Abstract
Abetalipoproteinemia (ABL) and chylomicron retention disease (CMRD) are extremely rare recessive forms of hypobetalipoproteinemia characterized by intestinal lipid malabsorption and severe vitamin E deficiency. Vitamin E is often supplemented in the form of fat-soluble vitamin E acetate, but fat malabsorption considerably limits correction of the deficiency. In this crossover study, we administered two different forms of vitamin E, tocofersolan (a water-soluble derivative of RRR-α-tocopherol) and α-tocopherol acetate, to three patients with ABL and four patients with CMRD. The aims of this study were to evaluate the intestinal absorption characteristics of tocofersolan versus α-tocopherol acetate by measuring the plasma concentrations of α-tocopherol over time after a single oral load and to compare efficacy by evaluating the ability of each formulation to restore vitamin E storage after 4 months of treatment. In patients with ABL, tocofersolan and α-tocopherol acetate bioavailabilities were extremely low (2.8% and 3.1%, respectively). In contrast, bioavailabilities were higher in patients with CMRD (tocofersolan, 24.7%; α-tocopherol acetate, 11.4%). Plasma concentrations of α-tocopherol at 4 months were not significantly different by formulation type in ABL or CMRD. This study provides new insights about vitamin E status in ABL and CMRD and suggests the potential of different formulations as treatment options.
Keywords: metabolic disease, lipid and lipoprotein metabolism, absorption, adipose tissue, hypocholesterolemia, Anderson disease, tocopherol
Hypobetalipoproteinemias (HBLs) represent a heterogeneous group of very rare diseases characterized by reduced plasma levels of LDL-cholesterol and apoB below the fifth age- and sex-specific percentile (1–3). Familial HBL (MIM 107730), the most frequent monogenic form of HBL with a codominant mode of inheritance, is mainly due to loss-of-function mutations in the APOB gene leading to a defect in the secretion of β-lipoproteins. The extremely rare (less than one in one million) recessive forms of primary monogenic HBL are represented by abetalipoproteinemia (ABL; MIM 200100) and chylomicron retention disease (CMRD; MIM 246700). ABL is due to mutations in the microsomal triglyceride transfer protein large subunit gene (MTTP), which encodes the MTP protein, the apoB chaperone protein, leading to a defect in apoB lipidation and, consequently, a lack of chylomicrons and VLDL (4, 5). CMRD is due to mutations in the SAR1B gene, which encodes the Sar1b protein involved in the control of the intracellular trafficking of chylomicrons in coat protein complex II (COPII)-coated vesicles (6).
The clinical phenotype of ABL, CMRD, and homozygous or compound heterozygous familial HBL is usually severe, being characterized by intestinal lipid malabsorption, steatorrhea, fat-soluble vitamin deficiency, and “failure to thrive” in the neonatal period (7). Most commonly, cases of ABL and homozygous or compound heterozygous familial HBL are complicated by retinal degeneration and ataxia beginning in the second decade of life if no treatment was initiated (3, 8). Oral α-tocopherol supplementation with high doses (50–200 IU/kg/day) has to be started as early as possible to prevent neurological and retinal disability and halt/abrogate progression of complications associated with this disease (9–11). However, despite initiation of vitamin treatment, fundoscopic and retinal changes may appear (12). Therefore, finding the most effective form and dose of tocopherol for treatment is of major interest because fat malabsorption considerably limits the correction of deficiencies with standard formulations of fat-soluble vitamins. In clinical nutrition, vitamin E is often supplemented in the form of vitamin E acetate. Tocofersolan, a water-soluble derivative of RRR-α-tocopherol, is a commercially available vitamin E supplement for lipid malabsorption syndromes, including chronic cholestasis and cystic fibrosis (13–15). To our knowledge, data on its effectiveness are scarce and the effects of this water-soluble derivative of tocopherol have never been studied in HBLs. Our hypothesis was that the water-soluble tocofersolan might be an effective form of oral vitamin E supplement in ABL and CMRD.
Furthermore, in these patients, plasma vitamin E concentration is significantly reduced due to defects of chylomicron assembly and secretion, which are necessary for its absorption. Vitamin E concentrations in adipose tissue (AT) and red blood cells (RBCs) have been suggested as potential relevant biomarkers of vitamin E status (16–18), but data on their reference values, particularly in children, are scarce in the scientific literature. In this context, we have previously established reference intervals for α-tocopherol in plasma, RBCs, and AT in healthy children aged 1 month to 18 years, and we have shown that they were suitable and relevant indicators to provide an overview of vitamin E status in CMRD (19).
The primary aim of this study was to evaluate the relative bioavailability of tocofersolan (Vedrop®) versus dl-α-tocopherol acetate (Toco 500®) and to propose a population pharmacokinetic (PK) model for plasma α-tocopherol concentrations after administration of either molecule in healthy volunteers (HVs) and in patients with ABL and CMRD. The secondary aims were: i) to compare the efficacy of a supplementation with tocofersolan versus α-tocopherol acetate to restore storage of vitamin E in plasma, RBCs, and AT after 4 months treatment in a randomized crossover clinical study; and ii) to evaluate the safety of the two molecules in these rare diseases.
MATERIALS AND METHODS
Subjects and study design
Hypocholesterolemia patients.
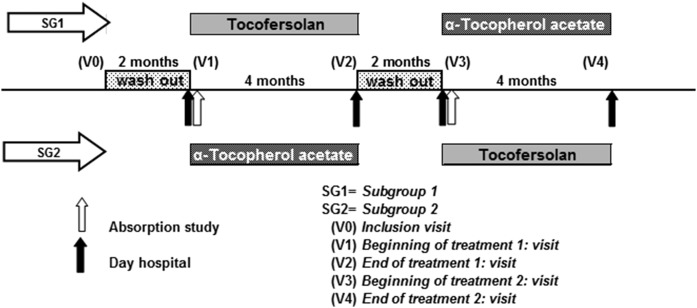
Seven patients with genetically proven HBL (three ABL and four CMRD) were included in the study (20, 21). A two-treatment two-period crossover randomized design was used (Fig. 1), assigning four of the subjects with the sequence α-tocopherol acetate followed by tocofersolan; the other three subjects were treated with the sequence of tocofersolan followed by α-tocopherol acetate. Assignment to each arm was double-blind. The protocol consisted of five visits: 1) an inclusion visit (V0) to initiate a washout period of 2 months without any α-tocopherol treatment; 2) a visit (V1) to administer an oral load of either α-tocopherol acetate or tocofersolan depending on the randomized group, followed by a 4 month period of treatment with the same molecule; 3) an end of treatment visit (V2) followed by a second 2 month washout; 4) a visit (V3) with an oral load and a 4 month treatment with the other molecule; and 5) an end of study visit (V4).
Fig. 1.
Study design.
The recommended total daily dose for Vedrop® is 17 mg/kg (= 25.33 IU/kg) of dl-α-tocopherol in the form of tocofersolan in pediatric patients suffering from congenital chronic cholestasis or hereditary chronic cholestasis. However, the recommended dosage for the prevention of abnormally low vitamin E levels in ABL is 50–200 mg/kg daily and 50 mg/kg/day in CMRD (8, 22, 23). Investigators therefore decided to administer an oral load of 100 IU/kg α-tocopherol acetate or tocofersolan during visits V1 and V3, and then 50 IU/kg daily for 4 months. Patients took the vitamin E twice a day with meals. Follow-up visits were scheduled to monitor safety and efficacy parameters as well as to assess compliance by counting returned empty bottles/box and measuring the remaining volume or capsules of Vedrop® or Toco 500®, respectively, if any.
HVs.
Five healthy adult volunteers (three men and two women from 32 to 62 years old) were recruited to study the PKs of tocofersolan and α-tocopherol acetate in a crossover design. Individuals were considered eligible for this study if they had no acute or chronic illness and were not taking vitamin or mineral supplements for more than 4 weeks before the study began. Each subject had an oral load with α-tocopherol acetate (1,000 IU) or tocofersolan (1,000 IU) in a fasting state, followed by a 1 month washout. Blood samples for determination of plasma α-tocopherol concentrations were collected at baseline (T0) and at time points 4, 8, 12, 24, 36, 48, 60, 72, 84, and 96 h. Blood samples were also collected over 96 h without vitamin E administration in order to assess natural intra-individual variations of α-tocopherol with food.
Ethical considerations
The study protocol was approved by the local ethics committee (March 2011, Hospices Civils de Lyon) and the French National Agency for Medicines and Health Products (A110125-36). It was conducted in accordance with the ethical principles of the Declaration of Helsinki. All patients or their parents gave written informed consent to participate and the clinical trial was registered at clinicaltrials.gov (NCT01457690).
Sample collection and processing
Hypocholesterolemic patients.
After the 2 month period of vitamin E washout, patients were hospitalized for 1 day during the V1 and V3 visits (Fig. 1). The morning of hospitalization, the participants’ AT was biopsied (see below). Then blood was collected at fasting state for determination of lipid profile (serum tubes, Becton Dickinson Vacutainer®, reference 369032) and α-tocopherol (lithium heparin tubes, Becton Dickinson Vacutainer®, reference 368496) in plasma and RBCs at baseline and after administration of α-tocopherol acetate or tocofersolan. The four following blood samples (at time points 4, 8, 12, and 24 h) were performed at the hospital. The subsequent samples were collected by a visiting nurse at home each morning in a fasting state and each evening at 8:00 PM for 4 days. Protected from light, samples were brought to the hospital laboratory every day after having verified the preanalytical stability of vitamin E under these conditions of transport (24). At the end of treatment, during visits V2 and V4, blood samples for lipid profile and α-tocopherol in plasma and RBCs, and AT biopsies were performed during the medical consultation.
HVs.
Six milliliters of blood (3 ml for lipid profile and 3 ml for plasma α-tocopherol) were collected at baseline before the single dose of tocofersolan or α-tocopherol acetate and at time points 4, 8, 12, 24, 36, 48, 60, 72, 84, and 96 h.
Biological studies
HPLC analysis of vitamin E.
Plasma, erythrocytes, and AT samples for determination of α-tocopherol concentrations were analyzed by HPLC using a Summit Dionex system (Thermo Fisher Scientific) and Chromeleon software (version 6.80; Thermo Fisher Scientific). Two levels of internal quality control were assayed at the beginning of each run. In addition, the laboratory participated regularly in external quality-assurance programs that provided monitoring of the measurement methods (bias and total error).
Plasma assay.
Briefly, after precipitation of plasma proteins by ethanol, α-tocopherol was extracted into hexane, evaporated under nitrogen, and the dried residue was dissolved in methanol/ethanol (85/15, v/v). The eluate was analyzed by HPLC at 292 nm. Separation was carried out on an Adsorbosphere HS C18 3 mm (Interchim) held at 37°C, using a gradient elution system starting with 100% methanol-acetonitrile (40/60, v/v) and ending with a 100% mixture of methanol-acetonitrile-dichloromethane (46/30/24, v/v), as described by Steghens et al. (25). Tocol was used as internal standard for measurement of vitamin E concentration to correct for losses during liquid/liquid extraction. The intra-assay coefficients of variation (CVs) for α-tocopherol in plasma were 2.4% and 1.1% at 17 and 40 μmol/l, respectively. The inter-assay CVs were 3.9% and 4%, respectively, at the same levels of concentration.
Erythrocyte assay.
Erythrocytes were prepared for tocopherol analysis as previously described (26). In brief, erythrocytes were washed three times in saline solution (9 g/l NaCl) containing pyrogallol (10 g/l) and resuspended in this solution to give a hematocrit of 50%, and 1 ml aliquots of washed erythrocytes were stored at less than −70°C until analysis. α-Tocopherol was analyzed as described above. The intra-assay CV was 4.1% at 5.3 μmol/l packed cells.
AT assay.
After local anesthesia (Xylocaine®), an AT sample was obtained by abdominal subcutaneous aspiration of AT and immediately frozen in liquid azote, and transfer in a −80°C refrigerator until analysis. Then, AT samples were removed from the connector, cut into smaller pieces, weighed, transferred to a ground glass tissue grinder containing ethanol and an internal standard (Tissue Grind Comp SZ 20; Kontes Kimble-Chase LLC) and chilled on ice. The AT was ground and produced a uniform homogenate. The homogenate was transferred to a 7 ml glass tube and α-tocopherol was extracted by hexane, as described above. The intra-assay CV was at 10.1% at 460 nmol of α-tocopherol per gram of AT.
Data analysis
Descriptive statistics.
Concentrations for α-tocopherol in plasma, RBCs, and AT in HVs and patients were reported using descriptive statistics [e.g., median and SD]. Areas under the curve (AUCs) for α-tocopherol acetate and tocofersolan in the PK assay were calculated according to the trapezoidal rule after calculating delta from baseline (T0) for each time point. Taking into account the distribution, Wilcoxon signed rank tests were performed to test the significance of differences in the same group (ABL, CMRD, or volunteers) and the Mann-Whitney test was performed to test the significance between groups. Statistical analyses were performed using GraphPad Prism (GraphPad Software). The differences were considered to be significant at the 95% confidence level (P < 0.05).
Population PK modeling of α-tocopherol plasma concentrations.
Plasma concentrations for α-tocopherol measured in HVs and in ABL and CMRD patients were analyzed using the so-called population approach, i.e., nonlinear mixed effect modeling. This approach allows the quantification of inter-individual variability and the estimation of PK parameters using all available data. Allometric scaling on clearance and volume of distribution (i.e., accounting for variability in PK processes due to body size, considering 0.75 and 1 exponent values for clearance and volume, respectively) allows model development for both adults (HVs) and hypocholesterolemic subjects (ABL and CMRD patients) (27, 28).
In order to allow a simultaneous analysis of all data, administered doses of α-tocopherol acetate and tocofersolan were converted into millimoles, and α-tocopherol observed concentrations were converted into micromoles per liter.
The typical kinetics of α-tocopherol was assumed to have a similar profile in all subgroups, but with different absorption parameters and basal endogenous production across pathologic conditions (HV, ABL, or CMRD). The absorption parameters (absorption rate and relative bioavailability) were also estimated to be different for tocofersolan and α-tocopherol acetate, while other parameters (volume of distribution, clearance, basal endogenous production) were assumed to be independent of the administered formulation.
Model development and parameter estimation were performed using NONMEM (version 7.3) (29). Model building was guided by parsimony principles in order to find the best compromise between the number of parameters to be estimated and model adjustment to data [based on the objective function value (OFV), as detailed below] and by model diagnostics (parameter precision and goodness-of-fit plots). Percent relative standard error (RSE) reports the precision on parameter estimated values; it can also be expressed in terms of 95% confidence interval of precision for each parameter estimate and calculated as [Parameter · (1±2 · RSE)].
Comparison of absorption characteristics.
The difference in absorption characteristics between α-tocopherol acetate and tocofersolan was evaluated by likelihood ratio test. Likelihood quantifies the quality of model agreement with data, and the OFV, defined as −2 log(likelihood), allows model comparison for nested models. Difference in OFV follows a chi-square distribution, with the degrees of freedom equal to the difference in the number of parameters. For instance, addition of one parameter is statistically significant if the OFV drop is greater than 3.84 (α risk 5%).
In order to compare bioavailability for α-tocopherol acetate and tocofersolan in each pathologic condition, model parameters were reestimated, constraining bioavailability of tocofersolan to be similar to α-tocopherol acetate. The likelihood ratio test was performed to evaluate the significance of the difference in bioavailability.
RESULTS
Physical and biochemical parameters of patients with ABL and CMRD
Three young adults with ABL (one girl, two boys; age = 18.3 ± 2.5 years; BMI 18.9 ± 1.9 kg/m2) and four with CMRD (three girls, one boy; age = 20.0 ± 2.8 years; BMI 19.8 ± 3.6 kg/m2) were included in the study.
While ABL patients presented lower LDL-cholesterol, triglycerides, and apoB concentrations than CMRD patients, both groups presented characteristic hypocholesterolemia (Table 1). Other biochemical parameters were close to normal values, except some hepatic biochemical parameters were slightly increased and CPK was dramatically increased in patients with CMRD, as previously described (30). Hemoglobin was decreased in two of three patients with ABL; their blood smear revealed many acanthocytes. Vitamin A was within the normal range with high-dose treatment (89,796 ± 85,675 IU/day), as well as vitamin D (85,714 ± 24,397 IU/month) and prothrombin ratio (14 ± 5 mg vitamin K per week).
TABLE 1.
Physical and biochemical parameters of patients with ABL and CMRD at baseline
| ABL | CMRD | |
| Number | 3 | 4 |
| Sex | 1 girl, 2 boys | 3 girls, 1 boys |
| Age (years) | 18.3 ± 2.52 | 20.0 ± 2.8 |
| Weight (kg) | 49.0 ± 4.0 | 56.3 ± 11.2 |
| Size (cm) | 155 ± 3.5 | 160 ± 9.9 |
| BMI (kg/m2) | 18.9 ± 1.9 | 19.8 ± 3.6 |
| Total cholesterol (mmol/l) | 1.04 ± 0.02 | 2.12 ± 0.8 |
| HDL cholesterol (mmol/l) | 1.02 ± 0.1 | 0.50 ± 0.05 |
| LDL-cholesterol (mmol/l) | 0.01 ± 0.02 | 1.26 ± 0.63 |
| Triglycerides (mmol/l) | 0.06 ± 0.01 | 0.61 ± 0.27 |
| ApoB (g/l) | 0.02 ± 0.0 | 0.37 ± 0.22 |
| Calcium (mmol/l) | 2.32 ± 0.05 | 2.28 ± 0.3 |
| Creatinin (μmol/l) | 40 ± 14.8 | 53.4 ± 14.2 |
| ASAT (U/l) | 52 ± 22.6 | 51.5 ± 8.0 |
| ALAT (U/l) | 86 ± 32.8 | 49.5 ± 5.5 |
| CPK (U/l) | 56 ± 22.5 | 523 ± 10.4 |
| GGT (U/l) | 93 ± 39.5 | 17.5 ± 16.8 |
| PAL (U/l) | 82 ± 7.21 | 103 ± 154 |
| Total bilirubin (μmol/l) | 20 ± 4.04 | 7 .5 ± 3.6 |
| Fer (pmol/l) | 17 ± 11.1 | 11 ± 4.0 |
| Ferritin (μg/l) | 34 ± 18.2 | 11 ± 5.3 |
| Hemoglobin (g/l) | 115 ± 36.1 | 136 ± 21 |
| Acanthocytes | +++ | + |
| Prothrombin ratio (%) | 82 ± 10.4 | 79.5 ± 6 |
| Vitamin A (μmol/l) | 1.98 ± 0.52 | 1.64 ± 0.55 |
| 25 OH vitamin D (nmol/l) | 204 ± 80.74 | 168 ± 82 |
Results are as expressed as median ± SD. ASAT, aspartate aminotransferase; ALAT, alanine aminotransferase; GGT, γ-glutamyl transferase; +, low number of acanthocytes (<10%); +++, high number of acanthocytes (> 35%).
Plasma α-tocopherol concentration profile
Descriptive results.
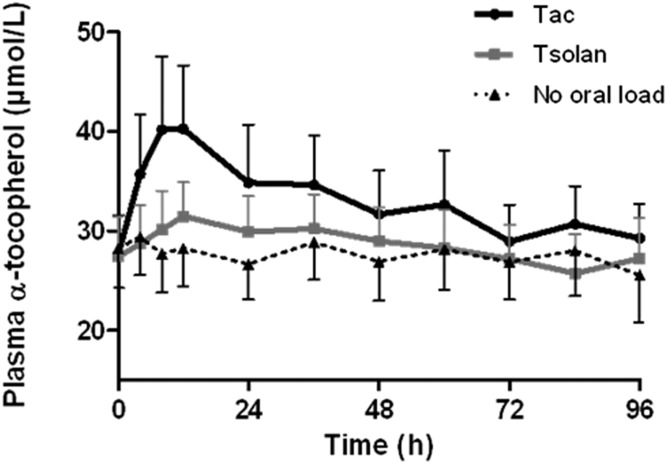
In healthy adult volunteers (Fig. 2), the AUC after a single administration of α-tocopherol acetate and tocofersolan (1,000 IU) was 56.7 and 15.9 μM·h, respectively. Both α-tocopherol acetate and tocofersolan were significantly better absorbed in HVs than in patients with ABL and CMRD (P < 0.05). In addition, α-tocopherol acetate was better absorbed than tocofersolan in HVs (P < 0.05).
Fig. 2.
Plasma α-tocopherol concentrations in HVs in the PK study. Five HVs received 1,000 UI of tocofersolan [RRR α-tocopheryl polyethylene glycol 1000 succinate (Tsolan)] or dl rac α-tocopheryl acetate (Tac) in a single oral load. Results as expressed as mean ± SEM.
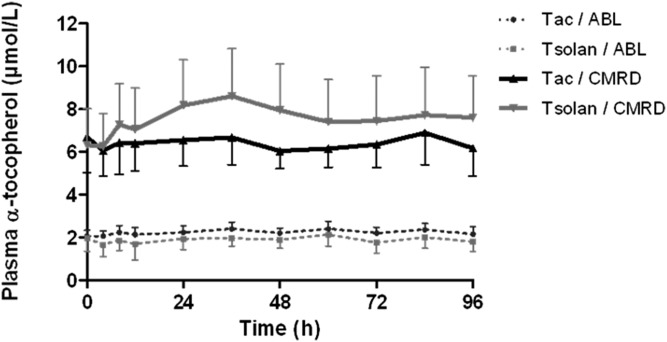
In ABL patients, the absorption of both molecules was almost nil (Fig. 3). No statistical analysis was performed due to the extremely low α-tocopherol concentration variations compared with the CV of the analytical method. In CMRD patients, interestingly, tocofersolan (AUC = 11.7 μM·h) seemed to be better absorbed than α-tocopherol acetate (AUC = 3.4 μM·h) (P < 0.05) (Fig. 3).
Fig. 3.
Plasma α-tocopherol concentrations in patients in the PK study. Three patients with ABL and four patients with CMRD received 100 UI/kg of tocofersolan [RRR α-tocopheryl polyethylene glycol 1000 succinate (Tsolan)] or dl rac α-tocopheryl acetate (Tac) in a single oral load. Results as expressed as mean ± SEM.
PK modeling.
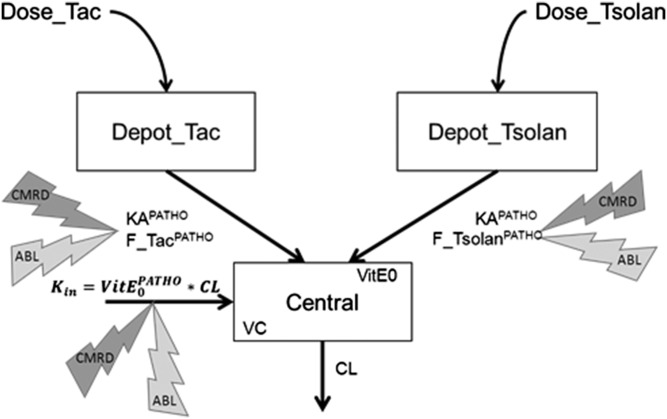
A PK model for plasma α-tocopherol concentrations after α-tocopherol acetate or tocofersolan administration was proposed. A schematic representation of the model is given in Fig. 4 and the corresponding set of equations in supplemental Table S1.
Fig. 4.
Schematic representation of the proposed model. Observations of plasma concentrations correspond to the central compartment with a volume of distribution (VC) and clearance (CL), while administered doses of Toco 500 and Vedrop are absorbed from the Depot_TOCO and Depot_VEDROP compartments respectively, at an absorption rate KAPATHO and relative bioavailability F_tocoPATHO and F_vedropPATHO. Kin is the endogenous production rate, depending on the basal concentration VitE0PATHO and clearance. ABL, abetalipoproteinemia; CMRD, chylomicron retention disease; PATHO, pathological condition.
Supplemental Fig. S1 presents the goodness-of-fit plots, i.e., observations versus population predictions (not accounting for inter-individual variability) and individual predictions (accounting for inter-individual variability). These graphics show a good agreement between observations and predictions, either considering the whole dataset or looking at subgroups (data collected after a single administration or after multiple administrations, stratified by pathologic conditions, stratified on the administered formulation, not shown). No trend with time or pathologic condition was found in the distribution of residuals (i.e., the difference between observations and predictions). Inter-individual variability was quantified for α-tocopherol acetate and tocofersolan bioavailability and for basal α-tocopherol concentration. Allometric scaling was implemented on clearance and volume of distribution, accounting for the influence of body size on kinetic parameters, and, therefore, takes into account the difference between adults and patients.
Table 2 presents the parameters estimated on the basis of all plasma α-tocopherol concentration values. All parameters were estimated with acceptable precision. RSEs are large for parameters related to ABL, but it should be remembered that only three ABL patients were considered in this analysis.
TABLE 2.
Table of parameter estimates
| Parameter | Typical Value (RSE %) | IIV [CV (RSE %)] |
| KA HV (h−1) | 0.241 (21%) | — |
| KA ABL | HV × 0.0394 (55%) = 0.0095 | — |
| KA CMRD | HV × 0.0167 (39%) = 0.0040 | — |
| Vc (liters/70 kg) | 184 (24%) | — |
| CL (l·h−1 / (70 kg0,75) | 5.43 (32%) | — |
| Relative bioavailability | ||
| Tac HV | 1 (−) | 77% (26%) |
| Tac ABL | HV × 3.08% (54%) = 3.08% | — |
| Tac CMRD | HV × 11.4% (28%) = 11.4% | — |
| Tsolan HV | 72.2% (17%) | 29.1% (29%) |
| Tsolan ABL | HV × 3.90% (20%) = 2.82% | — |
| Tsolan CMRD | HV × 34.2% (24%) = 24.7% | — |
| VitE0 (μmol·l−1) | ||
| VitE0 HV | 26.5 (12%) | 37.8% (13%) |
| VitE0 ABL | HV × 0.0643 (23%) = 1.70 | — |
| VitE0 CMRD | HV × 0.205 (26%) = 5.43 | — |
| Residual error | ||
| Additive (μmol·l−1) | 0.297 (25%) | — |
| Propotional (%) | 8.46 (12%) | — |
Bioavailability was calculated relatively to the group of HVs receiving α-tocopherol acetate (where 100% bioavailability is assumed). Bioavailability and basal level of plasma α-tocopherol in ABL and CMRD patients were estimated relatively to the corresponding value in HVs. IIV, interindividual variability (reported as CV); Tac, α-tocopherol acetate; Tsolan, tocofersolan; CL, clearance; Vc, volume of distribution; VitE0, basal level of plasma α-tocopherol.
Different basal levels of α-tocopherol plasma concentrations were estimated for HVs [typical value (95% confidence interval for precision): 26.5 μmol/l (20.14–32.86)], CMRD patients [5.43 μmol/l (2.61–8.25)], and ABL patients [1.70 μmol/l (0.92–2.48)]. The absorption rate constant (KA) was found to be different across pathologic conditions (the smaller the KA, the later the concentration peak), but not different among patients with the same pathology between α-tocopherol acetate or tocofersolan formulations.
Relative bioavailability, i.e., the relative fraction of the administered dose reaching plasma compared with a reference bioavailability of 1 for α-tocopherol acetate administered in HVs, was lower in HVs for tocofersolan [72.2% (47.7–96.7)] than for α-tocopherol acetate [100% (fixed)]. In ABL patients, the bioavailability was found to be low both for α-tocopherol acetate [3.1% (0–6.4)] and tocofersolan [2.8% ([1.7–3.9)]. In contrast, in CMRD patients, the bioavailability was less than half for α-tocopherol acetate [11.4% (5.0–17.8)] compared with tocofersolan [24.7% (12.8–36.6)]. In addition, the variability in α-tocopherol acetate bioavailability was very large (77% CV), while for tocofersolan it was 29%. Because it is not possible to distinguish inter-individual and inter-occasion variability, it could be expected that α-tocopherol plasma concentration profiles would be more reproducible after tocofersolan administration.
In order to compare absorption properties of tocofersolan and α-tocopherol acetate in each pathological condition, a likelihood ratio test was performed to evaluate model deterioration (based on OFV) when bioavailability was constrained to be similar for both formulations. Model agreement to the data was significantly impaired when bioavailability was constrained to be similar for α-tocopherol acetate and tocofersolan for the three pathologic conditions (supplemental Table S2).
Storage of vitamin E
After 2 month washout.
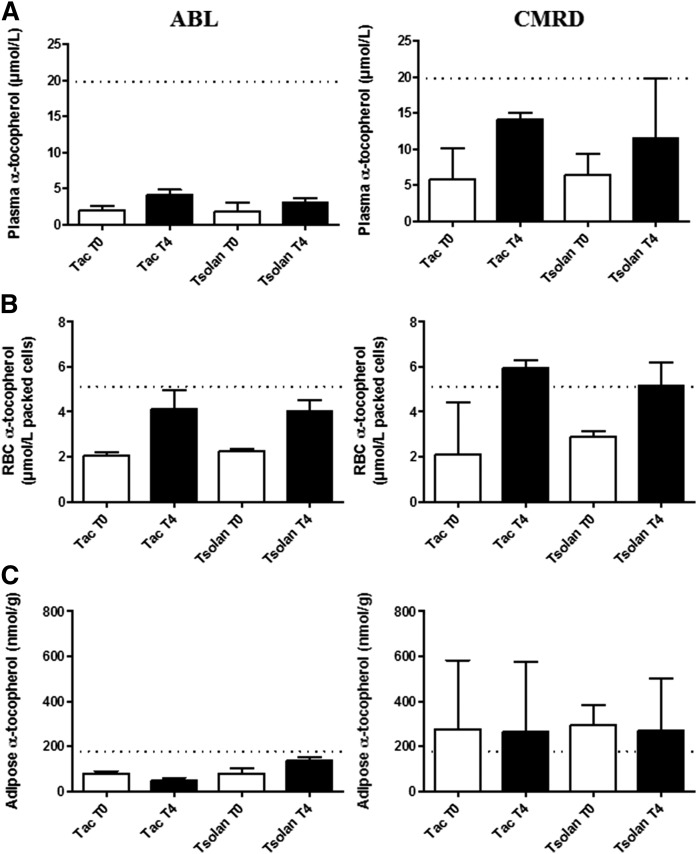
In ABL patients after the 2 month washout periods, α-tocopherol plasma concentrations were dramatically low (median ± SD = 1.8 ± 0.8 μmol/l after the first washout (WO1) and 1.9 ± 0.8 μmol/l after the second washout (WO2) (Fig. 5). In RBCs, the values were very similar to the lower normal value (WO1 = 2.19 ± 0.7 μmol/l; WO2 = 2.05 ± 0.6 μmol/l). In AT, α-tocopherol concentrations were very low (90 ± 12 nmol/g) compared with the usual values previously described, ranging from 60 to 573 nmol/g with a mean of 258 nmol/g (19).
Fig. 5.
Evolution of vitamin E concentrations after 4 months treatment. Three patients with ABL and four patients with CMRD were treated for 4 months with 50 UI/kg of tocofersolan (RRR α-tocopheryl polyethylene glycol 1000 succinate (Tsolan)] or dl rac α-tocopheryl acetate (Tac). α-Tocopherol concentrations in plasma (A), RBCs (B), and AT (C). Results are expressed as median with interquartile range. Dotted lines indicate mean values observed in healthy children (12–18 years).
In CMRD patients at baseline, α-tocopherol concentrations were low in plasma (WO1 = 5.8 ± 3.2 μmol/l; WO2 = 6.4 ± 3.9 μmol/l) and in RBCs (WO1 = 2.43 ± 1.9 μmol/l; WO2 = 2.72 ± 0.2 μmol/l), but significantly higher than in ABL patients (P < 0.05). In AT, concentrations were within the normal range (327 ± 214 nmol/g) and significantly higher than those in ABL (90 ± 12 nmol/g) (P < 0.05).
After 4 month treatment.
After 4 months of daily administration of 50 IU/kg of tocopherol acetate or tocofersolan, α-tocopherol concentrations increased significantly in plasma and RBCs in all patients, independently of the vitamin E formulation they were given (P < 0.05) (Fig. 5).
In ABL patients, α-tocopherol plasma concentrations only increased from 1.9 ± 0.5 μmol/l to 4.10 ± 1.7 μmol/l and from 1.8 ± 1.1 μmol/l to 3.0 ± 0.4 μmol/l after 4 month treatment with α-tocopherol acetate and tocofersolan, respectively. The α-tocopherol RBC concentrations were respectively increased from 2.05 ± 0.6 μmol/l to 4.13 ± 1.8 μmol/l and from 2.2 ± 0.7 μmol/l to 4.02 ± 1.3 μmol/l packed cells with α-tocopherol acetate and tocofersolan, respectively. In AT, α-tocopherol concentrations remained stable after both 4 month treatment periods, and close to the lower normal reference value in ABL (72 ± 52 nmol/g).
In CMRD patients, plasma α-tocopherol concentrations increased from 5.1 ± 3.7 μmol/l to 14.1 ± 3.4 μmol/l and from 6.4 ± 3.4 μmol/l to 11.45 ± 6.4 μmol/l after treatment by α-tocopherol acetate and tocofersolan, respectively. Concentrations in RBCs increased from 2.1 ± 1.9 μmol/l to 5.9 ± 1.4 μmol/l and from 2.9 ± 0.3 μmol/l to 5.14 ± 0.8 μmol/l packed cells with α-tocopherol acetate and tocofersolan, respectively; these values are close to the mean concentration in healthy children. (Statistical analyses were not performed due to the small number of patients in each group.) In AT, α-tocopherol concentrations remained stable after both 4 month treatment periods, but they were higher than in ABL patients and close to the mean normal reference value defined in healthy children (276 ± 183 nmol/g).
Interestingly, after 4 months, there was no statistical difference between the α-tocopherol concentrations in plasma, RBCs, or AT, whether patients had been treated with α-tocopherol acetate or tocofersolan (50 IU/kg/day).
Safety and compliance
No adverse events were observed in HVs or in hypocholesterolemic patients with tocofersolan, either during the oral load or during the 4 month treatment period. There was no significant change in any of the biochemical parameters used to monitor safety. In particular, coagulation and plasma creatinine values remained stable over time (Table 3).
TABLE 3.
Evolution of biological parameters after 4 month treatment with tocofersolan (50 IU/kg)
| ABL | CMRD | |||
| T0 | 4 Months | T0 | 4 Months | |
| Sodium (mmol/l) | 138 ± 2 | 138 ± 1 | 139 ± 2 | 139 ± 1 |
| Potass ium (mmol/l) | 4.2 ± 0.4 | 3.7 ± 0.3 | 4.0 ± 0.4 | 3.8 ± 0.2 |
| Creatinin (μmol/l) | 43 ± 14 | 45 ± 14 | 56 ± 13 | 56 ± 16 |
| Urea (mmol/l) | 3.9 ± 0.3 | 2.9 ± 0.6 | 2.8 ± 1.6 | 2.4 ± 2.2 |
| ASAT (U/l) | 56 ± 22 | 43 ± 3 | 51 ± 9 | 53 ± 8 |
| ALAT (U/l) | 86 ± 32 | 42 ± 7 | 51 ± 12 | 45 ± 13 |
| ALP (U/l) | 86 ± 9 | 64 ± 23 | 111 ± 112 | 109 ± 93 |
| GGT (U/l) | 93 ± 63 | 58 ± 62 | 16 ± 13 | 14 ± 22 |
| Total bilirubin (μmol/l) | 20 ± 5 | 19 ± 4 | 8 ± 3 | 8 ± 3 |
| Hemoglobin (g/l) | 115 ± 34 | 120 ± 25 | 135 ± 16 | 132 ± 23 |
| Prothrombin ratio (%) | 82 ± 10 | 87 ± 9 | 75 ± 15 | 81 ± 6 |
Results are expressed as median ± SD. ASAT, aspartate aminotransferase; ALAT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, γ-glutamyl transferase.
DISCUSSION
This study is the first to extensively evaluate intestinal absorption of vitamin E and to develop a PK model in very rare patients with hypocholesterolemia caused by ABL and CMRD.
Absorption of tocofersolan versus α-tocopherol acetate after single administration
The purpose of this study was to evaluate the absorption of tocofersolan versus α-tocopherol acetate in patients with ABL and CMRD in order to optimize vitamin E supplementation in these severely vitamin E-deficient patients.
In ABL patients, the vitamin E bioavailability was found to be extremely low for both α-tocopherol acetate [3.1% (0–6.4)] and tocofersolan [2.8% (1.7–3.9)]. These results are consistent with those reported by Traber et al. (31), who showed that ABL patients have a limited ability to absorb and transport vitamin E. In their study, plasma from ABL patients who received 7.4 g of labeled tocopherol contained about 1–10% of the labeled α-tocopherol concentrations of normal subjects who had only 0.30 g of α-tocopherol. In contrast, in CMRD patients, although the absorption of both molecules was very low considering the high doses administered, tocofersolan seemed to be better absorbed than α-tocopherol acetate. In our study, the population PK model showed that the bioavailability was more than doubled for tocofersolan [24.7% (12.8–36.6)] compared with α-tocopherol acetate [11.4% (5.0–17.8)]. The advantages of PK modeling compared with descriptive statistics are that: i) it allows simultaneous analysis of all data across pathologic conditions and across administered formulations; ii) it increases the power to estimate absorption parameters; and iii) it allows identification and quantification of inter-individual variability.
In HVs, α-tocopherol acetate was better absorbed than tocofersolan after a single oral dose [100% (reference for relative bioavailability) vs. 72.2% (47.7–96.7) according to PK model]. These results are similar to those reported by Dimitrov et al. (32), who showed that supplementation with tocofersolan had minimal effect on plasma α-tocopherol in normal individuals, whereas similar doses of α-tocopherol acetate resulted in a significant increase in plasma α-tocopherol. Several studies (13, 15, 33, 34) reported that intestinal absorption of tocofersolan is superior to that of dl-α-tocopherol acetate during severe cholestasis due to its unique structure incorporating a molecule of water-soluble PEG 1000, creating an amphipathic structure that presumably assumes a micellar form able to go through the unstirred water layer in the absence of bile acids. Only a few studies are available regarding tocofersolan in cystic fibrosis and the findings are contradictory, probably due to the design of the studies, and supplementation or no supplementation with pancreatic enzymes (14, 15, 35). The mechanism by which tocofersolan corrects these digestive (digestion impaired), but not intestinal (absorption of enterocytes normal), diseases is easily understandable. Interestingly, Traber et al. (36) also reported that tocofersolan can be an effective vitamin E supplement in short-bowel syndrome, despite severe fat malabsorption, and this case report has encouraged us to test the hypothesis that tocofersolan could be of interest in genetic syndromes associated with congenital lipid malabsorption due to impaired enterocytes.
Absorption and storage of α-tocopherol after a 4 month treatment
The secondary endpoint was to study the effect of tocofersolan or α-tocopherol acetate to improve storage of vitamin E. After a 4 month treatment period with each molecule (50 IU/kg/day) in a randomized crossover design, the concentrations of α-tocopherol were measured in plasma, RBCs, and AT. Paradoxically to the results obtained after administration of a single dose, after 4 months, there was no statistical difference between the α-tocopherol concentrations in plasma, RBCs, or AT, whether participants had been treated with α-tocopherol acetate or tocofersolan (50 IU/kg/day) in both pathologies. Different reasons could explain such a result: i) first, it is not impossible that, in the body, vitamin E is very quickly consumed because of the important oxidative stress in these patients (37); ii) second, it cannot be excluded that a part of the TPGS is hydrolyzed at the intestinal level in tocopherol, known to be poorly absorbed in the patients with ABL and CMRD (32). We could hypothesize that larger doses of both molecules and/or a longer treatment period could have increased concentrations in plasma and RBCs more with tocofersolan than with α-tocopherol acetate, particularly in CMRD patients, considering that tocofersolan seemed to be better absorbed in the PK assay in those patients. However, it is more likely that the nonsignificant difference that was seen might be related to the very small number of CMRD patients included in this study.
The link to pathophysiology
Although the results of the study did not show any difference between the two molecules in the long term storage of vitamin E, the design of the study provides an interesting overview on vitamin E status in these diseases.
In CMRD, α-tocopherol concentrations at baseline are 213 ± 28% higher than in ABL in plasma (P < 0.05), 45 ± 54% in RBCs (P = NS), and 375 ± 22% in AT (P < 0.05). In CMRD, vitamin E deficiency in plasma and RBCs was better improved than in ABL after 4 months of treatment. In plasma, although vitamin E concentrations increased mildly in CMRD, they were far from reaching the reference values in ABL due to defects of chylomicron assembly. Interestingly, RBC vitamin E concentrations increased to normal values after 4 months of supplementation, suggesting that RBC α-tocopherol concentrations, compared with plasma α-tocopherol concentrations, could be a good marker of compliance to treatment, even if they do not allow evaluation of the reserves of potentially deficient tissues, such as myelin or retina. AT vitamin E concentrations were stable over the study period and not influenced by the administration of the two molecules for 4 months. In ABL, AT α-tocopherol concentrations remained low despite long-term treatment with 50 IU/kg/day, confirming that higher doses for supplementation are required in ABL, although Kayden, Hatam, and Traber (16) reported that there appears to be no simple dose-to-tissue level relationship in ABL patients. In contrast, in CMRD, AT α-tocopherol concentrations were within the normal reference values, supporting the hypotheses that: i) long-term supplementation might allow correction of α-tocopherol depletion in AT despite low plasma values; and ii) 50 IU/kg/day is likely to be sufficient to treat CMRD (23). These observations are also consistent with the fact that complications are generally less severe in patients with CMRD than in patients with ABL and, particularly, the fact that retinitis pigmentosa and cirrhosis have not been reported in patients with CMRD (38). Some evidence in the literature suggests that minimal secretion of chylomicrons cannot be ruled out in CMRD (39–41). In particular, we cannot exclude that Sar1b’s paralogue protein [Sar1a, which shares 91% homology with Sar1b and is also expressed in the small intestine (6, 42)] could partially compensate the defect in Sar1b and allow minimal absorption of α-tocopherol via the chylomicron pathway (42). In contrast, in ABL, vitamin E is only exported via the HDL pathway.
Interestingly, patients who had the lowest concentrations of plasma α-tocopherol also had the lowest concentrations in RBCs and AT. Among the seven patients: i) All had a normal ophthalmological examination except for one ABL patient who had a normal visual field, color vision, electroretinogram, and electro-oculogram, but a moderately abnormal evoked potential test; this patient had the lowest AT α-tocopherol concentration. ii) All had a normal neurological examination except the previously mentioned patient, who had normal deep tendon reflexes and normal brain magnetic resonance imaging, but a sensory axonal neuropathy revealed by electromyogram, and another patient who was not always compliant with treatment.
In conclusion, we quantified the bioavailability and storage of vitamin E depending on galenic form (acetate and tocofersolan) in these very rare genetic hypocholesterolemias. Although α-tocopherol concentrations were not significantly different whether ABL and CMRD patients had been treated with one or the other formulation for 4 months, tocofersolan could be of greater advantage because: i) it was well tolerated both after a single administration and during 4 months of treatment in these patients, as has been reported in patients with other pathologies (13, 15, 43); ii) it allows easier administration in young children due to its water solubility and liquid form.
Acknowledgments
The authors are grateful to all patients and volunteers for agreeing to participate in this study. They also thank the Clinical Research and Information Department of Hospices Civils de Lyon as promoter and Barbara Baker for proofreading and improvement of English.
Footnotes
Abbreviations:
- ABL
- abetalipoproteinemia
- AT
- adipose tissue
- AUC
- area under the curve
- CMRD
- chylomicron retention disease
- CV
- coefficient of variation
- HBL
- hypobetalipoproteinemia
- HV
- healthy volunteer
- KA
- absorption rate constant
- OFV
- objective function value
- PK
- pharmacokinetic
- RBC
- red blood cell
- RSE
- relative standard error
- WO1
- first washout
- WO2
- second washout
This study was supported by the Association Lyonnaise de Logistique Posthospitalière (ALLP), a young investigator award from Hospices Civils de Lyon, and Orphan Europe Company. The funding organizations played no role in the design of the study, selection of subjects, review and interpretation of data, or preparation or approval of the manuscript.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Tarugi P., and Averna M.. 2011. Hypobetalipoproteinemia: genetics, biochemistry, and clinical spectrum. Adv. Clin. Chem. 54: 81–107. [PubMed] [Google Scholar]
- 2.Hooper A. J., and Burnett J. R.. 2014. Update on primary hypobetalipoproteinemia. Curr. Atheroscler. Rep. 16: 423. [DOI] [PubMed] [Google Scholar]
- 3.Levy E. 2015. Insights from human congenital disorders of intestinal lipid metabolism. J. Lipid Res. 56: 945–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharp D., Blinderman L., Combs K. A., Kienzle B., Ricci B., Wager-Smith K., Gil C. M., Turck C. W., Boumas M. E., Rader D. J., et al. . 1993. Cloning and gene defects in microsomal triglyceride transfer protein associated with abetalipoproteinaemia. Nature. 365: 65–69. [DOI] [PubMed] [Google Scholar]
- 5.Shoulders C. C., Brett D. J., Bayliss J. D., Narcisi T. M., Jarmuz A., Grantham T. T., Leoni P. R. D., Bhattacharya S., Pease R. J., and Cullen P. M.. 1993. Abetalipoproteinemia is caused by defects of the gene encoding the 97 kDa subunit of a microsomal triglyceride transfer protein. Hum. Mol. Genet. 2: 2109–2116. [DOI] [PubMed] [Google Scholar]
- 6.Jones B., Jones E. L., Bonney S. A., Patel H. N., Mensenkamp A. R., Eichenbaum-Voline S., Rudling M., Myrdal U., Annesi G., Naik S., et al. . 2003. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat. Genet. 34: 29–31. [DOI] [PubMed] [Google Scholar]
- 7.Di Filippo M., Moulin P., Roy P., Samson-Bouma M. E., Collardeau-Frachon S., Chebel-Dumont S., Peretti N., Dumortier J., Zoulim F., Fontanges T., et al. . 2014. Homozygous MTTP and APOB mutations may lead to hepatic steatosis and fibrosis despite metabolic differences in congenital hypocholesterolemia. J. Hepatol. 61: 891–902. [DOI] [PubMed] [Google Scholar]
- 8.Zamel R., Khan R., Pollex R. L., and Hegele R. A.. 2008. Abetalipoproteinemia: two case reports and literature review. Orphanet J. Rare Dis. 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller D. P., Lloyd J. K., and Bird A. C.. 1977. Long-term management of abetalipoproteinaemia. Possible role for vitamin E. Arch. Dis. Child. 52: 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller D. P., and Lloyd J. K.. 1982. Effect of large oral doses of vitamin E on the neurological sequelae of patients with abetalipoproteinemia. Ann. N. Y. Acad. Sci. 393: 133–144. [DOI] [PubMed] [Google Scholar]
- 11.Runge P., Muller D. P., McAllister J., Calver D., Lloyd J. K., and Taylor D.. 1986. Oral vitamin E supplements can prevent the retinopathy of abetalipoproteinaemia. Br. J. Ophthalmol. 70: 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowers I., Banin E., Merin S., Cooper M., and Granot E.. 2001. Long-term assessment of combined vitamin A and E treatment for the prevention of retinal degeneration in abetalipoproteinaemia and hypobetalipoproteinaemia patients. Eye (Lond.). 15: 525–530. [DOI] [PubMed] [Google Scholar]
- 13.Sokol R. J., Butler-Simon N., Conner C., Heubi J. E., Sinatra F. R., Suchy F. J., Heyman M. B., Perrault J., Rothbaum R. J., Levy J., et al. . 1993. Multicenter trial of d-alpha-tocopheryl polyethylene glycol 1000 succinate for treatment of vitamin E deficiency in children with chronic cholestasis. Gastroenterology. 104: 1727–1735. [DOI] [PubMed] [Google Scholar]
- 14.Papas K., Kalbfleisch J., and Mohon R.. 2007. Bioavailability of a novel, water-soluble vitamin E formulation in malabsorbing patients. Dig. Dis. Sci. 52: 347–352. [DOI] [PubMed] [Google Scholar]
- 15.Jacquemin E., Hermeziu B., Kibleur Y., Friteau I., Mathieu D., Le Coz F., Moyse D., Gérardin M., Jacqz-Aigrain E., and Munck A.. 2009. Bioavailability of oral vitamin E formulations in adult volunteers and children with chronic cholestasis or cystic fibrosis. J. Clin. Pharm. Ther. 34: 515–522. [DOI] [PubMed] [Google Scholar]
- 16.Kayden H. J., Hatam L. J., and Traber M. G.. 1983. The measurement of nanograms of tocopherol from needle aspiration biopsies of adipose tissue: normal and abetalipoproteinemic subjects. J. Lipid Res. 24: 652–656. [PubMed] [Google Scholar]
- 17.Bieri J. G., and Poukka R. K.. 1970. Red cell content of vitamin E and fatty acids in normal subjects and patients with abnormal lipid metabolism. Int. Z. Vitaminforsch. 40: 344–350. [PubMed] [Google Scholar]
- 18.Hatam L. J., and Kayden H. J.. 1979. A high-performance liquid chromatographic method for the determination of tocopherol in plasma and cellular elements of the blood. J. Lipid Res. 20: 639–645. [PubMed] [Google Scholar]
- 19.Cuerq C., Restier L., Drai J., Blond E., Roux A., Charriere S., Michalski M. C., Di Filippo M., Levy E., Lachaux A., et al. . 2016. Establishment of reference values of alpha-tocopherol in plasma, red blood cells and adipose tissue in healthy children to improve the management of chylomicron retention disease, a rare genetic hypocholesterolemia. Orphanet J. Rare Dis. 11: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chardon L., Sassolas A., Dingeon B., Michel-Calemard L., Bovier-Lapierre M., Moulin P., and Lachaux A.. 2009. Identification of two novel mutations and long-term follow-up in abetalipoproteinemia: a report of four cases. Eur. J. Pediatr. 168: 983–989. [DOI] [PubMed] [Google Scholar]
- 21.Charcosset M., Sassolas A., Peretti N., Roy C. C., Deslandres C., Sinnett D., and Lachaux A.. 2008. Anderson or chylomicron retention disease: molecular impact of five mutations in the SAR1B gene on the structure and the functionality of Sar1b protein. Mol. Genet. Metab. 93: 74–84. [DOI] [PubMed] [Google Scholar]
- 22.Muller D. P., Lloyd J. K., and Wolff O. H.. 1985. The role of vitamin E in the treatment of the neurological features of abetalipoproteinaemia and other disorders of fat absorption. J. Inherit. Metab. Dis. 8(Suppl 1): 88–92. [DOI] [PubMed] [Google Scholar]
- 23.Peretti N., Sassolas A., Roy C. C., Deslandres C., Charcosset M., Castagnetti J., Pugnet-Chardon L., Moulin P., Labarge S., Bouthillier L., et al. . 2010. Guidelines for the diagnosis and management of chylomicron retention disease based on a review of the literature and the experience of two centers. Orphanet J. Rare Dis. 5: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuerq C., Peretti N., Chikh K., Mialon A., Guillaumont M., Drai J., and Blond E.. 2015. Overview of the in vitro stability of commonly measured vitamins and carotenoids in whole blood. Ann. Clin. Biochem. 52: 259–269. [DOI] [PubMed] [Google Scholar]
- 25.Steghens J. P., Van Kappel A. L., Riboli E., and Collombel C.. 1997. Simultaneous measurement of seven carotenoids, retinol and alpha-tocopherol in serum by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 694: 71–81. [DOI] [PubMed] [Google Scholar]
- 26.Bieri J. G., Tolliver T. J., and Catignani G. L.. 1979. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am. J. Clin. Nutr. 32: 2143–2149. [DOI] [PubMed] [Google Scholar]
- 27.Liu T., Ghafoori P., and Gobburu J. V.. 2017. Allometry is a reasonable choice in pediatric drug development. J. Clin. Pharmacol. 57: 469–475. [DOI] [PubMed] [Google Scholar]
- 28.Holford N., Heo Y. A., and Anderson B.. 2013. A pharmacokinetic standard for babies and adults. J. Pharm. Sci. 102: 2941–2952. [DOI] [PubMed] [Google Scholar]
- 29.Beal S., Sheiner L., Boeckmann A., and Bauer R.. 2009. NONMEM User’s Guides (1989–2009). Icon Development Solutions, Ellicott City, MD. [Google Scholar]
- 30.Silvain M., Bligny D., Aparicio T., Laforet P., Grodet A., Peretti N., Ménard D., Djouadi F., Jardel C., Bégué J. M., et al. . 2008. Anderson’s disease (chylomicron retention disease): a new mutation in the SARA2 gene associated with muscular and cardiac abnormalities. Clin. Genet. 74: 546–552. [DOI] [PubMed] [Google Scholar]
- 31.Traber M. G., Rader D., Acuff R. V., Brewer H. B., and Kayden H. J.. 1994. Discrimination between RRR- and all-racemic-alpha-tocopherols labeled with deuterium by patients with abetalipoproteinemia. Atherosclerosis. 108: 27–37. [DOI] [PubMed] [Google Scholar]
- 32.Dimitrov N. V., Meyer-Leece C., McMillan J., Gilliland D., Perloff M., and Malone W.. 1996. Plasma alpha-tocopherol concentrations after supplementation with water- and fat-soluble vitamin E. Am. J. Clin. Nutr. 64: 329–335. [DOI] [PubMed] [Google Scholar]
- 33.Traber M. G., Sokol R. J., Ringel S. P., Neville H. E., Thellman C. A., and Kayden H. J.. 1987. Lack of tocopherol in peripheral nerves of vitamin E-deficient patients with peripheral neuropathy. N. Engl. J. Med. 317: 262–265. [DOI] [PubMed] [Google Scholar]
- 34.Socha P., Koletzko B., Pawlowska J., Proszynska K., and Socha J.. 1997. Treatment of cholestatic children with water-soluble vitamin E (alpha-tocopheryl polyethylene glycol succinate): effects on serum vitamin E, lipid peroxides, and polyunsaturated fatty acids. J. Pediatr. Gastroenterol. Nutr. 24: 189–193. [DOI] [PubMed] [Google Scholar]
- 35.Melhorn D. K. 1973. Vitamin E: who needs it? II. Diseases associated with vitamin E deficiency. Ohio State Med. J. 69: 830–833. [PubMed] [Google Scholar]
- 36.Traber M. G., Schiano T. D., Steephen A. C., Kayden H. J., and Shike M.. 1994. Efficacy of water-soluble vitamin E in the treatment of vitamin E malabsorption in short-bowel syndrome. Am. J. Clin. Nutr. 59: 1270–1274. [DOI] [PubMed] [Google Scholar]
- 37.Calzada C., Vericel E., Colas R., Guillot N., El Khoury G., Drai J., Sassolas A., Peretti N., Ponsin G., Lagarde M., et al. . 2013. Inhibitory effects of in vivo oxidized high-density lipoproteins on platelet aggregation: evidence from patients with abetalipoproteinemia. FASEB J. 27: 2855–2861. [DOI] [PubMed] [Google Scholar]
- 38.Sassolas A., Di Filippo M., Aggerbeck L. P., Peretti N., and Samson-Bouma M. E.. 2012. Anderson’s disease/chylomicron retention disease and mutations in the SAR1B gene. In Mutations in Human Genetic Disease. D. Cooper and J-M. Chen, editors. InTechOpen, London, UK. Chapter 13, 251–272. [Google Scholar]
- 39.Berriot-Varoqueaux N., Dannoura A. H., Moreau A., Verthier N., Sassolas A., Cadiot G., Lachaux A., Munck A., Schmitz J., Aggerbeck L. P., et al. . 2001. Apolipoprotein B48 glycosylation in abetalipoproteinemia and Anderson’s disease. Gastroenterology. 121: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 40.Bouma M. E., Beucler I., Aggerbeck L. P., Infante R., and Schmitz J.. 1986. Hypobetalipoproteinemia with accumulation of an apoprotein B-like protein in intestinal cells. Immunoenzymatic and biochemical characterization of seven cases of Anderson’s disease. J. Clin. Invest. 78: 398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy C. C., Levy E., Green P. H., Sniderman A., Letarte J., Buts J. P., Orquin J., Brochu P., Weber A. M., Morin C. L., et al. . 1987. Malabsorption, hypocholesterolemia, and fat-filled enterocytes with increased intestinal apoprotein B. Chylomicron retention disease. Gastroenterology. 92: 390–399. [DOI] [PubMed] [Google Scholar]
- 42.Georges A., Bonneau J., Bonnefont-Rousselot D., Champigneulle J., Rabes J. P., Abifadel M., Aparicio T., Guenedet J. C., Bruckert E., Boileau C., et al. . 2011. Molecular analysis and intestinal expression of SAR1 genes and proteins in Anderson’s disease (Chylomicron retention disease). Orphanet J. Rare Dis. 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Restellini S., Alaei M., Matthes T., Kherad O., Moschetta A., and Spahr L.. 2015. Effect of hydrosoluble vitamin E on erythrocyte membrane lipid composition in patients with advanced cirrhosis: an open-label pilot trial. Hepatol. Res. 45: 890–897. [DOI] [PubMed] [Google Scholar]