Enterococcus faecalis is a common cause of health care-associated infections in humans, largely due to its ability to persist in the hospital environment, colonize patients, acquire antimicrobial resistance, and form biofilms. Understanding how enterococci evolve in health care settings provides insight into factors affecting enterococcal survival and persistence. Macaques used in neuroscience research have long-term cranial implants that, despite best practices, often become colonized by E. faecalis. This provides a unique opportunity to noninvasively examine the evolution of enterococci on a long-term indwelling device. We collected E. faecalis strains from cephalic implants over a 7-year period and characterized the sequence type, antimicrobial resistance, virulence factors, biofilm production, and hypermutator phenotypes. Improved antimicrobial stewardship allowed a less-antimicrobial-resistant E. faecalis strain to predominate at the implant interface, potentially improving antimicrobial treatment outcomes if future clinical infections occur. Biofilm formation appears to play an important role in the persistence of the E. faecalis strains associated with these implants.
KEYWORDS: Enterococcus faecalis, animal models, antibiotic resistance, biofilms, implant, macaque, one health
ABSTRACT
Enterococcus faecalis is a common opportunistic pathogen that colonizes cephalic recording chambers (CRCs) of macaques used in cognitive neuroscience research. We previously characterized 15 E. faecalis strains isolated from macaques at the Massachusetts Institute of Technology (MIT) in 2011. The goal of this study was to examine how a 2014 protocol change prohibiting the use of antimicrobials within CRCs affected colonizing E. faecalis strains. We collected 20 E. faecalis isolates from 10 macaques between 2013 and 2017 for comparison to 4 isolates previously characterized in 2011 with respect to the sequence type (ST) distribution, antimicrobial resistance, biofilm formation, and changes in genes that might confer a survival advantage. ST4 and ST55 were predominant among the isolates characterized in 2011, whereas the less antimicrobial-resistant lineage ST48 emerged to dominance after 2013. Two macaques remained colonized by ST4 and ST55 strains for 5 and 4 years, respectively. While the antimicrobial resistance and virulence factors identified in these ST4 and ST55 strains remained relatively stable, we detected an increase in biofilm formation ability over time in both isolates. We also found that ST48 strains were typically robust biofilm formers, which could explain why this ST increased in prevalence. Finally, we identified mutations in the DNA mismatch repair genes mutS and mutL in separate ST55 and ST4 strains and confirmed that strains bearing these mutations displayed a hypermutator phenotype. The presence of a hypermutator phenotype may complicate future antimicrobial treatment for clinically relevant E. faecalis infections in macaques.
IMPORTANCE Enterococcus faecalis is a common cause of health care-associated infections in humans, largely due to its ability to persist in the hospital environment, colonize patients, acquire antimicrobial resistance, and form biofilms. Understanding how enterococci evolve in health care settings provides insight into factors affecting enterococcal survival and persistence. Macaques used in neuroscience research have long-term cranial implants that, despite best practices, often become colonized by E. faecalis. This provides a unique opportunity to noninvasively examine the evolution of enterococci on a long-term indwelling device. We collected E. faecalis strains from cephalic implants over a 7-year period and characterized the sequence type, antimicrobial resistance, virulence factors, biofilm production, and hypermutator phenotypes. Improved antimicrobial stewardship allowed a less-antimicrobial-resistant E. faecalis strain to predominate at the implant interface, potentially improving antimicrobial treatment outcomes if future clinical infections occur. Biofilm formation appears to play an important role in the persistence of the E. faecalis strains associated with these implants.
INTRODUCTION
Enterococcus faecalis, a Gram-positive, facultative anaerobe, is the sixth most common cause of health care-associated infections (1). Most of these infections result from the ability of enterococci to persist in the hospital environment, acquire antimicrobial resistance genes, and form biofilms, adhering to medical devices such as catheters (2–4).
Macaques used in cognitive neuroscience research are often surgically implanted with cephalic recording chambers (CRCs), which can become colonized by multiple bacterial species, including E. faecalis (5). While the majority tolerate polymicrobial colonization without complications, cases of peri-implant skin infections, meningitis, and brain abscessation are occasionally noted, necessitating identification of the bacterial species and antimicrobial resistance to optimize treatment (6). In 2011, aerobic culture identified Staphylococcus aureus, E. faecalis, and Proteus spp. as the most prevalent bacteria colonizing CRCs (5).
Due to the high levels of antimicrobial resistance observed for E. faecalis isolates, we previously characterized 15 strains from 2011 by multilocus sequence typing (MLST), antimicrobial susceptibility (MIC) testing, biofilm formation, and sequencing of select genomes (5). Among 15 isolates from 11 macaques, we identified two predominant sequence types (STs; ST4 and ST55), as well as genes associated with antimicrobial resistance, virulence, and biofilm formation (5).
The 2011 analysis highlighted the importance of refining and standardizing CRC sanitization procedures. These procedural changes introduced in 2014 included prohibition of prophylactic topical antimicrobial use within chambers (including oxytetracycline-polymyxin B and bacitracin-neomycin-polymyxin B ointments, gentamicin sulfate solution, and dilute injectable enrofloxacin) in favor of standardized sanitization procedures that use sterile 0.9% saline and 1 to 2% povidone-iodine solution. The goals of this study were to identify the impact of these changes on the dominant E. faecalis lineages colonizing implanted macaques and to examine how E. faecalis persists in implanted research macaques over time spans as long as 7 years. Specifically, for macaques with persistent CRC colonization, we determined if there were changes in sequence types, antimicrobial resistance profiles, biofilm production, or other genetic changes conferring increased growth or survival after implementation of the updated CRC sanitization protocols. Our results identified an overall shift in the enterococcal sequence type to a less resistant but strong biofilm-forming lineage (ST48). Our data suggest that improved antimicrobial stewardship has allowed a less resistant ST to supplant previously dominant, resistant strains associated with long-term CRC implants. This information provides new insight into how colonization and survival are affected by chronic exposure to antimicrobials and disinfectants, of relevance to both the research vivarium and hospital settings.
RESULTS
E. faecalis isolates and sequence types.
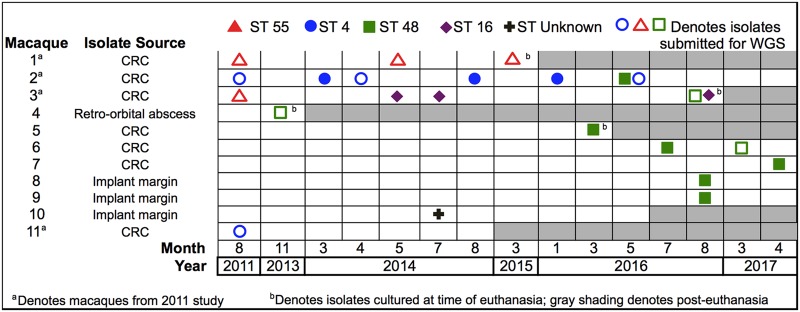
To identify the genetic lineages of the E. faecalis isolates colonizing research macaques, we compared the MLST STs of 20 strains isolated from 10 macaques over a 7-year period to those of 4 strains isolated in 2011 (Fig. 1). From the 10 more recently sampled macaques, we identified four dominant E. faecalis sequence types: ST4, ST16, ST48, and ST55. Macaques 1 and 2 remained colonized by ST55 and ST4 strains, respectively, over the 4- to 5-year course of sampling. The May 2016 sample from macaque 2 was colonized by both ST4 and ST48 strains. Macaque 3, initially colonized with an ST55 strain in 2011, yielded only an ST16 strain in May and July 2014. Samples collected at the time of euthanasia of macaque 3 in August 2016 yielded both ST16 and ST48 strains. E. faecalis strains belonging to ST48 were first identified in our macaque colony in a sample collected from macaque 4 in November 2013. Subsequent samples from macaques 5 to 9 showed that ST48 became the predominant lineage identified in 2016 and 2017. We also identified one unknown sequence type from macaque 10. This isolate is a single-locus pstS variant of ST594 containing the pstS 21 allele rather than the pstS 12 allele found in ST594. Isolates from macaques 8, 9, and 10 were collected from swabs of the skin margin surrounding the CRC implant.
FIG 1.
Sequence types of E. faecalis isolates collected from research macaques between 2011 and 2017. Each ST is designated by a colored symbol, and each row represents an individual macaque. Macaques included in the prior 2011 study and isolates collected at the time of euthanasia are designated with superscripts a and b, respectively. Gray shading indicates time points after the euthanasia date. WGS, whole-genome sequencing.
Antimicrobial susceptibility.
To determine changes in antimicrobial resistance over time for ST55 and ST4 strains and to evaluate the resistance profiles of the more recent ST16 and ST48 strains, we determined the MICs of a diverse panel of antimicrobials (Tables 1 and 2). ST55 isolates were susceptible to ampicillin and enrofloxacin, intrinsically resistant to gentamicin, resistant to bacitracin, erythromycin, and tetracycline, and resistant to high levels of streptomycin and neomycin. There were no remarkable changes in the MICs for ST55 isolates from macaque 1 over a 4-year period. ST4 isolates showed susceptibility to ampicillin, bacitracin, and erythromycin, intrinsic gentamicin resistance, resistance to enrofloxacin and tetracycline, and high-level resistance to streptomycin. The only change in MICs noted for ST4 isolates over the 5-year sampling period was an increase in neomycin resistance, from 128 μg/ml in the 2011 isolate to 256 μg/ml in the April 2014 isolate and a further increase to 2,048 μg/ml in the 2016 isolate. ST16 isolates displayed susceptibility to ampicillin and bacitracin, resistance to enrofloxacin and erythromycin, and high-level aminoglycoside resistance, including high-level gentamicin resistance. ST48 isolates expressed resistance to fewer antimicrobials, showing susceptibility to ampicillin, bacitracin, enrofloxacin, erythromycin, and tetracycline and only intrinsic aminoglycoside resistance (Table 2). We also examined the effectiveness of povidone-iodine by evaluating both MICs and minimum bactericidal concentrations (MBCs) (7, 8). The MIC for povidone-iodine for all ST4, ST48, and ST55 isolates was 0.3125%, and the minimum bactericidal concentration was 0.625%.
TABLE 1.
MIC testing of ST55 and ST4 E. faecalis strains isolated from macaques 1 and 2 over time
| Macaque | Mo and yr of isolate collection | ST | MIC (μg/ml)a |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | Bacitracin | Enrofloxacin | Erythromycin | Gentamicin | Neomycin | Streptomycin | Tetracycline | |||
| 1 | Aug. 2011 | 55 | 1–2 | >128 | 1 | >64 | 16–64 | >2,048 | >2,048 | 64 |
| May 2014 | 55 | 1 | >128 | 1 | >64 | 32 | >2,048 | >2,048 | 64 | |
| March 2015 | 55 | 1 | >128 | 1 | >64 | 32 | >2,048 | >2,048 | 64 | |
| 2 | Aug. 2011 | 4 | 1–2 | 8–16 | 64 | 0.25 | 8–16 | 128 | >2,048 | 128–256 |
| April 2014 | 4 | 1 | 4 | 64 | 0.25 | 16 | 256 | >2,048 | 256 | |
| May 2016 | 4 | 1 | 8 | 64 | 0.5 | 16–32 | 2,048 | >2,048 | 256 | |
| CLSI resistance breakpoints | ≤16 | NA | NA | ≥8 | Low-level IR; HLAR, >500 | Low-level IR; HLAR, NA | Low-level IR; HLAR, >1,000 | ≥16 | ||
TABLE 2.
MIC testing of ST48 and ST16 E. faecalis strains
| Macaque | Mo and yr of isolate collection | ST | MIC (μg/ml)a |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | Bacitracin | Enrofloxacin | Erythromycin | Gentamicin | Neomycin | Streptomycin | Tetracycline | |||
| 4 | Nov. 2013 | 48 | 1 | 16 | 1 | 0.25 | 8 | 256 | 128 | 2 |
| 5 | March 20 16 | 48 | 2 | 16 | 1 | 0.25 | 16 | 128 | 128 | 2 |
| 2 | May 2016 | 48 | 1 | 8 | 1 | 0.25 | 16 | 64 | 64 | 2 |
| 6 | July 2016 | 48 | 1 | 16 | 1 | 0.25 | 16 | 128 | 64 | 2 |
| 3 | Aug. 2016 | 48 | 2 | 16 | 2 | 0.25 | 128 | 512 | 128 | 2 |
| 8 | Aug. 2016 | 48 | 1 | 16 | 1 | 0.25 | 32 | 128 | 64 | 2 |
| 9 | Aug. 2016 | 48 | 2 | 8 | 2 | 0.25 | 8 | 64 | 64 | 1 |
| 6 | March 2017 | 48 | 1 | 16 | 0.5 | 0.125 | 16 | 128 | 128 | 1 |
| 7 | April 2017 | 48 | 1 | 16 | 1 | 0.125 | 16 | 64 | 64 | 0.5 |
| 3 | May 2014 | 16 | 0.5 | 4 | 32 | >64 | >2,048 | >2,048 | >2,048 | 256 |
| July 2014 | 16 | 1 | 8 | 32 | >64 | >2,048 | >2,048 | >2,048 | 128 | |
| Aug. 2016 | 16 | 1 | 8 | 64–128 | >64 | >2,048 | >2,048 | >2,048 | 256 | |
| CLSI resistance breakpoint | ≤16 | NA | NA | ≥8 | Low-level IR; HLAR, >500 μg/ml | Low-level IR; HLAR, NA | Low-level IR; HLAR, >1,000 μg/ml | ≥16 | ||
Antimicrobial resistance genes.
Genome sequences were examined to identify antimicrobial resistance genes present in ST4, ST55, and ST48 strains and to determine if there were changes in the antimicrobial resistance genotypes over time in sequential isolates from the same macaque. Known antibiotic resistance genes were identified by employing the ResFinder web tool and the specialty gene tool on the Pathosystems Resource Integration Center (PATRIC) website (9). The antimicrobial resistance genes identified in the genome sequences are displayed in Table 3. All sequenced ST55, ST4, and ST48 isolates possessed the lsaA gene encoding an ABC efflux pump that confers low-level macrolide resistance; however, only ST55 isolates displayed phenotypic resistance to erythromycin (3). Erythromycin resistance in ST55 isolates therefore is most likely attributable to the uniquely occurring plasmid-encoded ermB identified in ST55 but not in ST4 or ST48 isolates.
TABLE 3.
Antimicrobial resistance genes from selected E. faecalis strains identified by whole-genome sequencing and PCR
| Antimicrobial | Gene | Detection of the indicated gene in isolates from: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Macaque 1 (ST55) |
Macaque 2 (ST4) |
Macaque 3 |
Macaque 4 (ST48), Nov. 2013 | Macaque 6 (ST48), March 2017 | ||||||
| Aug. 2011 | May 2014 | March 2015 | Aug. 2011 | May 2016 | ST16,a May 2014 | ST48, Aug. 2016 | ||||
| Macrolides/lincosamides | lsaA | + | + | + | + | + | NAb | + | + | + |
| ermB | + | + | + | − | − | NA | − | − | − | |
| Tetracycline | tetL | − | − | − | + | + | NA | − | − | − |
| tetM | + | + | + | + | + | + | − | − | − | |
| tetS | + | + | + | − | − | + | − | − | − | |
| Bacitracin | bcrABR | + | + | + | − | − | NA | bcrA only | bcrA only | bcrA only |
| Chloramphenicol | cat | + | + | + | + | + | NA | − | − | − |
| Aminoglycoside | str | − | − | − | + | + | + | − | − | − |
| ant(6)-Ia | + | + | + | − | − | NA | + | + | + | |
| aph(3′)-IIIa | + | + | + | − | − | + | − | − | − | |
| aac(6′)-aph(2″) | − | − | − | − | − | + | − | − | − | |
| Fluoroquinolone | parC | − | − | − | S82I | S82I | NA | − | − | − |
| gyrA | − | − | − | S84I | S84I | NA | − | − | − | |
Antimicrobial resistance genes were determined by PCR.
NA, not assessed.
The tetracycline resistance of ST55 and ST4 isolates could be attributed to the chromosomal tetM and tetS genes found in the ST55 strains and plasmid-encoded tetL and tetM genes detected in the ST4 strains. Selected PCR on the May 2014 ST16 isolate confirmed the presence of tetM and tetS as the likely origin for tetracycline resistance in this strain. Both ST4 and ST55 isolates possessed the chloramphenicol acetyltransferase gene cat on a plasmid; while we did not assess the chloramphenicol MICs for new isolates, we previously confirmed chloramphenicol resistance in the 2011 ST4 and ST55 isolates (5). The plasmid-encoded aminoglycoside resistance genes identified included ant(6)-Ia and aph(3′)-IIIa in ST55 isolates, str in ST4 isolates, and ant(6)-Ia in ST48 isolates. The marked neomycin resistance in ST55 isolates was most likely due to the aph(3′)-IIIa gene, while high-level streptomycin resistance likely resulted from str and ant(6)-Ia genes (10). PCR was used to confirm the presence of the str, aph(3′)-IIIa, and aac(6')-aph(2″) in the May 2014 ST16 isolate from macaque 3, with high-level gentamicin resistance most likely being conferred by the aac(6')-aph(2″) gene (10).
Resistance to fluoroquinolones, including enrofloxacin, typically arises from mutations in DNA supercoiling enzymes. We confirmed the stable persistence of previously identified mutations in the genes for topoisomerase IV (parC) and DNA gyrase subunit A (gyrA), which are known to contribute to fluoroquinolone resistance (5, 11, 12), in ST4 isolates. Additionally, a 16-bp deletion upstream of a MATE family efflux pump was identified in the 2016 ST4 strain, which might explain the increased neomycin resistance that we observed in this lineage over time.
Virulence factor genes and biofilm production.
We examined the strains that were sequenced for genes for previously identified (5) virulence factors (Table 4). The profiles were largely similar across the strains, with variability being noted in the presence of the aggregation substance gene agg, the gelatinase gene gelE, the serine protease gene sprE, the quorum-sensing locus fsr, the cytolysin operon, and the hyaluronidase gene hylB. All sequenced ST55 and ST4 strains contained the E. faecalis pathogenicity island, which harbored the cytolysin operon in the ST4 strains (13). The pathogenicity island was not present in the ST48 strains that were sequenced. Phenotypically, all ST4 strains displayed beta-hemolysis on sheep blood agar plates. This was presumably due to the cytolysin operon but cannot be stated with certainty since hemolysis of ovine erythrocytes by the cytolysin is difficult to detect (14). A bacteriocin harbored by a plasmid was also identified in the ST4 strains, possibly contributing to the hemolytic activity observed in this strain. Finally, no changes in virulence factor genes were observed over time within isolates from the same macaque.
TABLE 4.
Virulence factor genes from selected E. faecalis strains identified by whole-genome sequencing
| Virulence factor function | Gene | Detection of the indicated gene in isolates from: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Macaque 1 (ST55) |
Macaque 2 (ST4) |
Macaque 4 (ST48), Nov. 2013 | Macaque 3 (ST48), Aug. 2016 | Macaque 6 (ST48), Mar. 2017 | |||||
| Aug. 2011 | May 2014 | Mar 2015 | Aug. 2011 | May 2016 | |||||
| Collagen adhesin precursor | ace | + | + | + | + | + | + | + | + |
| Aggregation substance | agg | − | − | − | + | + | + | + | + |
| Endocarditis and biofilm-associated pilus genes | ebpA | + | + | + | + | + | + | + | + |
| ebpB | + | + | + | + | + | + | + | + | |
| ebpC | + | + | + | + | + | + | + | + | |
| Cell wall adhesin expressed in serum | efaAfs | + | + | + | + | + | + | + | + |
| Enterococcal surface protein | esp | + | + | + | + | + | + | + | + |
| Gelatinase toxin | gelE | − | − | − | + | + | + | + | + |
| Serine protease | sprE | − | − | − | + | + | + | + | + |
| Sortase A | srtA | + | + | + | + | + | + | + | + |
| Quorum-sensing locus | fsrABDC | − | − | − | − | − | + | + | + |
| Cytolysin (hemolysin-bacteriocin) | cylL | − | − | − | + | + | − | − | − |
| Posttranslational cytolysin modification | cylM | − | − | − | + | + | − | − | − |
| Transport of cytolysin | cylB | − | − | − | + | + | − | − | − |
| Activation of cytolysin | cylA | − | − | − | + | + | − | − | − |
| Sex pheromone | cad | + | + | + | + | + | + | + | + |
| Sex pheromone cAM373 precursor | camE | + | + | + | + | + | + | + | + |
| Sex pheromone | cCF10 | + | + | + | + | + | + | + | + |
| Sex pheromone | cOB1 | + | + | + | + | + | + | + | + |
| Enterococcal Rgg-like regulator | elrA | + | + | + | + | + | + | + | + |
| Hyaluronidase | hylA | + | + | + | + | + | + | + | + |
| hylB | + | + | + | − | − | + | + | + | |
| Thiol peroxidase | tpx | + | + | + | + | + | + | + | + |
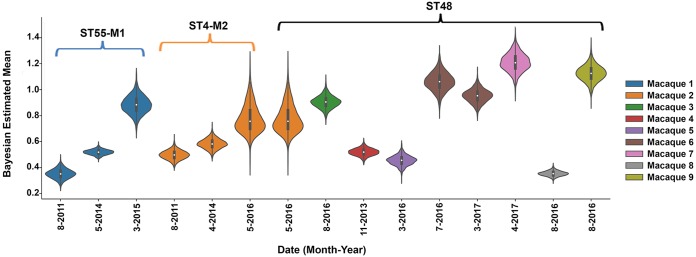
Next, we examined if the ability of E. faecalis to persistently colonize the CRCs was associated with the ability to form biofilms by measuring the biofilm-forming capacity of 15 strains (Fig. 2). Posterior credible intervals from Bayesian estimation modeling identified a subtle increase over time in biofilm formation among ST55 strains from the same animal and a more substantial increase in the 2016 ST4 isolate compared to that in prior isolates from the same macaque. ST48 isolates showed variable biofilm-forming capabilities; however, nearly all isolates formed more biofilm than the ST55 and ST4 strains from 2011 and 2014 (Fig. 2). This finding suggests that increased biofilm formation may have contributed to the strain succession leading to ST48 predominance following the change in the CRC maintenance protocol.
FIG 2.
Posterior credible intervals of biofilm production from 15 E. faecalis strains. Biofilm was measured using a crystal violet assay (47). Each macaque is represented in a different color, and the STs are indicated. Experiments were performed a minimum of three times on different days using 6 to 12 replicates per strain. Violin plots show the Bayesian estimated posterior of the mean OD570 readings; the interquartile range is indicated by the thicker black bars inside each violin, and the median is indicated by the white circle. For strains with completely overlapping violin plots, there is no way to distinguish between biofilm-forming ability, while strains with completely disparate violin plots can be interpreted to have differences in biofilm-forming ability. M1 and M2, macaques 1 and 2, respectively.
Gene and mobile element differences between strains.
To identify if E. faecalis strains of the same ST were isogenic with one another, we compared annotated gene lists from PATRIC. Comparison of ST55 annotated gene lists from macaque 1 between the 2011 and 2014 strains identified only one difference for a gene not annotated as a hypothetical protein (a terminase small subunit in the 2014 strain); thus, these two strains could be considered isogenic. The 2015 ST55 strain from macaque 1 had multiple differences in annotated genes compared to the 2011 and 2014 isolates and was not considered isogenic to these isolates. For ST4 strains, multiple differences in annotated genes were identified between the 2011 and 2016 strains from macaque 2; thus, these isolates were not isogenic. Comparison of the ST48 annotated gene lists between the 2013 isolate from macaque 4 and the 2017 isolate from macaque 6 did not reveal differences in the annotated genes; therefore, these two isolates could be considered isogenic. Comparison of the ST48 annotated gene lists between the 2016 isolate from macaque 3 and the 2013 and 2017 isolates from macaques 4 and 6 revealed multiple differences in annotated genes; thus, these strains were not considered isogenic.
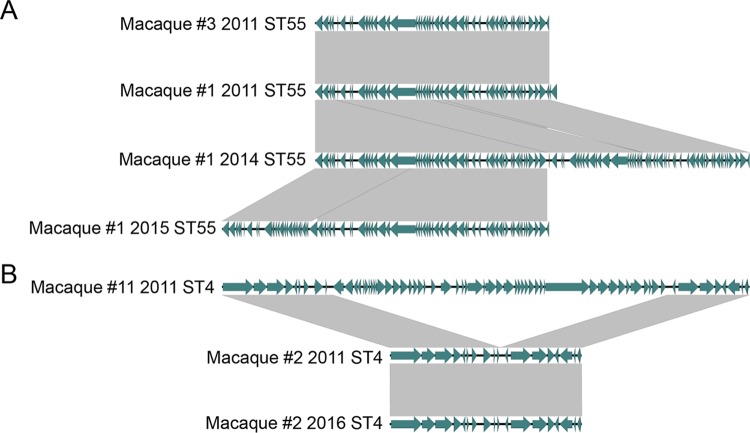
To understand how the E. faecalis strains differed from one another in their mobile genetic element content both within and between macaques, we analyzed the plasmid and prophage content of all sequenced strains. All sequenced ST55 strains harbored a plasmid similar to pB of E. faecalis strain CLB21560 (99% identity over 74% coverage; GenBank accession number CP019514.1). All sequenced ST4 strains harbored a plasmid that was similar to pEF123 of E. faecalis strain EF123 (99% identity over 85% coverage; GenBank accession number KX579977.1). ST48 strains isolated in 2013 and 2017 both contained a presumed plasmid most closely related (97% identity over 59% coverage) to pBEE99 of E. faecalis strain E99 (15); however, no known rep genes were identified. Sequential isolates from the same macaque displayed only minor changes in their plasmid gene contents over time, and the same plasmids from the ST55, ST4, and ST48 strains were all found in more than one animal, suggesting that they were stably maintained.
In contrast to the relatively stable plasmid maintenance in strains from the same ST, the prophage content was found to be more dynamic between strains (Fig. 3). The two ST55 strains isolated from different macaques in 2011 contained an identical prophage region; however, this region appeared to be duplicated in the 2014 strain and further shuffled in the 2015 strain from macaque 1 (Fig. 3A). Separately, a prophage region detected in the ST4 strain isolated from macaque 11 in 2011 was absent from the sequenced ST4 strains of macaque 2 (Fig. 3B). The ST48 strains contained multiple small, phage-like elements, one of which is found in all E. faecalis strains (16). We evaluated the possibility that one of these elements might have contributed to the sequence type shift within the macaque colony by attempting to induce the release of lysogenic bacteriophages from ST48 isolates and looking for activity against ST55 and ST4 isolates. Unfortunately, neither exposure to UV light nor addition of norfloxacin succeeded at inducing a phage that would plaque on the 2011 isolates of ST55 and ST4 from macaques 1 and 2, respectively.
FIG 3.
Differences in prophage content between ST55 (A) and ST4 (B) strains. Phages were compared to one another using analysis with the BLAST program, and plots were generated using the Easyfig application (48). Gray shading indicates regions with >95% nucleotide identity.
Variant analysis identifies transient hypermutator strains.
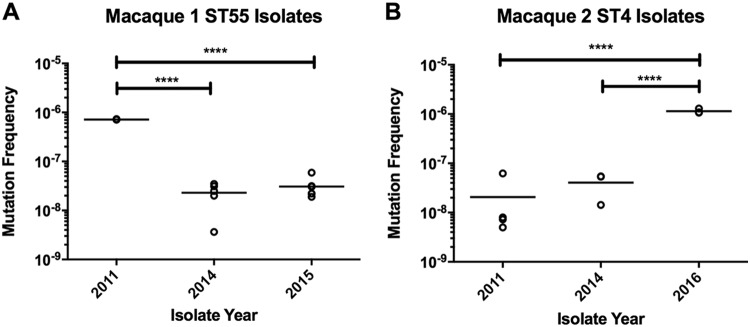
We next identified variants between strains of the same ST and evaluated changes in genes that might affect survival. The classification and the numbers of variants are listed in Table 5, and the identified variants are listed in Table S1 in the supplemental material. Overall, ST4 isolates had a higher average number of variants detected (both within and between macaques; n = 44) than ST55 and ST48 isolates (23 and 28, respectively). In all cases, approximately half of all variants detected were nonsynonymous changes, with the remaining variants being divided between synonymous mutations, intergenic variants, and insertion/deletion variants. Both ST4 and ST55 isolates had nonsynonymous variants identified within a transcription regulator containing a diacylglycerol kinase domain, the phosphate transport ATP-binding protein pstB, and DNA mismatch repair genes (mutL for the 2016 ST4 isolate and mutS for the 2011 ST55 isolate). We hypothesized that strains with mutations in DNA mismatch repair genes might have higher spontaneous mutation rates. We measured mutagenicity in ST55 and ST4 strains with a standard rifampin mutagenicity assay and confirmed a hypermutator phenotype in the 2011 ST55 isolate and the 2016 ST4 isolate (Fig. 4) (17). The median mutation frequencies for the 2011 ST55 isolate and 2016 ST4 isolate were approximately 30-fold and 40-fold higher, respectively, than those for isolates with wild-type versions of mutL and mutS from the same macaque. Representative mutagenicity data are shown in Fig. 4.
TABLE 5.
Numbers and types of variants identified within and between E. faecalis strains
| Reference genome (yr), comparison genome | Comparison genome yr of isolation | No. of variants with the following changes: |
||||
|---|---|---|---|---|---|---|
| Total | Nonsynonymous | Synonymous | Intergenic | Insertions/deletions | ||
| Macaque 1 (2011) | ||||||
| Macaque 1 | 2014 | 21 | 12 | 4 | 5 | 0 |
| 2015 | 19 | 10 | 4 | 5 | 0 | |
| Macaque 3 | 2011 | 28 | 13 | 6 | 5 | 4 |
| Macaque 2 (2011) | ||||||
| Macaque 2 | 2016 | 44 | 31 | 8 | 5 | 0 |
| Macaque 11 | 2011 | 43 | 26 | 10 | 4 | 3 |
| Macaque 4 (2013), macaque 6 | 2017 | 28 | 16 | 7 | 3 | 2 |
FIG 4.
Rifampin mutagenicity assay for ST55 and ST4 isolates over time. (A) ST55 isolates from 2011 to 2015 from macaque 1. (B) ST4 isolates from 2011 to 2016 from macaque 2. Data from a representative experiment are shown; bars represent sample means. ****, P < 0.0001, one-way analysis of variance with Holm-Sidak's multiple-comparison test.
DISCUSSION
Sequential sampling of 10 macaques over a 7-year time span allowed a unique opportunity to observe how E. faecalis persists within a polymicrobial community associated with a chronic implant. This has high relevance to human infections, where E. faecalis often persists on implanted medical devices, such as intravascular catheters, ureteral stents, and intraocular lens materials (2, 18, 19). Genome sequencing and phenotypic characterization uncovered two surprising findings. First, the minimization of antimicrobial pressure appears to have driven the displacement of more drug-resistant strains by a less resistant, stronger biofilm-forming lineage. Second, transient hypermutator strains arose in two different STs in two different animals that were persistently colonized by the same strain.
Analysis of cultures of CRC specimens collected in 2011 identified 15 E. faecalis isolates from 11 macaques, with ST4 and ST55 being the predominant sequence types (n = 7 each) (5). Both ST4 and ST55 have been identified among human clinical isolates (20). Interestingly, the majority of recorded ST4 isolates were identified in samples from Asia, while ST55 strains were more commonly isolated from patients in Europe and the United States (21). Rhesus macaques natively inhabit a wide territory, ranging from Afghanistan to China and India (22). While the majority of our research colony originates from primate centers located in the United States, these colonies originated from primates imported from Asia. Due to the length of time that animals are present in the MIT vivarium, it is difficult to determine whether strains colonizing macaque CRCs originated as commensal gastrointestinal strains arriving with the macaques or whether the strains were acquired after arriving at our vivarium. The relatively low diversity of the strains detected and the presence of the same strain in multiple animals suggest that at least some animals became colonized at MIT.
Analysis of E. faecalis isolates collected since initial sampling in 2011 revealed different colonization patterns in individual animals. Macaque 1 remained colonized by the ST55 lineage from 2011 to 2015, while macaque 2 remained colonized by ST4 from 2011 to 2016 and then gained ST48 prior to the August 2016 sampling. Both animals appear to have been colonized by the same strain over a 4- to 5-year time span, since fewer than 50 variants were observed between strains from the same animal. Strains of the same ST isolated from different animals were also very closely related to one another, suggesting that animals either transmitted strains to one another or acquired them from a common source. Macaque 3 was colonized by an ST55 strain in 2011, but subsequent sampling identified only ST16 isolates in 2014, with the addition of an ST48 strain in 2016. While an ST48 strain was first isolated in 2013 from macaque 4, our data indicate an increased prevalence of an ST48 isolate in samples collected in the 2016-2017 time frame, coincident with the decrease in the incidence of ST4 and ST55 strains in more recently implanted macaques. In the MLST database, only a single isolate of ST48 has been reported and was identified from a fecal surveillance sample in a hospitalized patient in Spain (20).
Antimicrobial resistance within the strains of the different STs remained relatively stable overall in both genotype and phenotype, with the exception of increased neomycin resistance in the ST4 strains over time. We also detected a more subtle increase in the gentamicin MIC in the same strains (Table 1). While we did not identify differences in antimicrobial resistance gene content within ST4 strains, we detected a 16-bp deletion upstream of a MATE family efflux pump in the 2016 isolate that had a higher neomycin MIC. This efflux pump showed similarity to the NorM pump, which has been shown to efflux aminoglycosides (23). We hypothesize that the observed deletion causes increased expression of the efflux pump and thereby increases neomycin resistance.
In September 2014, CRC sanitization procedures were updated to prohibit antimicrobial use within CRCs. Removal of this selective pressure for resistant strains corresponded with the appearance of ST48 isolates. It is likely that prior antimicrobial use within CRCs prevented ST48 strains from colonizing at a high density because this lineage is so much more susceptible to antimicrobials than the ST4 and ST55 lineages. Despite the standardized use of 1 to 2% povidone-iodine as part of routine cleaning procedures, E. faecalis has clearly persisted within and around the skin implant margin of CRCs. This persistence likely arises from the inability of the disinfectant to penetrate biofilms within the time duration of application, as the MIC and MBC for ST4, ST48, and ST55 strains were less than the current povidone-iodine concentration used (0.3125% and 0.625%, respectively). Additionally, the polymethylmethacrylate acrylic used for anchoring CRCs to the skin margin represents a likely reservoir for strains. This area is difficult to effectively sanitize with topical disinfectants, and polymethylmethacrylate has previously been demonstrated to permit E. faecalis biofilm formation (18).
The virulence factor genotypes remained stable over time within the sequence types; however, crystal violet assays suggested an increase in biofilm production noted over time in ST4 and ST55 isolates. The 2016 ST4 isolate, in particular, was found to produce significantly more biofilm than the 2011 or 2014 strains from the same animal. While no differences in virulence factor genes were observed in this strain, the increased biofilm formation in the 2016 strain could be due to one or more of the variants identified in the strain or to a recombination event detected within the gene for the enterococcal surface protein (esp), a gene that has previously been shown to play a role in biofilm formation in E. faecalis (24). Additionally, we determined that seven of nine tested ST48 strains were robust biofilm formers. Genome sequencing confirmed the presence of multiple biofilm-associated genes in select ST48 isolates, including the quorum-sensing operon fsrABDC, which was not present in ST4 and ST55 isolates. The fsrABDC operon controls gene expression of the secreted proteases gelatinase (gelE) and serine protease (sprE) (25–27). Gelatinase contributes to virulence and biofilm production via cleavage of host cell proteins to aid in surface attachment and dissemination based on bacterial density (25). An additional difference in virulence factor gene content between strains is that the ST4 isolates possessed the hemolysin-bacteriocin operon encoding the cytolysin within their pathogenicity island, which was not identified in ST48 or ST55 isolates (14). While the presence of the cytolysin toxin has previously been established to be a marker for increased virulence, morbidity, and mortality in both human enterococcal infections and animal models (28–32), no additional signs of disease were observed in the animal colonized with this ST4 strain.
Analysis of single nucleotide variants (SNVs) within STs over time revealed three gene families with variants in common between ST4 and ST55 isolates: a transcriptional regulator containing a diacylglycerol kinase domain, the phosphate transport ATP binding protein pstB, and DNA mismatch repair genes mutL and mutS.
Because hypermutator phenotypes have been observed before in infection contexts (33), we were interested in determining whether the identified mutL and mutS mutations affected strain mutagenicity. The 2016 ST4 isolate from macaque 2 was identified to have a C1999T transition in mutL, resulting in a Q667* nonsense mutation in the protein. The 2011 ST55 isolate from macaque 1 was found to have a 10-bp deletion causing a frameshift in the mutS protein, resulting in mutations at protein positions 437 to 439 (QWL → RS*) and premature truncation of the protein. To evaluate the effects of these identified mutations, we performed rifampin mutagenicity assays and confirmed that the 2011 ST55 and 2016 ST4 isolates displayed a hypermutator phenotype. Previous work has evaluated the effects of mutated mutS and mutL genes in emerging antimicrobial resistance in Enterococcus faecium with mixed conclusions (33, 34). While one study did not identify a hypermutator phenotype in two E. faecium strains with mutL and mutS mutations that developed linezolid resistance during therapy, another case presentation described recJ and mutL mutations in a daptomycin-nonsusceptible E. faecium blood culture isolate from an endocarditis patient (33, 34).
We would expect that isolates with a hypermutator phenotype would be more likely to accumulate nucleotide polymorphisms. When comparing the number of variants between isolates from the same macaque, we did identify more variants when comparing the 2016 ST4 isolate to the 2011 ST4 isolate from macaque 2 (44 variants total) than when comparing ST55 isolates from macaque 1 (21 and 19 SNVs for the 2014 and 2015 isolates, respectively). We also noticed that 90% of the variants detected in the 2016 ST4 isolate were single nucleotide polymorphisms, whereas 75 to 80% of the variants in the other comparisons were single nucleotide polymorphisms. While the ST55 hypermutator phenotype did not persist, future antimicrobial therapy for macaque 2 could be complicated by the presence of the ST4 hypermutator isolate. While limited studies have examined hypermutator E. faecalis strains, hypermutator phenotypes in chronic Pseudomonas aeruginosa infections contribute to the development of antimicrobial resistance in cystic fibrosis patients (35, 36).
Overall, our study provides insight into how different E. faecalis lineages have both persisted and been succeeded by other lineages within polymicrobially contaminated cranial implants in research macaques over a 7-year period. Our data suggest that improved antimicrobial stewardship has allowed the persistence of less resistant, more robust biofilm-forming ST48 strains within research macaques at MIT. In two macaques monitored sequentially since 2011, ST48 strains were able to colonize in a polymicrobial milieu of CRCs that were previously colonized with strains belonging to other, more drug-resistant STs. We have also demonstrated that these strains can maintain persistent colonization over time within CRCs with little change in antimicrobial resistance and that the ability to form biofilms likely contributes to this persistence. Future work will aim to develop a better understanding of the bacterial community within CRCs and to evaluate alternative techniques in treating E. faecalis biofilms.
MATERIALS AND METHODS
Animals.
Ten rhesus macaques (Macaca mulatta; 8 males, 2 females) with cephalic implants at MIT were sampled. Macaque cohort and implant parameters are listed in Table 6. All macaques were housed in an AAALAC International-accredited facility under standards outlined by the 8th edition of the Guide for the Care and Use of Laboratory Animals (37). Briefly, husbandry parameters included a 12-h light–12-h dark cycle and a diet of commercial primate biscuits (Purina 5038) supplemented with fruits, vegetables, nuts, and cereal. Macaques were housed in pairs, with exceptions being made for animals showing incompatibility with conspecifics. Macaque CRC and implant margin samples were obtained from chair-restrained macaques during routine implant sanitization procedures. Briefly, the chamber was opened and a sterile polyester-tipped applicator swab (Puritan Medical Products, Guilford, ME) was used to gently sample the chamber and exudate present. Implant margin swabs were obtained by application of the swab around the base of the CRC acrylic and/or the restraint pedestal. The MIT Committee on Animal Care (CAC) approved all study procedures. Macaques remained on study for the purpose of their CAC-approved cognitive neuroscience research protocol. No macaques were euthanized for the purposes of this study, but three macaques were euthanized for medical reasons with intravenous sodium pentobarbital (86 mg/kg of body weight; Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI). Macaque 1 was euthanized for seizures, macaque 4 was euthanized for meningitis and retro-orbital abscessation (S. aureus, E. faecalis, Fusobacterium nucleatum, and Prevotella spp. were cultured), and macaque 5 was euthanized as a result of surgical complications. Macaques 3, 7, and 10 were euthanized at their research endpoint. Macaques 1, 2, and 3 are the same individuals as macaques 6, 9, and 8, respectively, in our previous study (5). We also compared genomic data from macaque 2 in this study to those from macaque 1 from our previous study (5); this macaque is designated macaque 11 in this paper.
TABLE 6.
Macaque demographicsa
| Macaque | Isolate source | Sex | Yr of birth | Yr of arrival | Date implanted (mo/yr) | No. of CRCs | Implant materials | CRC antimicrobials; packing materials; sanitization solutions prior to 2014 | Euthanasia date (mo/yr) | Identifier in 2011 study |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CRC | M | 1998 | 2006 | 4/2008 | 3 | Ultem, PMMA, titanium screws | T-PB; nonwoven sponge balls; HP, PI, CHX, saline | 3/2015 | Macaque 6 |
| 2 | CRC | M | 1998 | 2007 | 4/2011 | 3 | Ultem, PMMA, titanium screws | T-PB; nonwoven sponge balls; HP, PI, CHX, saline | NA | Macaque 9 |
| 3 | CRC | M | 1998 | 2006 | 11/2008 | 2 | Ultem, PMMA, titanium screws | T-PB; nonwoven sponge balls; HP, PI, CHX, saline | 8/2016 | Macaque 8 |
| 4 | Abscess | M | 2005 | 2006 | 6/2013 | 1 | CILUX, PMMA, ceramic screws | No antimicrobials; petroleum jelly; CHX, saline | 11/2013 | NA |
| 5 | CRC | M | 2007 | 2010 | 3/2016 | 1 | Ultem, PMMA, titanium screws | NA | 3/2016 | NA |
| 6 | CRC | M | 2008 | 2011 | 6/2016 | 1 | PEEK, PMMA, titanium straps and screws | NA | NA | NA |
| 7 | CRC | F | 2009 | 2016 | 10/2016 | 1 | Ultem, PMMA, ceramic screws | NA | 6/2017 | NA |
| 8 | Restraint pedestal | M | 2007 | 2012 | 9/2014 | NA | Titanium | NA | NA | NA |
| 9 | Restraint pedestal | F | 2009 | 2013 | 12/2015 | NA | Titanium | NA | NA | NA |
| 10 | restraint pedestal | M | 1997 | 2006 | 6/2007 | NA | Titanium | NA | 6/2016 | NA |
| 11 | CRC | F | 1996 | 2000 | 6/2006 | 1 | Delrin, PMMA, ceramic screws | G; no packing material; PI, saline | 1/2015 | Macaque 1 |
M, male; F, female; Ultem (Curbell Plastics), a polyethermide plastic; PMMA, polymethylmethacrylate bone cement; CILUX (Crist Instrument Company), a proprietary plastic; PEEK, polyetheretherketone thermoplastic; Delrin (Grainger Industrial Supply), a polyoxymethylene plastic; G, 0.3% gentamicin sulfate; T-PB, 5 mg/g oxytetracycline–10,000 U/g polymyxin B; HP, hydrogen peroxide; PI, povidone iodine; CHX, chlorhexidine solution; NA, not applicable.
Cephalic recording chamber implant maintenance.
Prior to 2014, routine CRC sanitization procedures varied between investigators, including cleaning with various combinations of saline, hydrogen peroxide, dilute chlorhexidine solution, and/or dilute povidone-iodine solution, application of 0.5% oxytetracycline with polymyxin B ointment and gentamicin sulfate solution, and packing with sterile nonwoven sponge balls soaked in dilute chlorhexidine solution (Table 6) (5). In September 2014, CRC sanitization protocols were updated and standardized to include cleaning with saline and 1 to 2% povidone-iodine solution within the chamber and dilute chlorhexidine solution around the implant skin margin only, and the use of topical antimicrobial agents inside the CRC was prohibited without explicit veterinary approval. To our knowledge, no antimicrobials were used inside CRCs after 2014 for the macaques included in this study.
Bacterial isolation.
Swabs were plated on tryptic soy agar (TSA) with 5% sheep blood and incubated overnight at 37°C in 5% CO2. When necessary, subcultures onto phenylethyl alcohol blood agar and/or bile esculin azide agar were used to facilitate isolation of colonies of Gram-negative species. Colonies that grew on bile esculin azide and/or with a visual appearance consistent with Enterococcus (light gray, alpha-hemolytic, catalase-negative colonies) were selected for further isolation.
DNA extraction.
Crude DNA extraction for 16S rRNA sequencing was performed by collecting colonies with a 1-μl disposable loop and placing them into 200 μl of a solution containing 5% Chelex 100 resin and 0.02% proteinase K. Samples were heated at 56°C for 1 h, briefly vortexed, and then heated at 95°C for 10 min. Samples were then centrifuged at 10,000 × g for 3 min and stored at −20°C until use. Genomic DNA was extracted from overnight broth cultures using a commercially available kit with enzymatic lysis, as previously described (5). DNA was eluted in 10 mM Tris-HCl, 0.5 mM EDTA, and pH 9.5 buffer.
PCR and MLST.
PCR amplification of the 16S rRNA gene, MLST housekeeping genes, tetS, tetM, aph(3′)-IIIa, and aac(6′)-aph(2″) was performed using previously published primers and protocols (Table 7) (38–42). PCR primers for mutL and str were designed using the Primer-BLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Thermocycler parameters are as follows: for mutL, 95°C for 4 min, 35 cycles of 95°C for 1 min, 58°C for 45 s, and 72°C for 1 min, and a final elongation at 72°C for 8 min; for tetS, str, and aph(3′)-IIIa, 95°C for 5 min, 30 cycles of 95°C for 1 min, 57°C for 1 min, and 72°C for 1 min, and a final elongation at 72°C for 8 min; and for tetM 95°C for 5 min, 30 cycles of 95°C for 1 min, 52°C for 1 min, and 72°C for 1 min, and a final elongation at 72°C for 8 min. PCR bands were separated by electrophoresis on a 1% gel at 100 to 120 V for 30 to 40 min and visualized with UV light following ethidium bromide staining. PCR products (5 μl) were purified by adding 2 μl of a mixture containing 0.4 U/μl exonuclease I and 0.4 U/μl shrimp alkaline phosphatase, and the mixture was incubated for 20 min at 37°C followed by 20 min at 80 to 85°C. PCR products were submitted for sequencing at a commercial laboratory (Quintara Biosciences, Cambridge, MA). Enterococcal sequence types were identified by using the E. faecalis MLST website (http://pubmlst.org/efaecalis) (20).
TABLE 7.
PCR primers used for gene identification
| Gene | Sequence (5′ to 3′) |
Amplicon size (bp) | Source or authors (reference) | |
|---|---|---|---|---|
| Forward primer | Reverse primer | |||
| mutL | TCGGTCAAATGCACGGAACT | TTAATGGGGGTCTTGAATGCGT | 569 | This paper |
| aac(6′)-aph(2″) | CCAAGAGCAATAAGGGCATA | CACTATCATAACCACTACCG | 222 | Khani et al. (40) |
| aph(3′)-IIIa | GGCTAAAATGAGAATATCACCGG | CTTTAAAAAATCATACAGCTCGCG | 523 | Emaneini et al. (41) |
| str | ATTGCTCTCGAGGGTTCAAG | CGTTGAGACACTCCAAAACTCA | 423 | This paper |
| tetM | GTTAAATAGTGTTCTTGGAG | CTAAGATATGGCTCTAACAA | 657 | Choi and Woo (42) |
| tetS | TGGAACGCCAGAGAGGTATT | ACATAGACAAGCCGTTGACC | 660 | Emaneini et al. (41) |
Whole-genome sequencing, assembly, and annotation.
E. faecalis genomic DNA from macaques 1, 2, 4, and 6 was sequenced on a single SMRT cell per isolate using a Pacific Biosciences (PacBio) RS2 sequencer at the University of Massachusetts Deep Sequencing Core Facility (Worcester, MA). E. faecalis genomic DNA from macaque 3 was sequenced using an Illumina MiSeq instrument at the MIT Biomicro Center. PacBio DNA libraries were prepared for sequencing with the SMRTbell template preparation kit (v1.0) and DNA/polymerase binding kit P6 (v2), according to the manufacturer's instructions (Pacific Biosciences, Menlo Park, CA). E. faecalis genomic DNA from macaque 3 was concentrated using Agencourt AMPure XP beads at a 1.8× ratio and eluted in 10 mM Tris-HCl, pH 8.5, buffer to remove EDTA from the DNA sample. The bacterial DNA library was prepared using a QIAseq FX DNA library kit (Qiagen) with 1 μg of DNA per the manufacturer's instruction. Fragmentation of DNA was performed at 32°C for 4 min. AMPure XP beads were used to perform library purification as indicated in the QIAseq FX DNA library kit manual. Barcoded and adapter-ligated DNA libraries were pooled with other DNA libraries. Quality control prior to sample loading included both sizing of the Illumina libraries with an advanced analytical fragment analyzer and quantification of the libraries by quantitative PCR (qPCR), performed using a Kapa SYBR Fast qPCR master mix (KM4114) on a Roche LightCycler 480 II instrument. Two × 300-base paired end reads were generated on an Illumina MiSeq instrument using a MiSeq reagent kit (v3; Illumina). PacBio genomes were assembled using the hierarchical genome assembly process (version 3.0) workflow hosted on the SMRT portal (v2.3). Reads, coverage, and annotation are found in Table 8. Illumina genome assembly was performed using the spades tool on the Pathosystems Resource Integration Center (PATRIC) website, v3.5.2 (9). Annotation on assembled genomes was performed using the RAST tool kit on the PATRIC website, v3.5.2 (9). Genes encoding antimicrobial resistance and virulence factors were identified using ResFinder, VirulenceFinder, and the PATRIC database specialty genes features (9, 43, 44). Identification threshold parameters were set at 98% identity over a minimum length of 60% for ResFinder and 95% identity over a minimum length of 60% for VirulenceFinder.
TABLE 8.
Whole-genome sequencing metadata
| Macaque | ST | WGSa technology | Isolate date | Genome size (bp) | Total no. of reads | No. of contigs | N50 | Avg reference coverageb | No. of genes annotated | NCBI accession no. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | PacBio | Aug. 2011 | 3,011,796 | 83,861 | 2 | 17,828 | 257.05 | 2,941 | QFYO00000000 |
| 55 | PacBio | May 2014 | 3,024,067 | 92,116 | 2 | 13,530 | 211.55 | 2,946 | QFYN00000000 | |
| 55 | PacBio | March 2015 | 3,037,432 | 81,501 | 3 | 16,398 | 226.35 | 2,995 | QFYM00000000 | |
| 2 | 4 | PacBio | Aug. 2011 | 2,983,064 | 88,233 | 2 | 15,312 | 239.31 | 2,881 | MCFW00000000 |
| 4 | PacBio | May 2016 | 2,986,755 | 65,930 | 5 | 7,003 | 74.75 | 2,907 | QFYL00000000 | |
| 3 | 55 | PacBio | Aug. 2011 | 3,015,699 | 86,533 | 3 | 24,224 | 338.83 | 2,939 | MCFV00000000 |
| 48 | Illumina | Aug. 2016 | 2,806,131 | 1,807,478 | 19 | 364,769 | 142.19 | 2,670 | QFYJ00000000 | |
| 4 | 48 | PacBio | Nov. 2013 | 2,919,427 | 95,407 | 2 | 15,106 | 269.22 | 2,786 | QFYK00000000 |
| 6 | 48 | PacBio | March 2017 | 2,920,131 | 86,807 | 2 | 16,627 | 260.61 | 2,775 | QFYI00000000 |
| 11 | 4 | PacBio | Aug 2011 | 3,044,487 | 87,196 | 3 | 24,838 | 327.48 | 2,962 | MCFU00000000 |
WGS, whole-genome sequencing.
Defined as bases/genome size (fold coverage).
Antimicrobial susceptibility testing.
MICs for selected antimicrobial agents were determined by the broth microdilution method in cation-adjusted Mueller-Hinton broth as recommended by the Clinical and Laboratory Standards Institute (CLSI), using CLSI breakpoints (the M100-2D [45] and M07-A9 [46] methods). All antibiotics used for broth microdilution were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). E. faecalis strains ATCC 29212 and ATCC 51299 were used as standard reference strains. MIC determinations were performed at least three times on separate experimental days. For the purposes of this study, intrinsic aminoglycoside resistance was assigned for MICs of >500 μg/ml for gentamicin, >1,000 μg/ml for streptomycin, and >1,000 μg/ml for neomycin.
For povidone-iodine, a 10% solution (Triadine; Triad Group Inc., Hartland, WI) was diluted with cation-adjusted Mueller-Hinton broth to test concentrations of 2.5% to 0.0098%. MICs were recorded 24 h following incubation. To determine the minimum bactericidal concentration for povidone-iodine, 5 μl from selected wells spanning the MIC was spotted onto 5% sheep blood TSA. Following overnight incubation at 37°C in 5% CO2, spots were examined for positive or negative growth.
Variant analysis.
The variant analysis service on the PATRIC website was used for variant identification using the BWA-mem aligner and FreeBayes single nucleotide polymorphism caller. For macaque 1, filtered subreads from E. faecalis strains isolated in May 2014 and March 2015 were individually compared to those from the 2011 isolate. For macaque 2, filtered subreads from strains isolated in April 2014 and May 2016 were individually compared to those from the 2011 isolate. To examine intermacaque variation, filtered subreads from the 2011 ST55 isolate from macaque 3 were compared to those from the 2011 ST55 isolate from macaque 1, filtered subreads from the 2011 ST4 isolate from macaque 2 were compared to those from the 2011 ST4 isolate from macaque 11, and filtered subreads from the 2017 ST48 isolate from macaque 6 were compared to those from the 2013 ST48 isolate from macaque 4. During genome analysis, it was determined that the genome of the April 2014 ST4 isolate from macaque 2 was likely mixed; thus, it was excluded from subsequent genome sequence-based analyses. Variant filtering was performed to exclude variants that had a <50% frequency, those covered by fewer than 10 reads, or those found in genes with three or more variants called, due to the possibility of mapping errors or recombination events. Putative de novo variants are listed in Table S1 in the supplemental material.
Biofilm analysis.
Biofilm production was assayed using the crystal violet method (47). Briefly, 5 μl of an overnight E. faecalis culture was inoculated into 195 μl of tryptic soy broth supplemented with 1% glucose in replicates of 6 to 12 in a 96-well plate. Following 22 to 24 h of static incubation at 37°C, the optical density at 600 nm (OD600) was measured to determine overnight colony growth using a microtiter plate spectrophotometer (Epoch; BioTek Instruments, Inc., Winooski, VT). Biofilm staining was performed according to previously published protocols (5). Biofilm production was normalized to bacterial growth by dividing the OD570 by the OD600, and the normalized OD570 values were used for statistical analysis.
Mutagenicity assay.
Mutagenicity was determined with slight modifications to previous protocols (17). Fifty to 125 μl of an overnight E. faecalis culture in brain heart infusion (BHI) broth was plated onto BHI agar impregnated with 50 μg/ml of rifampin in replicates of 3 to 5 plates. To determine the concentration of the overnight inoculum, serial dilutions of the overnight culture inoculum were spotted onto TSA with 5% sheep blood (Remel). Colonies were enumerated following overnight incubation at 37°C in 5% CO2, and the concentration of the plated inoculum was determined. The mutation frequency (μ) was calculated as follows: μ = (number of rifampin-resistant colonies)/(inoculum plated).
Overnight cultures of ST48 isolates from macaques 3, 4, and 5 and the 2017 isolate from macaque 6 were adjusted to an OD600 of approximately 0.15 in duplicate in 3 ml of fresh brain heart infusion broth (BHI) and incubated at 37°C with shaking at 150 rpm for 90 min. Norfloxacin (3 μg/ml) was added to one set of tubes, and the cultures were incubated for another 2.5 h at 37°C with continued shaking. The second set of tubes was centrifuged to pellet the cells. The pellets were resuspended in 2 ml 10 mM to 1 mM Tris-EDTA (TE) buffer and then respun, and the pellets were then resuspended in 2 ml TE buffer with 1 mM CaCl2. The resuspended cultures were transferred to a 6-well plate and exposed to 254-nm UV light (8-W bulb) for 45 s. One milliliter of UV-exposed cultures was added to 4 ml of fresh BHI with 100 mM CaCl2 and incubated at 37°C with shaking at 150 rpm for 2 h. After incubation, the tubes were centrifuged and the supernatant was passed through a 0.2-μm-pore-size polyethersulfone filter. Fifty microliters of supernatant was mixed with 50 μl of overnight donor cultures (2011 isolates from macaques 1 and 2) and incubated for 5 min at 25°C. Five milliliters of 0.25% molten BHI agar with 100 mM MgCl2 and 100 mM CaCl2 was added, and 1 ml was added onto 1.5% bottom BHI agar with 100 mM MgCl2 and 100 mM CaCl2 in duplicate in 6-well plates. After top agar solidification, the plates were incubated at 37°C overnight and examined for plaques on the following day.
Statistics.
Biofilm measurement data were analyzed using Bayesian estimation, implemented in the Python programming language, using the PyMC3 program. Mutagenicity data were analyzed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA), with a P value of <0.05 being considered significant.
Accession number(s).
All code and data for making the violin plots are archived online on GitHub (https://github.com/ericmjl/mia-stats/releases/tag/mia-mbio) and Zenodo (https://zenodo.org/account/settings/github/repository/ericmjl/mia-stats; DOI, doi:10.5281/zenodo.1248852). This whole-genome shotgun project has been deposited into DDBJ/ENA/GenBank under accession numbers QFYI00000000, QFYJ00000000, QFYK00000000, QFYL00000000, QFYM00000000, QFYN00000000, and QFYO00000000. The DDBJ/ENA/GenBank accession numbers of the versions described in the paper are QFYI01000000, QFYJ01000000, QFYK01000000, QFYL01000000, QFYM01000000, QFYN01000000, and QFYO01000000. Genomes from the 2011 ST4 isolates from macaque 2 and macaque 11 and the 2011 ST55 isolate from macaque 3 are found under DDBJ/ENA/GenBank accession numbers MCFW00000000 (https://www.ncbi.nlm.nih.gov/nuccore/MCFW00000000), MCFU00000000 (https://www.ncbi.nlm.nih.gov/nuccore/MCFU00000000), and MCFV00000000 (https://www.ncbi.nlm.nih.gov/nuccore/MCFV00000000), respectively.
Supplementary Material
ACKNOWLEDGMENTS
The contributions of M.T.L. and J.D.-F. were supported by NIH grants T32 OD010978 and P30 ES002109 (to J.G.F.). The contributions of D.V.T. and M.S.G. were supported by PHS grant AI072360, AI108710, and the Harvard-wide Program on Antibiotic Resistance AI083214.
The authors declare no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01336-18.
REFERENCES
- 1.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S, National Healthcare Safety Network ( NHSN) Team and Participating NHSN Facilities. 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol 34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 2.Sandoe JA, Witherden IR, Cove JH, Heritage J, Wilcox MH. 2003. Correlation between enterococcal biofilm formation in vitro and medical-device-related infection potential in vivo. J Med Microbiol 52:547–550. doi: 10.1099/jmm.0.05201-0. [DOI] [PubMed] [Google Scholar]
- 3.Hollenbeck BL, Rice LB. 2012. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 3:421–569. doi: 10.4161/viru.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards MJ, Edwards JR, Culver DH, Gaynes RP. 1999. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med 27:887–892. [DOI] [PubMed] [Google Scholar]
- 5.Woods SE, Lieberman MT, Lebreton F, Trowel E, de la Fuente-Núñez C, Dzink-Fox J, Gilmore MS, Fox JG. 2017. Characterization of multi-drug resistant Enterococcus faecalis isolated from cephalic recording chambers in research macaques (Macaca spp.). PLoS One 12:e0169293. doi: 10.1371/journal.pone.0169293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leblanc M, Berry K, McCort H, Reuter JD. 2013. Brain abscess in a rhesus macaque (Macaca mulatta) with a cephalic implant. Comp Med 63:367–372. [PMC free article] [PubMed] [Google Scholar]
- 7.Kunisada T, Yamada K, Oda S, Hara O. 1997. Investigation on the efficacy of povidone-iodine against antiseptic-resistant species. Dermatology 195(Suppl 2):S14–S18. doi: 10.1159/000246025. [DOI] [PubMed] [Google Scholar]
- 8.Anderson MJ, Horn ME, Lin YC, Parks PJ, Peterson ML. 2010. Efficacy of concurrent application of chlorhexidine gluconate and povidone iodine against six nosocomial pathogens. Am J Infect Control 38:826–831. doi: 10.1016/j.ajic.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 9.Wattam AR, Abraham D, Dalay O, Disz TL, Driscoll T, Gabbard JL, Gillespie JJ, Gough R, Hix D, Kenyon R, Machi D, Mao C, Nordberg EK, Olson R, Overbeek R, Pusch GD, Shukla M, Schulman J, Stevens RL, Sullivan DE, Vonstein V, Warren A, Will R, Wilson MJ, Yoo HS, Zhang C, Zhang Y, Sobral BW. 2014. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res 42:D581–D591. doi: 10.1093/nar/gkt1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw KJ, Rather PN, Hare RS, Miller GH. 1993. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev 57:138–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen A, Jensen LB. 2004. Analysis of gyrA and parC mutations in enterococci from environmental samples with reduced susceptibility to ciprofloxacin. FEMS Microbiol Lett 231:73–76. doi: 10.1016/S0378-1097(03)00929-7. [DOI] [PubMed] [Google Scholar]
- 12.Onodera Y, Okuda J, Tanaka M, Sato K. 2002. Inhibitory activities of quinolones against DNA gyrase and topoisomerase IV of Enterococcus faecalis. Antimicrob Agents Chemother 46:1800–1804. doi: 10.1128/AAC.46.6.1800-1804.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shankar N, Baghdayan AS, Gilmore MS. 2002. Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis. Nature 417:746–750. doi: 10.1038/nature00802. [DOI] [PubMed] [Google Scholar]
- 14.Coburn PS, Gilmore MS. 2003. The Enterococcus faecalis cytolysin: a novel toxin active against eukaryotic and prokaryotic cells. Cell Microbiol 5:661–669. doi: 10.1046/j.1462-5822.2003.00310.x. [DOI] [PubMed] [Google Scholar]
- 15.Tendolkar PM, Baghdayan AS, Shankar N. 2006. Putative surface proteins encoded within a novel transferable locus confer a high-biofilm phenotype to Enterococcus faecalis. J Bacteriol 188:2063–2072. doi: 10.1128/JB.188.6.2063-2072.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McBride SM, Fischetti VA, LeBlanc DJ, Moellering RC, Gilmore MS. 2007. Genetic diversity among Enterococcus faecalis. PLoS One 2:e582. doi: 10.1371/journal.pone.0000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du X, Hua X, Qu T, Jiang Y, Zhou Z, Yu Y. 2014. Molecular characterization of Rifr mutations in Enterococcus faecalis and Enterococcus faecium. J Chemother 26:217–221. doi: 10.1179/1973947813Y.0000000137. [DOI] [PubMed] [Google Scholar]
- 18.Kobayakawa S, Jett BD, Gilmore MS. 2005. Biofilm formation by Enterococcus faecalis on intraocular lens material. Curr Eye Res 30:741–745. doi: 10.1080/02713680591005959. [DOI] [PubMed] [Google Scholar]
- 19.Keane PF, Bonner MC, Johnston SR, Zafar A, Gorman SP. 1994. Characterization of biofilm and encrustation on ureteric stents in vivo. Br J Urol 73:687–691. doi: 10.1111/j.1464-410X.1994.tb07557.x. [DOI] [PubMed] [Google Scholar]
- 20.Jolley KA, Maiden MC. 2010. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawalec M, Pietras Z, Daniłowicz E, Jakubczak A, Gniadkowski M, Hryniewicz W, Willems RJL. 2007. Clonal structure of Enterococcus faecalis isolated from Polish hospitals: characterization of epidemic clones. J Clin Microbiol 45:147–153. doi: 10.1128/JCM.01704-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magden ER, Mansfield KG, Simmons JH, Abee CR. 2015. Nonhuman primates. In Fox JG, Anderson LC, Otto G, Pritchett-Corning KR, Whary MT (ed), Laboratory animal medicine, 3rd ed Elsevier, Inc., Waltham, MA. [Google Scholar]
- 23.Morita Y, Kodama K, Shiota S, Mine T, Kataoka A, Mizushima T, Tsuchiya T. 1998. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob Agents Chemother 42:1778–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tendolkar PM, Baghdayan AS, Gilmore MS, Shankar N. 2004. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect Immun 72:6032–6039. doi: 10.1128/IAI.72.10.6032-6039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hancock LE, Perego M. 2004. The Enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J Bacteriol 186:5629–5639. doi: 10.1128/JB.186.17.5629-5639.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin X, Singh KV, Weinstock GM, Murray BE. 2001. Characterization of fsr, a regulator controlling expression of gelatinase and serine protease in Enterococcus faecalis OG1RF. J Bacteriol 183:3372–3382. doi: 10.1128/JB.183.11.3372-3382.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Papa MF, Perego M. 2011. Enterococcus faecalis virulence regulator FsrA binding to target promoters. J Bacteriol 193:1527–1532. doi: 10.1128/JB.01522-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huycke MM, Spiegel CA, Gilmore MS. 1991. Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob Agents Chemother 35:1626–1634. doi: 10.1128/AAC.35.8.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyazaki S, Ohno A, Kobayashi I, Uji T, Yamaguchi K, Goto S. 1993. Cytotoxic effect of hemolytic culture supernatant from Enterococcus faecalis on mouse polymorphonuclear neutrophils and macrophages. Microbiol Immunol 37:265–270. doi: 10.1111/j.1348-0421.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]
- 30.Chow JW, Thal LA, Perri MB, Vazquez JA, Donabedian SM, Clewell DB, Zervos MJ. 1993. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob Agents Chemother 37:2474–2477. doi: 10.1128/AAC.37.11.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jett BD, Jensen HG, Nordquist RE, Gilmore MS. 1992. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun 60:2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens SX, Jensen HG, Jett BD, Gilmore MS. 1992. A hemolysin-encoding plasmid contributes to bacterial virulence in experimental Enterococcus faecalis endophthalmitis. Invest Ophthalmol Vis Sci 33:1650–1656. [PubMed] [Google Scholar]
- 33.Matono T, Hayakawa K, Hirai R, Tanimura A, Yamamoto K, Fujiya Y, Mawatari M, Kutsuna S, Takeshita N, Mezaki K, Ohmagari N, Miyoshi-Akiyama T. 2016. Emergence of a daptomycin-non-susceptible Enterococcus faecium strain that encodes mutations in DNA repair genes after high-dose daptomycin therapy. BMC Res Notes 9:197. doi: 10.1186/s13104-016-2003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willems RJ, Top J, Smith DJ, Roper DI, North SE, Woodford N. 2003. Mutations in the DNA mismatch repair proteins MutS and MutL of oxazolidinone-resistant or -susceptible Enterococcus faecium. Antimicrob Agents Chemother 47:3061–3066. doi: 10.1128/AAC.47.10.3061-3066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciofu O, Riis B, Pressler T, Poulsen HE, Hoiby N. 2005. Occurrence of hypermutable Pseudomonas aeruginosa in cystic fibrosis patients is associated with the oxidative stress caused by chronic lung inflammation. Antimicrob Agents Chemother 49:2276–2282. doi: 10.1128/AAC.49.6.2276-2282.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macia MD, Blanquer D, Togores B, Sauleda J, Perez JL, Oliver A. 2005. Hypermutation is a key factor in development of multiple-antimicrobial resistance in Pseudomonas aeruginosa strains causing chronic lung infections. Antimicrob Agents Chemother 49:3382–3386. doi: 10.1128/AAC.49.8.3382-3386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 38.Coenye T, Falsen E, Vancanneyt M, Hoste B, Govan JR, Kersters K, Vandamme P. 1999. Classification of Alcaligenes faecalis-like isolates from the environment and human clinical samples as Ralstonia gilardii sp. nov. Int J Syst Bacteriol 49(Pt 2):405–413. doi: 10.1099/00207713-49-2-405. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz-Garbajosa P, Bonten MJ, Robinson DA, Top J, Nallapareddy SR, Torres C, Coque TM, Canton R, Baquero F, Murray BE, del Campo R, Willems RJ. 2006. Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination. J Clin Microbiol 44:2220–2228. doi: 10.1128/JCM.02596-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khani M, Fatollahzade M, Pajavand H, Bakhtiari S, Abiri R. 2016. Increasing prevalence of aminoglycoside-resistant Enterococcus faecalis isolates due to the aac(6′)-aph(2″) gene: a therapeutic problem in Kermanshah, Iran. Jundishapur J Microbiol 9:e28923. doi: 10.5812/jjm.28923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emaneini M, Aligholi M, Aminshahi M. 2008. Characterization of glycopeptides, aminoglycosides and macrolide resistance among Enterococcus faecalis and Enterococcus faecium isolates from hospitals in Tehran. Pol J Microbiol 57:173–178. [PubMed] [Google Scholar]
- 42.Choi JM, Woo GJ. 2015. Transfer of tetracycline resistance genes with aggregation substance in food-borne Enterococcus faecalis. Curr Microbiol 70:476–484. doi: 10.1007/s00284-014-0742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joensen KG, Scheutz F, Lund O, Hasman H, Kaas RS, Nielsen EM, Aarestrup FM. 2014. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J Clin Microbiol 52:1501–1510. doi: 10.1128/JCM.03617-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing, 28th ed CLSI document M100-2D. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 46.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th ed CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 47.Stepanović S, Vuković D, Hola V, Bonaventura G. 2007. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 115:891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.