Most filamentous fungi produce a vast number of extracellular enzymes that are used commercially for biorefineries of plant biomass to produce biofuels and value-added chemicals, which might promote the transition to a more environmentally friendly economy. The expression of these extracellular enzyme genes is tightly controlled at the transcriptional level, which limits their yields. Hitherto our understanding of the regulation of expression of plant biomass-degrading enzyme genes in filamentous fungi has been rather limited. In the present study, regulatory roles of a key regulator, PoxNsdD, were further explored in the soil fungus Penicillium oxalicum, contributing to the understanding of gene regulation in filamentous fungi and revealing the biotechnological potential of P. oxalicum via genetic engineering.
KEYWORDS: Penicillium oxalicum, transcription factor, PoxNsdD, starch-degrading enzyme, conidiation, pigment biosynthesis
ABSTRACT
Soil fungi produce a wide range of chemical compounds and enzymes with potential for applications in medicine and biotechnology. Cellular processes in soil fungi are highly dependent on the regulation under environmentally induced stress, but most of the underlying mechanisms remain unclear. Previous work identified a key GATA-type transcription factor, Penicillium oxalicum NsdD (PoxNsdD; also called POX08415), that regulates the expression of cellulase and xylanase genes in P. oxalicum. PoxNsdD shares 57 to 64% identity with the key activator NsdD, involved in asexual development in Aspergillus. In the present study, the regulatory roles of PoxNsdD in P. oxalicum were further explored. Comparative transcriptomic profiling revealed that PoxNsdD regulates major genes involved in starch, cellulose, and hemicellulose degradation, as well as conidiation and pigment biosynthesis. Subsequent experiments confirmed that a ΔPoxNsdD strain lost 43.9 to 78.8% of starch-digesting enzyme activity when grown on soluble corn starch, and it produced 54.9 to 146.0% more conidia than the ΔPoxKu70 parental strain. During cultivation, ΔPoxNsdD cultures changed color, from pale orange to brick red, while the ΔPoxKu70 cultures remained bluish white. Real-time quantitative reverse transcription-PCR showed that PoxNsdD dynamically regulated the expression of a glucoamylase gene (POX01356/Amy15A), an α-amylase gene (POX09352/Amy13A), and a regulatory gene (POX03890/amyR), as well as a polyketide synthase gene (POX01430/alb1/wA) for yellow pigment biosynthesis and a conidiation-regulated gene (POX06534/brlA). Moreover, in vitro binding experiments showed that PoxNsdD bound the promoter regions of the above-described genes. This work provides novel insights into the regulatory mechanisms of fungal cellular processes and may assist in genetic engineering of P. oxalicum for potential industrial and medical applications.
IMPORTANCE Most filamentous fungi produce a vast number of extracellular enzymes that are used commercially for biorefineries of plant biomass to produce biofuels and value-added chemicals, which might promote the transition to a more environmentally friendly economy. The expression of these extracellular enzyme genes is tightly controlled at the transcriptional level, which limits their yields. Hitherto our understanding of the regulation of expression of plant biomass-degrading enzyme genes in filamentous fungi has been rather limited. In the present study, regulatory roles of a key regulator, PoxNsdD, were further explored in the soil fungus Penicillium oxalicum, contributing to the understanding of gene regulation in filamentous fungi and revealing the biotechnological potential of P. oxalicum via genetic engineering.
INTRODUCTION
Soil-plant-atmosphere carbon cycling is an important feature of terrestrial ecosystems, in which soil microbes act as decomposers and play crucial roles in regulating carbon flux between the biosphere and the atmosphere (1, 2). Minor changes in the balance between soil carbon storage and release might affect the global climate and contribute to the greenhouse effect. Among soil microbes, fungi efficiently consume soil organic carbon stored in polysaccharides from plant biomass by secreting carbohydrate-active enzymes (CAZymes) (3). However, this process operates at a relatively low level in native ecosystems and is limited by enzyme production and biomass architecture (4). Cellulase, xylanase, and amylase have been employed in industrial applications, including biorefining of plant biomass into biofuels and/or high-value-added chemicals that are otherwise expensive to produce.
Soil fungi produce a wide range of chemical compounds that have been utilized by humans for many years, such as monascorubrin and its derivatives from Monascus species, which serve as natural red colorants for food (5), and penicillin and derived β-lactam antibiotics produced by Penicillium chrysogenum (6). Soil fungi can also produce mycotoxins that contaminate human and animal food and that represent a threat to human and animal health (7). Genomic data on soil fungi have revealed numerous unexplored secondary metabolites, for example, 68 secondary metabolite gene clusters were identified in Talaromyces pinophilus 1-95 alone (8).
Specific transcription factors (TFs) tightly regulate the production of plant biomass-degrading enzymes and/or the biosynthesis of secondary metabolites in host cells at the transcriptional level. Most studies on the identification of such TFs and the exploration of their regulatory mechanisms have focused mainly on cellulolytic filamentous fungi, such as Trichoderma, Aspergillus, and Penicillium (9–11). Studies on such TFs mainly include the transcription activators CLR-2/ClrB of Aspergillus nidulans FGSC A4 (12) and Penicillium oxalicum 114-2 (13) and HP7-1 (14), XYR1/XLR-1/XlnR of Trichoderma reesei QM9136 (15) and P. oxalicum 114-2 (13), AmyR of P. oxalicum 114-2 (13) and Aspergillus niger CICC2462 (11), and LaeA of P. oxalicum 114-2 (16) and T. reesei QM9414 (17) and the carbon catabolite repressor CreA/CRE1/CRE-1 of A. nidulans FGSC A4 (18) and T. reesei QM9414 (19). Interestingly, expression of genes encoding plant biomass-degrading enzymes and genes involved in asexual reproduction is coregulated by some TFs, such as BrlA (10), ClrC (20), and FlbC (21) of P. oxalicum 114-2.
NsdD in Aspergillus spp. and its orthologs, such as Pro44 in Sordaria macrospora, Ltf1 in Botrytis cinerea, Csm1 in Fusarium fujikuroi, SUB-1 in Neurospora crassa, and SsNsd1 in Sclerotinia sclerotiorum, regulate development and/or biosynthesis of secondary metabolites (22–28). Additional functions involving stress tolerance, light response, and virulence have been observed in the plant pathogens B. cinerea, F. fujikuroi, and/or N. crassa (23, 26, 27). NsdD is positioned between FLB (fluffy low BrlA expression) and BrlA and functions as a repressor by downregulating brlA in Aspergillus (25, 29). The P. oxalicum NsdD gene (PoxNsdD) was initially found to regulate the expression of cellulase and xylanase genes in P. oxalicum (30). However, the details of the regulatory roles of PoxNsdD in P. oxalicum merit further investigation.
In the present study, we investigated the regulatory roles of PoxNsdD in P. oxalicum through global RNA sequencing and biochemical and microbiological techniques.
RESULTS
Transcriptomic analyses reveal that PoxNsdD regulates the expression of genes involved in plant biomass-degrading enzymes, conidiation, and pigment biosynthesis in P. oxalicum.
A previous study reported that PoxNsdD positively regulated the production of cellulase and xylanase when P. oxalicum was grown in medium containing Avicel as the sole carbon source (30). To further explore the regulatory roles of PoxNsdD in P. oxalicum, deep RNA sequencing was employed to analyze the transcriptomic profiling of a PoxNsdD deletion mutant (ΔPoxNsdD) grown on Avicel, with the parental strain ΔPoxKu70 used as a control. In total, ∼23 million clean reads with a length of 100 bp (accession number SRA493765 in the Sequence Read Archive [SRA] database) were generated from each sample, indicating an average of 76-fold coverage (see Table S1 in the supplemental material). Over 90% of clean reads were successfully mapped to the genome of the wild-type strain HP7-1 (14). Pearson's correlation coefficients (r) for three biological replicates for each sample were subsequently calculated, and the results displayed high r values (>0.90), which illustrated that these transcriptomic data were reliable (Fig. S1).
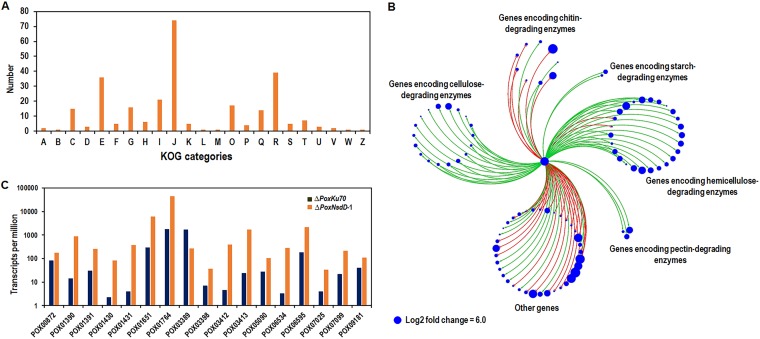
Using a probability of ≥0.8 and a ∣log2 fold change∣ of ≥1 as the threshold, transcripts of 761 genes were found to be altered significantly in the ΔPoxNsdD strain compared to those in the ΔPoxKu70 strain (Data Set S1), and these were defined as the PoxNsdD regulon. Among them, 308 were downregulated (−10.6 < log2 fold change < −1.0), and 453 were upregulated (1.0 < log2 fold change < 9.1). Eukaryotic orthologous group (KOG) annotation revealed that most of the 761 differentially expressed genes (DEGs) were mainly involved in primary and secondary metabolism (42%), specifically in category E (amino acid transport and metabolism) and category Q (secondary metabolite biosynthesis, transport, and catabolism) (Fig. 1A).
FIG 1.
Transcriptomic profiling of the ΔPoxNsdD mutant and the ΔPoxKu70 parental strain during growth in the presence of Avicel as the carbon source. (A) Eukaryotic orthologous group (KOG) annotation of 716 genes in the PoxNsdD regulon (a |log2 fold change| of ≥1 and a probability of ≥0.8 were used as thresholds). (B) Regulation of 95 genes encoding carbohydrate-active enzymes by PoxNsdD. Red lines represent negative regulation, and green lines with arrowheads represent positive regulation. Blue circles show the degree of regulation. (C) Regulation of 17 genes putatively involved in asexual development (conidiation) in P. oxalicum by PoxNsdD.
In the PoxNsdD regulon, 95 DEGs were annotated to encode CAZymes, including enzymes from 35 glycoside hydrolase families, 2 glycosyltransferase families, 7 carbohydrate esterase families, 6 auxiliary activity families, and 7 carbohydrate-binding module families, as well as 1 polysaccharide lyase. Among them, 63 DEGs were downregulated with log2 fold changes between −6.4 and −1.1, including 47 genes encoding plant cell wall-degrading enzymes (CWDEs) and two major amylase genes, POX01356/Amy15A and POX09352/Amy13A (Fig. 1B). Notably, the major CWDE genes in the P. oxalicum genome were detected in the PoxNsdD regulon, including two cellobiohydrolase genes (cbh genes; POX04786/Cel6A and POX05587/Cel7A-2), nine endo-β-1,4-glucanase genes (eg genes; POX01166/Cel5B, POX01896/Cel5C, POX02740, POX04137, POX05570/Cel45A, POX05571/Cel7B, POX06147/Cel5A, POX06983, and POX07535/Cel12A), four β-1,4-glucosidase genes (bgl genes; POX00968, POX03062, POX03641, and POX06079), three xylanase genes (xyn genes; POX00063/Xyn10A, POX06601, and POX06783/Xyn11A), and two genes (POX02308/Cel61A and POX08897) encoding lytic polysaccharide monooxygenases. Conversely, 32 DEGs were upregulated with log2 fold changes between 1.1 and 7.0, including five chitinase genes (POX00089, POX01447, POX03021, POX06241, and POX08171) involved in the degradation of fungal cell walls, a xyn gene (POX05916), and a polygalacturonase gene (POX01225) (Fig. 1B; Data Set S1).
Interestingly, 22 DEGs encoding putative TFs were also detected in the PoxNsdD regulon, among which 16 were upregulated, with log2 fold changes of 1.02 to 6.43, and 6 were downregulated (−10.6 < log2 fold change < −1.07). Five regulatory genes (POX00972/clrC, POX04420/PoxCxrB, POX04860/PDE_07199, POX05726, and POX06534/brlA) were known to regulate the expression of cellulase genes in P. oxalicum (10, 20, 30). In addition, the POX06534/brlA, POX07025/abaA, and POX07099/flbD genes were known to positively regulate conidiation in filamentous fungi (10, 25, 29).
In addition, in the PoxNsdD regulon, 16 DEGs (POX06534/brlA, POX07025/abaA, POX07099/flbD, POX01390/aygA, POX01391/abrA, POX01430/alb1/wA, POX01431/abrB/yA, POX03412/arpA, POX03413/arpB, POX01651/rodA-like gene, POX01764/rodA, POX06595/rodB, POX03398/axl2, POX05090/tmpA, POX00872/nimX, and POX09181/vosA) were predicted to be involved in conidiogenesis (10), including 15 that were upregulated (1.1 < log2 fold change < 6.6) and one (POX03389/axl2) that was downregulated (log2 fold change = −2.65) (Fig. 1C), which indicated that PoxNsdD also affected conidiation of P. oxalicum.
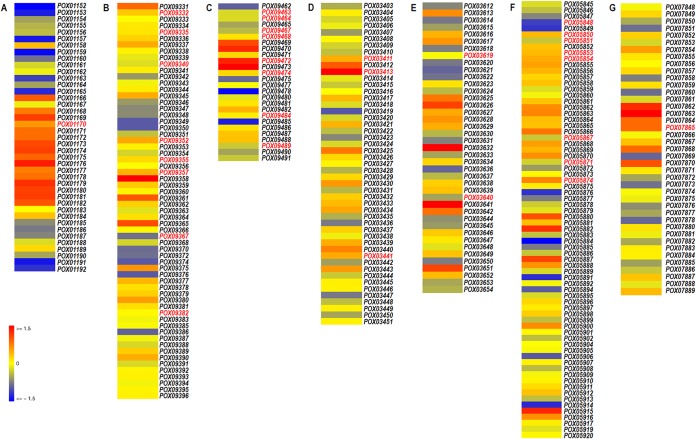
We next determined the number of DEGs involved in secondary metabolism of P. oxalicum. A total of 230 DEGs covering 69 putative biosynthetic gene clusters (BGCs) was identified (Data Set S1), of which 90 were downregulated (−4.4 < log2 fold change < −1.0) and 140 were upregulated (1.0 < log2 fold change < 9.0) in the ΔPoxNsdD strain. Sixty-four of the predicted BGCs were classified into 13 different types, as follows: 34 cf_putative gene clusters, 7 terpene gene clusters, 5 nonribosomal peptide synthase (Nrps) gene clusters, 4 T1 polyketide synthase (T1pks)-Nrps gene clusters, 3 T1pks gene clusters, 2 cf_fatty acid gene clusters, 2 cf_saccharide gene clusters, and 2 indole gene clusters, as well as single representatives of cf_fatty acid-Nrps, T3pks, indole-terpene-Nrps, indole-Nrps, and cf_fatty acid-T1pks-Nrps gene clusters. The remaining five gene clusters appeared to synthesize other, unknown secondary metabolites. Furthermore, compared to known secondary metabolite gene clusters, 11 were predicted to produce the following, with gene similarities ranging from 10 to 100%: aspyridone (clusters 10 and 28), emericellin (cluster 11), citrinin (cluster 17), leucinostatins (cluster 29), roquefortine C/meleagrin (cluster 33), beauvericin (cluster 49), cytochalasin (clusters 51 and 54), malbrancheamide (cluster 72), and viridicatumtoxin (cluster 88).
Among these clusters, a total of 33 core biosynthetic genes distributed among 22 BGCs were detected, including seven known BGCs in clusters 11, 29, 33, 49, 51, 72, and 88 (Fig. 2; Data Set S1). All the core biosynthetic genes in known BGCs were upregulated (1.6 < log2 fold change < 6.4) in the ΔPoxNsdD mutant in comparison to the ΔPoxKu70 strain (Fig. 2).
FIG 2.
Putative biosynthetic gene clusters of known secondary metabolites for which core biosynthetic genes are regulated by PoxNsdD. (A) Cluster 11 = emericellin; (B) cluster 29 = leucinostatins; (C) cluster 33 = roquefortine C/meleagrin; (D) cluster 49 = beauvericin; (E) cluster 51 = cytochalasin; (F) cluster 72 = malbrancheamide; (G) cluster 88 = viridicatumtoxin. Gene IDs labeled in red represent core biosynthetic genes.
Deletion of PoxNsdD from P. oxalicum results in significant reduction of production of enzymes degrading raw cassava starch and soluble starch.
Transcriptomic assays revealed that PoxNsdD positively regulates the expression of two important amylase genes, POX01356/Amy15A and POX09352/Amy13A, indicating that PoxNsdD might affect amylase production of P. oxalicum. A mycelial shift experiment from medium containing glucose to medium containing soluble corn starch over 2 to 4 days was performed, and the results showed that the ΔPoxNsdD deletion mutant lost 52.6 to 78.8% and 43.9 to 68.8% of raw cassava starch-degrading enzyme (RCSDE) activity and soluble starch-degrading enzyme (SSDE) activity compared to those of the ΔPoxKu70 parental strain (P < 0.05; Student's t test). Complementation of the ΔPoxNsdD mutant (30) mostly restored the RCSDE and SSDE activities to the levels of the ΔPoxKu70 strain under the induction conditions with soluble corn starch (Fig. 3).
FIG 3.
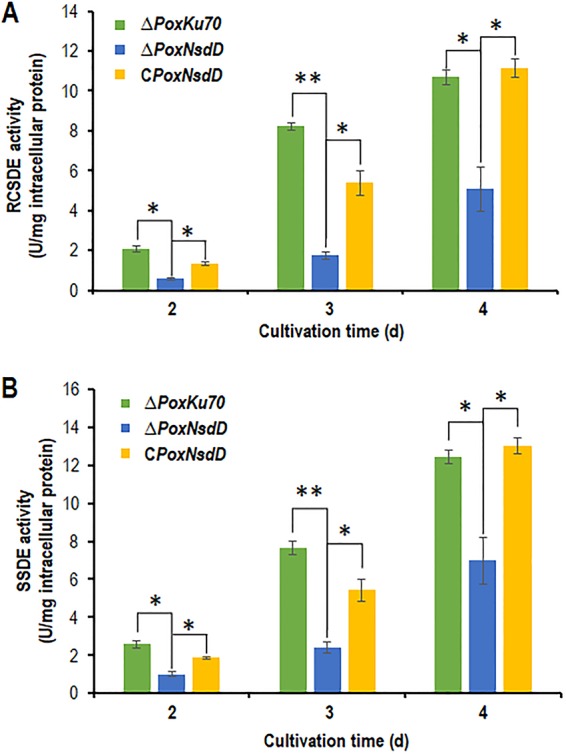
Activities of crude enzymes from the PoxNsdD deletion mutant of P. oxalicum following a shift in growth from glucose to soluble starch. Crude enzymes were produced by P. oxalicum strains grown in 1% soluble corn starch as the sole carbon source after a shift from glucose. Enzymatic activity was measured 2 to 4 days after the shift. (A) Raw cassava starch-degrading enzyme (RCSDE) activities. (B) Soluble starch-degrading enzyme (SSDE) activities. Asterisks indicate significant differences (**, P < 0.01; *, P < 0.05) between the ΔPoxNsdD mutant and the ΔPoxKu70 parental strain or the complementary strain (CPoxNsdD), as assessed by Student's t test.
PoxNsdD is involved in mycelial growth and conidiation in P. oxalicum.
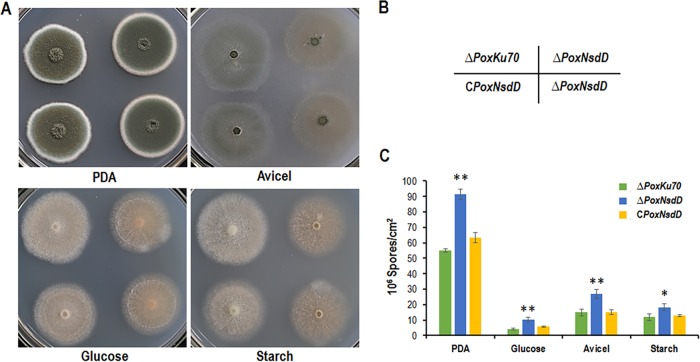
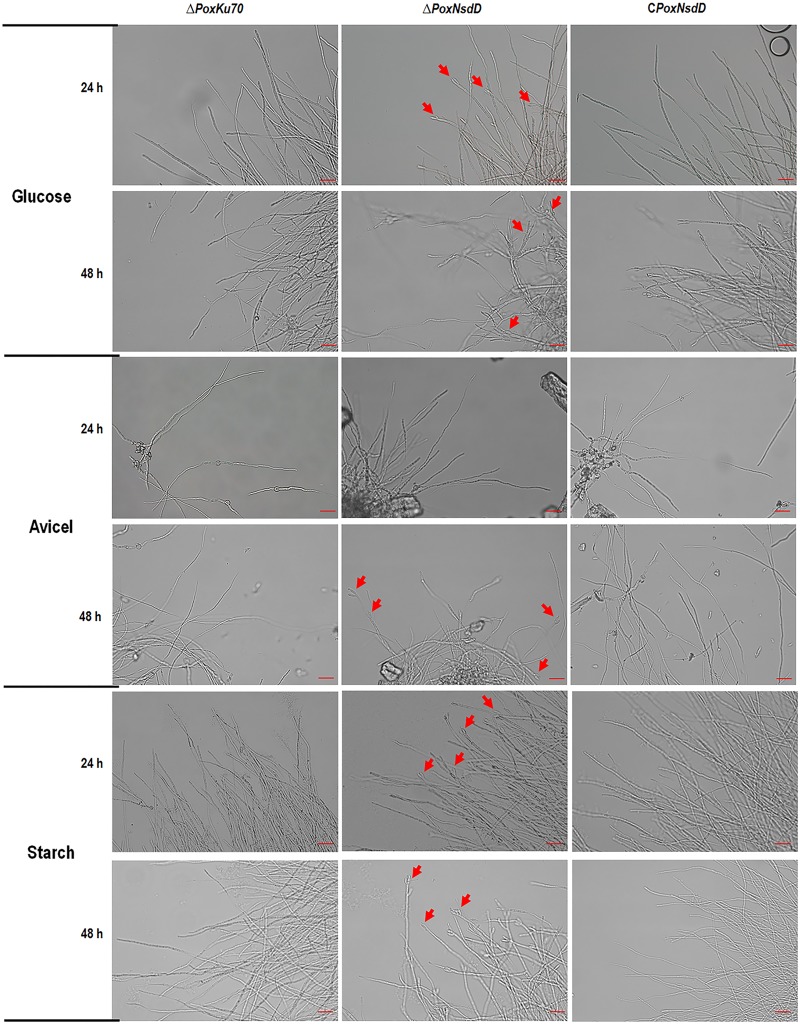
The ΔPoxNsdD mutant was inoculated onto solid medium plates containing glucose, Avicel, soluble corn starch, or potato dextrose agar (PDA) for 2 to 4 days. Three days after inoculation, ΔPoxNsdD colonies were bigger and greener than those of the ΔPoxKu70 parental strain on PDA plates, whereas the mycelial growth of the ΔPoxNsdD mutant was significantly retarded on all plates containing glucose, Avicel, or starch (Fig. 4A and B). Quantitative analysis of asexual spores revealed that the ΔPoxNsdD mutant had a significantly increased number of asexual spores compared to that for the ΔPoxKu70 strain on all plates (P < 0.05; Student's t test) (Fig. 4C). Furthermore, microscopy revealed that the ΔPoxNsdD mutant promoted phialide development to a greater degree than that with the ΔPoxKu70 strain (Fig. 5). The complemented strain, CPoxNsdD, showed physiological features, such as colony color, number of asexual spores, and phialide development, similar to those of the ΔPoxKu70 parental strain (Fig. 4 and 5).
FIG 4.
Phenotypic comparison of the ΔPoxNsdD mutant, the complementary strain (CPoxNsdD), and the ΔPoxKu70 parental strain on solid medium plates. (A) Colonies on plates containing PDA, glucose, Avicel, or starch were inoculated at 28°C for 4 days. (B) Schematic diagram of the P. oxalicum strains in panel A. (C) Quantitative analysis of conidiation on solid medium plates containing different carbon sources. P. oxalicum strains were grown on PDA plates at 28°C for 3 days, on glucose plates for 15 days, and on Avicel and starch plates for 10 days. Asterisks indicate significant differences (**, P < 0.01; *, P < 0.05; Student's t test) between the ΔPoxNsdD mutant and the ΔPoxKu70 parental strain or the complementary strain (CPoxNsdD).
FIG 5.
Microscopy of hyphae in liquid media containing glucose, Avicel, or starch as the carbon source. Conidiophores are marked by red arrowheads. Bars = 20 μm.
PoxNsdD is involved in pigment biosynthesis in P. oxalicum.
During measurement of the cellulase and xylanase activities of the ΔPoxNsdD mutant cultivated in medium containing Avicel after a transfer from glucose, the ΔPoxNsdD mutant biosynthesized colorful pigments, but the ΔPoxKu70 parental strain did not (data not shown). When the ΔPoxNsdD mutant was directly inoculated into Avicel medium for 1 to 6 days, the culture color of the ΔPoxNsdD mutant changed to pale orange, in contrast to that of the ΔPoxKu70 strain. Additionally, a similar phenomenon was observed for the ΔPoxNsdD mutant when it was directly inoculated into medium containing glucose or soluble corn starch and cultured for 1 to 6 days. Complementation of the ΔPoxNsdD mutant changed the pigment biosynthesis of the ΔPoxNsdD mutant to that of the ΔPoxKu70 parental strain (Fig. 6).
FIG 6.
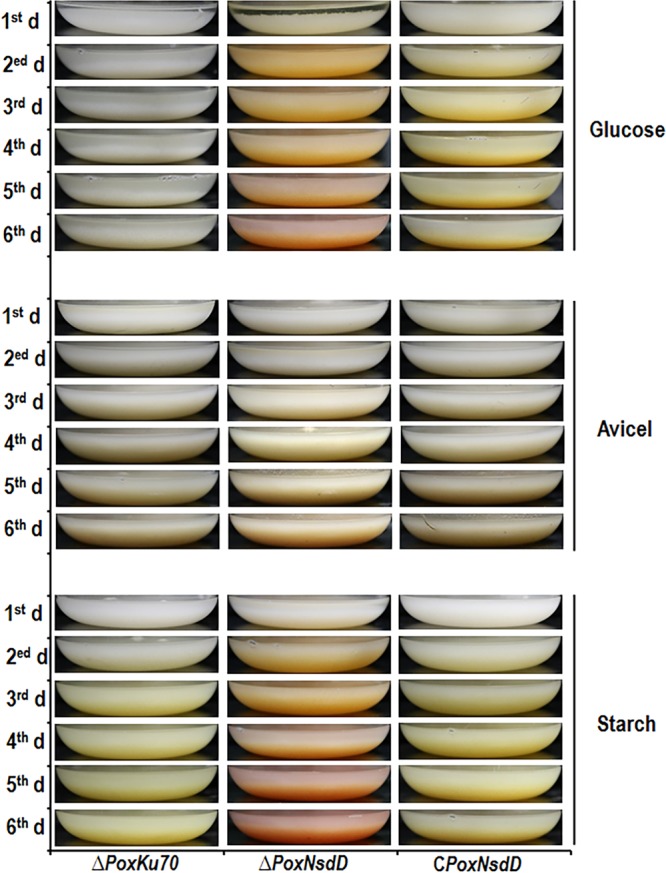
Pigment biosynthesis by the ΔPoxNsdD mutant, the complementary strain (CPoxNsdD), and the ΔPoxKu70 parental strain grown on liquid media. P. oxalicum strains were inoculated directly into liquid modified minimal medium containing glucose, Avicel, or soluble corn starch as the sole carbon source and then cultured at 28°C for 1 to 6 days.
Kinetics of PoxNsdD regulation of genes involved in starch degradation and pigment biosynthesis as well as TF-encoding genes in P. oxalicum.
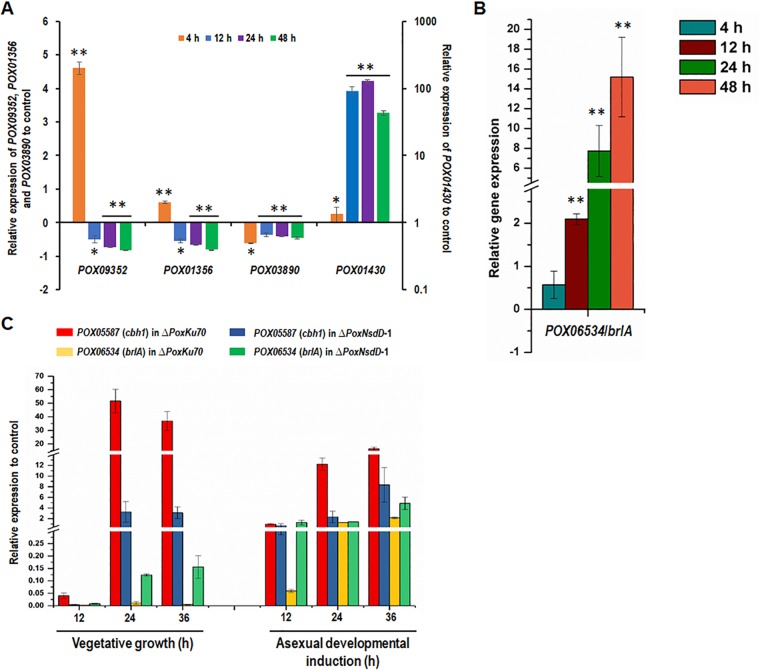
To further confirm the regulatory roles of PoxNsdD in P. oxalicum, real-time quantitative reverse transcription-PCR (RT-qPCR) was employed to measure the expression of target genes 4, 12, 24, and 48 h after a shift from glucose to soluble corn starch. Target genes included the glucoamylase gene POX01356/Amy15A, the α-amylase gene POX09352/Amy13A, and their transcriptional activator gene POX03890/amyR, as well as the yellow pigment biosynthesis polyketide synthase gene POX01430/alb1/wA. The results showed that expression of all three genes involved in starch degradation was downregulated in the ΔPoxNsdD mutant compared to that in the ΔPoxKu70 strain during the whole induction period, except for expression of POX01356/Amy15A and POX09352/Amy13A at 4 h. Specifically, the POX01356/Amy15A, POX09352/Amy13A, and POX03890/amyR genes were significantly downregulated, by 35.9 to 82.8% (P < 0.05; Student's t test), at 12, 24, and 48 h in the ΔPoxNsdD mutant compared to those in the ΔPoxKu70 parental strain. Conversely, POX01356/Amy15A and POX09352/Amy13A were significantly upregulated, from 60.5% to 460.4%, at 4 h in the ΔPoxNsdD mutant (P < 0.01; Student's t test) (Fig. 7A). In addition, transcription of the key gene POX01430/alb1/wA, involved in pigment biosynthesis, was increased 1.33- to 128.1-fold during the entire starch induction period (P < 0.05; Student's t test) (Fig. 7A).
FIG 7.
Kinetics of regulation of gene expression by PoxNsdD in P. oxalicum as revealed by real-time quantitative reverse transcription-PCR. (A) Gene expression under induction by soluble corn starch as the sole carbon source at four different time points (4, 12, 24, and 48 h) after shifting of the carbon source. Expression levels were normalized against those of the ΔPoxKu70 parental strain. Asterisks indicate significant differences (**, P < 0.01, *, P < 0.05) between the tested samples and those of the ΔPoxKu70 parental strain, as assessed by Student's t test. (B) Expression of the POX06534/brlA gene in the presence of Avicel. Expression levels were normalized against those of the ΔPoxKu70 parental strain. Asterisks indicate significant differences (**, P < 0.01) between the tested samples and those of the ΔPoxKu70 parental strain, as assessed by Student's t test. (C) Gene expression during vegetative growth and induction of asexual development. For vegetative growth, gene expression was measured at three different time points (12, 24, and 36 h) after the inoculation of spores into medium containing Avicel as the sole carbon source. For asexual development, hyphae grown for 36 h were transferred onto solid medium plates containing Avicel, and gene expression was measured at three different time points (12, 24, and 36 h) after the shift. Expression levels were normalized against those for spores of the ΔPoxNsdD mutant and the ΔPoxKu70 parental strain.
PoxNsdD positively regulates genes involved in biomass degradation during both vegetative growth and conidiation but negatively regulates genes involved in conidiation only during vegetative growth in P. oxalicum.
RT-qPCR was continuously employed to measure the expression of the conidiation-regulated gene POX06534/brlA in the ΔPoxNsdD mutant 4, 12, 24, and 48 h after a shift from glucose to Avicel. The results showed that the transcription level of POX06534/brlA increased, by 209.6% to 1,519.4%, in the ΔPoxNsdD mutant during almost the whole induction period in comparison to that in the ΔPoxKu70 strain (P < 0.01; Student's t test) (Fig. 7B).
As shown in Fig. 7C, POX06534/brlA expression was barely noticeable in the ΔPoxKu70 strain under vegetative growth conditions, but its expression increased rapidly 12 h after induction of conidiation. Deletion of PoxNsdD resulted in a slight increase in POX06534/brlA expression during vegetative growth and in high levels 12, 24, and 36 h after induction of conidiation.
In addition, expression of the cbh1 gene POX05587/Cel7A-2 was also investigated during both vegetative growth and conidiation. POX05587/Cel7A-2 expression during vegetative growth was significantly higher than that during conidiation in the ΔPoxKu70 parental strain, and deletion of PoxNsdD decreased the accumulation of POX05587/Cel7A-2 mRNA throughout the whole growth cycle (Fig. 7C).
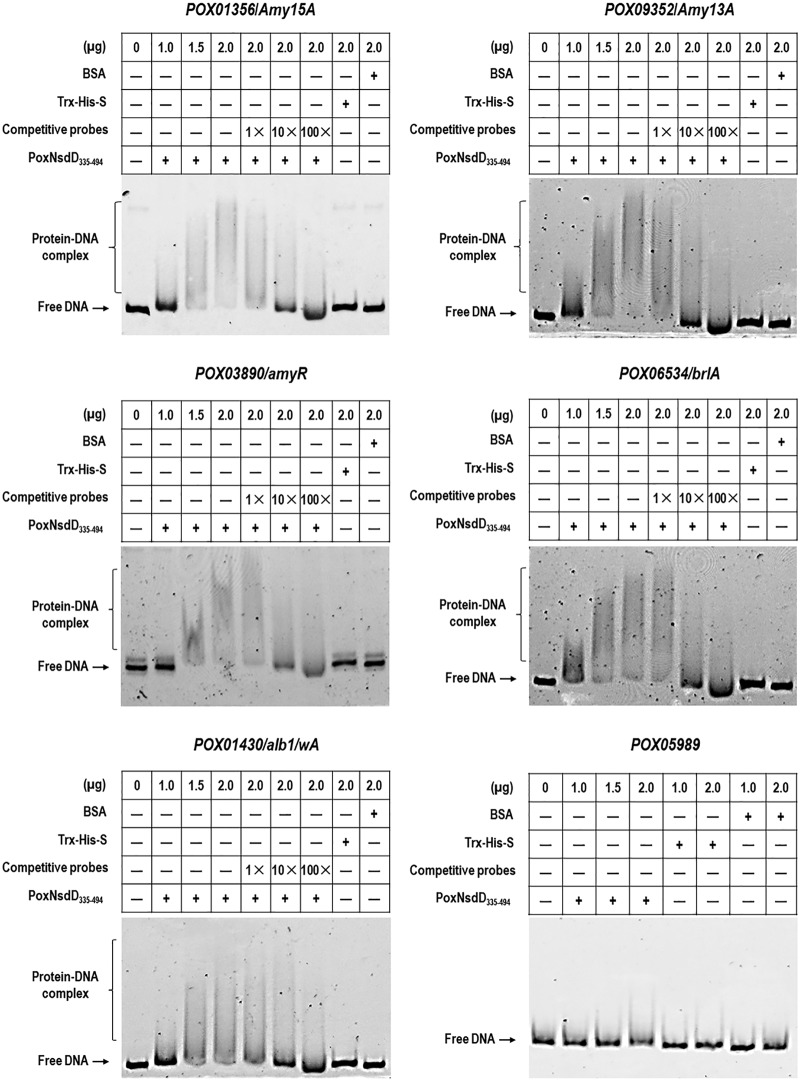
PoxNsdD binds the promoter regions of major genes involved in starch degradation, conidiation, and pigment biosynthesis and of TF-encoding genes in vitro.
Analysis using the SMART website (http://smart.embl-heidelberg.de/) revealed that PoxNsdD contains a GATA-type zinc finger (ZnF_GATA) that specifically binds the DNA sequence NGATAR (N = A, T, C, or G; R = A or G) (29, 31). Screening of the major genes involved in starch degradation, conidiation, and pigment biosynthesis, including POX01356/Amy15A, POX09352/Amy13A, POX03890/amyR, POX06534/brlA, and POX01430/alb1/wA, revealed an average of 7.6 PoxNsdD-binding sites within 2 kb of their 5′ upstream regions (Fig. S2).
To confirm whether PoxNsdD indirectly or directly regulated expression of the tested genes described above, an in vitro electrophoretic mobility shift assay (EMSA) was performed. 6-Carboxyfluorescein (FAM)-tagged DNA fragments (about 1,000 bp) from the promoter regions of POX01356/Amy15A, POX09352/Amy13A, POX03890/amyR, POX06534/brlA, and POX01430/alb1/wA were amplified (Fig. S2), purified, and used as probes for EMSA, and the promoter region of POX05989, encoding β-tubulin, was used as the negative control. The putative DNA-binding domain PoxNsdD335–494 was fused to thioredoxin (Trx), His, and S tags and recombinantly expressed and purified as described previously (30). As shown in Fig. 8, complexes of PoxNsdD335–494 and the promoters of the target genes, POX01356/Amy15A, POX09352/Amy13A, POX03890/amyR, POX06534/brlA, and POX01430/alb1/wA, were observed, and the concentrations of complexes gradually increased with increasing amounts of fusion proteins (1.0 to 2.0 μg). The complexes were not observed with Trx-His-S or bovine serum albumin (BSA) alone, with only the promoters of the target genes, or with the promoter of POX05989 (Fig. 8). When protein-binding DNA fragments lacking the FAM label were used as competitive probes, the concentrations of the complexes gradually decreased with increasing amounts of the competitive probes (Fig. 8), suggesting that PoxNsdD bound specifically to the promoters of POX01356/Amy15A, POX09352/Amy13A, POX03890/amyR, POX06534/brlA, and POX01430/alb1/wA.
FIG 8.
In vitro analysis of binding between the DNA-binding domain of PoxNsdD and the promoter sequences of target genes by electrophoretic mobility shift assay. Each reaction mixture contained 0 to 2.0 μg of Trx-His-S-tagged PoxNsdD335–494 and about 40 ng of each tested probe. As negative controls, the same amount of purified Trx-His-S fusion protein and BSA were used. POX05989, encoding β-tubulin, was used as a control probe.
DISCUSSION
Previous work identified the key TF PoxNsdD in P. oxalicum HP7-1 by comparative transcriptomic profiling and genetic analyses (30). PoxNsdD shares 57 to 64% identity with NsdD in A. nidulans FGSC A4 (accession number XP_660756.1), Aspergillus flavus NRRL3357 (accession number XP_002376041.1), and Aspergillus fumigatus AF293 (accession number XP_754237.1). PoxNsdD contributes to the production of cellulase and xylanase under induction by Avicel. In the present study, the regulatory roles of PoxNsdD were further explored in detail, and the results showed that PoxNsdD not only repressed conidiation in P. oxalicum, like its homologue NsdD in Aspergillus, but also regulated plant biomass-degrading enzyme production, including amylase production and pigment biosynthesis. Importantly, PoxNsdD could directly regulate the expression of the major genes involved in these processes.
NsdD and its orthologues are identified as TFs with the conserved DNA-binding domain ZnF_GATA in many filamentous fungi (22–29). Hitherto in the reported literature and this work, NsdD and its orthologues primarily played six roles, including (i) repressing asexual development in Aspergillus spp. (25, 29), F. fujikuroi (27), S. sclerotiorum (28), and P. oxalicum; (ii) activating sexual development in Aspergillus nidulans (22), S. sclerotiorum (28), and S. macrospora (24); (iii) affecting the production of secondary metabolites, such as the dark mycelial pigment and gliotoxin in Aspergillus spp., under specific conditions (25, 29); synthesizing the polyketide synthase (PKS)-derived pigments in F. fujikuroi (27) and P. oxalicum; (iv) regulating light responses in N. crassa (23) and B. cinerea (26); (v) regulating fungal virulence in the necrotrophic plant pathogen B. cinerea (26); and (vi) regulating the production of plant biomass-degrading enzymes and the expression of these enzyme genes in P. oxalicum, as identified previously (30) and in this study.
Conidiation is well known to be strictly controlled by a central regulatory pathway of three TFs, BrlA, AbaA, and WetA, acting in concert with other genes, such as FLBs, as an integrative part of the fungal life cycle (32). NsdD plays a negative regulatory role in conidiation via repressing brlA expression in conidia and growing hyphae in Aspergillus. In contrast, in developing cells, the removal of NsdD with the addition of VosA activates brlA expression, leading to further activation of conidiation (25, 29). Similarly, in the present study, both RT-qPCR and RNA sequencing revealed that POX06354/brlA expression was also derepressed in the ΔPoxNsdD mutant compared to that in the ΔPoxKu70 parental strain. Furthermore, by screening of the PoxNsdD regulon, ∼40% (17/41 DEGs) of DEGs were predicted to be involved in conidiogenesis in P. oxalicum, including 16 markedly upregulated genes and 1 downregulated gene (POX03389). Of these 16 genes, three key TF genes (POX06534/brlA, POX07025/abaA, and POX07099/flbD) are known to activate fungal conidiation (32), six (POX01390/aygA, POX01391/abrA, POX01430/alb1/wA, POX01431/abrB/yA, POX03412/arpA, and POX03413/arpB) are involved in pigment biosynthesis (33, 34), three (POX01651/rodA-like gene, POX01764/rodA, and POX06595/rodB) encode hydrophobins (35), two (POX03398/axl2 and POX05090/tmpA) encode transmembrane proteins (36), one (POX00872/nimX) controls cell division (37), and one encodes the velvet protein VosA, which represses fungal conidiation (32). The remaining DEG, POX03389, is an ortholog of phiA that is involved in phialide development in A. nidulans. However, how expression of these genes is regulated by PoxNsdD in P. oxalicum needs further study.
This study revealed that PoxNsdD regulates the production of plant biomass-degrading enzymes in filamentous fungi. Exactly how PoxNsdD simultaneously regulates such disparate processes is of significant interest. Throughout the life cycle of P. oxalicum grown on Avicel, the cbh1 gene POX05587/Cel7A-2 is expressed at high levels in order to provide nutrients for growth and/or substrates for other cellulases by degrading Avicel. In contrast, the key activator gene brlA, involved in asexual reproduction, is expressed at high levels only during conidiation. Accordingly, PoxNsdD activates the expression of genes encoding plant biomass-degrading enzymes, such as CBH1, and represses the expression of genes involved in fungal asexual development, such as brlA. Lee et al. (29) reported that NsdD encodes two distinct peptides, NsdDα and NsdDβ, in Aspergillus. Western blotting confirmed that NsdDα specifically accumulates in hyphae, whereas NsdDβ is expressed constitutively throughout the life cycle. Whether PoxNsdD similarly encodes two peptides which separately regulate the expression of genes involved in plant biomass degradation and asexual reproduction in P. oxalicum merits further study.
Studies on the regulatory mechanism for amylase genes in fungi are minimal. In P. oxalicum, the major amylase gene, Amy15A, is positively regulated by the G protein PGA3 (38), the casein kinase CK2 (39), the heterochromatin protein Hep1 (40), and the TFs LaeA (16), PrtT for activating the expression of genes encoding extracellular proteinases (41) and AmyR (13). However, it is unknown whether these regulators, except for AmyR, directly or indirectly regulate amylase genes. In the present study, under induction by soluble starch, PoxNsdD positively regulated the expression of the α-amylase gene POX09352/Amy13A, the glucoamylase gene POX01356/Amy15A, and their activator gene, POX03890/amyR, via direct binding to their promoters.
When cultivated in media containing different carbon sources, such as glucose, Avicel, or soluble corn starch, ΔPoxNsdD cultures became colorful, suggesting that PoxNsdD repressed the expression of genes involved in pigment biosynthesis, which might be similar to the effect of Csm1 of F. fujikuroi (27). Mining of the whole genome of P. oxalicum HP7-1 by using antibiotics and secondary metabolite analysis shell (antiSMASH) (42) revealed 93 putative BGCs for secondary metabolites (J. X. Feng et al., unpublished data). Approximately one-third of BGCs were found in the PoxNsdD regulon, indicating that the ΔPoxNsdD mutant might biosynthesize a larger number of emericellin, leucinostatin, roquefortine C/meleagrin, beauvericin, cytochalasin, malbrancheamide, and viridicatumtoxin compounds. Remarkably, PKSs contribute to pigment biosynthesis in filamentous fungi, such as that of the pigments melanin, mitorubrinol, and mitorubrinic acid, as well as monascorubrin, citrinin, and ankaflavin, in Talaromyces (formerly Penicillium) marneffei (43). The transcriptional levels of two genes in cluster 14, POX01430/alb1/wA and POX01431/abrB/yA, increased 36.5- and 95.9-fold in the ΔPoxNsdD mutants; these genes encode the putative polyketide synthase Alb1/wA and the laccase AbrB/yA, which likely contribute to the colorful phenotype of the ΔPoxNsdD mutant. POX01430/alb1/wA produced the yellow heptaketide naphthopyrone YWA1 intermediate asexual spore pigment in A. nidulans (44). In addition, P. oxalicum was predicted to biosynthesize citrinin as the secondary metabolite encoded by BGC cluster 17. Deletion of PoxNsdD significantly affected the expression of POX01612 (log2 fold change = −1.25), POX01614 (log2 fold change = −1.39), and POX01615 (log2 fold change = 5.97) under induction by Avicel.
Bioinformatics analysis revealed that POX01612 contains an HPP motif and may be a transporter protein, while POX01615 is a mannosyltransferase 1 and POX01614 is a hypothetical protein. Changes in the expression of these genes might enhance citrinin biosynthesis, thereby affecting culture color. In general, natural pigments secreted by fungi are complex mixtures. For example, the red pigment of T. marneffei contains more than 16 chemical compounds, including amino acid conjugates of monascorubrin and rubropunctatin (45). Therefore, the regulation of genes involved in pigment biosynthesis by PoxNsdD merits further study in P. oxalicum.
The gene regulatory network is integrative in cells but is complex and multidegree owing to specific ecological niches, requiring regulation of the signaling pathway for vegetative growth, secretion of extracellular enzymes, and the conidiation pathway. This results in cogovernance by various regulators, such as velvet proteins, G-proteins, and RAS proteins, that have global regulatory roles (32). Similarly, PoxNsdD coregulates the production of enzymes involved in biomass degradation, conidiation, and pigmentation in P. oxalicum.
In conclusion, the PoxNsdD gene of P. oxalicum was characterized as an ortholog of the NsdD gene encoding a GATA-type zinc finger TF. The present study not only provides evidence consistent with the general roles of NsdD orthologs in regulating asexual development and the production of secondary metabolites but also confirms that PoxNsdD plays a key role in regulating the production of enzymes that digest plant biomass into simple sugars, such as glucose, to be used as nutrients by host cells. These observations provide novel insights into the molecular mechanisms of transcriptional regulation of genes encoding plant biomass-degrading enzymes and of the elaborate cross-regulation between the signaling pathway for vegetative growth and the conidiation pathway in filamentous fungi.
MATERIALS AND METHODS
Fungal strains and culture conditions.
The P. oxalicum wild-type strain HP7-1 (China General Microbiological Culture Collection Center [CGMCC] no. 10781) was isolated from subtropical forest soil in the Guangxi Zhuang Autonomous Region, China (46). The ΔPoxKu70 (CGMCC no. 3.15650) and ΔPoxNsdD (CGMCC no. 12967) mutants were constructed by knocking out the PoxKu70 gene in HP7-1 and the PoxNsdD gene in the ΔPoxKu70 strain, respectively (14, 30). All P. oxalicum strains were maintained on potato dextrose agar (PDA) plates at 4°C. Fungal spores were harvested 6 days after inoculation onto PDA plates at 28°C and then resuspended in 0.1% Tween 80 for subsequent reproduction.
For enzymatic activity and RT-qPCR assays, 1 × 108 spores were precultured at 28°C for 24 h in liquid medium consisting of 100 ml of modified minimal medium (MMM) containing 4 g/liter (NH4)2SO4, 4 g/liter KH2PO4, 0.6 g/liter CaCl2, 0.6 g/liter MgSO4·7H2O, 0.005 g/liter FeSO4·7H2O, 0.0016 g/liter MnSO4, 0.0017 g/liter ZnCl2, 0.002 g/liter CoCl2, and 1 ml of Tween 80 supplemented with 1% (wt/vol) glucose. Pregrown hyphae were transferred to MMM containing 1% (wt/vol) soluble corn starch for 2 to 4 days (for enzymatic activity assay) or 4, 12, 24, and 48 h (for RT-qPCR) at 28°C.
For phenotypic investigation, a certain number of spores were inoculated onto solid medium plates containing MMM plus agar and 1% (wt/vol) glucose, Avicel, or soluble corn starch at 28°C for 2 to 4 days, and PDA plates were used as the control. In addition, P. oxalicum strains were inoculated into liquid medium under similar conditions to investigate pigment biosynthesis.
For RNA sequencing of P. oxalicum strains under noninduced conditions, 1 ml of spore suspension at a concentration of 1 × 108 cells per ml was used to inoculate 100 ml of MMM containing 1% (wt/vol) Avicel as the sole carbon source. Inoculated cultures were shaken at 180 rpm and 28°C for 72 h.
Extraction of total DNA and RNA.
Extraction of total DNA and RNA from P. oxalicum strains was performed according to the method described by Zhao et al. (14). Briefly, a lysate reagent (40 mM Tris-HCl, 10 mM EDTA, 20 mM sodium acetate, and 1% sodium dodecyl sulfate, pH 8.0) was added to grind mycelial powder at a ratio of 1:100 (vol:wt), incubated at room temperature for 10 min, and then centrifuged at 11,300 × g for 10 min to collect total DNA. Total RNA was extracted using a TRIzol RNA kit (Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instructions. The concentrations of extracted total DNA and RNA were determined by measuring the A260 and by electrophoresis on a 1% agarose gel.
Light microscopy.
Colonies were photographed using a Canon EOS 6D digital camera (Canon, Beijing, China). For light or fluorescence microscopy, harvested hyphae were transferred into fresh MMM on a microscope slide and covered with a coverslip. Slides were analyzed using an Olympus DP480 microscope (Olympus Corporation, Tokyo, Japan). Photomicrographs were taken and analyzed using cellSens Dimension digital imaging software (Olympus).
Enzymatic activity assay.
The activities of raw cassava starch-degrading enzymes and soluble starch-degrading enzymes were tested by measuring the reducing sugars released during hydrolysis. The 500-μl reaction mixtures consisted of 450 μl of 1% raw cassava flour or soluble potato starch in 0.1 M citrate-phosphate buffer (pH 4.5) and 50 μl of diluted enzyme solution. Reaction mixtures were incubated at 65°C for 30 min and then transferred to boiling water for 10 min to stop the reaction. Inactivated enzymes were used as a control. The concentration of reducing sugars released was determined using the 3,5-dinitrosalicylic acid method (47). One unit of enzymatic activity was defined as the amount of enzyme required to produce 1 μmol of reducing sugars per min from the reaction substrates. Triplicate independent experiments were performed for each sample.
RNA sequencing.
Total RNA sequencing of P. oxalicum strains was performed as described by Zhao et al. (14). Briefly, a cDNA library with an average length of 100 bp was constructed for each sample and evaluated with an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and an ABI StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA, USA). The constructed cDNA libraries were sequenced by use of an Illumina HiSeq 4000 system, and generated reads were mapped onto the genome of the wild-type strain HP7-1 for functional annotation using BWA v0.7.10-r789 (http://sourceforge.net/projects/bio-bwa/files/) and Bowtie2 v2.1.0 (48). Expression levels (fragments per kilobase of exon per million mapped reads [FPKM]) were analyzed using RAEM v1.2.12 software (49) and the NOISeq tool (http://www.bioconductor.org/packages/release/bioc/html/NOISeq.html). Genes with log2 ratios (ΔPoxNsdD_FPKM/ΔPoxKu70_FPKM) of ≥1 and probabilities of ≥0.8 were defined as significantly differentially expressed. Three biological replicates were analyzed for each sample.
Quantitative reverse transcription-PCR.
The RT-qPCR assay was performed based on a previously described method (14). First-stand cDNA was synthesized using total RNAs from P. oxalicum strains as templates, using a PrimeScript RT reagent kit (TaKaRa Bio Inc., Dalian, China). Each RT-qPCR mixture contained 0.8 μl of 10 μM primers (Table 1), 0.2 μl of first-strand cDNA as the template, and 10 μl of SYBR Premix Ex Taq II (TaKaRa Bio Inc.). All reactions were run for 40 cycles of 3 s at 95°C and 30 s at 60°C. The fluorescence signal was measured at the end of each 80°C extension step. The relative expression of target genes was calculated using the actin gene (POX09428) as a control, and expression was normalized against that for the ΔPoxKu70 parental strain. All RT-qPCRs were repeated at least in triplicate.
TABLE 1.
Primers used in this study
| Primer name | Sequence (5′ to 3′) |
|---|---|
| Primers used for RT-qPCR | |
| POX06534/brlA-F | CCAGTTGCCTGTTTCGTCAG |
| POX06534/brlA-R | GGTAAGGGAATGTCGGGTGTT |
| POX05587/Cel7A-2-F | GTACTTGCGATCCTGATGGG |
| POX05587/Cel7A-2-R | CCACGGTGAAGGGAGACTTG |
| POX01356/Amy15A-F | GCAAGGCCGTATCTGTCG |
| POX01356/Amy15A-R | GGGAGGTGCTGGTAATGG |
| POX01430/abl1/wA-F | TGGATGGTCCGCTGGTGG |
| POX01430/abl1/wA-R | GTAAAGGCGACGGGGTAG |
| POX03890/amyR-F | ACCCAGCCAGGGAACCAC |
| POX03890/amyR-R | CATTCCGATGCCGTGAGC |
| POX09352/Amy13A-F | CAGTGTCTTCAAGCCATTCAG |
| POX09352/Amy13A-R | CCCAAGCCAACAGTCCTC |
| Primers used for probe amplification for EMSA | |
| POX09352-Amy13-F | TCGGACCAACCCATAAGG |
| POX09352-Amy13-R | FAM-TGCCTCCTGATGATACCACA |
| POX01356-Amy15-F | ATGAAGGATCTCCAAGTGTAGC |
| POX01356-Amy15-R | FAM-AGTGATGAGCCTGGTAGAAGAA |
| POX03890-AmyR-F | TCGATCATTGGATCTCGCC |
| POX03890-AmyR-R | FAM-CGTTCAGGGAAGGAGAGAAAGTC |
| POX06534/brlA-F | GGAACTATAAACCCGCCCT |
| POX06534/brlA-R | FAM-CATGTCGTCGAGTTCTTCAATT |
| POX01430/abl1/wA-F | AAAGGGTGTGCCTGTTCAA |
| POX01430/abl1/wA-R | FAM-AAGAAGGAAGTCCGTTGTTGA |
| POX5989/tubulin-F | ACCTCACTTGCTCCGCTCTG |
| POX5989/tubulin-R | FAM-ACAAACTTCATAGATGGAGTGGACA |
Recombinant expression of the sequence encoding the DNA-binding domain (PoxNsdD335–494) and electrophoretic mobility shift assays.
Recombinant expression of the sequence encoding the putative DNA-binding domain (PoxNsdD335–494) in Escherichia coli, purification of the translated product, and EMSAs were performed as previously described (30). The DNA sequence encoding PoxNsdD335–494, flanked by SalI and XhoI restriction enzyme sites, was inserted into the expression vector pET-32a (+), which was digested with the same restriction enzymes. Cells harboring the resultant plasmid were induced with 1 mM isopropyl-β-d-thiogalactopyranoside and cultured at 20°C for 20 h. The fusion protein containing PoxNsdD335–494 with Trx, His, and S tags was purified using Ni-nitrilotriacetic acid (Ni-NTA) resin. Trx-His-S purified from E. coli cells harboring the empty vector pET-32a (+) and bovine serum albumin (BSA) alone were used as negative controls. Meanwhile, ∼1,000-bp fragments upstream of the ATG start codons of target genes, labeled with 6-carboxyfluorescein (FAM) at the 3′ terminus, were used as probes for EMSA, and a 500-bp DNA fragment from the promoter region of POX05989, encoding β-tubulin, was used as a negative control. The same DNA fragments without the FAM label were used as competitive probes following amplification with the corresponding primers (Table 1).
For in vitro binding experiments, ∼40 ng of probe was mixed with various amounts (0 to 2.0 μg) of purified proteins in binding buffer (0.1 mg/ml BSA, 20 mM Tris-HCl, pH 8.0, 5% glycerol, 50 mM KCl, 1 mM dithiothreitol [DTT], and 0.5 μg sheared salmon sperm DNA) at 30°C for 30 min. For competitive binding experiments, a known amount of binding protein was mixed with various amounts of probes under the same conditions as those described above. Protein-DNA complexes were separated by 4% polyacrylamide–Tris-acetic acid-EDTA (TAE) gel electrophoresis and visualized with a Bio-Rad ChemiDoc MP imaging system at an excitation wavelength of 489 to 506 nm.
Statistical analysis.
The Student t test (two-tailed) was used for statistical analysis in Microsoft Excel (Office 2016; Microsoft, Redmond, WA, USA).
Accession number(s).
All transcriptomic data are available from the Sequence Read Archive database under accession number SRA493765. The DNA sequence of PoxNsdD is available from the GenBank database under accession number KY368171.
Supplementary Material
ACKNOWLEDGMENTS
We thank Shang-Bo Xie and Da-Jie Zhou at BGI-Shenzhen, China, for their assistance with RNA sequencing and transcriptomic data analysis.
This work was financially supported by grants from the National Natural Science Foundation of China (grants 31760023 and 31260017) to J.-X.F. and by the One Hundred Person Project of Guangxi to S.Z.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01039-18.
REFERENCES
- 1.Zhu YG, Miller RM. 2003. Carbon cycling by arbuscular mycorrhizal fungi in soil-plant systems. Trends Plant Sci 8:407–409. doi: 10.1016/S1360-1385(03)00184-5. [DOI] [PubMed] [Google Scholar]
- 2.Johnston CA, Groffman P, Breshears DD, Cardon ZG, Currie W, Emanuel W, Gaudinski J, Jackson RB, Lajtha K, Nadelhoffer K, Nelson D, Post WM, Retallack G, Wielopolski L. 2002. Carbon cycling in soil. Front Ecol Environ 2:522–528. doi: 10.1890/1540-9295(2004)002[0522:CCIS]2.0.CO;2. [DOI] [Google Scholar]
- 3.Schimel JP, Schaeffer SM. 2012. Microbial control over carbon cycling in soil. Front Microbiol 3:348. doi: 10.3389/fmicb.2012.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding SY, Liu YS, Zeng YN, Himmel ME, Baker JO, Bayer EA. 2012. How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 338:1055–1060. doi: 10.1126/science.1227491. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, Shao Y, Chen F. 2012. Monascus pigments. Appl Microbiol Biotechnol 96:1421–1440. doi: 10.1007/s00253-012-4504-3. [DOI] [PubMed] [Google Scholar]
- 6.Peng Q, Yuan YH, Gao MY, Chen XP, Liu B, Liu PM, Wu Y, Wu DD. 2014. Genomic characteristics and comparative genomics analysis of Penicillium chrysogenum KF-25. BMC Genomics 15:144. doi: 10.1186/1471-2164-15-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puel O, Galtier P, Oswald IP. 2010. Biosynthesis and toxicological effects of patulin. Toxins (Basel) 2:613–631. doi: 10.3390/toxins2040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li CX, Zhao S, Zhang T, Xian L, Liao LS, Liu JL, Feng JX. 2017. Genome sequencing and analysis of Talaromyces pinophilus provide insights into biotechnological applications. Sci Rep 7:490. doi: 10.1038/s41598-017-00567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amore A, Giacobbe S, Faraco V. 2013. Regulation of cellulase and hemicellulase gene expression in fungi. Curr Genomics 14:230–249. doi: 10.2174/1389202911314040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin Y, Bao L, Gao M, Chen M, Lei Y, Liu G, Qu Y. 2013. Penicillium decumbens BrlA extensively regulates secondary metabolism and functionally associates with the expression of cellulase genes. Appl Microbiol Biotechnol 97:10453–10467. doi: 10.1007/s00253-013-5273-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Wang S, Zhang XX, Ji W, Song FP, Zhao Y, Li J. 2016. The amyR-deletion strain of Aspergillus niger CICC2462 is a suitable host strain to express secreted protein with a low background. Microb Cell Fact 15:68. doi: 10.1186/s12934-016-0463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coradetti ST, Xiong Y, Glass NL. 2013. Analysis of a conserved cellulase transcriptional regulator reveals inducer-independent production of cellulolytic enzymes in Neurospora crassa. Microbiologyopen 2:595–609. doi: 10.1002/mbo3.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li ZH, Yao GS, Wu RM, Gao LW, Kan QB, Liu M, Yang P, Liu GD, Qin YQ, Song X, Zhong YH, Fang X, Qu YB. 2015. Synergistic and dose-controlled regulation of cellulase gene expression in Penicillium oxalicum. PLoS Genet 11:e1005509. doi: 10.1371/journal.pgen.1005509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao S, Yan YS, He QP, Yang L, Yin X, Li CX, Mao LC, Liao LS, Huang JQ, Xie SB, Nong QD, Zhang Z, Jing L, Xiong YR, Duan CJ, Liu JL, Feng JX. 2016. Comparative genomic, transcriptomic and secretomic profiling of Penicillium oxalicum HP7-1 and its cellulase and xylanase hyper-producing mutant EU2106, and identification of two novel regulatory genes of cellulase and xylanase gene expression. Biotechnol Biofuels 9:203. doi: 10.1186/s13068-016-0616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichius A, Bidard F, Buchholz F, Le Crom S, Martin J, Schackwitz W, Austerlitz T, Grigoriev IV, Baker SE, Margeot A, Seiboth B, Kubicek CP. 2015. Genome sequencing of the Trichoderma reesei QM9136 mutant identifies a truncation of the transcriptional regulator XYR1 as the cause for its cellulase-negative phenotype. BMC Genomics 16:326. doi: 10.1186/s12864-015-1526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YN, Zheng XJ, Zhang XJ, Bao LF, Zhu YY, Qu YB, Zhao J, Qin YQ. 2016. The different roles of Penicillium oxalicum LaeA in the production of extracellular cellulase and β-xylosidase. Front Microbiol 7:2091. doi: 10.3389/fmicb.2016.02091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiboth B, Karimi RA, Phatale PA, Linke R, Hartl L, Sauer DG, Smith KM, Baker SE, Freitag M, Kubicek CP. 2012. The putative protein methyltransferase LAE1 controls cellulase gene expression in Trichoderma reesei. Mol Microbiol 84:1150–1164. doi: 10.1111/j.1365-2958.2012.08083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huberman LB, Liu J, Qin LN, Glass NL. 2016. Regulation of the lignocellulolytic response in filamentous fungi. Fungal Biol Rev 30:101–111. doi: 10.1016/j.fbr.2016.06.001. [DOI] [Google Scholar]
- 19.Antonieto ACC, Castro LD, Silva-Rocha R, Persinoti GF, Silva RN. 2014. Defining the genome-wide role of CRE1 during carbon catabolite repression in Trichoderma reesei using RNA-Seq analysis. Fungal Genet Biol 73:93–103. doi: 10.1016/j.fgb.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Lei Y, Liu G, Yao G, Li Z, Qin Y, Qu Y. 2016. A novel bZIP transcription factor ClrC positively regulates multiple stress responses, conidiation and cellulase expression in Penicillium oxalicum. Res Microbiol 167:424–435. doi: 10.1016/j.resmic.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Yao GS, Li ZH, Wu RM, Qin YQ, Liu GD, Qu YB. 2016. Penicillium oxalicum PoFlbC regulates fungal asexual development and is important for cellulase gene expression. Fungal Genet Biol 86:91–102. doi: 10.1016/j.fgb.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Han KH, Han KY, Yu JH, Chae KS, Jahng KY, Han DM. 2001. The nsdD gene encodes a putative GATA-type transcription factor necessary for sexual development of Aspergillus nidulans. Mol Microbiol 41:299–309. doi: 10.1046/j.1365-2958.2001.02472.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen CH, Ringelberg CS, Gross RH, Dunlap JC, Loros JJ. 2009. Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO J 28:1029–1042. doi: 10.1038/emboj.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowrousian M, Teichert I, Masloff S, Kuck U. 2012. Whole-genome sequencing of Sordaria macrospora mutants identifies developmental genes. G3 (Bethesda) 2:261–270. doi: 10.1534/g3.111.001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee MK, Kwon NJ, Choi JM, Lee IS, Jung S, Yu JH. 2014. NsdD is a key repressor of asexual development in Aspergillus nidulans. Genetics 197:159–173. doi: 10.1534/genetics.114.161430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schumacher J, Simon A, Cohrs KC, Viaud M, Tudzynski P. 2014. The transcription factor BcLTF1 regulates virulence and light responses in the necrotrophic plant pathogen Botrytis cinerea. PLoS Genet 10:e1004040. doi: 10.1371/journal.pgen.1004040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niehaus EM, Schumacher J, Burkhardt I, Rabe P, Spitzer E, Munsterkotter M, Guldener U, Sieber CMK, Dickschat JS, Tudzynski B. 2017. The GATA-type transcription factor Csm1 regulates conidiation and secondary metabolism in Fusarium fujikuroi. Front Microbiol 8:1175. doi: 10.3389/fmicb.2017.01175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li JT, Mu WH, Veluchamy S, Liu YZ, Zhang YH, Pan HY, Rollins JA. 2018. The GATA-type IVb zinc-finger transcription factor SsNsd1 regulates asexual-sexual development and appressoria formation in Sclerotinia sclerotiorum. Mol Plant Pathol 19:1679–1689. doi: 10.1111/mpp.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee MK, Kwon NJ, Lee IS, Jung S, Kim SC, Yu JH. 2016. Negative regulation and developmental competence in Aspergillus. Sci Rep 6:28874. doi: 10.1038/srep28874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan YS, Zhao S, Liao LS, He QP, Xiong YR, Wang L, Li CX, Feng JX. 2017. Transcriptomic profiling and genetic analyses reveal novel key regulators of cellulase and xylanase gene expression in Penicillium oxalicum. Biotechnol Biofuels 10:279. doi: 10.1186/s13068-017-0966-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gronenborn AM. 2005. The DNA-binding domain of GATA transcription factors—a prototypical type IV Cys2-Cys2 zinc finger, p 26–30. In Iuchi S, Kuldell N (ed), Zinc finger proteins: from atomic contact to cellular function. Molecular Biology Intelligence Unit, Springer, Boston, MA. [Google Scholar]
- 32.Park HS, Yu JH. 2012. Genetic control of asexual sporulation in filamentous fungi. Curr Opin Microbiol 15:669–677. doi: 10.1016/j.mib.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Mayorga ME, Timberlake WE. 1990. Isolation and molecular characterization of the Aspergillus nidulans wA gene. Genetics 126:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abad A, Fernandez-Molina JV, Bikandi J, Ramirez A, Margareto J, Sendino J, Hernando FL, Ponton J, Garaizar J, Rementeria A. 2010. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev Iberoam Micol 27:155–182. doi: 10.1016/j.riam.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Paris S, Debeaupuis JP, Crameri R, Carey M, Charles F, Prevost MC, Schmitt C, Philippe B, Latge JP. 2003. Conidial hydrophobins of Aspergillus fumigatus. Appl Environ Microbiol 69:1581–1588. doi: 10.1128/AEM.69.3.1581-1588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Si HY, Rittenour WR, Xu KM, Nicksarlian M, Calvo AM, Harris SD. 2012. Morphogenetic and developmental functions of the Aspergillus nidulans homologues of the yeast bud site selection proteins Bud4 and Axl2. Mol Microbiol 85:252–270. doi: 10.1111/j.1365-2958.2012.08108.x. [DOI] [PubMed] [Google Scholar]
- 37.McGuire SL, Roe DL, Carter BW, Carter RL, Grace SP, Hays PL, Lang GA, Mamaril JL, McElvaine AT, Payne AM, Schrader MD, Wahrle SE, Young CD. 2000. Extragenic suppressors of the nimX2(cdc2) mutation of Aspergillus nidulans affect nuclear division, septation and conidiation. Genetics 156:1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y, Liu G, Li Z, Qin Y, Qu Y, Song X. 2013. G protein-cAMP signaling pathway mediated by PGA3 plays different roles in regulating the expressions of amylases and cellulases in Penicillium decumbens. Fungal Genet Biol 58–59:62–70. doi: 10.1016/j.fgb.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Lei Y, Liu G, Li Z, Gao L, Qin Y, Qu Y. 2014. Functional characterization of protein kinase CK2 regulatory subunits regulating Penicillium oxalicum asexual development and hydrolytic enzyme production. Fungal Genet Biol 66:44–53. doi: 10.1016/j.fgb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Qu Y, Qin Y. 2016. Expression and chromatin structures of cellulolytic enzyme gene regulated by heterochromatin protein 1. Biotechnol Biofuels 9:206. doi: 10.1186/s13068-016-0624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Zou G, Zhang L, de Vries RP, Yan X, Zhang J, Liu R, Wang C, Qu Y, Zhou Z. 2014. The distinctive regulatory roles of PrtT in the cell metabolism of Penicillium oxalicum. Fungal Genet Biol 63:42–54. doi: 10.1016/j.fgb.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Blin K, Wolf T, Chevrette MG, Lu X, Schwalen CJ, Kautsar SA, Suarez Duran HG, de Los Santos ELC, Kim HU, Nave M, Dickschat JS, Mitchell DA, Shelest E, Breitling R, Takano E, Lee SY, Weber T, Medema MH. 2017. antiSMASH 4.0—improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res 45:W36–W41. doi: 10.1093/nar/gkx319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tam EW, Tsang CC, Lau SK, Woo PC. 2015. Polyketides, toxins and pigments in Penicillium marneffei. Toxins (Basel) 7:4421–4436. doi: 10.3390/toxins7114421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe A, Fujii I, Sankawa U, Mayorga ME, Timberlake WE, Ebizuka Y. 1999. Re-identification of Aspergillus nidulans wA gene to code for a polyketide synthase of naphthopyrone. Tetrahedron Lett 40:91–94. doi: 10.1016/S0040-4039(98)80027-0. [DOI] [Google Scholar]
- 45.Woo PC, Lam CW, Tam EW, Lee KC, Yung KK, Leung CK, Sze KH, Lau SK, Yuen KY. 2014. The biosynthetic pathway for a thousand-year-old natural food colorant and citrinin in Penicillium marneffei. Sci Rep 4:6728. doi: 10.1038/srep06728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z, Liu JL, Lan JY, Duan CJ, Ma QS, Feng JX. 2014. Predominance of Trichoderma and Penicillium in cellulolytic aerobic filamentous fungi from subtropical and tropical forests in China, and their use in finding highly efficient beta-glucosidase. Biotechnol Biofuels 7:107. doi: 10.1186/1754-6834-7-107. [DOI] [Google Scholar]
- 47.Miller GL. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 48.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li B, Dewey CN. 2011. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.