Abstract
Introduction
African American (AA) smokers are at disproportionate risk of tobacco dependence, utilizing smoking to regulate stress, and poor cessation outcomes. Positive emotional traits may function as coping factors that buffer the extent to which dependence increases vulnerability to adverse responses to acute tobacco abstinence (ie, tobacco withdrawal). This laboratory study examined subjective happiness (SH; dispositional orientation towards frequent and intense positive affect [PA] and life satisfaction) as a moderator of the relation between tobacco dependence and subjective and behavioral abstinence effects among AA smokers.
Methods
AA smokers (N = 420, 39.0% female) completed self-report measures of tobacco dependence and SH followed by two counterbalanced experimental sessions (nonabstinent vs. 16-hour abstinent) involving self-report measures of composite withdrawal, urge to smoke, and mood, and a behavioral smoking task in which participants could: (1) earn money to delay smoking reinstatement, and (2) subsequently purchase cigarettes to smoke.
Results
Tobacco dependence was positively associated with increased abstinence effects in composite withdrawal, urge to smoke, PA, and latency to smoking reinstatement (ps < .04). SH significantly moderated the relation between dependence and abstinence-induced increases in composite withdrawal (β = −.17, p < .001), such that the predictive power of dependence on withdrawal severity grew proportionately weaker as levels of SH increased.
Conclusions
SH may insulate against adverse effects of dependence on withdrawal during acute smoking abstinence, particularly withdrawal symptom clusters that are craving- and mood-based. Consideration of positive emotional traits as stress-coping factors in the dependence-withdrawal link may be warranted in research and practice with AA smokers.
Implications
The current study contributes to a growing body of literature examining the potentially advantageous role of positive emotional traits to smokers. We do so by identifying a relatively understudied psychological construct within tobacco research—subjective happiness—that may suppress the extent to which more severe tobacco dependence increases risk for subjective withdrawal-related distress during acute smoking abstinence in AA smokers. In doing so, the study provides a primer for future targeting of subjective happiness and other positive emotional traits as means to understand and treat acute tobacco abstinence effects among dependent AA smokers.
Introduction
African American (AA) smokers are at disproportionate risk of poor cessation outcomes and tobacco-related disease and mortality compared to smokers of other racial backgrounds.1 One means of advancing the development of more effective, ethno-culturally specific cessation strategies in this community is to identify mechanisms underlying the etiology and persistence of tobacco dependence that have salience to AA smokers.
Evidence suggests that AA (vs. European American [EA]) smokers experience greater tobacco dependence. For example, AA (vs. EA) smokers smoke their first cigarette in the morning sooner, exhibit higher concentrations of biomarkers of nicotine and carcinogen exposure per cigarette smoked, metabolize nicotine slower, and prefer cigarettes with properties known to amplify risk of tobacco dependence (eg, menthol).2,3 One core expression of the tobacco dependence process is tobacco withdrawal4—a series of changes that arise during acute smoking abstinence following chronic tobacco exposure5 that are both subjective (eg, urge to smoke, decreased positive affect [PA], increased negative affect [NA], difficulty concentrating) and behavioral (eg, motivation to reinstate smoking). Acute abstinence-induced tobacco withdrawal may perpetuate habitual smoking and interfere with cessation,6 given that the desire to suppress aversive withdrawal symptoms and act on urge states may produce a strong motivation to resume smoking.7 For the dependent smoker, acute abstinence is a major stressor characterized by neuroendocrine changes that resemble stress-like psychophysiological response profiles predictive of smoking withdrawal severity and relapse.8 Thus, factors related to stress-coping during abstinence may be pertinent to consider in the dependence-withdrawal link.
Compared to their EA counterparts, AA smokers experience greater chronic and acute stress related to discrimination and poverty and may consequently develop a stronger reliance on smoking to cope with stress.9 As such, prior work has utilized the social stress model of substance abuse (ie, smoking is exacerbated by high stress, low social support, and insufficient material resources)10 and the stress-coping model (ie, smoking is a stress-related coping strategy)11 as theoretical frameworks for examining tobacco-related disparities in AA smokers.12 These tenets suggest that individuals from backgrounds of chronic stress who utilize smoking as a primary method of stress management may be especially affected by the sudden inability to rely on familiar smoking reinforcement-mediated coping strategies during periods of acute abstinence. This, in turn, could result in especially aversive perceptions of adverse reactions to abstinence.13 These mechanisms may be particularly salient in smokers who are heavily tobacco dependent or who lack dispositional qualities that contribute to effective appraisal of distressing situations, both of which influence withdrawal severity.8 Hence, identifying psychological factors with stress-buffering dimensions that may mitigate dependence-linked abstinence effects could advance etiologic models of tobacco addiction and smoking cessation clinical targets, particularly among priority populations with backgrounds of high chronic stress and poor cessation outcomes.1,9
One relatively understudied construct in tobacco research that may protect against the adverse effects of dependence on withdrawal severity is subjective happiness (SH). Believed to be a key component of positive mental health, SH is a disposition towards experiencing more frequent and intense PA and life satisfaction.14 Prior work indicates that PA and other SH-related traits counteract adverse physiological effects of negative emotions15 and buffer the acute stress response provoked by a variety of conditions.16 SH is also associated with lower perceived stress, reduced physiological and emotional stress reactivity, and greater utilization of adaptive coping strategies in response to acute stressors.17–19 These data suggest that happier (vs. less happy) people may have a dampened sensitivity to the psychobiological stress response or a natural tendency towards adaptive cognitive processing mechanisms (eg, appraisal of distressing situations as less menacing/more controllable). This may result in a more protective psycho-somatic adjustment to acute stress.
SH also appears to benefit smokers, as it is inversely associated with smoking status and persistence.20–22 Critically, elements of SH are also associated with reduced severity of both tobacco dependence and acute tobacco abstinence effects.20–23 Evidence that SH, withdrawal, and dependence each bivariately associate with one another gives rise to questions about how they intersect. Given the suggestive evidence of a stress-coping dimensionality to the SH trait,15–19 it is plausible that affective and behavioral impairment in response to acute tobacco abstinence in dependent smokers could be suppressed for those high in SH. This could result in less severe withdrawal symptoms and reduced motivation to reinstate smoking for dependent smokers who also carry the buffering trait of SH. Moreover, the role of SH as a potential buffer against abstinence-provoked withdrawal distress in highly-dependent smokers may be particularly relevant in the AA smoker population, given that they may be more vulnerable to tobacco dependence,2,3 chronic stress,9 and certain abstinence-induced mood changes than smokers of other racial backgrounds.24
The current study tested the hypothesis that SH suppresses the relation between tobacco dependence and severity of acute subjective and behavioral abstinence effects in AA smokers. We do so using a controlled laboratory design that assessed SH and tobacco dependence as baseline predictors of the difference in composite withdrawal symptoms, mood, urge to smoke, and performance on a behavioral measure of smoking reinstatement motivation between experimentally manipulated abstinent and nonabstinent conditions. Consistent with prior literature linking SH with protective psycho-somatic adjustment to acute stress15–19 and a range of favorable smoking-related outcomes,20–23 we hypothesize that the positive relation of tobacco dependence with subjective and behavioral abstinence effects indicative of heightened distress, mood dysregulation, and motivation to smoke will be suppressed in individuals endorsing higher levels of SH.
Methods
Participants
The current report is a secondary analysis of data from AA nontreatment-seeking daily smokers (N = 420) recruited from the Los Angeles area via advertisements for a laboratory study on individual differences that moderate responses to acute tobacco abstinence. The University of Southern California Internal Review Board approved all procedures, and all participants completed informed consent. Inclusion criteria were: (1) ≥ 18 years of age; (2) self-report as having Non-Hispanic AA ancestry in both biological parents; (3) regular smoker (≥ 2 years, ≥ 10 cigarettes/day); and (4) fluency in English. Exclusion criteria included: (1) current non-nicotine substance dependence (based on Diagnostic and Statistical Manual of Mental Disorders [DSM-IV25] criteria); (2) baseline breath carbon monoxide (CO) levels of < 10 ppm; (3) a desire to reduce or quit smoking in the next 30 days; (4) current, regular use of other tobacco products, nicotine replacement therapy, or psychiatric medications; and (5) current breast feeding or pregnancy.
Procedure
Following a preliminary telephone eligibility screening, individuals who met inclusion criteria (N = 574) attended an in-person baseline session involving informed consent, breath alcohol and CO analyses, psychiatric interview, and the substance use disorder module of the Structured Clinical Interview for DSM-IV Non-Patient Edition25 to determine study eligibility. Of these, 51 were ineligible because of low baseline CO (n = 25), current psychiatric disorder or use of psychiatric medications (n = 9), or other criteria (n = 17). Eligible participants completed the baseline session, which included self-report measures of tobacco dependence and SH. Of the 523 eligible participants, 55 dropped out after study entry and 48 did not meet abstinence criteria at the abstinent session (see below), leaving a final sample of 420.
Following the baseline session, participants completed two counterbalanced, in-person experimental sessions (abstinent vs. nonabstinent) that began at noon. Participants were instructed to stop smoking 16 hours prior to their abstinent session and to smoke normally prior to their nonabstinent session. The mean number of days between the baseline session and first experimental session and experimental session two and three was 6.43 (SD = 6.03; range = 1–47 days) and 6.70 (SD = 5.49; range = 1–35 days), respectively. Because acute alcohol consumption can influence the magnitude and emotional valence of subjective abstinence effects,26 experimental sessions began with a breath alcohol analysis to confirm 24 hours abstinence from alcohol. Nonabstinent and abstinent session procedures were identical, except that participants were administered a cigarette of their preferred brand following the breath alcohol analysis at the nonabstinent session to standardize abstinence levels across the sample. Following the breath alcohol analysis at the abstinent session and the cigarette administration at the nonabstinent session, participants were given a CO assessment. Adhering to published recommendations that expired CO concentrations of ≤8 ppm are indicative of recent abstinence,27 participants with CO measurements >8 ppm at their abstinent session were considered nonabstinent and were not included in final analyses (n = 48). At both experimental sessions, participants completed subjective measures of composite withdrawal, urge to smoke, and affect, followed by a behavioral smoking lapse task that began 75 minutes after the session outset.
Measures—Baseline Session
Predictor: Fagerström Test for Nicotine Dependence (FTND28)
The FTND is a 6-item self-report measure of tobacco dependence severity. Scores range from 0 to 10, with higher scores reflecting greater tobacco dependence.
Moderator: Subjective Happiness Scale (SHS14)
The SHS is a 4-item self-report measure of dispositional happiness that uses 7-point Likert scales, with higher scores indicating greater overall happiness. Participants rate their happiness levels both generally and relative to others. Participants also rate the extent to which characterizations of chronically happy and unhappy people describe them.
Descriptive Measures and Covariates
The following covariates were included to determine whether tobacco dependence and SH predicted tobacco abstinence effects over and above depressive symptoms, sensation seeking, and demographic variables, which have been linked to key study variables in past work.29–32 An author-constructed questionnaire assessed demographic and smoking characteristics. We also administered the 20-item Inventory for Depression and Anxiety Symptoms-General Depression subscale (IDAS-GD33) and the 12-item UPPS-P-Sensation Seeking subscale (UPPS-P-SS34). Item scores on both the IDAS-GD and the UPPS-P-SS range from 1 to 5, with higher scores indicating greater depressive symptomatology and greater disposition towards pursuing exciting activities, respectively.
Measures—Experimental Sessions
Minnesota Nicotine Withdrawal Scale (MNWS35)
The current study used an 11-item MNWS variant that assessed symptoms of craving, anger/irritability, anxiety/tension, difficulty concentrating, restlessness, impatience, excessive hunger, physical symptoms, increased eating, drowsiness, and headache experienced “so far today” on 6-point Likert scales. Higher scores indicate greater severity of subjective withdrawal. The MNWS is robustly sensitive to abstinence effects.7,36
The Brief Questionnaire of Smoking Urges (QSU37)
The QSU is a 10-item measure that assesses urge to smoke. Participants rate their agreement to self-statement urge experiences “right now” on 6-point Likert scales. Higher scores indicate greater immediate urge to smoke. The QSU is highly sensitive to abstinence effects.24,38
The Profile of Mood States (POMS39)
The POMS is a multidimensional affect scale that is sensitive to abstinence effects.24,38 Participants rated their affect state “right now” using 5-point Likert scales across 72 high- and low-arousal PA/NA adjectives. Composite PA and NA subscales were calculated by taking the mean of the subscale scores for friendliness, vigor, and elation and subscale scores for anger, depression, anxiety, confusion, and fatigue, respectively.
The Smoking Lapse Analogue Task40 measures acute smoking reinstatement motivation. Responses on this task appear to be modulated by tobacco abstinence.38 Participants received eight preferred brand cigarettes and an ashtray. In the “delay period,” participants were told they could smoke any time during the next 50 minutes but for each 5 minutes they delayed smoking, they could earn $0.20 up to a maximum of $2.00. The delay period ended when participants decided to smoke or at the end of the 50 minutes (latency to smoking reinstatement range: 0–50 minutes). Afterwards, participants entered a 60-minute “self-administration period,” where they were told they had a $1.60 credit from which they could pay $0.20 for each cigarette they wished to smoke. The selected $0.20 incentive is based off piloting conducted in prior samples from our study population, which are socioeconomically similar to the current study, that showed adequate variance in lapse behaviors at the $0.20 (n = 300) level,7,38,41 but not at the $1.00 (n = 15; ie, incentive validated by McKee et al.42) or $0.50 (n = 15) levels. During the delay and self-administration periods, eating, sleeping, drinking, and using personal belongings were not permitted. Following the self-administration period, participants entered a rest period for the remainder of the session, during which they were not allowed to smoke. This served to standardize session length for participants who chose not to fully delay, as well as to minimize the influence of participants’ impending ability to smoke following session completion on lapse behaviors during the task (rest time range: 60–110 minutes).
Analytical Approach
Data were analyzed using SPSS Version (v.24) and the PROCESS macros were used for moderation analyses.43 All statistical tests were two-tailed and used a significance level of 5%, except in cases requiring Bonferroni corrections.
Preliminary Analyses
Preliminary analyses involved reporting sample descriptives, correlations between key measures, and Cronbach’s α coefficients. Abstinence-induced change scores were calculated for each outcome (ie, MNWS, QSU, POMS-PA, POMS-NA, latency to smoking reinstatement, and number of cigarettes smoked) by subtracting the non-abstinent session score from the corresponding abstinent session score for each measure (reported as M(SD) with Cohen’s d effect size estimates for the abstinence-status effect). Single-sample t tests were then carried out for each abstinence-induced change score outcome to examine whether smoking abstinence effects departed from zero.
Primary Analyses
Using linear regression models, we first tested for main effects of both tobacco dependence (FTND) and SH (SHS) as simultaneous predictors of abstinence-induced change scores, with separate models for each outcome. We then added the FTND × SHS interaction term to each model to determine whether the FTND-abstinence-effect association was moderated by SHS. All models adjusted for the nonabstinent (baseline) score for the respective outcome and planned covariates (ie, gender, age, depressive symptoms, and sensation seeking). Follow-up simple slopes analyses were performed to deconstruct the direction of significant interactions. Results are presented as standardized parameter estimates (β). p -Values below a Bonferroni correction for multiple testing of six separate outcomes (.05/6; p < .008) were considered to have strong support. Tests that yielded p-values ranging .008 to .05 were considered to provide suggestive support and are interpreted with caution.
Supplementary Analyses
Separate, individual MNWS item analyses were performed for each symptom to determine which specific features or symptoms of the composite withdrawal measure were most impacted by the hypothesized buffering influence of SH (ie, affective vs. cognitive vs. physiological).
Results
Preliminary Analyses
Descriptive statistics, internal consistency estimates, and correlations among demographic/smoking characteristics and all baseline measures are reported in Table 1. Table 2 reports the results of single-sample t tests conducted to determine effects of smoking abstinence on outcomes. Smoking abstinence significantly affected all outcomes in the expected direction and the effect magnitudes ranged from medium to large as evidence by Cohen’s d statistics (Table 2). For the delay period of the Smoking Lapse Analogue Task, 30.3% smoked immediately (47.2% Abstinent, 13.4% Non-abstinent) and 46.4% waited the full 50 minutes (31.5% Abstinent, 61.3% Non-Abstinent). For the self-administration period of the Smoking Lapse Analogue Task, 15.3% smoked 0 cigarettes (11.5% Abstinent, 19.0% Non-Abstinent) and 0.1% smoked all 8 cigarettes (0.0% Abstinent, 0.20% Nonabstinent).
Table 1.
Descriptive Statistics and Correlations of Key Variables
| Key variables | Mean (SD) or % | Correlations (r) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| 1. Age (years) | 49.03 (10.73) | |||||||||
| 2. Gender (female) | 39.4 | .16*** | ||||||||
| 3. Baseline FTND | 5.46 (1.98) | .06 | −.07 | (.60) | ||||||
| 4. Baseline SHS | 5.36 (1.25) | .07 | −.10** | −.11** | (.68) | |||||
| 5. Baseline IDAS-GD | 1.91 (0.66) | −.10** | −.04 | .12*** | −.40*** | (.90) | ||||
| 6. Baseline UPPS-P-SS | 2.64 (0.57) | .23*** | −.05 | −.06 | −.08* | −.15*** | (.76) | |||
| 7. Baseline CO level (parts per million) | 18.46 (8.07) | −.06 | −.08* | .14*** | −.04 | .01 | −.07 | |||
| 8. Age of smoking onset (≥1 cigarette/day) | 17.37 (5.99) | −.01 | .03 | −.16*** | −.03 | .03 | .03 | −.04 | ||
| 9. Average cigarettes/day | 15.29 (7.40) | .11** | .07 | .45*** | −.13*** | .02 | −.01 | .16*** | −.05 | |
| 10. Menthola | 61.4 | −.09** | −.14*** | −.01 | .01 | .04 | −.11*** | −.02 | .00 | −.10** |
Sample size varies across analyses (Ns 516–523 due to missing data). Cronbach’s α for baseline measures shown in parentheses on the diagonal. Correlations among continuous variables are Pearson correlation coefficients. Correlations between continuous and dichotomous variables are point-biserial correlation coefficients. Women were coded as 0, and men were coded as 1. CO = Carbon Monoxide; FTND = Fagerström Test of Nicotine Dependence (range 0–10); IDAS-GD = Inventory for Depression and Anxiety Symptoms—General Depression Subscale (range 1–5); SHS = Subjective Happiness Scale (range 1–7); UPPS-P-SS = UPPS-P Impulsive—Behavior Sensation Seeking Subscale (range 0–5).
aMentholated cigarette preference (nonmentholated preference coded as 0, mentholated preference coded as 1).
* p < .10.
** p < .05.
*** p < .01.
Table 2.
Effects of Abstinence on Subjective Withdrawal and Smoking Lapse Behavior Outcomes
| Nonabstinent | Abstinent | Abstinence-induced change score | Abstinence effect | ||||
|---|---|---|---|---|---|---|---|
| M (SD) | α | M (SD) | α | M (SD) | t | d | |
| CO Levels (parts per million) | 19.43 (8.67) | - | 4.35 (2.07) | - | −15.02 (8.16) | −39.12 | −1.84† |
| QSU | 1.18 (1.25) | .95 | 3.03 (1.27) | .92 | 1.86 (1.37) | 29.21 | 1.36† |
| MNWS | 0.78 (0.78) | .84 | 2.24 (1.50) | .88 | 1.37 (1.38) | 18.26 | 0.99† |
| POMS | |||||||
| Positive Affect | 2.18 (0.85) | .95 | 1.74 (0.89) | .96 | −0.43 (0.76) | −12.20 | −0.57† |
| Negative Affect | 0.53 (0.52) | .95 | 0.68 (0.61) | .97 | 0.15 (0.50) | 6.49 | 0.30† |
| Latency to Smoking Reinstatement (minutes) | 35.78 (19.89) | - | 18.65 (22.52) | - | −17.43 (23.50) | −15.96 | −0.74† |
| Number of Cigarettes Smoked | 1.28 (1.12) | - | 1.47 (0.96) | - | 0.19 (1.04) | 3.77 | 0.18† |
Sample size varies across analyses (Ns 444–523 due to missing data). CO = Carbon Monoxide; MNWS = Minnesota Nicotine Withdrawal Scale (range 0–5); POMS = Profile of Mood States (range 0–5); Latency to Smoking Reinstatement (range 0–50 min for abstinent and nonabstinent score means and −50 to 50 min for abstinence-induced change score mean); QSU = Questionnaire of Smoking Urges (range 0–5); Number of Cigarettes Smoked (range 0–8 for abstinent and nonabstinent score means and −8 to 8 cigarettes for abstinence-induced change score mean); Abstinence-Induced Change Score = Score in Abstinent Condition—Score in Non-Abstinent Condition.
† p < .001.
Primary Analyses
Associations of Dependence and SH with Abstinence-Induced Changes in Study Outcomes
As illustrated in Table 3, higher FTND score was significantly associated with larger abstinence-induced increases in MNWS and QSU, and decreases in POMS-PA (ps < .008). FTND was also marginally associated with abstinence-induced decreases in latency to smoking reinstatement (p = .04). No associations between FTND and abstinence-induced changes in POMS-NA or number of cigarettes smoked were found (Table 3). No significant associations emerged between SH and any outcome.
Table 3.
Standardized Parameter Estimates for Main Effect Linear Regression and Moderation Analyses
| FTND main effects | SHS main effects | FTND × SHS moderation | ||||
|---|---|---|---|---|---|---|
| Abstinence-Induced Change Measure | β | p | β | p | β | P |
| Subjective Data | ||||||
| MNWS | .25 | <.001* | .06 | .20 | −.16 | <.001* |
| QSU | .17 | <.001* | .09 | .03 | −.07 | .08 |
| POMS Negative Affect | .07 | .14 | −.003 | .95 | −.05 | .30 |
| POMS Positive Affect | −.13 | .002* | .06 | .20 | −.06 | .19 |
| Behavioral Data | ||||||
| Latency to Smoking Reinstatement (min) | −.10 | .03 | −.06 | .21 | −.002 | .96 |
| Number of Cigarettes Smoked | .07 | .06 | −.03 | .43 | −.04 | .26 |
Sample size varies across analyses (Ns 433–457 due to missing data). Abstinence-Induced Change Measure = Score in Abstinent Condition—Score in Non-Abstinent Condition; FTND = Fagerström Test for Nicotine Dependence; MNWS = Minnesota Nicotine Withdrawal Scale; POMS = Profile of Mood States; QSU = Questionnaire of Smoking Urges; SHS = Subjective Happiness Scale. Models adjusted for respective non-abstinent scores, age, sex, Inventory for Depression and Anxiety Symptoms—General Depression subscale score, and UPPS-P Impulsive Behavior—Sensation Seeking subscale score.
*Significant after Bonferroni correction for multiple testing.
SH as A Moderator of the Association of Dependence with Abstinence-Induced Changes in Study Outcomes
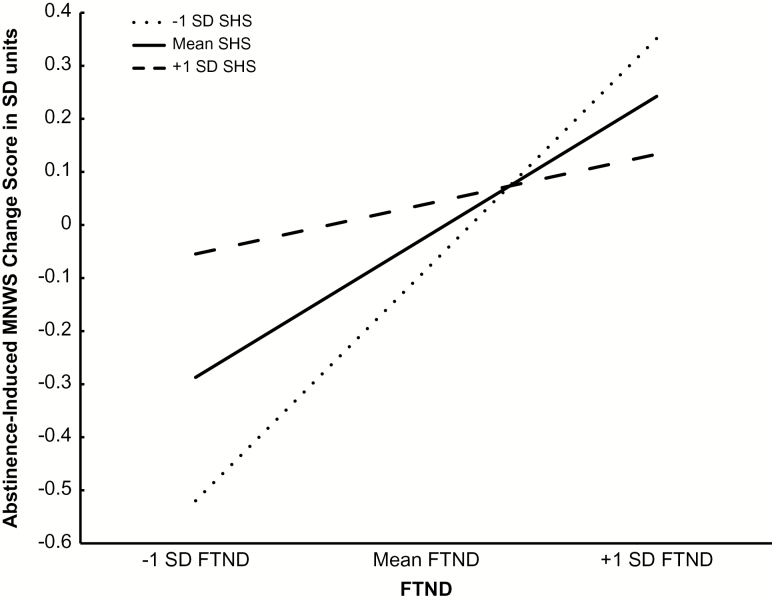
SH moderated the relationship between FTND and abstinence-induced increases in MNWS as evidenced by a significant FTND × SHS interaction term (Table 3). The FTND × SHS interaction term was not significant for other outcomes. Simple slopes analysis illustrated that the predictive effect of FTND on abstinence-induced increases in MNWS was weakened with increasing levels of SH. The relation of FTND and abstinence-induced changes in MNWS was β = .45 (p < .001) for individuals 1 SD below the mean SHS level, β = .25 (p < .001) for individuals at the mean SHS level, and β = .07 (p = .49) for individuals 1 SD above the mean SHS level (Figure 1).
Figure 1.
Simple slopes of tobacco dependence (Fagerstrom Test of Nicotine Dependence; FTND) predicting abstinence-induced changes in composite withdrawal symptoms (Minnesota Nicotine Withdrawal Scale; MNWS) by subjective happiness level (Subjective Happiness Scale; SHS). Reported slopes from adjusted model, which included FTND as the independent variable, abstinence-induced changes in MNWS as the dependent variable, and age, sex, depression, sensation seeking, and respective non-abstinent outcome scores as covariates. Lines represent 1 SD below the mean, the mean, and 1 SD above the mean scores on the SHS. All measures were standardized (M = 0, SD = 1) to facilitate ease of interpretation. Abstinence-Induced Change Score = Score in Abstinent Condition—Score in Non-Abstinent Condition.
Supplementary Analyses
Individual MNWS Item Analysis
Individual MNWS item analyses demonstrated main effects of tobacco dependence on all 11 MNWS symptoms (βs = .10−.27, ps < .04) apart from excessive hunger and drowsiness (ps > .06) and no main effects of SH. Additionally, significant FTND × SHS interaction terms were found for six individual withdrawal symptoms: craving, irritability/anger, anxiety/tension, difficulty concentrating, restlessness, and increased eating (βs = −.09−−.18, ps ≤ .03).
Discussion
To our knowledge, this is the first study to examine SH in relation to tobacco withdrawal using a laboratory design with an experimental abstinence manipulation. The results reported here contribute to a small, but emergent body of tobacco research aiming to identify the advantageous role of positive emotional traits to smokers. Main effect analyses indicated that tobacco dependence was associated with greater sensitivity to several subjective and behavioral abstinence effects, extending previous findings in racially-heterogeneous samples4,7 to an AA sample of daily smokers. We also found support for the hypothesized buffering role of SH, as SH suppressed the positive relation of tobacco dependence to abstinence-induced increases in a composite index of tobacco withdrawal amalgamating various affective, cognitive, and physiological symptoms. Specifically, for individuals with average to below average levels of SH, a positive association occurred between tobacco dependence and abstinence-induced increases in composite withdrawal. For individuals with above average levels of SH, however, this positive association was suppressed.
Factor-analytic assessments across versions of the MNWS generally conclude that “craving” is an important single-item withdrawal domain.5,36 Moreover, four out of the other five individual MNWS symptoms that reached significance in our model—irritability/anger, anxiety, difficulty concentrating, and restlessness—cluster together in a manner that resembles a 4-item MNWS “Mood” domain identified in prior factor-analytic work.5 Apart from “increased eating,” no physiological symptoms (ie, “excessive hunger,” “somatic complaints,” “drowsiness,” or “headaches”) were found to be significant. Thus, while SH moderated dependence-provoked increases in a broad composite measure of withdrawal spanning multiple dimensions (ie, affective, cognitive, physiological), individual item analyses suggest that SH may have only buffered against dependence-withdrawal associations in our sample that are specific to craving- and mood-based symptom clusters.
Results from individual MNWS item analyses also provide insight into why the hypothesized moderation only extended to the composite measure of withdrawal. Each individual withdrawal outcome (QSU, POMS-PA, POMS-NA) differ from the MNWS in their narrow focus and detailed assessment of a single aspect of the tobacco withdrawal experience. For example, although both the POMS and the MNWS contain items designed to assess “withdrawal-related mood,” the mood-related MNWS items that were significant in the moderation model (eg, “restlessness,” “difficulty concentrating,” “anxiety/tension”) capture aspects of mood that are conceptually related yet distinct from the affect-based POMS items that were found to be nonsignificant (eg, “overjoyed,” “unhappy”). It is possible that the mood- and craving-related items found on the broad composite MNWS measure more keenly capture the distinct features of withdrawal that are most salient to our sample of AA smokers. Alternatively, the moderating influence of SH may not have mapped onto the distinct components of withdrawal that are captured by the POMS, QSU, or Smoking Lapse Analogue Task. Further support for the specificity of our results to the MNWS measure may follow from the fact that it is the only withdrawal outcome tested to include all six of the tobacco withdrawal symptoms that have been found to correlate most strongly with tobacco dependence, our predictor (ie, craving, insomnia, anxiety, irritability, difficulty concentrating, and restlessness).
Broadly, our findings suggest that SH may act as a preventive buffer against a detrimental consequence of tobacco dependence—the experience of severe withdrawal symptoms upon smoking abstinence—in a subgroup who is at disproportionate risk of tobacco dependence and smoking-related health problems.1 Our results cohere with prior work documenting the stress-buffering influence of traits that comprise positive mental health.16 The findings also corroborate the distinct value of the SH trait to understanding the correlates of smoking, contributing to prior findings of bivariate inverse associations of aspects of SH (ie, PA, life satisfaction) with various smoking characteristics.20–23 The current report, however, is the first to our knowledge to illustrate how SH moderates the relationship between two factors that prolong smoking and amplify risk for tobacco-related disease: tobacco dependence and tobacco withdrawal symptomatology. Prior evidence of increased odds of successful smoking cessation experienced by individuals with higher levels of SH20,22 may be explained by the protective effect of SH against dependence-provoked tobacco withdrawal symptoms that often serve as a powerful deterrent to a successful cessation attempt. We believe these results support a conceptual model in which SH may function as a stress-buffering coping factor during acute smoking abstinence that offsets the expression of dependence in terms of severe withdrawal.
Although the current findings could potentially benefit all heavily tobacco-dependent smokers, we did not have comparison groups of non-AA smokers in our study. Thus, it is unclear if the reported interaction would remain in a racially diverse sample. However, the apparent advantage of SH against dependence-related tobacco withdrawal documented here may be of particular value to AA smokers. Compared to other racial groups, AAs may be more culturally socialized toward expressing emotional disturbance in terms of diminished PA instead of heightened NA44 and may be more motivated to smoke for PA enhancement.45 Although this study did not find that SH buffered against abstinence-induced deficits in PA, another study found that AA (vs. Non-Hispanic and Hispanic) smokers experienced greater reductions in PA after acute tobacco abstinence.24 Overall, this trend is suggestive that positive emotional states and traits may be particularly important to understanding and treating tobacco dependence in AA smokers.
One limitation of the study’s sample is the inclusion of only nontreatment-seeking smokers during a brief period of abstinence that is externally-imposed instead of intrinsically initiated. Although evidence suggests that tobacco withdrawal during experimentally-imposed abstinence is predictive of withdrawal following a self-initiated quit attempt within a naturalistic environment,46 it is possible that the results of this study may not generalize to smokers whose efforts to abstain are self-motivated. Additionally, while it is unclear whether these findings will generalize to tobacco withdrawal symptoms experienced after periods of abstinence that exceed 16 hours, withdrawal symptoms tend to be most severe and relapses occur most often during the first 48 hours of acute smoking abstinence.47 Furthermore, the exclusion of individuals who smoked ≤10 cigarettes per day may limit the generalizability of the current findings to AA smokers outside of our sample, who may be light and intermittent smokers.48 A further limitation to the study is the sole measurement of SH at single time point, as psychological well-being and SH may be influenced by one’s mood at the time of assessment.49 Lastly, a post-hoc “floor and ceiling effect” analysis revealed that the monetary incentives utilized for the self-administration period of the Smoking Lapse Analogue Task may not have been salient to participants in our sample, which may have contributed to the limited variance in the number of cigarettes participants were willing to smoke.
Despite these limitations, the current findings—that SH may protect against the severity of abstinence-induced and dependence-linked withdrawal symptoms among AA smokers—have implications for research and practice. If these results are deemed to be causal and generalizable to clinical settings, they raise the possibility that heavily-dependent AA smokers may benefit from smoking cessation interventions that target (1) SH or other positive emotional characteristics related to psychological well-being and (2) the cultivation of stress-coping strategies. Such treatments may perhaps offset the degree to which highly-dependent AA smokers experience adverse reactions to the stress brought on by subjective withdrawal during a quit attempt, which could in turn enhance cessation outcomes.
One set of treatment approaches that could be incorporated into standard cessation interventions to advance this end is positive psychological interventions (PPIs; eg, mood management, mindfulness-based stress reduction).50 We believe that this study provides a primer for future research investigating the effectiveness of PPI’s and or stress-reduction interventions among heavily dependent AA smokers and other groups subject to tobacco-related disparities. If this vein of ethno-culturally tailored intervention research were to demonstrate promise towards preventing relapse to smoking among AA smokers, it may be a productive step towards reducing the prevalence of smoking and its associated morbidity within the AA smoker community as well as the overall public health burden posed by tobacco dependence.
Funding
This work was supported by funds from the National Institute on Drug Abuse at the National Institutes of Health (grant number K01-DA040043) and the American Cancer Society (grant number RSG-13-163-01).
Declaration of Interests
None declared.
References
- 1. Vidrine JI, Reitzel LR, Wetter DW. Smoking and health disparities. Curr Cardiovasc Risk Rep. 2009;3(6):403–408. [Google Scholar]
- 2. Branstetter SA, Mercincavage M, Muscat JE. Predictors of the nicotine dependence behavior time to the first cigarette in a multiracial cohort. Nicotine Tob Res. 2015;17(7):819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pérez-Stable EJ, Benowitz NL. Do biological differences help explain tobacco-related disparities?Am J Health Promot. 2011;25(5 Suppl):S8–10. [DOI] [PubMed] [Google Scholar]
- 4. Shiffman S. Tobacco “chippers”–individual differences in tobacco dependence. Psychopharmacology (Berl). 1989;97(4):539–547. [DOI] [PubMed] [Google Scholar]
- 5. Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal: a replication and extension. Arch Gen Psychiatry. 1991;48(1):52–59. [DOI] [PubMed] [Google Scholar]
- 6. Nakajima M, al’Absi M. Predictors of risk for smoking relapse in men and women: a prospective examination. Psychol Addict Behav. 2012;26(3):633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aguirre CG, Madrid J, Leventhal AM. Tobacco withdrawal symptoms mediate motivation to reinstate smoking during abstinence. J Abnorm Psychol. 2015;124(3):623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59(3):218–227. [DOI] [PubMed] [Google Scholar]
- 9. Feigelman W, Gorman B. Toward explaining the higher incidence of cigarette smoking among black Americans. J Psychoactive Drugs. 1989;21(3):299–305. [DOI] [PubMed] [Google Scholar]
- 10. Lindenberg CS, Gendrop SC, Reiskin HK. Empirical evidence for the social stress model of substance abuse. Res Nurs Health. 1993;16(5):351–362. [DOI] [PubMed] [Google Scholar]
- 11. Wills TA, Hirky AE. Coping and substance abuse: a theoretical model and review of the evidence. In: Zeichnec M, Eudler NS, eds. Handbook of Coping: Therapy, Research, and Applications. New York, NY: Wiley; 1996:279–302. [Google Scholar]
- 12. Webb MS, Carey MP. Tobacco smoking among low-income Black women: demographic and psychosocial correlates in a community sample. Nicotine Tob Res. 2008;10(1):219–229. [DOI] [PubMed] [Google Scholar]
- 13. Leventhal AM. The sociopharmacology of tobacco addiction: implications for understanding health disparities. Nicotine Tob Res. 2016;18(2):110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lyubomirsky S, Lepper HS. A measure of subjective happiness: preliminary reliability and construct validation. Soc Indic Res. 1999;46(2):137–155. [Google Scholar]
- 15. Ong AD, Allaire JC. Cardiovascular intraindividual variability in later life: the influence of social connectedness and positive emotions. Psychol Aging. 2005;20(3):476–485. [DOI] [PubMed] [Google Scholar]
- 16. Pressman SD, Cohen S. Does positive affect influence health?Psychol Bull. 2005;131(6):925–971. [DOI] [PubMed] [Google Scholar]
- 17. Lyubomirsky S, Tucker KL. Implications of individual differences in subjective happiness for perceiving, interpreting, and thinking about life events. Motiv Emot. 1998;22(2):155–186. [Google Scholar]
- 18. Gomez M, Vincent A, Toussaint LL. Correlates of resilience in adolescents and adults. Int J Clin Psychiatry Ment Health. 2013;1(1):18–24. [Google Scholar]
- 19. Brummett BH, Boyle SH, Kuhn CM, Siegler IC, Williams RB. Positive affect is associated with cardiovascular reactivity, norepinephrine level, and morning rise in salivary cortisol. Psychophysiology. 2009;46(4):862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haller CS. Trajectories of smoking behavior as a function of mood and satisfaction with life: what matters most?J Affect Disord. 2016;190(15):407–413. [DOI] [PubMed] [Google Scholar]
- 21. Grassi MC, Alessandri G, Pasquariello S et al. Association between positivity and smoking cessation. Biomed Res Int. 2014;2014:780146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. Nicotine Tob Res. 2008;10(3):507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine Tob Res. 2004;6(1):39–47. [DOI] [PubMed] [Google Scholar]
- 24. Bello MS, Pang RD, Cropsey KL et al. Tobacco withdrawal amongst African American, Hispanic, and White smokers. Nicotine Tob Res. 2015;18(6):1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. First MB, Spitzer RL, Gibbon M, Williams JBW.. Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders, Research Version, Non-Patient Edition (SCID-I/NP). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 26. Sayette MA, Martin CS, Wertz JM, Perrott MA, Peters AR. The effects of alcohol on cigarette craving in heavy smokers and tobacco chippers. Psychol Addict Behav. 2005;19(3):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benowitz NL, Jacob P III, Ahijevych K et al. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. [DOI] [PubMed] [Google Scholar]
- 28. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The fagerström test for nicotine dependence: a revision of the fagerström tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 29. Doran N, Cook J, McChargue D, Spring B. Impulsivity and cigarette craving: differences across subtypes. Psychopharmacology (Berl). 2009;207(3):365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boden JM, Fergusson DM, Horwood LJ. Cigarette smoking and depression: tests of causal linkages using a longitudinal birth cohort. Br J Psychiatry. 2010;196(6):440–446. [DOI] [PubMed] [Google Scholar]
- 31. Kandel D, Chen K, Warner LA, Kessler RC, Grant B. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the U.S. population. Drug Alcohol Depend. 1997;44(1):11–29. [DOI] [PubMed] [Google Scholar]
- 32. Lucas RE, Gohm CL. Age and sex differences in subjective well-being across cultures. In: Diener E, Suh EM, eds. Culture and Subjective Well-being. Vol. 3 Cambridge, Mass: MIT Press; 2000:91–317. [Google Scholar]
- 33. Watson D, O’Hara MW, Simms LJ et al. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychol Assess. 2007;19(3):253–268. [DOI] [PubMed] [Google Scholar]
- 34. Whiteside SP, Lynam DR. The five factor model and impulsivity: using a structural model of personality to understand impulsivity. Pers Individ Dif. 2001;30(4):669–689. [Google Scholar]
- 35. Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. [DOI] [PubMed] [Google Scholar]
- 36. Toll BA, O’Malley SS, McKee SA, Salovey P, Krishnan-Sarin S. Confirmatory factor analysis of the Minnesota Nicotine Withdrawal Scale. Psychol Addict Behav. 2007;21(2):216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. [DOI] [PubMed] [Google Scholar]
- 38. Pang RD, Leventhal AM. Sex differences in negative affect and lapse behavior during acute tobacco abstinence: a laboratory study. Exp Clin Psychopharmacol. 2013;21(4):269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McNair DM, Lorr M, Droppelman LF.. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services; 1971. [Google Scholar]
- 40. McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addict Biol. 2009;14(1):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ameringer KJ, Leventhal AM. Psychological symptoms, smoking lapse behavior, and the mediating effects of nicotine withdrawal symptoms: a laboratory study. Psychol Addict Behav. 2015;29(1):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology (Berl). 2006;189(2):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. New York, NY: Guilford Press; 2013, http://www.personal.psu.edu/jxb14/M554/specreg/templates.pdf. [Google Scholar]
- 44. Consedine NS, Magai C. The uncharted waters of emotion: ethnicity, trait emotion and emotion expression in older adults. J Cross Cult Gerontol. 2002;17(1):71–100. [DOI] [PubMed] [Google Scholar]
- 45. Lam CY, Robinson JD, Carter BL, Wetter DW, Minnix JA, Cinciripini PM. Nicotine differentially inhibits the acoustic startle reflex in African American and Caucasian American smokers. Addict Behav. 2008;33(12):1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Strong DR, Leventhal AM, Evatt DP et al. Positive reactions to tobacco predict relapse after cessation. J Abnorm Psychol. 2011;120(4):999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shiffman S. Relapse following smoking cessation: a situational analysis. J Consult Clin Psychol. 1982;50(1):71–86. [DOI] [PubMed] [Google Scholar]
- 48. Pulvers K, Romero DR, Blanco L, Sakuma KL, Ahluwalia JS, Trinidad DR. Light and intermittent smoking among California Black, Hispanic/Latino, and non-Hispanic White men and women. Nicotine Tob Res. 2015;17(6):755–759. [DOI] [PubMed] [Google Scholar]
- 49. Schwarz N, Bohner G. Feelings and their motivational implications: moods and the action sequence. In: Gollwitzer PM, Bargh JA, eds. The Psychology of Action: Linking Cognition and Motivation to Behavior. New York, NY: Guilford Press; 1996:119–145. https://pub.uni-bielefeld.de/publication/1938409. [Google Scholar]
- 50. Bolier L, Haverman M, Westerhof GJ, Riper H, Smit F, Bohlmeijer E. Positive psychology interventions: a meta-analysis of randomized controlled studies. BMC Public Health. 2013;13(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]