Abstract
Total cholesterol to HDL cholesterol ratio (TC/HDL) is an important prognostic factor for CVD. This study used restricted cubic spline modeling to investigate the dose-response associations between TC/HDL and both CVD hospitalization and CVD rehospitalization in two independent prospective cohorts. The East Cambridgeshire and Fenland cohort includes 4,704 patients with T2D from 18 general practices in Cambridgeshire. The Randomized controlled trial of Peer Support In type 2 Diabetes cohort comprises 1,121 patients with T2D with posttrial follow-up data. TC/HDL and other demographic and clinical measurements were measured at baseline. Outcomes were CVD hospitalization over 2 years and CVD rehospitalization after 90 days of the prior CVD hospitalization. Modeling showed nonlinear relationships between TC/HDL and risks of CVD hospitalization and rehospitalization consistently in both cohorts (all P < 0.001 for linear tests). The lowest risks of CVD hospitalization and rehospitalization were consistently found for TC/HDL at 2.8 (95% CI: 2.6–3.0) in both cohorts and both overall and by gender. This is lower than the current lipid control target, 4.0 of TC/HDL. Reducing the TC/HDL target to 2.8 would include a further 33–44% patients with TC/HDL in the 2.8–4.0 range. Studies are required to assess the effectiveness and cost-effectiveness of the earlier introduction of, and more intensive, lipid-lowering treatment needed to achieve this new lower TC/HDL target.
Keywords: diabetes population, rehospitalization, lipid
The prevalence and cost of diabetes is growing rapidly worldwide (1). People with diabetes are twice as likely to be admitted to the hospital, and at least 10% of those in the hospital have diabetes at any one time (2). In some locations and age groups, it is as many as one in five (3). The associated costs of excess admissions, as well as increased costs per admission, are significant contributors to the financial burden borne by healthcare systems from diabetes and often reflect preventable morbidity suffered by patients (4).
As one of the most dominant risk factors, dyslipidemia has been found to be associated with coronary heart disease (CHD) among people with T2D in large prospective studies such as the UK Prospective Diabetes Study (UKPDS) (1, 2). Both total and LDL cholesterol have been found to correlate with risk of CHD consistently over different studies (5). Among various lipid profile measurements, the total cholesterol to HDL cholesterol ratio (TC/HDL) has been widely used as a prognostic factor to predict the risk of CVD both in general (3) and diabetes populations, as applied in the UKPDS score, reflecting its association both with CHD (4) and stroke in people with T2D.
CVD hospitalization and rehospitalization are important components of the increased costs of diabetes and the preventable morbidity suffered by people with diabetes (4). The TC/HDL management target among those with diabetes is an important risk factor for CVD hospitalization and rehospitalization and might be useful in defining population risk of morbidity and increased health costs (6). Few studies have set out to investigate the associations between TC/HDL level and risk of CVD hospitalization and rehospitalization in people with T2D.
In our previous risk score to predict CVD hospitalization and rehospitalization, TC and HDL were incorporated as four separated polynomial fractional terms to achieve a better model discrimination and calibration, which did not allow further examination of the association between the TC/HDL ratio and the risks of CVD hospitalization and rehospitalization (7). For example, it is unclear whether or not there is a dose-response relationship between TC/HDL and the risk of hospitalization in people with T2D and, if so, whether this is linear. If there is a nonlinear relationship, and a potential threshold exists between TC/HDL and CVD hospitalization, it could inform lipid management among people with T2D in the primary care setting, thereby reducing hospitalization and health payments.
The aim of this study was to investigate the dose-response relationships between TC/HDL and risks of CVD hospitalization over the subsequent 2 years, and CVD rehospitalization up to 90 days, following a prior CVD-related hospital stay in two independent prospective cohort studies.
METHODS
Data source and study population
ECF cohort.
In the East Cambridgeshire and Fenland (ECF) cohort, patient lists from 18 general practices across Cambridgeshire, England, in 2008/2009 were collated and linked with hospital admissions (Secondary Uses Service) data as part of an evaluation of diabetes care across the county by the local health board, the National Health Service (NHS) Cambridgeshire. This cohort was limited to volunteer practices using the Egton Medical Information Systems (EMIS) general practitioner (GP) software system, from which a predefined set of data could be extracted. There was no systematic selection process for these surgeries, and data extracted were for their entire diabetes population. T2D was defined based on GP diagnosis (8). All patients with diabetes had follow-up hospitalization data to 2010–2011. Hospital admissions to NHS and private hospitals within and outside Cambridgeshire were followed up. No personal identifiers were released to researchers, and all subsequent analyses were conducted on anonymized datasets.
RAPSID cohort.
The design and methods of the Randomized controlled trial of Peer Support In type 2 Diabetes (RAPSID) trial have been published previously (6), as have its CONSORT (Consolidated Standards of Reporting Trials) diagram and the results of its primary outcomes (9). Briefly, RAPSID was a 2 × 2 factorial cluster randomized controlled trial comparing four groups: controls, 1:1 (individual) peer support, group peer support, and combined 1:1 and group peer support among patients with T2D. Participants had their diabetes for at least 12 months, and those with dementia or psychotic illness were excluded. T2D was defined based on GP diagnosis. Participants were recruited from communities across Cambridgeshire and neighboring areas of Essex and Hertfordshire. Follow-up data were only available for participants in Cambridgeshire and neighboring areas of Hertfordshire that are served by the Cambridgeshire and Peterborough Clinical Commissioning Group (CCG). Clusters were defined by local government (“parish council”) boundaries. The intervention was developed following a pilot (10), using a framework defined by Peers for Progress (11). Peers facilitating peer support were termed peer support facilitators, and their selection, training, and support and the overall program are described elsewhere (12). The intervention lasted 8–12 months and was commenced and concluded, cluster by cluster, between 02/06/11 and 12/04/12.
At baseline, demographic data, blood pressure, and hemoglobin A1c (HbA1c) and lipid profile were collected. Each participant was followed up until June 2015 (0.91–4.07 years follow-up from beginning/entry into the trial). Hospitalization (NHS hospitals and private hospitals), Accident and Emergency, and outpatient visits within/outside Cambridgeshire and the included areas of Hertfordshire were completely collected through Cambridgeshire and Peterborough CCG (13), including the elective/nonelective status and International Classification of Diseases 10 (ICD-10) codes (8).
Defining CVD hospitalization and rehospitalization
The primary outcome of the study was having at least one hospitalization with CVD as the primary diagnosis (ICD-10: I20–I25, I60–I69, and I73 in the first ICD field) over the 2-year follow-up and having at least one CVD rehospitalization after 90 days of prior CVD hospitalization.
Clinical measurements and missing data
Objective clinical measurements were used as predictors in the model, including BMI and blood pressure [systolic blood pressure (SBP) and diastolic blood pressure (DBP)] and the metabolic variables glycated hemoglobin (HbA1c) and lipid profile. We also included demographic characteristics (age and gender) and whether the patient was on lipid-lowering treatment. Patients with diabetes were invited to have their blood pressure and metabolic variables measured at least once a year after the diagnosis of diabetes, and the most recent was taken before 1 April 2009 (a minimum of 50 days before the first admission). Diabetes duration was not universally recorded, and hence was not usefully available for analysis. Diabetes therapy was not included in the dataset. The TC/HDL was defined as the ratio of total cholesterol to HDL cholesterol.
The ECF cohort had missing information on BMI (3.17%), SBP (9.95%), DBP (9.95%), total cholesterol (12.35%), HDL (14.56%), and LDL (16.27%). We used multiple imputation to replace missing values by using a chained equation approach based on all candidate predictors and outcomes. We created 16 imputed datasets for missing variables that were then combined across all datasets by using Rubin’s rule to obtain final model estimates. Limited information was missing (<1%) in RAPSID, and the complete dataset was used in our analysis.
Ethical approval
The derivation cohort work had approval from the Cambridgeshire research ethics committee as part of a wider service evaluation. Ethics approval for the validation cohort was received from the Cambridgeshire REC2 Committee (10/H0308/72), and signed consent included agreement for access to hospital data.
Statistical analysis
We used “incidence occurrence of CVD hospitalization after the first 90 days since the start of follow-up” and the “incident occurrence of CVD rehospitalization” as binary outcome measures. A multivariable logistic regression model was used to explore the prospective association between TC/HDL and risks of CVD hospitalization and rehospitalization with adjustment of covariables presented in Table 1. The adjusted incidence rates ratio was estimated as , with estimated regression coefficients (β) from the multivariable logistic regression model.
TABLE 1.
. Baseline characteristics of study populations in the ECF and RAPSID cohorts
| ECF cohort | RAPSID cohort | |
| n | 4,704 | 1,121 |
| CVD hospitalization, n (%) | 588 (12.5) | 183 (16.3) |
| CVD rehospitalization, n (%) | 316 (6.7) | 78 (7.0) |
| Female, n (%) | 1,919 (40.8) | 444 (39.6) |
| Lipid-lowering treatment, n (%) | 3,342 (71.4) | 731 (65.2) |
| Age, years | 65.0 (56.0 –77.0) | 65.8 (60.0–72.1) |
| BMI, kg/m2 | 30.8 (26.2–34.3) | 32.3 (28.0–35.4) |
| SBP, mmHg | 135.0 (125.0–143.0) | 139.3 (128.3–151.0) |
| DBP, mmHg | 76.5 (70.0–82.0) | 75.5 (69.0–82.3) |
| HbA1c, mmol/mol | 61.6 (49.7–70.5) | 56.5 (48.0–63.0) |
| Total cholesterol, mmol/l | 4.3 (3.6–5.0) | 4.2 (3.6–5.0) |
| HDL, mmol/l | 1.3 (1.0 –1.6) | 1.2 (1.0–1.4) |
| LDL, mmol/l | 2.5 (1.7 –3.3) | 2.4 (1.5–2.7) |
| TC/HDL | 3.1 (2.6 –4.5) | 3.7 (2.8–4.4) |
Age, gender, SBP, DBP, BMI, HbA1c, LDL cholesterol, and lipid-lowering treatment were adjusted. Categorical variable is presented as n (%). Continuous variable is presented as median (interquartile rage).
The dose-response relationships between TC/HDL and risks of CVD hospitalization and rehospitalization were estimated using a linear model, a natural cubic spline model with three equally spaced knots determined from the levels of TC/HDL measures, and a quadratic spline model. The natural cubic spline model was chosen as the best-fit model for the relationship curve by its minimum Akaike information criterion (AIC) compared with the linear model or quadratic spline model. The linear test was used in the natural cubic spline model to test the linearity of the relationship. The break-point test11 was carried out to target the potential thresholds (P5–P95 of TC/HDL measures) by incorporating the piecewise term into the cubic spline model. The threshold with a significant break in the regression coefficients, and achieving the minimum AIC was chosen as the final threshold. The 95% CI of the threshold was obtained from 1,000 bootstrap samples. As the most important confounder, the role of the lipid-lowering agents in the association between TC/HDL and risks of CVD hospitalization and rehospitalization was also presented. In the first sensitivity analyses, all analyses were carried out in the continuous-measurement data-rich range (covering > 95% people). In the second analysis, all analyses were carried out in men and women separately.
RESULTS
Study participants
In the ECF cohort, we analyzed information on 4,704 T2D patients with 588 CVD hospitalizations within 2 years and 316 rehospitalizations after 90 days since a prior CVD hospitalization. Our RAPSID cohort had information on 1,121 T2D patients with 183 CVD hospitalizations and 78 rehospitalizations. Table 1 summarizes the basic characteristics and potential predictors of the study population at baseline. Patients with T2D in both cohorts had similar age, gender, blood pressure, and total cholesterol. Patients in the RAPSID cohort had a higher level of HDL, LDL, and HbA1c. Compared with the ECF cohort, those in the RAPSID cohort were more likely to be prescribed lipid-lowering medicine and had more CVD hospitalization and rehospitalization.
Dose-response relationships between TC/HDL and CVD hospitalization and rehospitalization
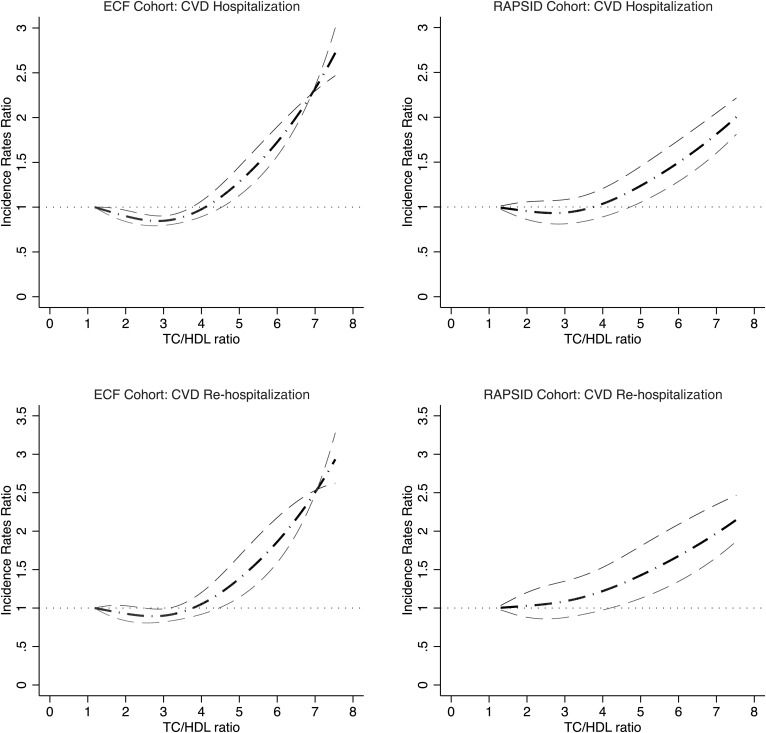
In both the ECF and RAPSID cohorts, nonlinear (“J-shape”) relationships were found between TC/HDL and risks of CVD hospitalization (both P values for linearity test < 0.0001) and rehospitalization (both P values for linearity test < 0.0001). Relationship curves were derived from the natural cubic spline models with adjustment of covariates in Fig. 1. Similar dose-response relationships were revealed in the sensitivity analyses modeling the associations within the data-rich range (5th–95th percentile of the above measurements) as shown in supplemental Fig. S1 for both the ECF and RAPSID cohorts. In another sensitivity analysis modeling the associations by gender, similar dose-response relationships were identified in men and women for both the ECF and RAPSID cohorts (supplemental Fig. S2).
Fig. 1.
Adjusted dose-response associations between TC/HDL cholesterol ratio and adjusted incidence rates ratios for CVD hospitalization and rehospitalization in the ECF and RAPSID cohorts
For both CVD hospitalization and rehospitalization, a TC/HDL below 2.8 (95% CI: 2.6–3.0) was estimated to be associated with the lowest risk of CVD hospitalization and rehospitalization both in ECF and RAPSID cohorts, as tested by linear threshold models. The thresholds were the same among men and women. Table 2 shows the CVD hospitalization and rehospitalization rates below and above the threshold. In the ECF cohort, the CVD hospitalization rates below and above the threshold were 9.8% (of 2,211 participants) and 14.9% (of 2,493 participants), respectively, and in the RAPSID cohort, 14.5% (of 269 patients) and 16.9% (of 852 patients), respectively. Similarly, CVD rehospitalization rates below and above the threshold were 4.1% and 9.0% in the ECF cohort and 6.6% and 7.2% in the RAPSID cohort. Table 2 also shows that the risks of CVD hospitalization and rehospitalization increase significantly with a 1 unit increase of TC/HDL above the TC/HDL threshold (2.8) in both the ECF and RAPSID cohorts: adjusted incidence rates ratio (IRR) per TC/HDL unit for CVD hospitalization 1.39 (95% CI: 1.37–1.41, P < 0.0001) in ECF and 1.18 (1.15–1.22, P = 0.012) in RAPSID; adjusted IRR for CVD rehospitalization 1.20 (1.17–1.23, P < 0.0001) in ECF and 1.17 (1.13–1.21, P = 0.040) in RAPSID. The risks of CVD hospitalization and rehospitalization did not increase significantly with a 1 unit increase of TC/HDL below the TC/HDL threshold (2.8) in either the ECF or RAPSID cohorts: adjusted IRR for CVD hospitalization was 1.05 (0.97–1.14, P = 0.062) in ECF and 1.00 (0.85–1.19, P = 0.595) in RAPSID; adjusted IRR for CVD rehospitalization was 1.04 (0.94–1.17, P = 0.272) in ECF and 0.90 (0.72–1.12, P = 0.385) in RAPSID. Findings were similar in men and women (supplemental table S1).
TABLE 2.
. Adjusted IRRs for CVD hospitalization and rehospitalization by 1 unit increase in TC/HDL ratio in groups classified by TC/HDL threshold (2.8) in the ECF and RAPSID cohorts
| ECF cohort | RAPSID cohort | |||||||
| TC/HDL ratio ≤ 2.8 [n = 2,211 (47.0%)] | P | TC/HDL ratio > 2.8 [n = 2,493 (53.0%)] | P | TC/HDL ratio ≤ 2.8 [n = 269 (24.0%)] | P | TC/HDL ratio > 2.8 [n = 852 (76.0%)] | P | |
| CVD hospitalization | ||||||||
| Hospitalization, n (%) | 216 (9.8) | — | 372 (14.9) | — | 39 (14.5) | 144 (16.9) | ||
| Adjusted IRRa | 1.09 (1.00–1.19) | 0.020 | 1.55 (1.53–1.57) | <0.0001 | 1.05 (0.86–1.27) | 0.890 | 1.24 (1.20–1.27) | 0.016 |
| Adjusted IRRb | 1.05 (0.97–1.14) | 0.062 | 1.39 (1.37–1.41) | <0.0001 | 1.00 (0.85–1.19) | 0.595 | 1.18 (1.15–1.22) | 0.012 |
| CVD rehospitalization | ||||||||
| Rehospitalization, n (%) | 91 (4.1) | — | 225 (9.0) | — | 18 (6.6) | 60 (7.2) | ||
| Adjusted IRRa | 1.08 (0.95–1.22) | 0.390 | 1.46 (1.43–1.49) | <0.0001 | 1.07 (0.98–1.18) | 0.932 | 1.30 (1.24–1.36) | 0.001 |
| Adjusted IRRb | 1.04 (0.94–1.17) | 0.272 | 1.20 (1.17–1.23) | <0.0001 | 0.90 (0.72–1.12) | 0.385 | 1.17 (1.13–1.21) | 0.040 |
Indicates age and gender were adjusted.
Indicates that age, gender, SBP, DBP, BMI, HbA1c, LDL cholesterol, and lipid-lowering treatment were adjusted.
Lipid-lowering agent was not a significant entrant into the model, and the distribution of the TC/HDL ratio was not significantly different between patients with and without lipid-lowering agents in each cohort (adjusted IRR for CVD hospitalization: 1.01 (0.96–1.06) and 0.99 (0.89–1.09) for ECF and RAPSID cohorts, respectively; IRR for rehospitalization: 0.99 (0.93–1.05) and 1.00 (0.98–1.01) for ECF and RAPSID cohorts, respectively). Similar findings were found in men and women: IRR for CVD hospitalization: 1.01 (0.90–1.14) and 1.01 (0.97–1.04) in men and 1.00 (0.99–1.01) and 0.99 (0.97–1.02) in women for ECF and RAPSID cohorts, respectively; IRR for CVD rehospitalization: 1.00 (0.98–1.01) and 1.00 (0.96–1.05) in men and 1.00 (0.99–1.02) and 0.98 (0.94–1.02) in women for ECF and RAPSID cohorts, respectively.
DISCUSSION
Our study was undertaken to relate TC/HDL to the risks of CVD hospitalization and rehospitalization in two independent cohorts of patients with T2D. We focused our investigation on the dose-response relationships assessing the evidence for nonlinear and particular in the existence of a threshold. In all our analyses, we found evidence that the associations are nonlinear. Threshold analysis provided evidence of a TC/HDL threshold: 2.8 (2.6–3.0). The significantly higher risks of CVD admissions and readmissions were found above 2.8 of TC/HDL.
Heart UK has recommended that a TC/HDL above 6 be regarded as a major risk factor for heart disease (14). However, Diabetes UK recommends a lower treatment goal of below 4 in diabetes patients (15). There are some other studies that set the TC/HDL ratio target below 4 for patients with T2D (16). However, based on our findings, comparing patients with TC/HDL at 2.8, for people with TC/HDL at 4.0, there was 55.2% and 24.0% increased risks of CVD hospitalization within 2 years and rehospitalization after 90 days of prior CVD hospitalization, respectively.
Our results extend previous findings, suggesting a “J-shaped” nonlinear association between TC/HDL and risks of both CVD hospitalization and rehospitalization among people with T2D. The existence of a nonlinear relationship between TC/HDL and CVD outcomes has not been investigated before. In most previous studies, the association between TC/HDL and CVD outcomes were analyzed by 1 unit or 1 SD increase, assuming linearity (consistent slope), which may have led to an underestimate of the risk of CVD events (17, 18). In other studies, the TC/HDL has been categorized into several groups based on percentiles, with the association analyzed by increases by 1 unit or 1 SD. The different slopes of association between TC/HDL and CVD outcomes have been presented over categories of TC/HDL, which also actually indicated that the association was nonlinear (19, 20). However, a threshold could not be identified by this strategy. Moreover, this strategy of categorized exposure is not recommended, as it leads to the loss of statistical power and the introduction of residual confounders. Therefore, in our study, TC/HDL was treated as a continuous variable, and nonlinear models were examined in an independent cohort study as the best-fitted model. The TC/HDL threshold of 2.8 was consistently identified in both cohorts for both genders and for both CVD hospitalization and rehospitalization.
Previous studies have not focused on CVD as both a major cause and cost for hospital admission among patients with diabetes. Understanding the potential risk of CVD hospitalization in the next year and the risk of a new episode (within 90 days) of a CVD event (rehospitalization) could be helpful for clinicians to facilitate tailored, more intensive management to those with high TC/HDL and to reduce hospitalization inpatient costs.
Our study has several advantages. We examined associations between TC/HDL and CVD hospitalization and rehospitalization in two independent prospective cohorts, which suggests that the findings in this study are reliable. The variables used in this study are from routinely recorded demographic and clinical measurements in primary care settings, which suggests that the findings in this study could increase the introduction of lipid-lowering treatment for people with T2D in clinical practice within countries that have routine recorded data accessible. We acknowledge that our study does not take into account diabetes duration, antidiabetes treatments, prior history of CVD, other diabetes complications (e.g., renal failure), lifestyle risk factors (like smoking), and other comorbidities due to limitations in the original data, but we feel that the clinical measurements included in our study could be proxies for missing predictors. A small minority of CVD events would have resulted in death, but data relating to mortality were not accessible due to linkage limitations. Based on the current study, the threshold is the same for the men and women. In this study, the event numbers are not enough for us to repeat the analyses by gender, which will be tested in future studies.
As far as we are aware, our study is the first study to investigate the associations between TC/HDL and the 2 year risk of CVD hospitalization and rehospitalization within 90 days of a previous hospitalization in two independent prospective cohort studies. Our study has two important implications for clinical practice. First, the relationship between TC/HDL and CVD outcomes are nonlinear, which suggests that the risk of CVD outcomes might be substantially underestimated by previous studies in which linear shapes were assumed. Second, our finding suggests that T2D patients with a TC/HDL ratio at 2.8 have the lowest risk of CVD outcomes, much lower than the 4.0 accepted in previous clinical guidelines. This suggests that 33% (ECF cohort) to 44% (RAPSID cohort) of patients whose TC/HDL are between 2.8 and 4.0 [similar by gender: 32% (ECF) to 45% (RAPSID) in men; 34% (ECF) to 46% (RAPSID) in women] may need more intensive lipid-lowering treatment, introduced at an earlier stage, to achieve this new optimal control target. Studies are required to assess the effectiveness and cost-effectiveness of these strategies.
Supplementary Material
Acknowledgments
The authors thank Toby Prevost, Chris Bunn, Simon Cohn, Sarah Donald, Charlotte Paddison, Candice Ward, Peers for Progress, West Anglia Comprehensive Local Research Network, Cambridgeshire and Peterborough Primary Care Trust, Primary Care Research Network-East of England, Eastern Diabetes Research Network, Medical Research Council Epidemiology Unit, participating general practices, Jackie Williams, Caroline Taylor, Kym Mercer, Kevin Baker, Ben Bowers, Kalsoom Akhter [Cambridge University Hospitals (CUH) Wolfson Diabetes and Endocrinology Clinic], James Brimicombe (Cambridge University), Kim Birch of Trumpington St. General Practice, CUH Wolfson Diabetes and Endocrinology Clinic Educators, The RAPSID Patient Committee (Phillip Jones, Liz Carvlin, and Roger Smith), and the peers and peer support participants.
Footnotes
Abbreviations:
- CHD
- coronary heart disease
- DBP
- diastolic blood pressure
- ECF
- East Cambridgeshire and Fenland
- GP
- general practitioner
- HbA1c
- hemoglobin A1c
- ICD
- International Classification of Diseases
- IRR
- incidence rates ratio
- NHS
- National Health Service
- RAPSID
- Randomized controlled trial of Peer Support In type 2 Diabetes
- SBP
- systolic blood pressure
- TC/HDL
- total cholesterol to HDL cholesterol ratio.
This work was supported by National Institute for Health Research (NIHR) Research for Patient Benefit Programme Grant PB-PG-0808-17303; and NIHR for Patient Benefit Programme Grant Ref PB-PG-0610-22311. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health. The authors declare that there are no conflicts of interest.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.American Diabetes Association. 2012. Standards of medical care in diabetes—2012. Diabetes Care. 35(Suppl 1): S11–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sampson M. J., Dozio N., Ferguson B., and Dhatariya K.. 2007. Total and excess bed occupancy by age, specialty and insulin use for nearly one million diabetes patients discharged from all English Acute Hospitals. Diabetes Res. Clin. Pract. 77: 92–98. [DOI] [PubMed] [Google Scholar]
- 3.Simmons D., English P., Robins P., Craig A., and Addicott R.. 2011. Should diabetes be commissioned through multidisciplinary networks, rather than Practice Based Commissioning? Prim. Care Diabetes. 5: 39–44. [DOI] [PubMed] [Google Scholar]
- 4.Vamos E. P., Millett C., Parsons C., Aylin P., Majeed A., and Bottle A.. 2012. Nationwide study on trends in hospital admissions for major cardiovascular events and procedures among people with and without diabetes in England, 2004–2009. Diabetes Care. 35: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holman R. R., Coleman R. L., Shine B. S., and Stevens R. J.. 2005. Non-HDL cholesterol is less informative than the total-to-HDL cholesterol ratio in predicting cardiovascular risk in type 2 diabetes. Diabetes Care. 28: 1796–1797. [DOI] [PubMed] [Google Scholar]
- 6.Simmons D., and Wenzel H.. 2011. Diabetes inpatients: a case of lose, lose, lose. Is it time to use a ‘diabetes-attributable hospitalization cost’ to assess the impact of diabetes? Diabet. Med. 28: 1123–1130. [DOI] [PubMed] [Google Scholar]
- 7.Yu D., Cai Y., Graffy J., Holman D., Zhao Z., and Simmons D.. 2018. Development and External Validation of Risk Scores for Cardiovascular Hospitalization and Rehospitalization in Patients With Diabetes. J. Clin. Endocrinol. Metab. 103: 1122–1129. [DOI] [PubMed] [Google Scholar]
- 8.Yu D., and Simmons D.. 2014. Association between blood pressure and risk of cardiovascular hospital admissions among people with type 2 diabetes. Heart. 100: 1444–1449. [DOI] [PubMed] [Google Scholar]
- 9.Simmons D., Prevost A. T., Bunn C., Holman D., Parker R. A., Cohn S., Donald S., Paddison C. A., Ward C., Robins P., et al. 2015. Impact of community based peer support in type 2 diabetes: a cluster randomised controlled trial of individual and/or group approaches. PLoS One. 10: e0120277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmons D., Cohn S., Bunn C., Birch K., Donald S., Paddison C., Ward C., Robins P., Prevost A. T., and Graffy J.. 2013. Testing a peer support intervention for people with type 2 diabetes: a pilot for a randomised controlled trial. BMC Fam. Pract. 14: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher E. B., Ayala G. X., Ibarra L., Cherrington A. L., Elder J. P., Tang T. S., Heisler M., Safford M. M., and Simmons D., and Peers for Progress Investigator Group. 2015. Contributions of Peer Support to Health, Health Care, and Prevention: Papers from Peers for Progress. Ann. Fam. Med. 13(Suppl 1): S2–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dale J. R., Williams S. M., and Bowyer V.. 2012. What is the effect of peer support on diabetes outcomes in adults? A systematic review. Diabet. Med. 29: 1361–1377. [DOI] [PubMed] [Google Scholar]
- 13.Simmons D., Yu D., and Wenzel H.. 2014. Changes in hospital admissions and inpatient tariff associated with a Diabetes Integrated Care Initiative: preliminary findings. J. Diabetes. 6: 81–89. [DOI] [PubMed] [Google Scholar]
- 14.Heart UK (n.d.) Cholesterol tests—know your number. Accessed July 9, 2018, at https://heartuk.org.uk/health-and-high-cholesterol/cholesterol-tests---know-your-number.
- 15.Diabetes.co.uk. 2018. Total cholesterol to HDL ratio calculator. Diabetes Digital Media Ltd. Accessed July 9, 2018, at https://www.diabetes.co.uk/cholesterol-to-hdl-ratio-calculator.html.
- 16.Braga M., Casanova A., Teoh H., Dawson K. C., Gerstein H. C., Fitchett D. H., Harris S. B., Honos G., McFarlane P. A., Steele A., et al. , and Diabetes Registry to Improve Vascular Events (DRIVE) Investigators. 2010. Treatment gaps in the management of cardiovascular risk factors in patients with type 2 diabetes in Canada. Can. J. Cardiol. 26: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng R. X., Li S., Zhang M. Z., Li X. L., Zhu C. G., Guo Y. L., Zhang Y., and Li J. J.. 2017. Remnant cholesterol predicts periprocedural myocardial injury following percutaneous coronary intervention in poorly-controlled type 2 diabetes. J. Cardiol. 70: 113–120. [DOI] [PubMed] [Google Scholar]
- 18.Smith K. J., Rabasa-Lhoret R., Strychar I., Karelis A. D., Clyde M., Levasseur J., Pinaroc C., Pedneault M., and Schmitz N.. 2014. Good vs. poor self-rated diabetes control: differences in cardiovascular risk and self-care activities. Exp. Clin. Endocrinol. Diabetes. 122: 236–239. [DOI] [PubMed] [Google Scholar]
- 19.Gudbjörnsdottir S., Eliasson B., Eeg-Olofsson K., Zethelius B., and Cederholm J., and National Diabetes Register (NDR). 2011. Additive effects of glycaemia and dyslipidaemia on risk of cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register. Diabetologia. 54: 2544–2551. [DOI] [PubMed] [Google Scholar]
- 20.Wan E. Y. F., Fong D. Y. T., Fung C. S. C., Yu E. Y. T., Chin W. Y., Chan A. K. C., and Lam C. L. K.. 2017. Prediction of five-year all-cause mortality in Chinese patients with type 2 diabetes mellitus—a population-based retrospective cohort study. J. Diabetes Complications. 31: 939–944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.