Abstract
We investigated the associations of ten previously identified high risk molecular lipid species and three ceramide ratios with the occurrence of major adverse cardiac events (MACEs) during a median follow-up of 4.7 years in patients with coronary artery disease (CAD). Between 2008 and 2011, 581 patients underwent diagnostic coronary angiography or percutaneous coronary intervention for stable angina pectoris (SAP) or acute coronary syndrome (ACS). Blood was drawn prior to the index procedure and lipid species were determined. The primary endpoint was the occurrence of a MACE, comprising all-cause mortality, nonfatal ACS, or unplanned coronary revascularization. The secondary endpoint comprised all-cause mortality or nonfatal ACS. During a median follow-up of 4.7 [IQR: 4.2–5.6] years, 155 patients (27%) had MACEs. In multivariable analyses, Cer(d18:1/16:0) concentration was associated with MACEs {hazard ratio 2.32; 95% CI [1.09–4.96] per natural logarithm (ln) (pmol/ml) P = 0.030} after adjustment for cardiac risk factors, clinical presentation, statin use at baseline, and admission nonHDL cholesterol level. Furthermore, after multivariable adjustment, concentrations of Cer(d18:1/16:0), Cer(d18:1/20:0), Cer(d18:1/24:1), and their ratios to Cer(d18:1/24:0) were associated with the composite endpoint death or nonfatal ACS. The data together show the circulating ceramide lipids we investigated here are associated with adverse cardiac outcome during long-term follow-up independent of clinical risk factors.
Keywords: lipidomics, ceramides, heart, atherosclerosis, vascular biology, follow-up, prognosis
Established lipid markers such as total cholesterol, LDL cholesterol, triglycerides (TGs), and HDL cholesterol have long formed the cornerstone of lipid-based risk stratification in coronary artery disease (CAD) (1–4). However these measures alone do not fully capture the complexity of the altered lipid metabolism in cardiovascular disease (2), and this may be the reason that they fail to identify a substantial proportion of patients at high risk for coronary events (1).
Lipidomics is a systems-based study of all lipids (5) that has been defined as the full characterization of lipid molecular species and their biological roles (6). In its most advanced form, lipidomics is able to quantify hundreds of diverse molecular lipid species across multiple lipid classes such as sphingolipids, phospholipids, sterol esters, and acylglycerols (7), many of which play an integral role in modulation of biological function such as formation of cellular membranes, energy storage, and cell signaling (8, 9). Because lipidomics provides such detailed lipid profiles, it may further improve risk stratification of CAD patients and provide novel mechanistic insights into CAD (4).
In line with this hypothesis, we have recently performed lipidomics in the Ludwigshafen Risk and Cardiovascular Health (LURIC) study and identified several molecular lipid species that are associated with fatal events in patients with CAD (1). In the current study, we hypothesized that these ten previously identified high risk molecular lipid species and three ceramide ratios are associated with occurrence of major adverse cardiac events (MACEs) during long-term follow-up.
METHODS
Study population and design
The design of the European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis (ATHEROREMO) has been described elsewhere in detail (10). In brief, from 2008 until 2011, 581 patients with an indication for diagnostic coronary angiography (CAG) and/or percutaneous coronary intervention (PCI) due to stable angina pectoris (SAP) or acute coronary syndrome (ACS) at the Erasmus MC, Rotterdam, The Netherlands, were included. Prior to the CAG or PCI procedure, blood samples were collected from the arterial sheath and were transported to the clinical laboratory of Erasmus MC within 2 h after blood collection for storage at −80°C. All included patients were 18 years or older. The ATHEROREMO study was approved by the medical ethics committee of Erasmus MC and was performed in accordance with the criteria described in the Declaration of Helsinki. Written informed consent was obtained from all included patients.
Serum concentrations of cholesterol and TGs
Levels of total cholesterol, LDL cholesterol, HDL cholesterol, and TGs were measured in the clinical laboratory of the Erasmus MC in serum samples using a Roche/Hitachi cobas c 701/702 analyzer (Roche Diagnostics, Indianapolis, IN) on the Cobas 8000 modular analyzer platform (Roche Diagnostics).
Plasma concentrations of molecular lipids
Molecular lipids and lipid ratios that were previously found to be associated with fatal cardiovascular outcome at a P < 0.05 level in the LURIC study were selected for evaluation in the current study (1). These included cholesteryl esters (CEs): CE 14:0, CE 18:3, CE 20:4, CE 20:5, and CE 22:5; ceramides (Cer): Cer(d18:1/16:0), Cer(d18:1/20:0), Cer(d18:1/24:0), and Cer(d18:1/24:1); ceramide ratios: Cer(d18:1/16:0)/Cer(d18:1/24:0), Cer(d18:1/20:0)/Cer(d18:1/24:0), and Cer(d18:1/24:1)/Cer(d18:1/24:0)), and lactosylceramide (LacCer): LacCer(d18:1/18:0).
Plasma samples for measurement of lipid concentrations were available for 574 patients. Stored plasma samples were subjected to lipid extraction at Zora Biosciences, Finland. Briefly, samples (10 μl) were spiked with known amounts of lipid-class specific, nonendogenous synthetic internal standards, D6-CE 18:0 (C/D/N Isotopes Inc.,Pointe-Claire, Quebec, Canada), Cer(d18:1/17:0) (Avanti Polar Lipids Inc., Alabaster, AL) and D3-LacCer(d18:1/16:0) (Matreya LLC, State College, PA). Lipid extraction was performed using chloroform (HPLC grade) (Rathburn Chemicals Ltd., Walkerburn, Scotland), methanol, and acetic acid (both LC-MS grade) (Sigma-Aldrich GmbH, Steinheim, Germany) (11). After lipid extraction, samples were reconstituted in chloroform-methanol (1:2, v/v) for sphingolipids analysis, and for molecular shotgun lipidomic analysis, the extracts were further diluted with chloroform-methanol (1:2, v/v) containing 5 mM ammonium acetate. Quality control samples were prepared along with the actual samples for lipidomic analyses to monitor the extraction and MS performance. The intra-day (n = 3) average coefficient of variation of sphingolipids and CEs was less than or equal to 6% and inter-day [n = 24 for Cer and LacCer; n = 23 for CE except for CE(22:5) n = 22] coefficient of variation was less than 21% for both sphingolipids and CE.
Sphingolipids were analyzed on a QTRAP® 5500 mass spectrometer (AB SCIEX, Concord, Canada) equipped with an ultra-high pressure liquid chromatography (UHPLC) system CTC PAL autosampler (Leap Technologies) and Accela 1250 Pump (Thermo Fisher Scientific, Agawam, MA). Chromatographic separation was performed on an Acquity BEH C18, 2.1 × 50 mm column with a particle size of 1.7 μm (Waters, Milford, MA). Mobile phases were 10 mM ammonium acetate in water with 0.1% formic acid (solvent A) and 10 mM ammonium acetate in acetonitrile-isopropanol (4:3, v/v) containing 0.1% formic acid (solvent B). Lipids were separated with linear gradient from 75% B to 100% B in 15 min. Flow rate was 500 µl/min and column temperature was 60°C. Data was collected using multiple reaction monitoring in positive ion mode (12). Curtain gas was set at 25, ion spray voltage was set at 5000, and ion source was heated to 400°C. Collision energy was optimized for each lipid class. Collision energy for Cer and LacCer was set to 40 and 45, respectively.
Shotgun lipidomics was performed to monitor CEs on a QTRAP® 5500 mass spectrometer (AB SCIEX) equipped with a robotic nanoflow ion source NanoMate HD (Advion, Ithaca, NY) as described (11). CEs were analyzed in positive ion mode using precursor ion scanning of 369.35 with collision energy 30 (13). Mass spectrometry data files were processed using MultiQuant™ 2.0.1 or LipidView™ 1.0 (AB SCIEX) (13). Identified lipids were quantified by normalizing against their respective internal standard and volume of plasma used for the extraction. The limit of quantification (LOQ) for Cer, LacCer, and CE in extract was 0.0004 µM, 0.0016 µM, and 0.012 µM, respectively. All lipids monitored were within the LOQ. The LOQ was defined as the lowest point in the calibration curve with a signal-to-noise ratio greater than or equal to 10.
Follow-up and study endpoints
Clinical and vital status of patients were collected from medical charts, civil registries, or by written or telephone contacts with the patients or relatives. All living patients participating in this study received a questionnaire consisting of queries regarding the occurrence of MACEs and readmissions. For patients with adverse events, hospital discharge letters were obtained and treating physicians or institutions were contacted if necessary for additional information.
The primary endpoint was the occurrence of a MACE comprising all-cause mortality, nonfatal ACS, or unplanned coronary revascularization. The secondary endpoint comprised all-cause mortality and nonfatal ACS. ACS was defined as the clinical diagnosis of ST-segment elevation myocardial infarction (STEMI), nonSTEMI, or unstable angina pectoris in accordance with the guidelines of the European Society of Cardiology (14, 15). Unplanned coronary revascularization was defined as unplanned repeated PCI or unplanned coronary artery bypass grafting (CABG). The endpoints were adjudicated according to their definitions by a clinical events committee that was blinded to the lipid data.
Statistical analysis
Categorical variables are presented as numbers and percentages. The distributions of continuous variables, including lipid concentrations and lipid ratios, were examined for normality by visual inspection of the histogram. Normally distributed continuous variables are presented as mean ± SD. Nonnormally distributed continuous variables (which included molecular lipid concentrations and lipid ratios) are presented as median (interquartile range [IQR]) and their natural logarithm (ln) was used for further analyses.
Patients lost during follow-up were considered at risk until the date of last contact, at which time-point they were censored. Cox proportional hazards models were used to evaluate the associations between molecular lipids and clinical study endpoints. For patients who experienced more than one event, the first was considered. The results are presented as hazard ratios (HRs) per unit increase in (ln-transformed) molecular lipid concentrations or lipid ratios, with 95% CIs. First, all analyses were performed univariably. In the multivariable analyses, gender, age, hypertension, hypercholesterolemia, diabetes mellitus, and statin use were considered as potential confounders and were entered as covariates. These covariates were chosen for etiologic reasons and were based on existing literature (16). To evaluate whether the associations between molecular lipids and the clinical endpoints are independent of serum LDL cholesterol levels or serum nonHDL cholesterol levels, baseline serum LDL cholesterol level and baseline serum nonHDL cholesterol level were additionally (and consecutively) added into the multivariable models. Serum nonHDL level was calculated by subtracting HDL cholesterol level from total cholesterol level. In the full cohort, indication for CAG (ACS versus SAP) was also entered as a covariate. Interaction terms were added to the model to account for possible effect modification by indication for baseline CAG. Subsequently, analyses were stratified on indication for CAG. All data were analyzed with SPSS software (SPSS 23.0 IBM Corp., Armonk, NY). All statistical tests were two-tailed and P-values < 0.05 were considered statistically significant.
RESULTS
Baseline characteristics
The baseline clinical characteristics and the lipid concentrations of the ATHEROREMO study are summarized in Table 1 and Table 2. In total, 574 patients were included. The mean age of the patients was 61.5 years and 75% were men. A total of 55% patients were diagnosed with ACS (28% STEMI and 26% nonSTEMI) and 46% patients with SAP. PCI was performed in 88% of the patients during the index procedure. Prior to the index procedure, median serum LDL cholesterol level was 2.71 [IQR: 2.12–3.54] mmol/l, median serum HDL cholesterol level was 1.04 [IQR: 0.87–1.29] mmol/l, median serum nonHDL cholesterol level was 3.23 [IQR: 2.54–4.00] mmol/l, and median serum TG level was 1.27 [IQR: 0.88–1.83] mmol/l in the full cohort. ACS patients had significantly higher serum LDL cholesterol level {(median: 3.10 [IQR: 2.32–3.87] mmol/l) P = < 0.001}, higher serum nonHDL cholesterol level {(median: 3.56 [2.81–4.36]mmol/l) P = < 0.001} and lower serum TG level {(median = 1.15 [IQR: 0.77–1.77] mmol/l) P = < 0.001)} compared with SAP patients (median: 2.37 [IQR: 1.94–2.99] mmol/l, 2.83 [2.35–3.56] mmol/l and 1.41 [IQR: 1.05–1.94] mmol/l, respectively). In addition, several other clinical characteristics were significantly different between the ACS patients and the SAP patients (Table 1). At the time of hospital admission, 89% of the patients in the full cohort used statins.
TABLE 1.
Clinical characteristics
| Clinical characteristics | Total (n = 574) | ACS patients (n = 313) | SAP patients (n = 261) | P |
| Age, years, mean ± SD | 61.5 ± 11.3 | 59.7 ± 11.9 | 63.6 ± 10.3 | <0.001 |
| Male, n (%) | 432 (75) | 230 (74) | 202 (77) | 0.279 |
| Diabetes mellitus, n (%) | 97 (17) | 40 (13) | 57 (22) | 0.004 |
| Hypertension, n (%) | 298 (52) | 137 (44) | 161 (62) | <0.001 |
| Hypercholesterolemia, n (%) | 318 (55) | 138 (44) | 180 (69) | <0.001 |
| Smoking, n (%) | 166 (29) | 116 (37) | 50 (19) | <0.001 |
| Positive family history of CAD, n (%) | 298 (52) | 145 (46) | 153 (59) | 0.004 |
| Previous MI, n (%) | 184 (32) | 80 (26) | 104 (40) | <0.001 |
| Previous PCI, n (%) | 184 (32) | 57 (18) | 127 (49) | <0.001 |
| Previous CABG, n (%) | 18 (3) | 7 (2) | 11 (4) | 0.176 |
| Previous stroke, n (%) | 26 (5) | 11 (4) | 15 (6) | 0.200 |
| Peripheral artery disease, n (%) | 35 (6) | 11 (4) | 24 (9) | 0.005 |
| History of heart failure, n (%) | 19 (3) | 6 (2) | 13 (5) | 0.041 |
| Serum LDL cholesterol, mmol/L | 2.71 [2.12–3.54] | 3.10 [2.32–3.87] | 2.37 [1.94–2.99] | <0.001 |
| Serum HDL cholesterol, mmol/L | 1.04 [0.87–1.29] | 1.05 [0.87–1.27] | 1.03 [0.86–1.30] | 0.80 |
| Serum nonHDL levels, mmol/L | 3.23 [2.54–4.00] | 3.56 [2.81–4.36] | 2.83 [2.35–3.56] | <0.001 |
| Serum TG, mmol/L | 1.27 [0.88-1.83] | 1.15 [0.77–1.77] | 1.41 [1.05–1.94] | <0.001 |
| Statin use at baseline, n (%) | 508 (89%) | 308 (98%) | 235 (90%) | 0.499 |
| Procedural characteristics | ||||
| Indication for CAG | ||||
| ACS, n (%) | 313 (55) | 313 (100) | 0 (0) | |
| STEMI, n (%) | 162 (28) | 162 (52) | 0 (0) | |
| Non-ST-elevation, n (%) | 151 (26) | 151 (48) | 0 (0) | |
| Stable angina pectoris, n (%) | 261 (46) | 0 (0) | 261 (100) | |
| PCI performed, n (%) | 505 (88) | 291 (93) | 214 (82) | |
| a | ||||
| No significant stenosis, n (%) | 42 (7) | 18 (6) | 24 (9) | |
| 1-vessel disease, n (%) | 304 (53) | 172 (55) | 132 (51) | |
| 2-vessel disease, n (%) | 167 (29) | 88 (28) | 79 (30) | |
| 3-vessel disease, n (%) | 61 (11) | 35 (11) | 26 (10) |
Continuous variables are presented as mean ± (SD) or median [IQR]. Categorical variables are presented in numbers (n) and percentages (%). P-value was obtained from Student’s t-test or Chi square test. ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CAG, coronary angiography; IQR, interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention; SAP, stable angina pectoris; TG, triglyceride.
A significant stenosis was defined as a stenosis ≥ 50% of the vessel diameter by visual assessment of the coronary angiogram.
TABLE 2.
Lipid concentrations in the full cohort, patients with MACEs during follow-up, and patients without MACEs during follow-up
| Lipid concentrations in the full cohort | Total (n = 574) | ACS patients (n = 313) | SAP patients (n = 261) | P |
| CE 14:0, pmol/µl | 21.7 [15.9–28.1] | 22.9 [16.5–30.5] | 21.2 [15.4–26.8] | 0.008 |
| CE 18:3, pmol/µl | 70.3 [51.8–90.7] | 72.3 [53.6–99.5] | 66.1 [50.3–85.2] | 0.003 |
| CE 20:4, pmol/µl | 386 [317–457] | 394 [324–453] | 374 [307–471] | 0.31 |
| CE 20:5, pmol/µl | 49.1 [36.3–72.6] | 49.2 [36.4–72.1] | 49.0 [35.9–74.7] | 0.69 |
| CE 22:5, pmol/µl | 2.65 [2.00–3.62] | 2.81 [2.12–3.77] | 2.53 [1.90–3.40] | 0.037 |
| Cer(d18:1/16:0) pmol/µl | 0.12 [0.10–0.15] | 0.13 [0.11–0.17] | 0.11 [0.09–0.13] | <0.001 |
| Cer(d18:1/20:0) pmol/µl | 0.11 [0.09–0.15] | 0.12 [0.10–0.16] | 0.11 [0.08–0.13] | <0.001 |
| Cer(d18:1/24:0) pmol/µl | 5.98 [4.72–7.49] | 6.43 [5.00–8.07] | 5.65 [4.49–6.61] | <0.001 |
| Cer(d18:1/24:1) pmol/µl | 1.79 [1.42–2.25] | 1.89 [1.52–2.44] | 1.67 [1.35–2.05] | <0.001 |
| LacCer(d18:1/18:0) pmol/µl | 0.13 [0.10–0.16] | 0.13 [0.11–0.16] | 0.12 [0.10–0.15] | 0.001 |
| Cer(d18:1/16:0)/Cer(d18:1/24:0) pmol/µl | 0.020 [0.018–0.024] | 0.021 [0.018–0.025] | 0.020 [0.017–0.023] | 0.001 |
| Cer(d18:1/20:0)/Cer(d18:1/24:0) pmol/µl | 0.019 [0.016–0.024] | 0.019 [0.015–0.024] | 0.019 [0.016–0.023] | 0.62 |
| Cer(d18:1/24:1)/Cer(d18:1/24:0) pmol/µl | 0.31 [0.26–0.36] | 0.31 [0.26–0.36] | 0.31 [0.26–0.36] | 0.65 |
| Lipid concentrations in those with MACEsa | Total (n = 155) | ACS patients (n = 65) | SAP patients (n = 90) | P |
| CE 14:0, pmol/µl | 22.6 [15.7–27.1] | 21.7 [15.5–30.3] | 22.7 [15.7–26.6] | 0.67 |
| CE 18:3, pmol/µl | 67.9 [51.2–90.3] | 70.3 [52.8–103] | 66.9 [50–84.1] | 0.17 |
| CE 20:4, pmol/µl | 381 [310–445] | 381 [310–432] | 379 [310–447] | 0.95 |
| CE 20:5, pmol/µl | 52.4 [37.4–74.5] | 52.6 [38.4–76.3] | 50.9 [36.6–74.4] | 0.73 |
| CE 22:5, pmol/µl | 2.57 [1.86–3.71] | 2.61 [2.04–3.97] | 2.51 [1.80–3.45] | 0.065 |
| Cer(d18:1/16:0) pmol/µl | 0.12 [0.10–0.16] | 0.15 [0.11–0.17] | 0.11 [0.09–0.13] | <0.001 |
| Cer(d18:1/20:0) pmol/µl | 0.11 [0.09–0.16] | 0.13 [0.10–0.17] | 0.11 [0.08–0.14] | 0.011 |
| Cer(d18:1/24:0) pmol/µl | 5.86 [4.65–7.48] | 6.44 [5.02–8.10] | 5.66 [4.54–6.58] | 0.063 |
| Cer(d18:1/24:1) pmol/µl | 1.78 [1.35–2.36] | 2.10 [1.66–2.84] | 1.62 [1.33–2.13] | 0.008 |
| LacCer(d18:1/18:0) pmol/µl | 0.13 [0.10–0.16] | 0.14 [0.10–0.18] | 0.13 [0.10–0.16] | 0.137 |
| Cer(d18:1/16:0)/Cer(d18:1/24:0) pmol/µl | 0.021 [0.018–0.025] | 0.022 [0.019–0.027] | 0.019 [0.017–0.024] | 0.006 |
| Cer(d18:1/20:0)/Cer(d18:1/24:0) pmol/µl | 0.020 [0.016–0.025] | 0.020 [0.016–0.024] | 0.020 [0.015–0.025] | 0.504 |
| Cer(d18:1/24:1)/Cer(d18:1/24:0) pmol/µl | 0.31 [0.27–0.37] | 0.33 [0.27–0.36] | 0.31 [0.26–0.37] | 0.222 |
| Lipid concentrations in those without MACEsa | Total (n = 411) | ACS patients (n = 242) | SAP patients (n = 169) | P |
| CE 14:0, pmol/µl | 21.5 [15.8–28.7] | 23 [16.5–30.5] | 20.7 [15.3–27] | 0.007 |
| CE 18:3, pmol/µl | 70.5 [51.7–91.3] | 73.8 [53.5–99] | 66 [50.3–86.4] | 0.011 |
| CE 20:4, pmol/µl | 391 [321–467] | 397 [310–432] | 374 [304–476] | 0.272 |
| CE 20:5, pmol/µl | 49.1 [35.6–70.5] | 49.2 [35.5–70.7] | 47.5 [35.6–70] | 0.575 |
| CE 22:5, pmol/µl | 2.69 [2.04–3.58] | 2.79 [2.12–3.70] | 2.54 [1.92–3.40] | 0.041 |
| Cer(d18:1/16:0) pmol/µl | 0.12 [0.10–0.15] | 0.13 [0.11–0.16] | 0.11 [0.09–0.13] | <0.001 |
| Cer(d18:1/20:0) pmol/µl | 0.12 [0.09–0.14] | 0.12 [0.09–0.15] | 0.11 [0.08–0.13] | <0.001 |
| Cer(d18:1/24:0) pmol/µl | 6 [4.75–7.47] | 6.39 [4.97–8.07] | 5.64 [4.49–6.64] | <0.001 |
| Cer(d18:1/24:1) pmol/µl | 1.78 [1.35–2.36] | 1.86 [1.51–2.33] | 1.68 [1.35–2.04] | <0.001 |
| LacCer(d18:1/18:0) pmol/µl | 0.13 [0.10–0.16] | 0.13 [0.11–0.16] | 0.12 [0.10–0.15] | 0.001 |
| Cer(d18:1/16:0)/Cer(d18:1/24:0) pmol/µl | 0.021 [0.018–0.025] | 0.021 [0.018–0.024] | 0.020 [0.017–0.023] | 0.017 |
| Cer(d18:1/20:0)/Cer(d18:1/24:0) pmol/µl | 0.020 [0.016–0.025] | 0.019 [0.015–0.024] | 0.019 [0.016–0.023] | 0.60 |
| Cer(d18:1/24:1)/Cer(d18:1/24:0) pmol/µl | 0.31 [0.27–0.37] | 0.30 [0.25–0.36] | 0.31 [0.26–0.36] | 0.77 |
Concentrations are presented in μM as median [IQR]. P-value was obtained from Student’s t-test for difference in ln-transformed mean lipid concentration. ACS, acute coronary syndrome; CE, cholesteryl ester; Cer, ceramide; LacCer, lactosylceramide; MACE, major adverse cardiac event; SAP, stable angina pectoris.
Information on MACEs was available in n = 566.
As shown in Table 2, ACS patients had significantly higher plasma concentrations of CE 14:0, CE 18:3, CE 22:5; Cer(d18:1/16:0), Cer(d18:1/20:0), Cer(d18:1/24:0), Cer(d18:1/24:1), LacCer(d18:1/18:0) and Cer(d18:1/16:0)/Cer(d18:1/24:0) as compared with SAP patients, both in the full cohort and in patients who remained free of MACEs. In patients with MACEs during follow-up, except plasma concentration of CE 14:0, CE 18:3, and LacCer(d18:1/18:0), all of the above-mentioned lipid species plasma concentrations were significantly higher in the ACS patients as compared with the SAP patients. In addition, in ACS patients, concentration of Cer(d18:1/16:0) tended to be higher (P = 0.054) in those with MACEs as compared with those without MACEs during follow-up.
Molecular lipids concentrations and cardiovascular outcome
In the full cohort (n = 574), vital status was acquired for 572 patients (99.7%). The follow-up questionnaire assessing the occurrence of MACEs was completed by 99% of the 574 patients. During a median follow-up time of 4.7 years (IQR: [4.2–5.6]) years, a total of 155 patients (27%) experienced at least one MACE (primary endpoint). In the ACS group, 65 patients (21%) experienced MACEs during long-term follow-up; in the SAP group, this was 90 patients (34%).
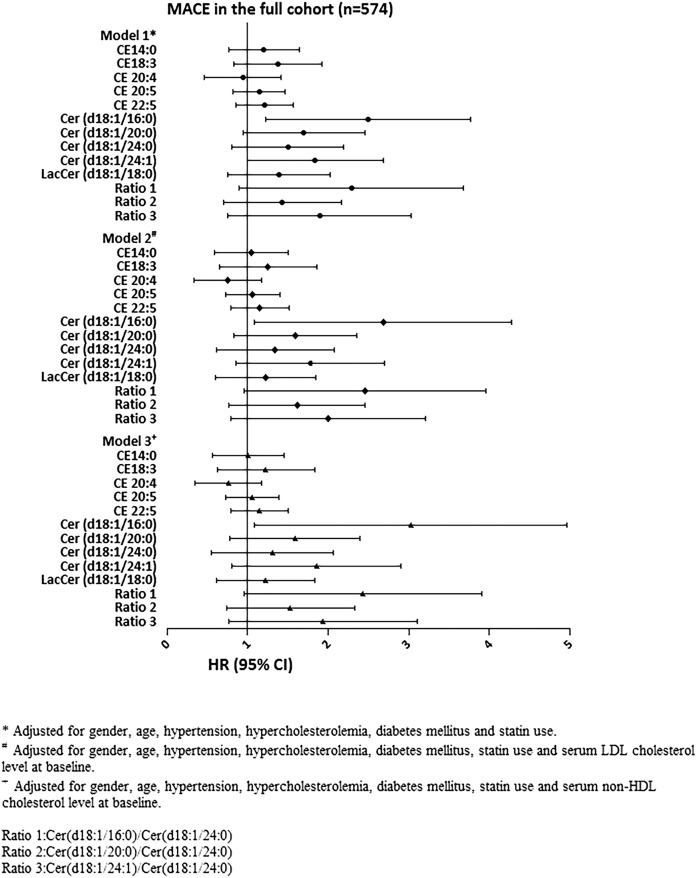
The results for the associations between the molecular lipids concentrations and MACEs are depicted in Fig. 1 and supplemental Table S1a. In multivariable analyses, after adjustment for cardiac risk factors, clinical presentation, and statin use at baseline, Cer(d18:1/16:0) concentration {HR: 2.14; 95% CI [1.22–3.76] per ln(pmol/ml) P = 0.008} and Cer(d18:1/24:1) concentration {HR: 1.64; 95% CI [1.00–2.68] per ln(pmol/ml) P = 0.049} were significantly associated with MACEs. After additional adjustment for admission serum LDL cholesterol level or serum nonHDL cholesterol level, only Cer(d18:1/16:0) concentration remained associated with MACE {HR: 2.16; 95% CI [1.09–4.27] per ln(pmol/ml), P = 0.027, and HR: 2.32; 95% CI [1.09–4.96] per ln(pmol/ml, P = 0.030, respectively}.
Fig. 1.
Association of plasma concentrations of molecular lipid species with MACEs. The results are presented as hazard ratios (HRs) per unit increase in (Ln-transformed) molecular lipid concentrations or lipid ratios, with 95% CIs. CE, cholesteryl ester; Cer, ceramide; LacCer, lactosylceramide; MACE, major adverse cardiac event.
The interaction term between Cer(d18:1/16:0) and indication for CAG was significant (P = 0.030) in the multivariable model. In ACS patients, higher Cer(d18:1/16:0) concentration was significantly associated with MACE, both in the uni- and multi-variable model {(HR adjusted for cardiac risk factors, statin use, and nonHDL cholesterol level at baseline: 6.13; 95% CI [1.65–22.8]) per ln(pmol/ml) P = 0.007} (supplemental Table S2a). In SAP patients, HRs were closer to the null and did not reach statistical significance (supplemental Table S2b). The interaction term between Cer(d18:1/16:0)/Cer(d18:1/24:0) and indication for CAG was also significant (P = 0.044). Its association with MACEs only reached statistical significance in univariable analysis in ACS patients (supplemental Table S2a).
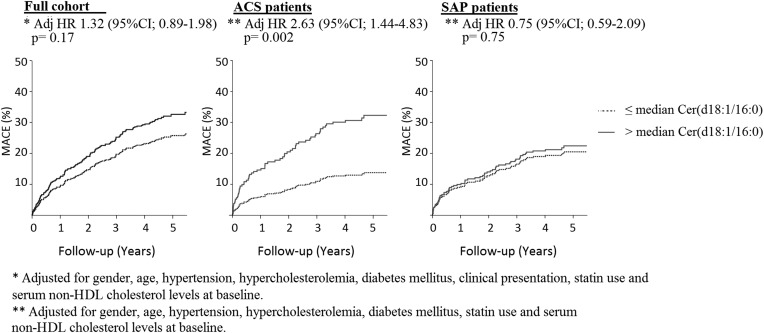
The incidence of MACEs for Cer(d18:1/16:0) levels above and below the median are depicted in Fig. 2. After adjustment for cardiac risk factors, statin use and baseline serum nonHDL cholesterol level, plasma Cer(d18:1/16:0) levels above vs below the median were significantly associated with MACEs (HR: 2.63; 95% CI [1.44- 4.83], per ln(pmol/ml) P = 0.002) in ACS patients. In the full cohort and in SAP patients significant associations between plasma Cer(d18:1/16:0) levels above vs below the median and MACEs could not be demonstrated (Fig. 2).
Fig. 2.
Association of plasma concentrations of Cer (d18:1/16:0) with MACE in the full cohort and in patients with ACS or SAP. The results are presented as hazard ratios (HRs) for Cer(d18:1/16:0) above versus below the median, with 95% CIs. ACS, acute coronary syndrome; Cer, ceramide; MACE, major adverse cardiac event; SAP, stable angina pectoris.
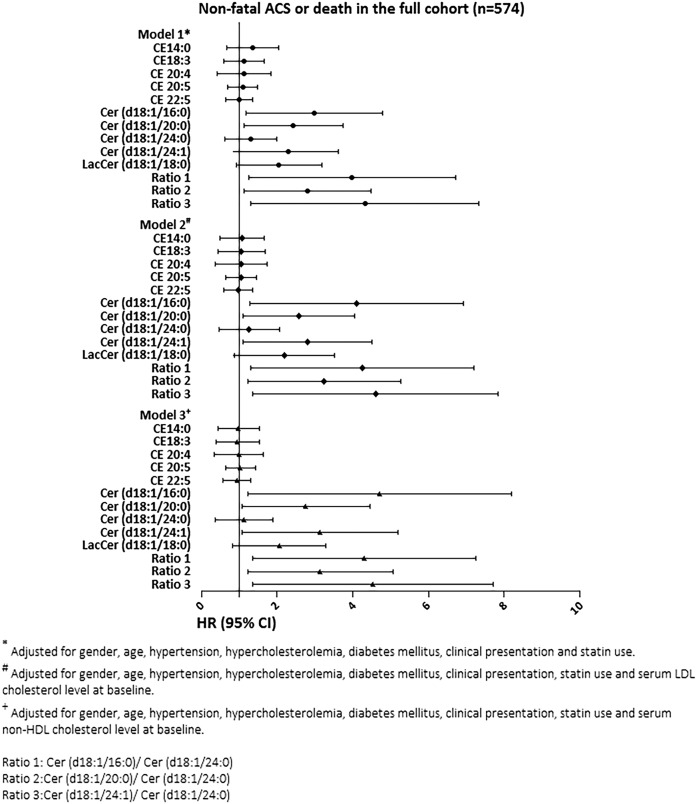
Several lipid species displayed associations with the secondary endpoint (Fig. 3 and Supplemental Table S1b); in univariable analysis, concentrations of Cer(d18:1/16:0), Cer(d18:1/20:0), Cer(d18:1/24:1), LacCer(d18:1/18:0), Cer(d18:1/16:0)/Cer(d18:1/24:0), Cer(d18:1/20:0)/Cer(d18:1/24:0), and Cer(d18:1/24:1)/Cer(d18:1/24:0) were significantly associated with the composite endpoint of death or nonfatal ACS (supplemental Table S1b). After multivariable adjustment for cardiac risk factors, indication for CAG, statin use at baseline, and serum LDL cholesterol level, except LacCer(d18:1/18:0) concentration, all of the above-mentioned lipid species remained significantly associated with the composite endpoint of death or nonfatal ACS. Results remained materially the same after adjusting the multivariable models for serum nonHDL cholesterol level instead of serum LDL cholesterol level. The interaction terms between indication for CAG and Cer(d18:1/16:0) (P = 0.006), Cer(d18:1/24:1) (P = 0.025), Cer(d18:1/16:0)/Cer(d18:1/24:0) (P = 0.004), and Cer(d18:1/24:1)/Cer(d18:1/24:0) (P = 0.006) were significant on univariable and multivariable adjustment. All these associations were driven by the ACS patients (supplemental Table S3a and S3b). Interaction terms between indication for CAG and the remaining molecular lipids and lipid ratios did not reach statistical significance.
Fig. 3.
Association of plasma concentrations of molecular lipid species with nonfatal ACS or death. The results are presented as hazard ratios (HRs) per unit increase in (Ln-transformed) molecular lipid concentrations or lipid ratios, with 95% CIs. ACS, acute coronary syndrome CE, cholesteryl ester; Cer, ceramide; LacCer, lactosylceramide.
DISCUSSION
We investigated the associations of ten previously identified high risk molecular lipid species and three ceramide ratios with clinical cardiovascular outcome during long-term follow-up in 581 patients. The main finding of our study was that higher Cer(d18:1/16:0) concentration and Cer(d18:1/24:1) concentration were significantly associated with MACEs after multivariable adjustment for cardiac risk factors, clinical presentation, and statin use at baseline. After additional adjustment for admission serum LDL cholesterol level or nonHDL cholesterol level, this association persisted only for Cer(d18:1/16:0) concentration. The latter association was driven by patients that presented with ACS. Another important finding of this study was that several lipid species were significantly and independently associated with the secondary endpoint, comprising the composite of all-cause mortality or nonfatal ACS. Likewise, several of these associations were driven by patients presenting with ACS, in whom we also observed higher plasma lipid concentrations as compared with patients with SAP.
Ceramides are a family of waxy lipid molecules and are composed of sphingosine and a fatty acid (17). They can be implicated in coronary artery disease through several mechanisms (3, 8). Ceramide is mainly produced through the SMase pathway, which breaks down sphingomyelin in the cell membrane and releases ceramides (17, 18). The production of ceramides can be increased by numerous cardiovascular risk factors such as oxidized-LDL and homocyteine (17). Moreover, inflammatory cytokines could also activate SMase and increase ceramide production mediated by increased reactive oxygen species (17), which include H2O2, superoxide, and hydroxyl radicals (17, 19). Ceramides can also act as signaling molecules regulating numerous cell responses and functions, including the differentiation, proliferation, apoptosis, and gene expression, such as cytokines (17). Some of these roles of ceramides are associated with the molecular mechanisms of atherosclerosis and with plaque vulnerability (1, 17, 20).
The molecular lipids species in this study were chosen from the LURIC lipidomic study (1). The LURIC lipidomic study compared 258 male CAD patients who died within 3 years of follow-up with 187 matched control patients with CAD who did not die during follow-up. The chosen molecular lipid species were associated with CAD outcome at the P < 0.05 level. Our present results in essence validate the previous LURIC study, albeit with a somewhat different endpoint that also contains nonfatal adverse cardiovascular events. Several molecular lipid species, such as CE 14:0, CE 18:3, CE 20:4, CE 20:5, CE 22:5, and Cer(d18:1/24:0), were protective in the LURIC study but were not associated with clinical outcome in the present study. Conversely, Cer(d18:1/16:0), Cer(d18:1/20:0), and Cer(d18:1/24:1) were associated with mortality in the LURIC study and with clinical outcome in our present study. In a previous study, ceramide long-chain-species were shown to mediate insulin resistance in mice and to be pro-apoptotic, whereas very-long-chain species were anti-apoptotic (21, 22). In our present study, Cer(d18:1/16:0) and Cer(d18:1/20:0) were independently associated with clinical outcome and more harmful than Cer(d18:1/24:0). Interestingly, Cer(d18:1/24:1) behaved contrarily when compared with Cer(d18:1/24:0). The reason for this difference remains to be investigated in further studies. Furthermore, in the LURIC study, LacCer(d18:1/18:0) was associated with mortality, whereas in the current study there was only an association with clinical outcome (supplemental Table S1b) in the unadjusted model. Based on previous observations, LacCer (a glycosphingolipid) and other glucosylceramides appear to influence the atherogenic process in the atherosclerotic plaque by suppressing the production of macrophage apolipoprotein E leading to an accumulation of cholesterol in macrophage foam cells (19, 23). Moreover, in aortic smooth muscle cell, LacCer is activated by oxidized-LDL and subsequently, LacCer enhances the activity of nicotinamide adenine dinucleotide phosphate oxidase to generate superoxide radicals, which in turn mediate p44MAPK activation to enhance nuclear transcription factor expression and to stimulate the proliferation of smooth muscle cells, thereby contributing to atherosclerosis (2, 23). Altogether, as increased levels of LacCer mediate plaque formation, a relationship between LacCer and cardiovascular clinical outcome is to be expected. However, in our study, there was no independent association. Further studies are needed to establish the biological mechanisms of LacCer in CAD patients.
In our previous report on the current study population, Cer(d18:1/16:0) was an independent predictor of MACEs during 1 year follow-up, and the three ceramide ratios were independent predictors of the composite endpoint of death or nonfatal ACS (20). Our current study confirms and extends the findings of our previous lipidomics analyses with shorter term follow-up (20).
Associations of lipids with incident acute MACEs (all-cause mortality and nonfatal ACS) were more prominent than those with ‘overall’ MACEs. Associations were also more prominent in patients presenting with ACS than in those with SAP; although in the stratified analyses, numbers of MACEs were limited (90 in SAP patients and 65 in ACS patients), which may have influenced statistical significance, hazard ratios in SAP patients were also clearly closer to the null and interaction terms were significant. These findings are in line with pathophysiological insights from earlier studies. Patients with ACS have been shown to exhibit an increased pro-inflammatory and oxidative state compared with SAP patients (24, 25). This state even persists after stabilization (26, 27). High lipid plasma concentrations induce oxidative stress mediated by reactive oxygen species (24, 28) and herewith, further increase the risk of incident ACS. Furthermore, in ACS patients, so-called high risk or vulnerable plaques have been shown to be more frequently present and are more likely to lead to plaque rupture and thus to acute cardiac events (29, 30). These plaques have been shown to carry large lipid cores and have been associated with high circulating lipid levels (20, 31).
In the last decade, lipid species have received considerable attention as potential biomarkers in several lipid-related diseases (9). Furthermore, several molecular lipid species have been associated with the composition of atherosclerotic plaque (20) and with cardiovascular events during short-term follow-up (20, 32). However, clinical studies in patients with CAD on the association of lipid molecular species with cardiovascular outcome during long term follow-up are scarce. Laaksonen et al. (32) examined several ceramides and ceramide ratios in the Corogene cohort (80 stable CAD patients who died and 80 matched controls, 2.5 years follow-up), as well as in the BECAC cohort (1,580 stable CAD patients, 81 of whom died during 4.6 years follow-up). In both cohorts Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/24:1), Cer(d18:1/16:0)/Cer(d18:1/24:0), Cer(d18:1/18:0)/Cer(d18:1/24:0), and Cer(d18:1/24:1)/Cer(d18:1/24:0) were associated with cardiovascular death. Although Laaksonen et al. only examined cardiovascular death and not (acute) MACEs, the results with regard to the corresponding ceramides and ceramide ratios were in line with our results, although the associations in our study were driven by patients presenting with ACS. Havulinna et al. (3), in the FINRISK cohort, measured four circulating ceramides in a healthy population, and within this population, they examined a subgroup with prevalent or incident MACEs. In these 396 patients, plasma Cer(d18:1/16:0) and Cer(d18:1)/24:1) concentrations were independent predictors of recurrent MACEs (n = 226) or fatal recurrent MACEs (n = 70) during a follow-up of 13 years. These results were in line with ours, although in our study, the association between Cer(d18:1/24:1) and MACEs was not independent of LDL or nonHDL cholesterol level. Conversely, the association of Cer(d18:1/24:1) with the secondary endpoint (nonfatal ACS or death) was independent of LDL or nonHDL cholesterol level. Other studies on long-term prognostic value of lipidomics have mostly used healthy populations. In the Bruneck study, Stegemann et al. (33) analyzed the association of 135 lipid species (including CEs) with incident CVD during 10 year follow-up in a prospective population-based survey. They demonstrated significant associations for 28 lipids. Among these lipid species, three were most informative for CVD risk: TAG(54:2), CE(16:1), and PE(36:5). The results with regard to the CEs that were also measured in this study were in line with our results; i.e., no significant associations were found. Moreover, Alshehry et al. (2) examined the prognostic value of 310 plasma lipid species (including CEs and CERs) for cardiovascular risk stratification in a case-cohort of 3,779 individuals with diabetes mellitus that included 698 patients with cardiovascular events and 355 patients with cardiovascular death. Multiple lipid species were significantly associated with cardiovascular events and cardiovascular death. In line with our study, a significant association was found between Cer(d18:1/24:1) and death. In contrast to our study, no association was found between plasma Cer(d18:1/16:0) and cardiovascular outcome. This may possibly be explained by differences in the study populations; in particular, the patients studied by Alshehry et al. were diagnosed with diabetes mellitus and had ≥ 1 additional cardiovascular risk factors. For the other lipid species, no significant associations were found.
Some limitations of this study need to be acknowledged. First, this study is an observational cohort study. Despite using multivariable analysis to adjust for possible confounders that may be related to the study outcomes, we cannot exclude the possibility of residual confounding. Second, a large proportion of the subjects were on lipid lowering medication, which would influence the plasma lipid concentrations in these individuals. However, all multivariable analyses were adjusted for statin use.
In conclusion, in patients with established CAD, plasma Cer(d18:1/16:0) was associated with MACEs during a median follow-up time of 4.7 years, independently from established cardiac factors, statin use, and serum LDL or nonHDL cholesterol level. Furthermore, after multivariable adjustment, concentrations of Cer(d18:1/16:0), Cer(d18:1/20:0), Cer(d18:1/24:1), and all ceramide ratios were associated with the secondary endpoint, comprising the composite of all-cause mortality or nonfatal ACS. Our results support the hypothesis that ceramide plasma concentrations and ratios predict long-term cardiovascular outcome, and therefore circulating molecular lipids, may further improve risk stratification of CAD patients.
Supplementary Material
Footnotes
Abbreviations:
- ACS
- acute coronary syndrome
- CABG
- coronary artery bypass grafting
- CAD
- coronary artery disease
- CAG
- coronary angiography
- CE
- cholesteryl ester
- Cer
- ceramide
- HR
- hazard ratio
- IQR
- interquartile range
- LacCer
- lactosylceramide
- ln
- natural logarithm
- LOQ
- limit of quantification
- MACE
- major adverse cardiac event
- PCI
- percutaneous coronary intervention
- SAP
- stable angina pectoris
- STEMI
- ST-segment elevation myocardial infarction
- TG
- triglyceride
The ATHEROREMO-IVUS study was funded by the European Commission, Seventh Framework Programme (Grant FP7-HEALTH-2007-2.4.2-1).M. Hilvo, D. Kauhanen, K. Koistinen, and R. Laaksonen are employed by Zora Biosciences, Espoo, Finland. The other authors declare no conflict of interest.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Tarasov K., Ekroos K., Suoniemi M., Kauhanen D., Sylvanne T., Hurme R., Gouni-Berthold I., Berthold H. K., Kleber M. E., Laaksonen R., et al. . 2014. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J. Clin. Endocrinol. Metab. 99: E45–E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alshehry Z. H., Mundra P. A., Barlow C. K., Mellett N. A., Wong G., McConville M. J., Simes J., Tonkin A. M., Sullivan D. R., Barnes E. H., et al. . 2016. Plasma lipidomic profiles improve on traditional risk factors for the prediction of cardiovascular events in type 2 diabetes mellitus. Circulation. 134: 1637–1650. [DOI] [PubMed] [Google Scholar]
- 3.Havulinna A. S., Sysi-Aho M., Hilvo M., Kauhanen D., Hurme R., Ekroos K., Salomaa V., and Laaksonen R.. 2016. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler. Thromb. Vasc. Biol. 36: 2424–2430. [DOI] [PubMed] [Google Scholar]
- 4.Ekroos K., Janis M., Tarasov K., Hurme R., and Laaksonen R.. 2010. Lipidomics: a tool for studies of atherosclerosis. Curr. Atheroscler. Rep. 12: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson A. D. 2006. Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J. Lipid Res. 47: 2101–2111. [DOI] [PubMed] [Google Scholar]
- 6.Roberts L. D., McCombie G., Titman C. M., and Griffin J. L.. 2008. A matter of fat: an introduction to lipidomic profiling methods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 871: 174–181. [DOI] [PubMed] [Google Scholar]
- 7.Jänis M. T., Laaksonen R., and Oresic M.. 2008. Metabolomic strategies to identify tissue-specific effects of cardiovascular drugs. Expert Opin. Drug Metab. Toxicol. 4: 665–680. [DOI] [PubMed] [Google Scholar]
- 8.Stock J. 2012. The emerging role of lipidomics. Atherosclerosis. 221: 38–40. [DOI] [PubMed] [Google Scholar]
- 9.Hu C., van der Heijden R., Wang M., van der Greef J., Hankemeier T., and Xu G.. 2009. Analytical strategies in lipidomics and applications in disease biomarker discovery. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 2836–2846. [DOI] [PubMed] [Google Scholar]
- 10.de Boer S. P., Cheng J. M., Garcia-Garcia H. M., Oemrawsingh R. M., van Geuns R. J., Regar E., Zijlstra F., Laaksonen R., Halperin E., Kleber M. E., et al. . 2014. Relation of genetic profile and novel circulating biomarkers with coronary plaque phenotype as determined by intravascular ultrasound: rationale and design of the ATHEROREMO-IVUS study. EuroIntervention. 10: 953–960. [DOI] [PubMed] [Google Scholar]
- 11.Heiskanen L. A., Suoniemi M., Ta H. X., Tarasov K., and Ekroos K.. 2013. Long-term performance and stability of molecular shotgun lipidomic analysis of human plasma samples. Anal. Chem. 85: 8757–8763. [DOI] [PubMed] [Google Scholar]
- 12.Merrill A. H. Jr., Sullards M. C., Allegood J. C., Kelly S., and Wang E.. 2005. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods. 36: 207–224. [DOI] [PubMed] [Google Scholar]
- 13.Ejsing C. S., Duchoslav E., Sampaio J., Simons K., Bonner R., Thiele C., Ekroos K., and Shevchenko A.. 2006. Automated identification and quantification of glycerophospholipid molecular species by multiple precursor ion scanning. Anal. Chem. 78: 6202–6214. [DOI] [PubMed] [Google Scholar]
- 14.Roffi M., Patrono C., Collet J. P., Mueller C., Valgimigli M., Andreotti F., Bax J. J., Borger M. A., Brotons C., Chew D. P., et al. . 2016. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 37: 267–315. [DOI] [PubMed] [Google Scholar]
- 15.Ibanez B., James S., Agewall S., Antunes M. J., Bucciarelli-Ducci C., Bueno H., Caforio A. L. P., Crea F., Goudevenos J. A., Halvorsen S., et al. . 2018. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 39: 119–177. [DOI] [PubMed] [Google Scholar]
- 16.Stone G. W., Maehara A., Lansky A. J., de Bruyne B., Cristea E., Mintz G. S., Mehran R., McPherson J., Farhat N., Marso S. P., et al. . 2011. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 364: 226–235. [DOI] [PubMed] [Google Scholar]
- 17.Bismuth J., Lin P., Yao Q., and Chen C.. 2008. Ceramide: a common pathway for atherosclerosis? Atherosclerosis. 196: 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merrill A. H. Jr., Schmelz E. M., Dillehay D. L., Spiegel S., Shayman J. A., Schroeder J. J., Riley R. T., Voss K. A., and Wang E.. 1997. Sphingolipids–the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol. Appl. Pharmacol. 142: 208–225. [DOI] [PubMed] [Google Scholar]
- 19.Garner B., Mellor H. R., Butters T. D., Dwek R. A., and Platt F. M.. 2002. Modulation of THP-1 macrophage and cholesterol-loaded foam cell apolipoprotein e levels by glycosphingolipids. Biochem. Biophys. Res. Commun. 290: 1361–1367. [DOI] [PubMed] [Google Scholar]
- 20.Cheng J. M., Suoniemi M., Kardys I., Vihervaara T., de Boer S. P., Akkerhuis K. M., Sysi-Aho M., Ekroos K., Garcia-Garcia H. M., Oemrawsingh R. M., et al. . 2015. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Atherosclerosis. 243: 560–566. [DOI] [PubMed] [Google Scholar]
- 21.Turpin S. M., Nicholls H. T., Willmes D. M., Mourier A., Brodesser S., Wunderlich C. M., Mauer J., Xu E., Hammerschmidt P., Bronneke H. S., et al. . 2014. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20: 678–686. [DOI] [PubMed] [Google Scholar]
- 22.Raichur S., Wang S. T., Chan P. W., Li Y., Ching J., Chaurasia B., Dogra S., Ohman M. K., Takeda K., Sugii S., et al. . 2014. CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 20: 687–695. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee S. B., Dey S., Shi W. Y., Thomas K., and Hutchins G. M.. 1997. Accumulation of glycosphingolipids in human atherosclerotic plaque and unaffected aorta tissues. Glycobiology. 7: 57–65. [DOI] [PubMed] [Google Scholar]
- 24.Libby P., Ridker P. M., and Maseri A.. 2002. Inflammation and atherosclerosis. Circulation. 105: 1135–1143. [DOI] [PubMed] [Google Scholar]
- 25.Lorgis L., Zeller M., Dentan G., Sicard P., Richard C., Buffet P., L’Huillier I., Beer J. C., Cottin Y., Rochette L., et al. . 2010. The free oxygen radicals test (FORT) to assess circulating oxidative stress in patients with acute myocardial infarction. Atherosclerosis. 213: 616–621. [DOI] [PubMed] [Google Scholar]
- 26.Ridker P. M., Rifai N., Pfeffer M., Sacks F., Lepage S., and Braunwald E.. 2000. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 101: 2149–2153. [DOI] [PubMed] [Google Scholar]
- 27.Patel P. J., Khera A. V., Jafri K., Wilensky R. L., and Rader D. J.. 2011. The anti-oxidative capacity of high-density lipoprotein is reduced in acute coronary syndrome but not in stable coronary artery disease. J. Am. Coll. Cardiol. 58: 2068–2075. [DOI] [PubMed] [Google Scholar]
- 28.Witztum J. L. 1994. The oxidation hypothesis of atherosclerosis. Lancet. 344: 793–795. [DOI] [PubMed] [Google Scholar]
- 29.Hong M. K., Mintz G. S., Lee C. W., Kim Y. H., Lee S. W., Song J. M., Han K. H., Kang D. H., Song J. K., Kim J. J., et al. . 2004. Comparison of coronary plaque rupture between stable angina and acute myocardial infarction: a three-vessel intravascular ultrasound study in 235 patients. Circulation. 110: 928–933. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein J. A., Demetriou D., Grines C. L., Pica M., Shoukfeh M., and O’Neill W. W.. 2000. Multiple complex coronary plaques in patients with acute myocardial infarction. N. Engl. J. Med. 343: 915–922. [DOI] [PubMed] [Google Scholar]
- 31.Falk E., Shah P. K., and Fuster V.. 1995. Coronary plaque disruption. Circulation. 92: 657–671. [DOI] [PubMed] [Google Scholar]
- 32.Laaksonen R., Ekroos K., Sysi-Aho M., Hilvo M., Vihervaara T., Kauhanen D., Suoniemi M., Hurme R., Marz W., Scharnagl H., et al. . 2016. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 37: 1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stegemann C., Pechlaner R., Willeit P., Langley S. R., Mangino M., Mayr U., Menni C., Moayyeri A., Santer P., Rungger G., et al. . 2014. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 129: 1821–1831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.