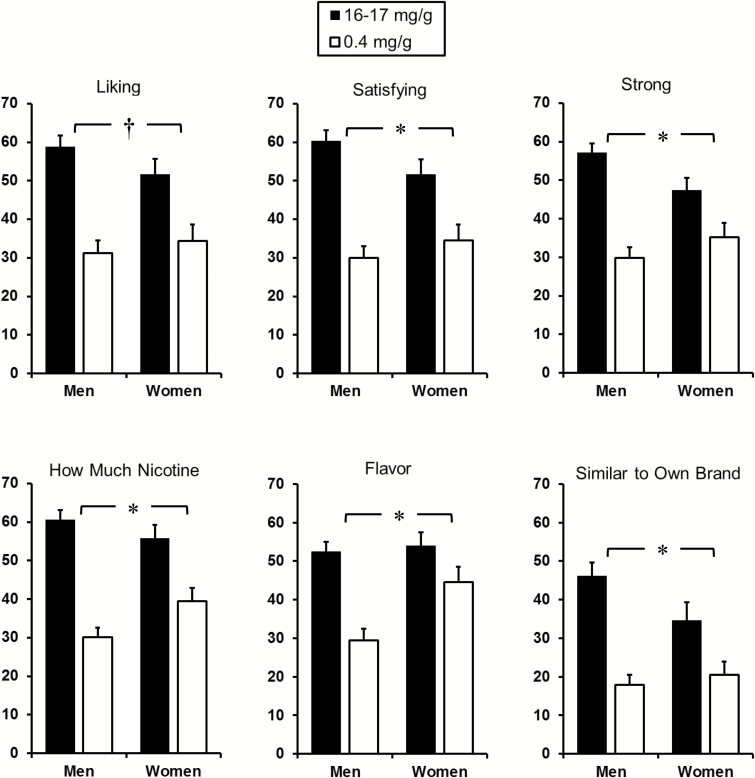
More importantly, the multivariate interaction of nicotine content by sex was significant, F(6,65) = 2.37, p < .05, η2p = 0.2, indicating differences in responding across the linear combination of subjective items between nicotine contents as a function of sex. Follow-up multivariate analyses showed that the main effect of nicotine content was highly significant for men, F(6,36) = 16.11, p < .001, η2p = 0.7, but only marginal for women, F(6,24) = 2.18, p = .08, η2p = 0.35. Figure 1 displays the mean (SEM) subjective responses by nicotine content and sex. In univariate comparisons, the interaction of nicotine content × sex was significant for satisfying, how much nicotine, strong, flavor, and similar to own brand, all F(1,70)’s > 5, p’s < .03, η2p’s = 0.1, although only marginal for liking, F(1,70) = 3.1, p < .10, η2p = 0.04.
Figure 1.
Mean (SE) 0–100 visual analog scale ratings on subjective perceptions of each Spectrum research cigarette by nicotine content, for men and women. (*p < .05; †p < .10 for the interaction of nicotine content by sex).
Discussion
We found differences between men and women in their sensitivity to the nicotine content of research cigarettes on ratings of their perceptions of those cigarettes. In multivariate analyses following up the significant overall interaction of nicotine content by sex, the effect of nicotine content on rated perceptions was highly significant in men but only marginal in women. This study goes beyond the few prior studies of sex differences in subjective responses to acute smoking by very carefully controlling puff topography and by closely manipulating nicotine delivery via use of Spectrum research cigarettes. They allow for better comparisons of responding to nicotine dosing per se via smoking since they have known, widely differing nicotine contents but are matched on non-nicotine constituents.
These results are consistent with research showing comparable sex differences in other responses to acute smoking exposure as a function of the manipulation of nicotine versus non-nicotine cigarette stimuli.16,18,44 They are also consistent with studies showing less sensitivity of women to relief from abstinence symptoms due to nicotine per se from acute smoking, that is, negative reinforcing effects.45 One prior study did not find sex differences in subjective effects from smoking Spectrum cigarettes, but it compared a broader variety of responses in nonabstinent smokers to cigarettes differing less markedly in nicotine content (11–12 vs. 0.4 mg/g) and with less control over topography of the single exposure to each,29 relative to the current study. Although speculative, these sex differences in sensitivity to pharmacological versus nonpharmacological factors in acute smoking may be one aspect of a much larger difference between men and women, which suggests women are more sensitive to exteroceptive cues for affect while men are more sensitive to interoceptive cues.46
With the recent availability through NIDA of these Spectrum research cigarettes differing in known nicotine contents, potential sex differences in other acute responses to smoked nicotine may warrant study. For example, sex differences in self-administration behavior may occur as a function of cigarette nicotine content, as previously seen in studies of cigarettes varying in nicotine yield21 or nicotine administered via nonsmoked means.15 A second example may be differential relief of withdrawal or craving due to acute exposure to these cigarettes under carefully controlled conditions.45 However, the chronology of onset for these smoking abstinence symptoms is much longer than for intermittent acute perceptual ratings of four puffs on a cigarette, as in this study. Thus, a test of abstinence relief effects due to different cigarettes administered individually likely requires separate sessions,45 which could attenuate differences in subjective responses to these cigarettes.21 We could not simultaneously assess withdrawal relief as well as the acute cigarette perception ratings, given our within-session comparison between cigarettes, but it is conceivable that those perception ratings may be affected by the concurrent relief of withdrawal the smoker is experiencing by smoking one cigarette or the other. Such differences in perceptions due to nicotine content also could vary when administered via commercial brand cigarettes, because those also may contain various additives affecting acute responses to smoking that were explicitly omitted in these Spectrum research cigarettes.27,29
Perhaps similar is the possibility that switching to long-term use of very low nicotine content cigarettes in an effort to eventually quit all combustible smoking28 may be differentially efficacious between women and men. Their quit success after transitioning to these cigarettes may depend on the relative degree to which persistent exposure to the nonpharmacological effects of smoking, while eliminating nicotine intake, facilitates eventual cessation. At the start rather than end of the dependence process, another future direction could explore when these sex differences in responses to nicotine versus non-nicotine stimuli of smoking become apparent during or after the onset of smoking. The focus here could be to gauge their rate of development over time and the degree to which they depend on the presence of nicotine dependence.47
Similar comparisons of sex differences in these subjective responses should be examined with other smoked or nonsmoked forms of acute doses of nicotine exposure, such as via hookah, smokeless, or electronic cigarettes. We assessed most of the self-report items from this study in an earlier comparison of nicotine versus placebo e-cigarettes, finding no sex differences in these responses.48 Yet, each e-cigarette was administered in separate sessions, which as noted may attenuate differential responding between cigarettes relative to differences to each when assessed intermittently within a single session.21 Also, that sample of male and female smokers with no history of regularly using e-cigarettes was small. Moreover, because women appear to be more sensitive to nonpharmacological stimuli accompanying nicotine intake via cigarette smoking, the form of nicotine administration used to assess sex differences in acute responding likely has to be one already very familiar to the participant, so that the accompanying stimuli have become secondary reinforcers of use.49
Regarding possible study limitations, these perceptual ratings of research cigarettes differing in nicotine content were made within one session, raising the potential for carryover effects between 15-minute trials. Consistent with similar acute research,29,32,33 minimizing possible carryover effects was one reason that exposure to each cigarette on each trial was limited to four controlled puffs, along with avoiding smoke toxicity across trials from the higher nicotine cigarette. If such carryover effects had occurred, differences in subjective perceptions of the cigarettes should have been attenuated, and the sex differences reported here would be even more substantial. Yet, we have observed greater differences in perceptions of cigarettes varying in nicotine when assessed concurrently in a single session, relative to testing of each cigarette in separate sessions,21 contrary to the notion of carryover effects. Similarly, if our procedure had involved greater numbers of puffs per exposure to each cigarette per trial, the sex differences in responding as a function of cigarette nicotine content could have been further enhanced, although risk of toxicity would have increased.
Among other limitations, men and women differed on likelihood of menthol preference, as commonly observed.31 Yet, we adjusted for this difference by using menthol preference as a blocking variable, and we previously reported no effects of menthol per se on subjective responses to Spectrum cigarettes.33 Women also had smaller puff volumes than men, as also observed previously,42,43 but exposure between cigarettes varying in nicotine content was the same within participants, further demonstrating the effectiveness of our puffing procedure instructions. Moreover, the roughly 20% less volume per puff in women smokers might be expected, based on their comparably lower body weights, relative to men.50 Blood samples were not assessed, partly due to the intermittent administration of the two cigarettes within one session, preventing us from confirming plasma nicotine levels from smoking these cigarettes. However, similar sex differences have been observed in nicotine administered by other means in doses that were corrected for body weight.7,13
In conclusion, men and women differ in response to perceptual ratings of cigarettes varying in known nicotine contents but very similar in other constituents, further supporting the idea that women, relative to men, are less sensitive to pharmacological factors (especially nicotine) and more sensitive to nonpharmacological factors (eg, visual, olfactory stimuli) in acute cigarette smoking. Future studies should examine other perceptions or behavioral responses to carefully controlled nicotine intake via smoking aside from those tested here that may differ due to cigarette nicotine content. Comparable comparisons between men and women in acute responses to other smoked and nonsmoked forms of nicotine intake are also warranted.
Funding
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under grant award DA035968. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Contributions
KAP designed the study, oversaw protocol development, and wrote the first draft of the manuscript. JLK oversaw statistical analyses and assisted with writing the manuscript. NK managed participant recruitment, data collection, and collected participant data during sessions.
Declaration of Interests
None declared.
Acknowledgments
The authors thank Melanie Kukich for her helpful assistance with data preparation.
References
- 1. Kalman D, Smith SS. Does nicotine do what we think it does? A meta-analytic review of the subjective effects of nicotine in nasal spray and intravenous studies with smokers and nonsmokers. Nicotine Tob Res. 2005;7(3):317–333. [DOI] [PubMed] [Google Scholar]
- 2. Pomerleau CS, Pomerleau OF. Euphoriant effects of nicotine in smokers. Psychopharmacology (Berl). 1992;108(4):460–465. [DOI] [PubMed] [Google Scholar]
- 3. Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111(1):33–51. [DOI] [PubMed] [Google Scholar]
- 4. Perkins KA. Baseline-dependency of nicotine effects: a review. Behav Pharmacol. 1999;10(6–7):597–615. [DOI] [PubMed] [Google Scholar]
- 5. Russell MAH. Subjective and behavioural effects of nicotine in humans: some sources of individual variation. In: A., Nordberg, K. Fuxe, B. Holmstedt, and A Sundwall, eds. Progress in Brain Research, vol. 79 Elsevier Sci Publ; 1989:289–302. [DOI] [PubMed] [Google Scholar]
- 6. Jensen KP, DeVito EE, Valentine G, Gueorguieva R, Sofuoglu M. Intravenous nicotine self-administration in smokers: dose-response function and sex differences. Neuropsychopharmacology. 2016;41(8):2034–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perkins KA. Nicotine discrimination in men and women. Pharmacol Biochem Behav. 1999;64(2):295–299. [DOI] [PubMed] [Google Scholar]
- 8. Addicott MA, Froeliger B, Kozink RV et al. Nicotine and non-nicotine smoking factors differentially modulate craving, withdrawal and cerebral blood flow as measured with arterial spin labeling. Neuropsychopharmacology. 2014;39(12):2750–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rose JE, Salley A, Behm FM, Bates JE, Westman EC. Reinforcing effects of nicotine and non-nicotine components of cigarette smoke. Psychopharmacology (Berl). 2010;210(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benowitz NL, Hatsukami D. Gender differences in the pharmacology of nicotine addiction. Addict Biol. 1998;3(4):383–404. [DOI] [PubMed] [Google Scholar]
- 11. Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P 3rd. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79(5):480–488. [DOI] [PubMed] [Google Scholar]
- 12. Cosgrove KP, Esterlis I, McKee SA et al. Sex differences in availability of β2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Arch Gen Psychiatry. 2012;69(4):418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeVito EE, Herman AI, Waters AJ, Valentine GW, Sofuoglu M. Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. Neuropsychopharmacology. 2014;39(6):1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fallon JH, Keator DB, Mbogori J, Taylor D, Potkin SG. Gender: a major determinant of brain response to nicotine. Int J Neuropsychopharmacol. 2005;8(1):17–26. [DOI] [PubMed] [Google Scholar]
- 15. Perkins KA. Sex differences in nicotine versus non-nicotine reinforcement as determinants of tobacco smoking. Exper Clin Psychopharmacol. 1996; 4: 166–177. [Google Scholar]
- 16. Perkins KA. Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking. In: Bevins R, Caggiula AR, eds. The Motivational Impact of Nicotine and its Role in Tobacco Use. New York: Springer-Verlag; 2009:143–169. [DOI] [PubMed] [Google Scholar]
- 17. Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. [DOI] [PubMed] [Google Scholar]
- 18. Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res. 2001;3(2):141–150. [DOI] [PubMed] [Google Scholar]
- 19. Chaudhri N, Caggiula AR, Donny EC et al. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl). 2005;180(2):258–266. [DOI] [PubMed] [Google Scholar]
- 20. Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula A. Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology (Berl). 2006;184(3–4):600–607. [DOI] [PubMed] [Google Scholar]
- 21. Perkins KA, Jacobs L, Sanders M, Caggiula AR. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology (Berl). 2002;163(2):194–201. [DOI] [PubMed] [Google Scholar]
- 22. Doran N. Sex differences in smoking cue reactivity: craving, negative affect, and preference for immediate smoking. Am J Addict. 2014;23(3):211–217. [DOI] [PubMed] [Google Scholar]
- 23. Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1485. [DOI] [PubMed] [Google Scholar]
- 24. Benowitz NL, Hall SM, Herning RI, Jacob P 3rd, Jones RT, Osman AL. Smokers of low-yield cigarettes do not consume less nicotine. N Engl J Med. 1983;309(3):139–142. [DOI] [PubMed] [Google Scholar]
- 25. Strasser AA, Ashare RL, Kozlowski LT, Pickworth WB. The effect of filter vent blocking and smoking topography on carbon monoxide levels in smokers. Pharmacol Biochem Behav. 2005;82(2):320–329. [DOI] [PubMed] [Google Scholar]
- 26. St Charles FK, Kabbani AA, Borgerding MF. Estimating tar and nicotine exposure: human smoking versus machine generated smoke yields. Regul Toxicol Pharmacol. 2010;56(1):100–110. [DOI] [PubMed] [Google Scholar]
- 27. U.S. Department of Health and Human Services (USDHHS). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [PubMed] [Google Scholar]
- 28. Donny EC, Denlinger RL, Tidey JW et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hatsukami DK, Heishman SJ, Vogel RI et al. Dose-response effects of Spectrum Research Cigarettes. Nicotine Tob Res. 2013;15:1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Villanti AC, Mowery PD, Delnevo CD, Niaura RS, Abrams DB, Giovino GA. Changes in the prevalence and correlates of menthol cigarette use in the USA, 2004–2014. Tob Control 2016;25(suppl 2):ii14–ii20. [DOI] [PubMed] [Google Scholar]
- 31. Tobacco Products Scientific Advisory Committee (TPSAC). Menthol Cigarettes and Public Health: Review of the Scientific Evidence and Recommendations. Rockville MD: US Food and Drug Administration; 2011. [Google Scholar]
- 32. Perkins KA, Kunkle N, Michael VC, Karelitz JL, Donny EC. Assessing discrimination of nicotine in humans via cigarette smoking. Nicotine Tob Res. 2016;18(9):1830–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perkins KA, Kunkle N, Karelitz JL. Threshold dose for behavioral discrimination of cigarette nicotine content in menthol vs. non-menthol smokers. Psychopharmacology (Berl). 2017;234(8):1255–1265. [DOI] [PubMed] [Google Scholar]
- 34. American Psychiatric Association (APA). Diagnostic and Statistical Manual-V. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 35. Breslau N, Kilbey MM, Andreski P. DSM-III-R nicotine dependence in young adults: prevalence, correlates and associated psychiatric disorders. Addiction. 1994;89(6):743–754. [DOI] [PubMed] [Google Scholar]
- 36. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 37. Strasser AA, Ashare RL, Kaufman M, Tang KZ, Mesaros AC, Blair IA. The effect of menthol on cigarette smoking behaviors, biomarkers and subjective responses. Cancer Epidemiol Biomarkers Prev. 2013;22(3):382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009;11(7):896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nicotine Tob Res 2012;14:490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hasenfratz M, Jacober A, Bättig K. Smoking-related subjective and physiological changes: pre- to postpuff and pre- to postcigarette. Pharmacol Biochem Behav. 1993;46(3):527–534. [DOI] [PubMed] [Google Scholar]
- 41. SRNT Subcommittee. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. [DOI] [PubMed] [Google Scholar]
- 42. Eissenberg T, Adams C, Riggins EC 3rd, Likness M. Smokers’ sex and the effects of tobacco cigarettes: subject-rated and physiological measures. Nicotine Tob Res. 1999;1(4):317–324. [DOI] [PubMed] [Google Scholar]
- 43. Melikian AA, Djordjevic MV, Hosey J et al. Gender differences relative to smoking behavior and emissions of toxins from mainstream cigarette smoke. Nicotine Tob Res. 2007;9(3):377–387. [DOI] [PubMed] [Google Scholar]
- 44. Barrett SP, Darredeau C. The acute effects of nicotine on the subjective and behavioural responses to denicotinized tobacco in dependent smokers. Behav Pharmacol. 2012;23(3):221–227. [DOI] [PubMed] [Google Scholar]
- 45. Perkins KA, Karelitz JL. Sex differences in acute relief of abstinence-induced withdrawal and negative affect due to nicotine content in cigarettes. Nicotine Tob Res. 2015;17(4):443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roberts TA, Pennebaker JW. Gender differences in perceiving internal states: toward a his-and-hers model of perceptual cues. Adv Exper Soc Psychol. 1995;27:143–175. [Google Scholar]
- 47. Kandel DB, Hu MC, Griesler PC, Schaffran C. On the development of nicotine dependence in adolescence. Drug Alcohol Depend. 2007;91(1):26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perkins KA, Karelitz JL, Michael VC. Reinforcement enhancing effects of acute nicotine via electronic cigarettes. Drug Alcohol Depend. 2015;153:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Caggiula AR, Donny EC, White AR et al. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70(4):515–530. [DOI] [PubMed] [Google Scholar]
- 50. Pisinger C, Jorgensen T. Waist circumference and weight following smoking cessation in a general population: the Inter99 study. Prev Med. 2007;44:290–295. [DOI] [PubMed] [Google Scholar]