Abstract
Simultaneous activation of bile acid receptors farnesoid X receptor (FXR) and G protein-coupled bile acid receptor 1 (TGR5) by INT-767 significantly reduces atherosclerotic formation. In this study, we investigated the effect of simultaneous inactivation of these bile acid receptors in atherosclerosis and which bile acid receptor mediates the anti-atherogenic effect of INT-767. To investigate the role of simultaneous inactivation of FXR and TGR5 in vivo, we generated LDL receptor knockout (LDLR) KO mice with FXR and TGR5 dual deficiency, which exhibited severe atherosclerosis and aortic inflammation through nuclear factor κΒ activation. The lipid-lowering effects of INT-767 were completely blocked by FXR single deficiency but not TGR5 single deficiency. INT-767 was able to block atherosclerotic formation and decrease levels of aortic cytokines and chemokines in LDLR KO mice under either FXR or TGR5 single deficiency. Dual deficiency of FXR and TGR5 completely blocked the anti-atherogenic and anti-inflammatory effects of INT-767 in LDLR KO mice. We demonstrated that 1) FXR and TGR5 dual deficiency exacerbated the development of atherosclerosis and 2) the anti-atherogenic effect of INT-767 requires the anti-inflammatory effect but not the lipid-lowering effect through the simultaneous activation of FXR and TGR5. Our results indicate that dual activation of FXR and TGR5 is a promising strategy for treating atherosclerosis.
Keywords: atherosclerosis, bile acid, inflammation
INT-767 is a potent dual activator for farnesoid X receptor (FXR) and G protein-coupled bile acid receptor 1 (TGR5), which are two major receptors responsible for bile acid signaling (1). A number of recent reports showed beneficial effects of INT-767 on cardiovascular and metabolic diseases (2–8). Because administration of several FXR-specific or TGR5-specifc molecules showed similar beneficial effects on atherosclerosis (9–12), we studied whether simultaneous activation of both FXR and TGR5 is required for the beneficial effects of INT-767.
FXR is selectively expressed in the liver, kidneys, and intestines (13), while TGR5 is mainly expressed in the intestines and immune cells, including macrophages (14). Many lines of evidence have demonstrated that activation of FXR by FXR-specific agonists inhibits the development of atherosclerosis, possibly through a potent lipid-lowering effect (10, 11, 15). TGR5 activation by TGR5-specific agonists such as INT-777 also significantly blocked atherosclerotic formation in LDLR KO mice through the inhibition of NF-κB-mediated macrophage inflammation (12). Contrarily, either FXR or TGR5 deficiency did not significantly aggravate atherosclerosis in LDLR KO mice, suggesting that loss of one bile receptor was compensated for by the other receptor (12, 16).
We previously reported that INT-767 administration significantly blocked atherosclerotic formation in ApoE KO and LDLR KO mice (4), which was recently confirmed by another group (7). In addition, INT-767 treatment showed potent lipid-lowering effects and anti-inflammatory effects (4). In this study, we examined 1) which bile acid receptor mediates the anti-atherogenic, lipid-lowering, and anti-inflammatory effects of INT-767, 2) whether the lipid-lowering effect, anti-inflammatory effect, or both is required for the anti-atherogenic effect of INT-767, and 3) whether simultaneous inhibition of FXR and TGR5 aggravates atherosclerosis in LDLR KO mice.
MATERIALS AND METHODS
Animals
FXR−/− and LDLR−/− mice on a C57BL/6J background were obtained from the Jackson Laboratory (4, 17). TGR5−/− mice on a C57BL/6J background were kindly provided by Dr. Galya Vassileva (Merck) (18). Male FXR+/−; TGR5+/−; LDLR−/− mice were mated to obtain LDLR−/− (LDLR KO), FXR−/−; LDLR−/− (FXR-DKO), TGR5−/−; LDLR−/− (TGR5-DKO), FXR−/−; TGR5−/−; LDLR−/− (TKO) mice. Male animals were fed a Western diet (TD88137) containing INT-767 (30 mg/kg body weight) for 16 weeks. The dose (30 mg/kg body weight) of INT-767 was calculated based on weekly body weight and food intake. There was no difference in food intake among experimental groups. Eight animals per group were used for all experiments, unless otherwise mentioned. All animals were euthanized by isoflurane overdose after a 4 h fasting period. Animal experiments were approved by the Institutional Animal Care and Research Advisory Committee of the University of Colorado at Denver. INT-767 was kindly provided by Intercept Pharmaceuticals Inc. (New York, NY).
Histological and biochemical analysis
En face and histological analyses in the aortic sinus were performed as we previously described (4). Immunofluorescence analysis for CD68 (Bio-rad, Clone FA-11) and MCP1 (abcam ab25124) in the aortic root was performed using a Life Technologies EVOS fluorescence microscope as we described previously (4). Fasted serum lipids samples were quantified using commercially available kits (4).
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) analysis was performed as previously described (4). The DNA binding activity of nuclear factor-κB (NF-κB) was assayed according to the protocol from Promega Corp. Briefly, the oligo with NF-κB consensus binding element (Promega) was end-labeled by T4 polynucleotide kinase (Promega) using [γP32]ATP (BioRad). Thirty micrograms of total tissue extract was isolated from the aorta using 1X passive lysis buffer (Promega) and mixed with radio-labeled oligo for binding. Unlabeled cold probe was used to compete with the radio-labeled probe to show binding specificity. The reaction mixture was loaded to a 5% polyacrylamide gel under nondenaturing conditions and was separated by electrophoresis at 4°C. The gel was then dried and exposed to X-ray film to visualize the binding of NF-κB onto the radio-labeled probe. The binding specificity was shown by blockage of binding with excessive competitive cold probe, and the position of the NF-κB p65/50 complex was confirmed using anti-p65 (#8242, Cell Signaling Technology) and anti-p50 antibodies (14-6732-81, ThermoFisher).
RNA analysis
Real-time quantitative PCR assays were performed using an Applied Biosystems StepOne qPCR instrument. Primer sequences that are fully validated were obtained from the Primer Bank (http://pga.mgh.harvard.edu/primerbank, Harvard University) and published previously (4).
Statistical analysis
Data were collected from more than two independent analyses and reported as the means ± SEM. Two-way ANOVA or one-way ANOVA with a Newman-Keuls posthoc test was used for multi-group comparison. Significance was accepted at P < 0.05.
RESULTS
FXR and TGR5 simultaneous deficiency induces atherosclerosis and blocks the anti-atherogenic effect of INT-767
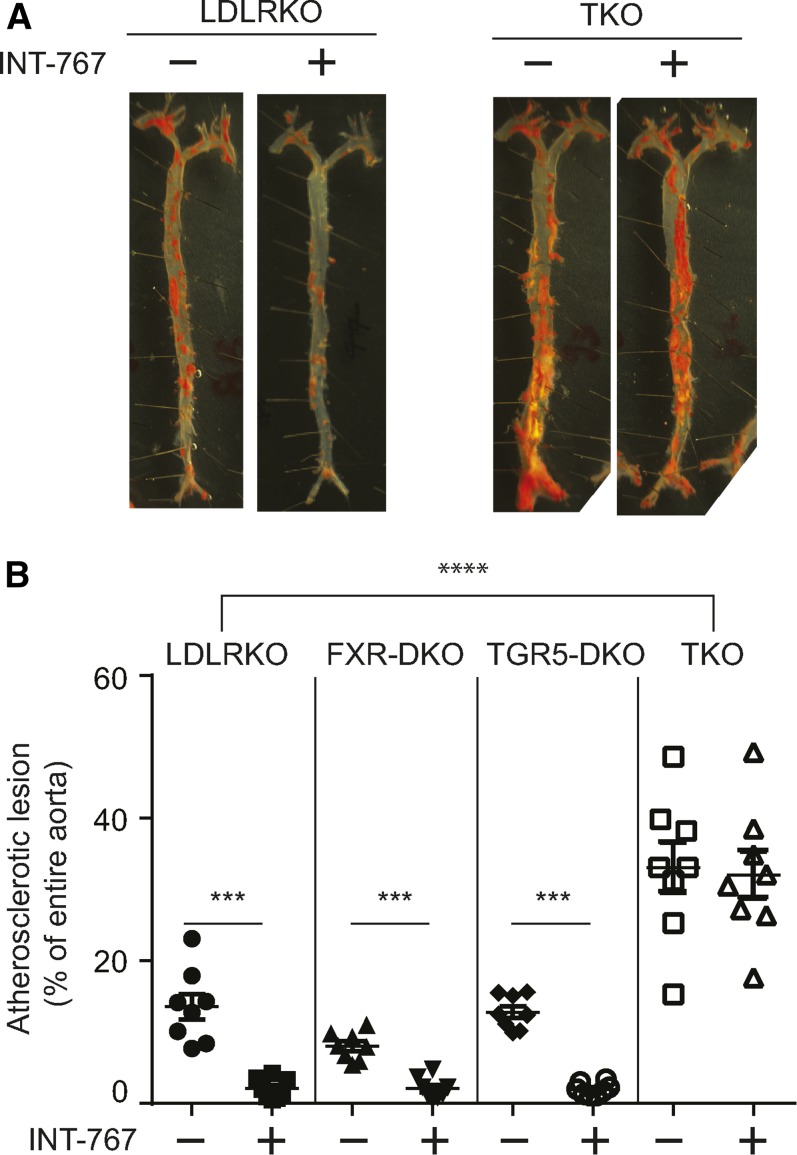
We previously reported that a potent FXR and TGR5 dual agonist, INT-767, blocked the development of atherosclerosis in ApoE KO and LDLR KO mice (4). A number of previous studies have reported that either FXR or TGR5 single activation by specific agonists was sufficient to reduce the development of atherosclerosis in mouse models such as ApoE KO and LDLR KO mice (10, 11, 15). FXR and TGR5 single deficiency, however, was not sufficient to aggravate atherosclerosis in LDLR KO mice (12, 16). We have examined 1) whether the anti-atherogenic effect of INT-767 requires activation of both FXR and TGR5 or whether one receptor is sufficient and 2) whether lack of major bile acid signaling aggravates atherosclerosis in murine models of atherosclerosis. We used LDLR KO male mice in this study because FXR and TGR5 dual deficiency caused embryonic lethal ApoE KO mice (data not shown) and INT-767 treatment was effective in preventing atherosclerosis equally in ApoE KO and LDLR KO mice (4). To determine which bile acid receptor mediates the anti-atherogenic effect of INT-767, 1) LDLR single KO mice, 2) FXR; LDLR double KO (FXR-DKO) mice, 3) TGR5; LDLR double KO (TGR5-DKO) mice, and 4) FXR; TGR5; LDLR triple KO (TKO) mice were treated with 30 mg/kg body weight INT-767. En face analysis clearly showed that FXR and TGR5 simultaneous deficiency remarkably aggravated atherosclerotic lesions of LDLR KO mice compared with LDLR KO, FXR-DKO, and TGR5-DKO (Fig. 1A, B). Consistent with a previous report (16), FXR single deficiency slightly reduced atherosclerotic lesions (P < 0.05 using Student’s t-test), whereas TGR5 single deficiency did not affect atherosclerosis in LDLR KO mice (Fig. 1B). In addition, either FXR or TGR5 single deficiency did not affect the anti-atherogenic effect of INT-767. INT-767 treatment reduced atherosclerotic lesions by 84%, 75%, and 84% in LDLR KO, FXR-DKO, and TGR5-DKO mice, respectively (Fig. 1B). On the other hand, FXR and TGR5 deficiency abrogated the anti-atherogenic effect of INT-767 (Fig. 1A, B). These data demonstrate that the anti-atherogenic effect of INT-767 occurs through activation of both FXR and TGR5.
Fig. 1.
FXR and TGR5 dual deficiency accelerates atherosclerosis in LDLR KO mice. A: Representative picture of en face analysis of atherosclerosis in LDLR KO, FXR-DKO, TGR5-DKO, and TKO male mice treated with INT-767. B: Quantification of en face analysis in mice treated with INT-767 for 16 weeks. ***P < 0.001, ****P < 0.0001.
FXR mediates the lipid-lowering effect of INT-767
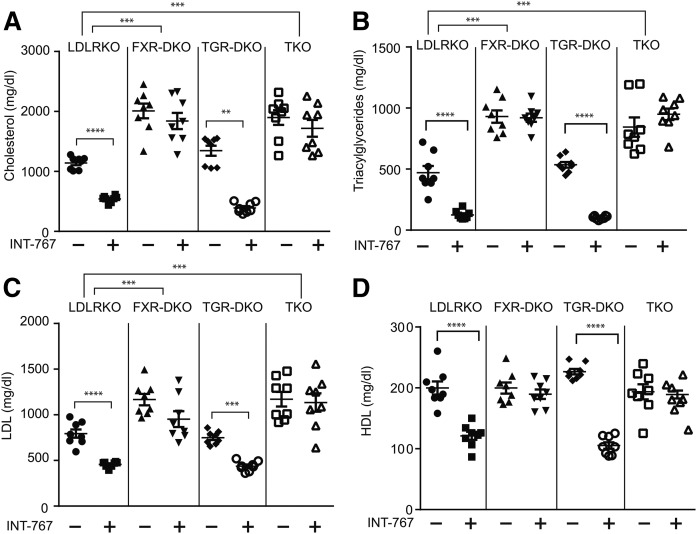
Consistent with our previous report (4), INT-767 elicited the potent lipid-lowering effect in male LDLR KO mice. Levels of serum total cholesterol, triacylglyceride, LDL-cholesterol, and HDL-cholesterol were reduced by 53%, 73%, 50%, and 39%, respectively, in LDLR KO mice treated with INT-767 (Fig. 1). TGR5 deficiency did not affect levels of serum lipids or the lipid-lowering effect of INT-767. INT-767 treatment reduced levels of serum total cholesterol, triacylglyceride, LDL-cholesterol, and HDL-cholesterol by 70%, 81%, 42%, and 33%. FXR-DKO and TKO mice had significantly higher levels of serum total cholesterol, triacylglycerides, and LDL-cholesterol compared with LDLR KO mice (Fig. 2). In addition, FXR single and FXR/TGR5 dual deficiency completely blocked the lipid-lowering effects of INT-767 (Fig. 2). These data suggest that INT-767 reduced serum lipids through FXR activation.
Fig. 2.
FXR single deficiency is sufficient to block the lipid-lowering effect of INT-767. Mice were treated with INT-767 for 16 weeks. Total cholesterol (A), triacylglycerides (B), LDL-cholesterol (C), and HDL-cholesterol (D) of LDLR KO, FXR-DKO, TGR5-DKO, and TKO mice treated with INT-767. **P < 0.01, ***P < 0.001, ****P < 0.0001.
FXR and TGR5 simultaneous deficiency aggravates aortic inflammation and blocks the-anti-inflammatory effect of INT-767 in LDLR KO mice
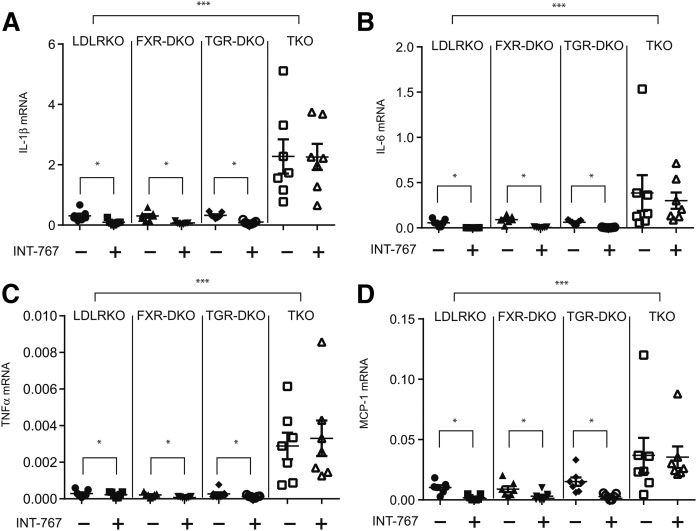
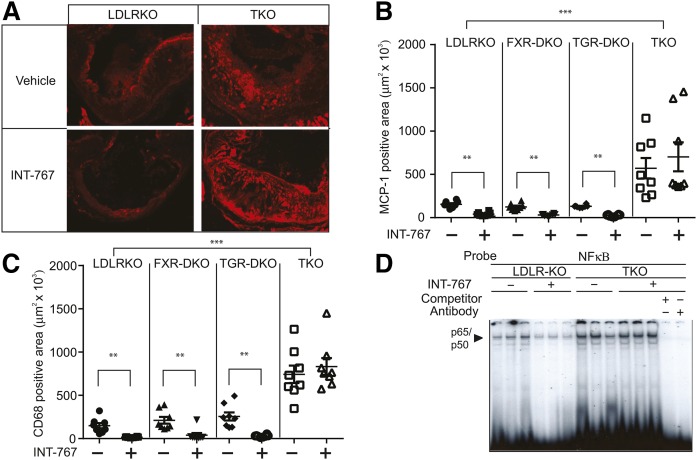
We previously reported that INT-767 reduced aortic cytokine and chemokine expression of ApoE−/− mice through the attenuation of NFκΒ activation (4). In this study, we examined which bile acid receptor mediates the anti-inflammatory effect of INT-767. While FXR and TGR5 single deficiency did not alter mRNA levels of cytokine and chemokines, FXR and TGR5 dual deficiency drastically increased levels of aortic IL-1β, IL-6, TNFα, and MCP-1 by 7.4-fold, 15.0-fold, 6.9-fold, and 7.4-fold (Fig. 3A–D). INT-767 treatment significantly reduced mRNA levels of aortic IL-1β, IL-6, TNFα, and MCP-1 in LDLR KO, FXR-DKO, and TGR5-DKO mice, whereas FXR and TGR5 dual deficiency completely blocked the effect of INT-767 (Fig. 3A–D). Consistent with quantitative PCR analysis, immunofluorescence microscopic analysis showed that FXR and TGR5 simultaneous deficiency but not FXR or TGR5 single deficiency, significantly increased C68-macrophages and MCP-1 positive areas in atherosclerotic lesions of LDLR KO mice (Fig. 4A–C). Consistent with a previous report (4), INT-767 treatment reduced CD68-macrophages and MCP-1 positive areas in atherosclerotic lesions of LDLR KO mice. While FXR and TGR single deficiency did not affect CD68 and MCP-1 positive lesions, FXR and TGR5 simultaneous deficiency completely blocked the effect of INT-767 on macrophage infiltration and MCP-1 expression (Fig. 4A–C). We previously showed that the anti-inflammatory effect of INT-767 occurs through inhibition of the aortic NFκΒ pathway (4). INT-767 significantly reduced levels of active NFκΒ p65/p50 complexes in the aortas of LDLR KO mice (Fig. 4D). FXR and TGR5 simultaneous deficiency increased levels of aortic active NFκΒ and also blocked the effect of INT-767 (Fig. 4D).
Fig. 3.
FXR and TGR5 dual deficiency induces aortic cytokine and chemokine expression. Mice were treated with INT-767 for 16 weeks. mRNA levels of aortic IL-1β (A), IL-6 (B), TNFα (C), and MCP-1 (D) in LDLR KO, FXR-DKO, TGR5-DKO, and TKO mice treated with INT-767. mRNA levels were analyzed by RT-qPCR. *P < 0.01, ***P < 0.001.
Fig. 4.
FXR and TGR5 dual deficiency induces NFκΒ-mediated inflammation. Mice were treated with INT-767 for 16 weeks. A: Representative picture of immunofluorescence analysis of MCP-1 in the aortas of LDLR KO and TKO mice treated with INT-767. Quantification of immunofluorescence analysis of MCP-1 (B) and CD68 (C) in the aortas of LDLRKO, FXR-DKO, TGR5-DKO, and TKO mice treated with INT-767. D: NFκΒ binding activity in the aortas of LDLR KO and TKO mice treated with INT-767. Treatment with either cold probe competitor or antibodies against p50/p65 completely blocked the specific binding of p50/p65 NFκΒ complex to the NFκΒ consensus oligonucleotide probe. **P < 0.01, ***P < 0.001.
DISCUSSION
We and other laboratories previously reported that treatment with an FXR and TGR5 dual bile acid receptor agonist, INT-767, reduced atherosclerotic lesions in murine models such as LDLR KO and ApoE KO mice (4, 7). In addition, INT-767 significantly reduced circulating lipids such as LDL-cholesterol and the expression of aortic inflammatory cytokines and chemokines through the inhibition of the NFκΒ pathway. However, because treatment with either FXR- or TGR5-specific agonists was sufficient to reduce the development of atherosclerosis in mouse models of atherosclerosis (10–12, 15), in this study, we examined which bile acid receptor activation mediates the anti-atherosclerotic effect, the lipid-lowering effect, and the anti-inflammatory effect of INT-767. Our current study clearly demonstrated that FXR and TGR5 simultaneous activation is required for the anti-atherosclerotic effect of INT-767. In addition, other bile acid-activated receptors, such as vitamin D receptor, pregnane X receptor, and muscarinic receptor (19–21), are not involved in the anti-atherosclerotic effect of INT-767.
The anti-atherosclerotic effect of INT-767 was not required to be present for the lipid-lowering effect to occur. While FXR sole deficiency was sufficient to block the lipid-lowering effect of INT-767, INT-767 was still able to reduce atherosclerotic formation in FXR-DKO mice, similar to LDLR KO mice. These results suggest that the presence of the anti-inflammatory effect of INT-767 is more crucial than the anti-atherosclerotic effect in order for the lipid-lowering effect of INT-767 to occur. Our previous in vitro studies and studies from others have suggested that the anti-inflammatory property of bile acids is mediated through TGR5 activation (4, 12). TGR5 is highly expressed in leukocytes such as macrophages (14), whereas FXR expression is low or undetectable in leukocytes (4, 16, 22). While FXR or TGR5 single deficiency did not affect the anti-inflammatory effect of INT-767, FXR and TGR5 simultaneous deficiency completely blocked the anti-inflammatory effect. Our data show that FXR and TGR5 activation both contribute to the anti-inflammatory effect of INT-767 in vivo. Because bone marrow cell transplants lacking both FXR and TGR5 did not induce atherosclerotic formation in irradiated LDLR KO mice (data not shown), we need to find tissues in which FXR and TGR5 activation is more critical for the development of atherosclerosis and aortic inflammation.
The current study is the first report showing that simultaneous inhibition of FXR and TGR5 is required for aggravating atherosclerotic formation in LDLR KO mice. A number of studies have shown that either FXR or TGR5 sole activation is sufficient to reduce the development of atherosclerosis. Paradoxically, however, either FXR or TGR5 sole deficiency was not sufficient to aggravate atherosclerosis in several mouse models, including LDLR KO mice (16, 22). In addition, FXR sole deficiency decreased atherosclerotic formation in male LDLR KO mice (16). These results suggest that there are cross-talks, synergistic alterations, and compensatory alterations between FXR and TGR5 signaling. In the intestine, FXR activation leads to activation of TGR5 through the alteration of gut microbiota and their bile acid metabolites (23). These results suggest that subsequent and indirect activation of TGR5 by FXR activation and vice versa may be required for the anti-atherosclerotic effects of FXR- and TGR5-specific agonists to occur. Hyperlipidemia and inflammation are major hallmarks of atherosclerosis. TKO mice showed severe hyperlipidemia and aortic inflammation. However, as INT-767 inhibits atherosclerosis in the presence of hyperlipidemia in FXR-DKO mice, the induction of aortic NFκΒ-mediated inflammation is more crucial for acclerating atherosclerosis by FXR and TGR5 simultaneous deficiency.
Our current study made four major findings: 1) Lacking major bile acid signaling, FXR and TGR5 aggravate aortic NFκΒ-mediated inflammation and atherosclerosis, 2) simultaneous activation of FXR and TGR5 is required for the anti-atherosclerotic and anti-inflammatory effects of INT-767 to occur, 3) the lipid-lowering effect of INT-767 occurs solely through the activation of FXR and 4) the anti-inflammatory effect of INT-767 is more critical than the lipid-lowering effect for the anti-atherosclerotic effect of INT-767 to occur. Taken together, these findings suggest that targeting both FXR and TGR5 simultaneously may be more effective for treatment of atherosclerosis than targeting either receptor alone.
Acknowledgments
The authors thank L. Adorini and M. Pruzanski (Intercept) for the INT-767 and G Vassileva (Merck) for the TGR5 knockout mice.
Footnotes
Abbreviations:
- DKO
- double KO
- FXR
- farnesoid X receptor
- LDLR
- LDL receptor
- NF-κΒ
- nuclear factor-κΒ
- TGR5
- G protein-coupled bile acid receptor 1
- TKO
- triple KO
This study was supported by National Institutes of Health Grants R01DK096030, R01HL117062, R01HL133545 and R01HL132318. The authors have declared that no conflict of interest exists.
REFERENCES
- 1.Rizzo G., Passeri D., De Franco F., Ciaccioli G., Donadio L., Orlandi S., Sadeghpour B., Wang X. X., Jiang T., Levi M., et al. . 2010. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol. Pharmacol. 78: 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baghdasaryan A., Claudel T., Gumhold J., Silbert D., Adorini L., Roda A., Vecchiotti S., Gonzalez F. J., Schoonjans K., Strazzabosco M., et al. . 2011. Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2−/− (Abcb4−/−) mouse cholangiopathy model by promoting biliary HCO output. Hepatology. 54: 1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMahan R. H., Wang X. X., Cheng L. L., Krisko T., Smith M., El Kasmi K., Pruzanski M., Adorini L., Golden-Mason L., Levi M., et al. . 2013. Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. J. Biol. Chem. 288: 11761–11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyazaki-Anzai S., Masuda M., Levi M., Keenan A. L., and Miyazaki M.. 2014. Dual activation of the bile acid nuclear receptor FXR and G-protein-coupled receptor TGR5 protects mice against atherosclerosis. PLoS One. 9: e108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beli E., Yan Y., Moldovan L., Vieira C. P., Gao R., Duan Y., Prasad R., Bhatwadekar A., White F. A., Townsend S., et al. . 2018. Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes. Epub ahead of print. April 30, 2018; doi: 10.2337/db18-0158 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth J. D., Feigh M., Veidal S. S., Fensholdt L. K., Rigbolt K. T., Hansen H. H., Chen L. C., Petitjean M., Friley W., Vrang N., et al. . 2018. INT-767 improves histopathological features in a diet-induced ob/ob mouse model of biopsy-confirmed non-alcoholic steatohepatitis. World J. Gastroenterol. 24: 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jadhav K., Xu Y., Xu Y., Li Y., Xu J., Zhu Y., Adorini L., Lee Y. K., Kasumov T., Yin L., et al. . 2018. Reversal of metabolic disorders by pharmacological activation of bile acid receptors TGR5 and FXR. Mol. Metab. 9: 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X. X., Wang D., Luo Y., Myakala K., Dobrinskikh E., Rosenberg A. Z., Levi J., Kopp J. B., Field A., Hill A., et al. . 2018. FXR/TGR5 dual agonist prevents progression of nephropathy in diabetes and obesity. J. Am. Soc. Nephrol. 29: 118–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanniman E. A., Lambert G., McCarthy T. C., and Sinal C. J.. 2005. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J. Lipid Res. 46: 2595–2604. [DOI] [PubMed] [Google Scholar]
- 10.Hartman H. B., Gardell S. J., Petucci C. J., Wang S., Krueger J. A., and Evans M. J.. 2009. Activation of farnesoid X receptor prevents atherosclerotic lesion formation in LDLR−/− and apoE−/− mice. J. Lipid Res. 50: 1090–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mencarelli A., Renga B., Distrutti E., and Fiorucci S.. 2009. Antiatherosclerotic effect of farnesoid X receptor. Am. J. Physiol. Heart Circ. Physiol. 296: H272–H281. [DOI] [PubMed] [Google Scholar]
- 12.Pols T. W., Nomura M., Harach T., Lo Sasso G., Oosterveer M. H., Thomas C., Rizzo G., Gioiello A., Adorini L., Pellicciari R., et al. . 2011. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 14: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bookout A. L., and Mangelsdorf D. J.. 2003. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl. Recept. Signal. 1: e012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawamata Y., Fujii R., Hosoya M., Harada M., Yoshida H., Miwa M., Fukusumi S., Habata Y., Itoh T., Shintani Y., et al. . 2003. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 278: 9435–9440. [DOI] [PubMed] [Google Scholar]
- 15.Hambruch E., Miyazaki-Anzai S., Hahn U., Matysik S., Boettcher A., Perovic-Ottstadt S., Schluter T., Kinzel O., Krol H. D., Deuschle U., et al. . 2012. Synthetic farnesoid X receptor agonists induce high-density lipoprotein-mediated transhepatic cholesterol efflux in mice and monkeys and prevent atherosclerosis in cholesteryl ester transfer protein transgenic low-density lipoprotein receptor (−/−) mice. J. Pharmacol. Exp. Ther. 343: 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Wang X., Vales C., Lee F. Y., Lee H., Lusis A. J., and Edwards P. A.. 2006. FXR deficiency causes reduced atherosclerosis in Ldlr−/− mice. Arterioscler. Thromb. Vasc. Biol. 26: 2316–2321. [DOI] [PubMed] [Google Scholar]
- 17.Sinal C. J., Tohkin M., Miyata M., Ward J. M., Lambert G., and Gonzalez F. J.. 2000. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 102: 731–744. [DOI] [PubMed] [Google Scholar]
- 18.Vassileva G., Golovko A., Markowitz L., Abbondanzo S. J., Zeng M., Yang S., Hoos L., Tetzloff G., Levitan D., Murgolo N. J., et al. . 2006. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem. J. 398: 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staudinger J. L., Goodwin B., Jones S. A., Hawkins-Brown D., MacKenzie K. I., LaTour A., Liu Y., Klaassen C. D., Brown K. K., Reinhard J., et al. . 2001. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. USA. 98: 3369–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makishima M., Lu T. T., Xie W., Whitfield G. K., Domoto H., Evans R. M., Haussler M. R., and Mangelsdorf D. J.. 2002. Vitamin D receptor as an intestinal bile acid sensor. Science. 296: 1313–1316. [DOI] [PubMed] [Google Scholar]
- 21.Raufman J. P., Chen Y., Cheng K., Compadre C., Compadre L., and Zimniak P.. 2002. Selective interaction of bile acids with muscarinic receptors: a case of molecular mimicry. Eur. J. Pharmacol. 457: 77–84. [DOI] [PubMed] [Google Scholar]
- 22.Guo G. L., Santamarina-Fojo S., Akiyama T. E., Amar M. J., Paigen B. J., Brewer B. Jr., and Gonzalez F. J.. 2006. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim. Biophys. Acta. 1761: 1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pathak P., Cen X., Nichols R. G., Ferrell J. M., Boehme S., Krausz K. W., Patterson A. D., Gonzalez F. J., and Chiang J. Y. L.. 2018. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology. Epub ahead of print. February 27, 2018; doi: 10.1002/hep.29857 . [DOI] [PMC free article] [PubMed] [Google Scholar]