The introduction of solid foods (complementary feeding or weaning) to infants leads to more-complex compositions of microbial communities (microbiota or microbiome) in the gut. In baby-led weaning (BLW), infants are given only finger foods that they can pick up and feed themselves—there is no parental spoon-feeding of puréed baby foods—and infants are encouraged to eat family meals. BLW is a new approach to infant feeding that is increasing in popularity in the United States, New Zealand, the United Kingdom, and Canada. We used mediation modeling, commonly used in health research but not in microbiota studies until now, to identify particular dietary components that affected the development of the infant gut microbiota.
KEYWORDS: alpha diversity, baby-led weaning, dietary fiber, fecal microbiota, fruit and vegetables, mediation analysis
ABSTRACT
The introduction of “solids” (i.e., complementary foods) to the milk-only diet in early infancy affects the development of the gut microbiota. The aim of this study was to determine whether a “baby-led” approach to complementary feeding that encourages the early introduction of an adult-type diet results in alterations of the gut microbiota composition compared to traditional spoon-feeding. The Baby-Led Introduction to SolidS (BLISS) study randomized 206 infants to BLISS (a modified version of baby-led weaning [BLW], the introduction of solids at 6 months of age, followed by self-feeding of family foods) or control (traditional spoon-feeding of purées) groups. Fecal microbiotas and 3-day weighed-diet records were analyzed for a subset of 74 infants at 7 and 12 months of age. The composition of the microbiota was determined by sequencing of 16S rRNA genes amplified by PCR from bulk DNA extracted from feces. Diet records were used to estimate food and dietary fiber intake. Alpha diversity (number of operational taxonomic units [OTUs]) was significantly lower in BLISS infants at 12 months of age (difference [95% confidence interval {CI}] of 31 OTUs [3.4 to 58.5]; P = 0.028), and while there were no significant differences between control and BLISS infants in relative abundances of Bifidobacteriaceae, Enterobacteriaceae, Veillonellaceae, Bacteroidaceae, Erysipelotrichaceae, Lachnospiraceae, or Ruminococcaceae at 7 or 12 months of age, OTUs representing the genus Roseburia were less prevalent in BLISS microbiotas at 12 months. Mediation models demonstrated that the intake of “fruit and vegetables” and “dietary fiber” explained 29% and 25%, respectively, of the relationship between group (BLISS versus control) and alpha diversity.
IMPORTANCE The introduction of solid foods (complementary feeding or weaning) to infants leads to more-complex compositions of microbial communities (microbiota or microbiome) in the gut. In baby-led weaning (BLW), infants are given only finger foods that they can pick up and feed themselves—there is no parental spoon-feeding of puréed baby foods—and infants are encouraged to eat family meals. BLW is a new approach to infant feeding that is increasing in popularity in the United States, New Zealand, the United Kingdom, and Canada. We used mediation modeling, commonly used in health research but not in microbiota studies until now, to identify particular dietary components that affected the development of the infant gut microbiota.
INTRODUCTION
The first year of life sees marked changes in the composition of the gut microbiota of infants as the diet changes from one that is entirely milk based to one that is similar to the family diet by 12 months or so of age (1). Although attention has been paid to the impact of breast milk and infant formula on the composition of the microbiota (2–8), less research has investigated the impact of the change in diet that occurs when infants are introduced to solid foods during the complementary feeding period (known in some countries as “weaning”) (9). To date, the majority of studies on this transition have been observational (10, 11), and the few randomized controlled trials (RCTs) have focused largely on the impact of iron fortification on the gut microbiota (12, 13).
Traditionally, parents have been encouraged to start spoon-feeding their infant puréed foods from around 6 months of age, progressing to mashed and then chopped foods in the hope that they will be eating family foods by around 12 months of age (1). However, an alternative method of complementary feeding, known as baby-led weaning (BLW), is becoming popular in New Zealand (14), the United Kingdom (15), the United States (16), and Canada (17). In BLW, infants are encouraged to feed themselves whole pieces of food from the family meal from 6 months of age, instead of being offered “baby food” (18). As a result, infants following BLW are more likely to eat the same foods as the rest of the family than their traditionally fed counterparts (14). Because the introduction of family foods is a major determinant of the development of the gut microbiota (19), it is possible that the more rapid transition to the family diet observed in baby-led approaches will differentially influence the gut microbiota in these children.
The Baby-Led Introduction to SolidS (BLISS) randomized controlled trial (20, 21) has investigated the impact of a modified version of BLW on growth (22), choking risk (23), and iron status (24) and provides an opportunity to investigate the impact of the early introduction of an adult-type diet on the developing infant gut microbiota. The aim of this study was to determine whether a “baby-led” approach to complementary feeding affects the fecal microbiota composition relative to traditional spoon-feeding and, if so, to use mediation analysis to associate dietary components with altered microbiota.
RESULTS
Study population.
Fecal samples were obtained from 73 participants at 7 months of age (data for 1 BLISS participant are missing) and 68 participants at 12 months of age (data for 3 control and 3 BLISS participants are missing) (Fig. 1). The baseline and early feeding characteristics of these participants are shown in Tables 1 and 2; infants were predominantly born by vaginal delivery to university-educated mothers. BLISS infants were introduced to complementary foods later than control infants. No differences were observed in the rates of breastfeeding, or the amounts of breast milk or infant formula consumed, at 7 or 12 months of age. Rates of recent antibiotic use were similar in control and BLISS infants at 7 months (16.7% versus 13.5%; P = 0.707) and 12 months (16.1% versus 8.8%; P = 0.371) of age. From the main-outcomes paper, BLISS infants had very good adherence to the baby-led approach and were significantly (P < 0.01) more likely to feed themselves most or all their food than control infants at every age (for example, 74% of BLISS infants at 7 months of age compared to 21% of control infants) (22). Using population-averaged generalized estimating equations with the full sample, BLISS infants were also significantly more likely to be eating the same foods as the rest of their family by 7 months of age, having 3.3 times the odds (95% confidence interval [CI], 2.0, 5.6; P < 0.001) of eating the same evening meal as the rest of the family compared to the control infants (data not shown).
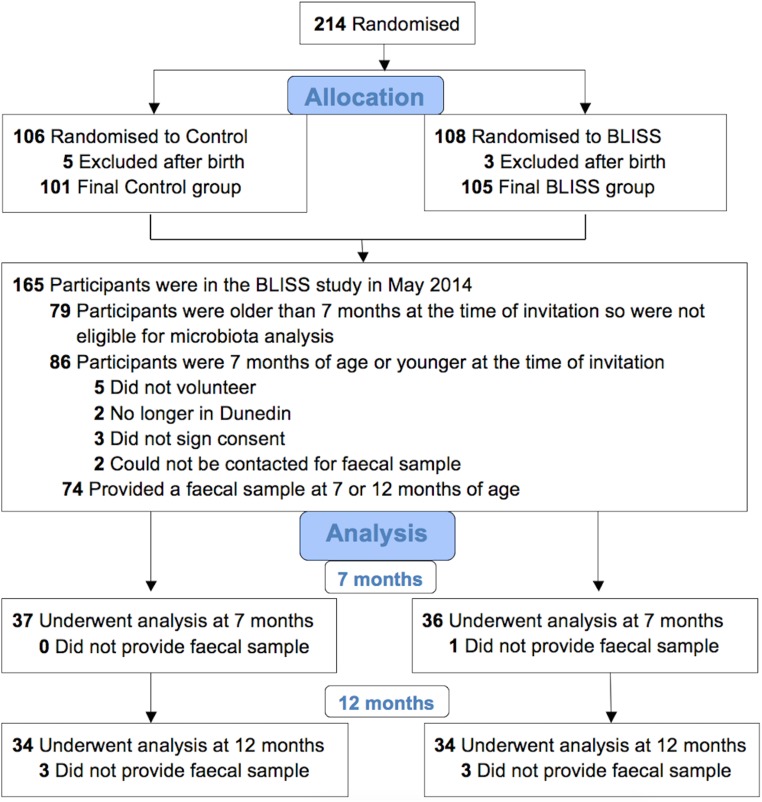
FIG 1.
Flow diagram for the microbiota component of the BLISS (Baby-Led Introduction to SolidS) study.
TABLE 1.
Characteristics of the BLISS study participants who agreed to provide a fecal samplea
| Variable | Value for group |
|
|---|---|---|
| Control (n = 37) | BLISS (n = 37) | |
| No. (%) of male participants | 19 (51.4) | 15 (40.5) |
| Mean birth wt (g) (SD) | 3,443 (531) | 3,507 (474) |
| No. (%) of participants with parity status | ||
| 1 | 15 (40.5) | 14 (37.8) |
| >1 | 22 (59.5) | 23 (62.2) |
| No. (%) of participants with mode of delivery | ||
| Vaginal | 27 (73.0) | 29 (78.4) |
| Caesarean section | 10 (27.0) | 8 (21.6) |
| Mean maternal age (yr) (SD) | 31.6 (6.0) | 31.7 (4.6) |
| Mean maternal self-reported BMI (kg/m2) (SD) | 25.3 (5.9) | 25.8 (6.2) |
| No. (%) of participants with maternal education of: | ||
| School or postschool onlyb | 15 (40.5) | 21 (56.8) |
| University | 22 (59.5) | 16 (43.2) |
| No. (%) of participants with household deprivation decile ofc: | ||
| 1–3 (low) | 11 (29.7) | 11 (29.7) |
| 4–7 | 14 (37.8) | 21 (56.8) |
| 8–10 (high) | 12 (32.4) | 5 (13.5) |
| No. (%) of participants with dietary data provided at 7 mo | 34 (92) | 32 (86) |
| No. (%) of participants with dietary data provided at 12 mo | 29 (78) | 29 (78) |
Data are presented as means (standard deviations) unless otherwise indicated. Data were missing for 2 participants for birth weight and for 3 participants for maternal self-reported BMI. P values were not calculated for baseline measurements, as the groups are randomized. Abbreviations: BLISS, Baby-Led Introduction to SolidS; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
School or postschool only includes primary school, secondary school, trade, certificates, and diplomas.
Determined using the 2013 New Zealand index of deprivation (47). The index combines 9 variables from the 2013 New Zealand National Census to provide a deprivation score for each mesh block (a geographical unit defined by Statistics New Zealand that contains approximately 81 people). The score reflects the extent of material and social deprivation and is used to construct deciles from 1 (low deprivation) to 10 (high deprivation).
TABLE 2.
Early diet characteristics of the BLISS study participants who agreed to provide a fecal samplea
| Variable | Value for group |
P value | |
|---|---|---|---|
| Control (n = 37) | BLISS (n = 37) | ||
| No. (%) of participants who had consumed infant formula by 7 mo | 20 (54) | 18 (50) | 0.729b |
| Median age at introduction (wk) (25th, 75th percentiles) | |||
| Infant formula | 4.0 (0.6, 26) | 4.5 (0.4, 28.2) | 0.454c |
| Any solids | 22.8 (21.7, 24.9) | 26.0 (23.8, 26) | 0.002c |
| Infant cereal | 23.8 (22.1, 25.5) | 26.0 (23.8, 26.4) | 0.032c |
n = 45 participants who provided data for age of introduction of infant formula, and n = 62 participants who provided data for age of introduction of all solids and infant cereal.
Determined by a chi-square test.
Determined by a log rank test.
Gut microbiota.
Figure 2 shows that alpha diversity (all samples rarefied to 30,000 sequences per sample), as measured by observed “species,” increased in both groups from 7 to 12 months of age (mean change [95% CI] of 77 OTUs [64, 90]; P < 0.001 from a paired t test), and at 12 months, the BLISS group had significantly lower alpha diversity than the control infants (P = 0.028). As shown in Table 3, no significant group differences were observed for the five measures of alpha diversity at 7 months (all P > 0.1), and the proportion of variance explained by group, parity, and maternal university education was low for observed species, phylogenetic diversity, and the Chao1 estimator (all <2%). For those measures of alpha diversity that included evenness (Simpson index and Shannon index), the proportion of variance explained was slightly higher (6.3% and 8.1%, respectively) although still low.
FIG 2.
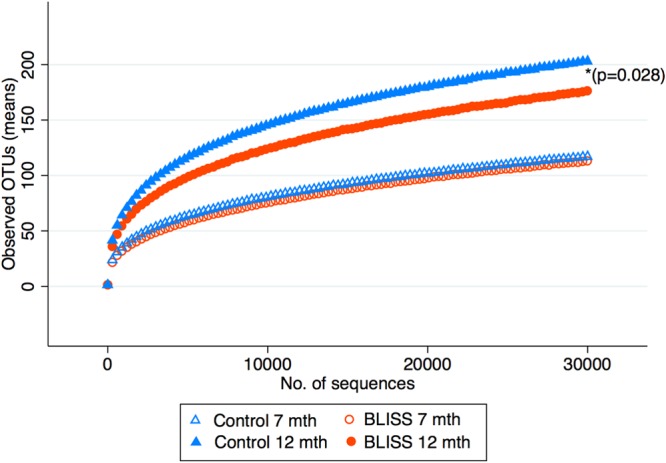
Rarefaction curves of observed OTUs against the number of sequences from control and BLISS groups at 7 and 12 months of age. OTUs, operational taxonomic units (a proxy for observed species and a measure of alpha diversity). *, P value between groups at 12 months.
TABLE 3.
Alpha diversity measures for control and BLISS groups at 7 and 12 months
| Parametera | Value for group |
Mean differenceb between groups (95% CI) | P valuec | Proportion of variance explained (R2) | |
|---|---|---|---|---|---|
| Control | BLISS | ||||
| 7 mo | |||||
| No. of participants | 37 | 36 | |||
| Alpha diversity measure | |||||
| Mean no. of observed species (no. of OTUs) (SD) | 117 (36) | 113 (30) | −5.2 (−21.2, 10.9) | 0.523 | 0.018 |
| Mean phylogenetic diversity (SD) | 10.7 (2.0) | 10.7 (1.8) | 0.04 (−0.88, 0.96) | 0.925 | 0.004 |
| Mean Chao1 estimator (SD) | 174 (46) | 173 (39) | −1.6 (−22.0, 18.8) | 0.877 | 0.012 |
| Mean Simpson index (SD) | 0.76 (0.11) | 0.74 (0.09) | −0.03 (−0.08, 0.02) | 0.187 | 0.063 |
| Mean Shannon index (SD) | 2.99 (0.72) | 2.79 (0.58) | −0.25 (−0.56, 0.06) | 0.109 | 0.081 |
| 12 mo | |||||
| No. of participants | 34 | 34 | |||
| Alpha diversity measure | |||||
| Mean no. of observed species (no. of OTUs) (SD) | 203 (58) | 176 (58) | −30.9 (−58.5, −3.4) | 0.028 | 0.171 |
| Mean phylogenetic diversity (SD) | 14.7 (2.8) | 13.8 (3.3) | −1.2 (−2.6, 0.2) | 0.094 | 0.175 |
| Mean Chao1 (SD) | 277 (69) | 246 (69) | −36.4 (−69.1, −3.8) | 0.029 | 0.164 |
| Mean Simpson index (SD) | 0.88 (0.06) | 0.83 (0.09) | −0.06 (−0.09, −0.02) | 0.004 | 0.185 |
| Mean Shannon index (SD) | 4.18 (0.73) | 3.72 (0.81) | −0.52 (−0.88, −0.16) | 0.006 | 0.210 |
Estimates were made at 30,000 sequences.
Linear regression adjusted for parity and maternal university education.
Boldface indicates statistically significant differences between variables.
At 12 months of age, however, the BLISS group had significantly lower alpha diversity than the control group. The BLISS group had a mean of 31 fewer operational taxonomic units (OTUs) (P = 0.028) and a 0.52-lower Shannon index (P = 0.006) than the control group. The proportion of variance explained at 12 months (between 16% and 21%) was higher than that at 7 months (between 0.4% and 8%) (Table 3). As observed species and Shannon index were the alpha diversity measures that covered richness as well as richness and evenness with the highest R2 values and had the strongest associations with the randomized group, subsequent analyses with alpha diversity were limited to these two measures.
Sensitivity analyses were carried out with the inclusion of antibiotic use in the days before the fecal sample was collected (n = 11 at 7 months; n = 8 at 12 months). No appreciable impact on the results at 7 months was seen, but the results at 12 months were slightly strengthened by the inclusion of antibiotic use in the model (mean difference [95% CI] for observed species of −33 OTUs [−62, −5] [R2 = 0.19]; mean difference [95% CI] for Shannon index values of −0.56 [−0.94, −0.19] [R2 = 0.23]).
Figure 3 illustrates the relative abundances of the 7 most abundant families (median of at least a 1% relative abundance), which in combination explained approximately 86% of the sequence data. There were no significant differences in the relative abundances of the 7 bacterial families between groups at either 7 or 12 months of age (see Table S1 in the supplemental material). However, for all infants combined, there was a decrease in the relative abundances of Bifidobacteriaceae, Enterobacteriaceae, and Veillonellaceae, with increases in the relative abundances of Lachnospiraceae and Ruminococcaceae at between 7 and 12 months of age (Table S2).
FIG 3.
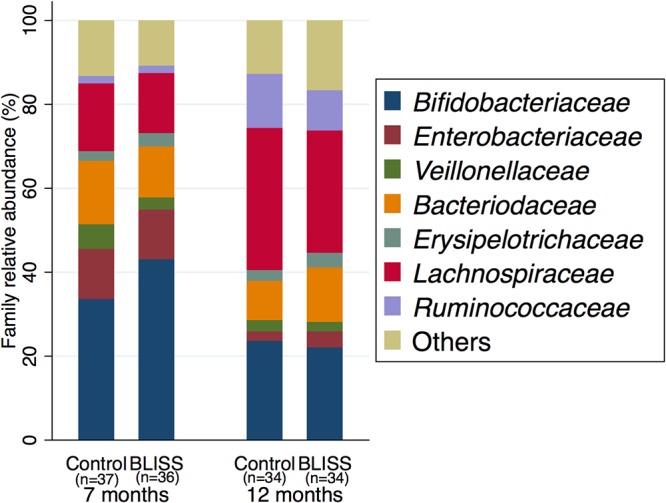
Relative abundances of the top (>1%) seven bacterial families in feces of control and BLISS infants at 7 and 12 months of age.
Beta diversity metrics (Bray-Curtis dissimilarity unweighted UniFrac, weighted UniFrac, and Jaccard distance) were determined, and permutational multivariate analysis of variance (PERMANOVA) was used to compare diet/time groups for each metric. The results indicated that the BLISS and control group microbiotas were different at 12 months but only when using presence/absence data (Jaccard distance and unweighted UniFrac; P = 0.028 and 0.037 and q = 0.0336 and 0.0444, respectively).
While it is tempting to think that if alpha and beta diversities differ between groups, there will be corresponding clear-cut differences in OTU distributions, this may not be the case because multiple communities may differ in diversity without having taxonomic differences in common. Nevertheless, we attempted to identify OTUs that were differentially represented in the control group relative to the BLISS group at 7 and 12 months of age using analysis of composition of microbiomes (ANCOM), gneiss, and multivariate association with linear models (MaAsLin). The original OTU table was split into two tables separated by sampling time in order to focus on differences due to diet rather than developmental age. Additionally, the full OTU table was retained where models could be applied to account for continuous variables (in this case, “time”) When the tables were split, no OTUs were reported to be differentially abundant for either time regardless of the method. When MaAsLin was employed with the full table and time was included as a forced predictor, six OTUs were predicted to be differentially abundant between the dietary groups (Table 4), and all were less abundant in the BLISS group. Three of these OTUs represented the genus Roseburia.
TABLE 4.
OTUs differentially abundant according to diet when controlling for timea
| Feature | Coefficientb | No. of OTUs | No. of OTUs not at 0 h | P value | q value |
|---|---|---|---|---|---|
| Firmicutes|Lachnospiraceae|Roseburia|faecis | 0.003716215 | 141 | 54 | 0.00013082 | 0.001252132 |
| Firmicutes|Lachnospiraceae|Eubacterium|rectale3 | 0.004406438 | 141 | 46 | 0.000499015 | 0.004179247 |
| Firmicutes|Lachnospiraceae|Roseburia|unculturedbacterium1 | 0.004087229 | 141 | 46 | 0.000621837 | 0.005048626 |
| Firmicutes|Lachnospiraceae|Roseburia|unculturedorganism1 | 0.001173604 | 141 | 64 | 0.001987778 | 0.013171758 |
| Firmicutes|Ruminococcaceae|Faecalibacterium|prausnitzi11 | 0.004271382 | 141 | 31 | 0.00311382 | 0.019974824 |
| Firmicutes|Lachnospiraceae|Clostridium2 | 0.004449289 | 141 | 69 | 0.007028392 | 0.041961589 |
The variable in all cases was diet, and the value used in all cases was Control diet.
A positive coefficient indicates a higher relative abundance in the control group.
An alternative approach was to search for differential prevalence by analyzing the OTU table using presence/absence data. A between-group difference of 10 participants with the presence of a species was decided to be a meaningful difference for this analytical approach (i.e., 10 more participants in the control group had a particular OTU present than in the BLISS group; this difference also represents the difference that we would give 80% power for detection to the 5% level). When applied to an OTU-level table, at 12 months, 21 OTUs were overrepresented in the control group, while two OTUs were overrepresented in the BLISS group. All OTUs in the control group were assigned to the order Clostridiales, and 7 OTUs were classified as belonging to the genus Roseburia. At 7 months, only three OTUs were overrepresented in the control group (Table S3).
Diet.
Energy intakes were similar in control and BLISS participants at 7 months of age (means [standard deviations {SD}] of 2,820 (470) kJ/day and 2,860 (440) kJ/day, respectively; P = 0.749) and also at 12 months of age (means [SD] of 3,460 (850) kJ/day and 3,430 [480] kJ/day, respectively; P = 0.881). However, the proportion of energy contributed by each food group changed considerably over time (Fig. 4). Milk (breast milk and infant formula) contributed approximately 78% of the energy for both groups at 7 months of age, decreasing to around 36% at 12 months.
FIG 4.
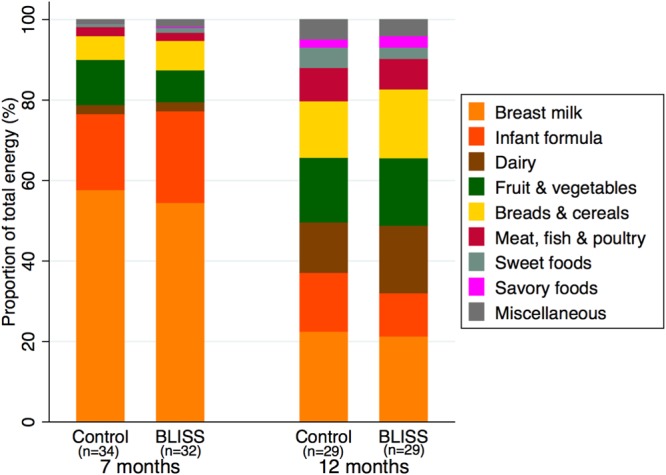
Proportions of total energy intake contributed by nine food groups at 7 and 12 months of age.
Predictors of alpha diversity.
Table 5 shows that the only variable associated with alpha diversity at 12 months of age, from a range of demographic variables, feeding characteristics, and antibiotic use, was parity: greater alpha diversity was observed in children with a higher birth order. The intake of “breads and cereals,” “fruit and vegetables,” and “dietary fiber” at 7 months of age were all positively and significantly associated with at least one measure of (higher) alpha diversity at 12 months of age (Table 6). For example, at 7 months, the intake of an additional 10 g of breads and cereals was associated with 9.5 more OTUs and a 0.14-higher Shannon index at 12 months. “Infant milk” and “meat” intake were also moderately associated with alpha diversity but not statistically significantly (P = 0.066 and 0.0.089, respectively, for the number of OTUs and P = 0.234 and 0.070, respectively, for the Shannon index).
TABLE 5.
Associations between alpha diversity (number of OTUs and Shannon index) and variables related to demography, change in feeding mode, and antibiotic use at 12 months (n = 68)
| Predictor variable | No. of OTUs |
Shannon index |
||
|---|---|---|---|---|
| Mean difference per unit of predictor variablea (95% CI) | P valueb | Mean difference per unit of predictor variablea (95% CI) | P valueb | |
| Parity (no. of children) | 23.5 (9.5, 37.5) | 0.001 | 0.31 (0.12, 0.49) | 0.002 |
| Mode of delivery (caesarian section compared to vaginal birth) | 14.1 (−19.2, 47.4) | 0.401 | 0.17 (−0.27, 0.61) | 0.440 |
| Maternal education (having university degree compared to not having one) | 0.3 (−28.9, 29.5) | 0.983 | −0.03 (−0.41, 0.36) | 0.890 |
| Household deprivation (deprivation category) | −13.6 (−34.0, 6.9) | 0.189 | −0.10 (−0.37, 0.17) | 0.473 |
| Consumption of infant formula (had consumed compared to had not) | 24.2 (−5.2, 53.7) | 0.105 | 0.12 (−0.28, 0.52) | 0.547 |
| Age at introduction of infant formula (wk) | 0.7 (−0.5, 1.9) | 0.265 | 0.01 (−0.002, 0.03) | 0.098 |
| Age at introduction of solids (wk) | −3.2 (−8.5, 2.1) | 0.237 | −0.03 (−0.10, 0.04) | 0.450 |
| Age at introduction of infant cereal (wk) | 0.7 (−2.6, 3.9) | 0.688 | −0.002 (−0.04, 0.04) | 0.931 |
| Infant antibiotic use at time of fecal sample (had consumed compared to had not) | 15.9 (−29.2, 61.1) | 0.483 | 0.29 (−0.30, 0.88) | 0.333 |
Unless otherwise indicated. Shown is linear regression with adjustment for group.
Boldface indicates statistically significant differences between variables.
TABLE 6.
Associations between dietary components at 7 months and alpha diversity at 12 months (number of OTUs and Shannon index) (n = 63)
| Dietary component(s) (unit used in regression) | Mean intake at 7 mo (g/day) (SD) | No. of OTUs |
Shannon index |
||
|---|---|---|---|---|---|
| Mean difference per unit of dietary component (95% CI)a | P valueb | Mean difference per unit of dietary component (95% CI)a | P valueb | ||
| Breast milk (100 g) | 565 (297) | −2.6 (−7.5, 2.3) | 0.300 | 0.01 (−0.06, 0.07) | 0.763 |
| Infant formula (100 g) | 192 (341) | 3.5 (−0.7, 7.8) | 0.100 | 0.01 (−0.05, 0.06) | 0.834 |
| Infant milk (breast or formula) (100 g) | 757 (135) | 9.8 (−0.7, 20.4) | 0.066 | 0.08 (−0.06, 0.22) | 0.234 |
| Breads and cereals (10 g) | 16.9 (14.9) | 9.5 (0.3, 18.7) | 0.042 | 0.14 (0.02, 0.26) | 0.025 |
| Fruit and vegetables (10 g) | 91.9 (74.7) | 1.8 (−0.2, 3.8) | 0.071 | 0.03 (0.0002, 0.05) | 0.048 |
| Meat (g) | 7.6 (9.6) | 1.3 (−0.2, 2.7) | 0.089 | 0.02 (−0.001, 0.04) | 0.070 |
| Dairyc (g) | 8.5 (10.4) | 0.4 (−0.9, 1.7) | 0.565 | 0.00 (−0.01, 0.02) | 0.696 |
| Sweet foods (g) | 1.2 (2.8) | 4.0 (−1.2, 9.2) | 0.127 | 0.03 (−0.04, 0.10) | 0.337 |
| Savory foods (g) | 0.3 (1.4) | 4.7 (−5.8, 15.3) | 0.371 | −0.01 (−0.15, 0.13) | 0.891 |
| Miscellaneous (10 g) | 49.1 (88.9) | 0.1 (−1.5, 1.7) | 0.933 | 0.00 (−0.02, 0.02) | 0.988 |
| Dietary fiber (g) | 3.1 (2.3) | 6.2 (−0.1, 12.5) | 0.054 | 0.10 (0.01, 0.18) | 0.024 |
| Fiber variety scored | 11.3 (4.3) | 1.03 (−2.8, 4.9) | 0.592 | 0.01 (−0.04, 0.06) | 0.672 |
Linear regression adjusted for group, parity, and maternal university education.
Boldface indicates statistically significant differences between variables.
n = 61. Two participants had very high dairy intake compared to the rest of the sample (98 g and 117 g) and influenced the regression (using the full sample, the mean difference in the number of OTUs per gram [95% CI] was 0.9 [0.2, 1.6] OTUs, and the mean difference in the Shannon index per gram [95% CI] was 0.01 [−0.002, 0.02]).
Determined by counting each different fiber-containing food (i.e., grain product, vegetable, or fruit) consumed over the 3 recording days (n = 56).
Because there were significant associations between diet at 7 months of age and alpha diversity at 12 months, we hypothesized that diet at 7 months might explain (mediate) the observed differences in alpha diversity between the BLISS and control groups. However, as there were no differences between groups for infant milk intake (mean difference [95% CI] of −4 [−73 to 65] g/day), bread and cereal intake (mean difference [95% CI] of 1.9 [−5.9 to 9.8] g/day), or meat intake (mean difference [95% CI] of −0.9 [−6.0 to 4.1] g/day), these foods were not considered to be mediators, leaving only the intake of fruit and vegetables and dietary fiber as potential mediators. Figure 5 illustrates the mediating effect that the intake of fruit and vegetables and dietary fiber at 7 months had on the relationship between group and alpha diversity at 12 months. The control group consumed a mean of 53 g/day more fruit and vegetables than the BLISS group, and this difference explained 29% and 27% of the associations between group and observed species and the Shannon index, respectively. Similarly, the control group consumed a mean of 1.3 g/day more fiber than the BLISS group, explaining 25% of the relationship between group and both observed species and the Shannon index. We also investigated the association of dietary intake at 12 months with alpha diversity; only the intake of breast milk and sweet foods showed a meaningful association, but these variables were not different between the groups and thus were unlikely to explain a meaningful proportion of the differences seen between the groups at 12 months.
FIG 5.
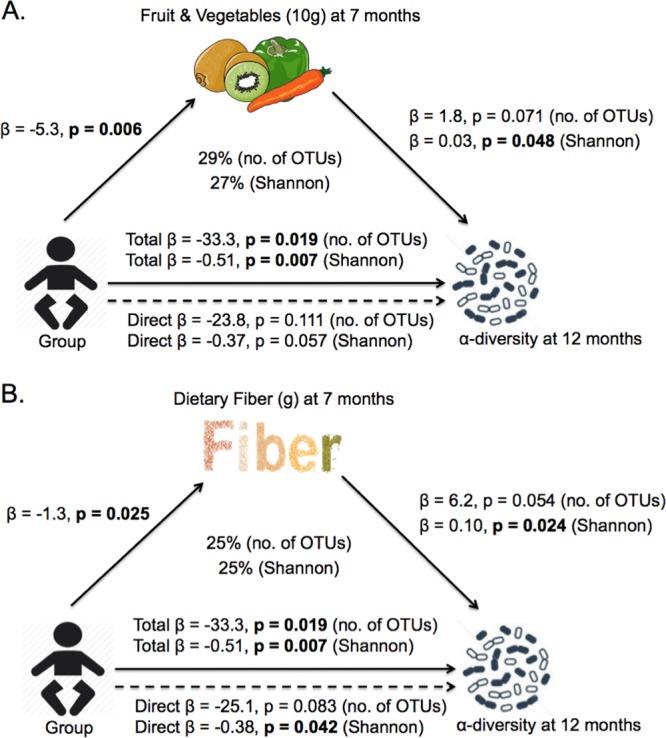
Observed relationship between infant group and alpha diversity (mediation model). (A) Fruit and vegetables (10 g for each). (B) Dietary fiber (for each gram) intake. (n = 63) (see Fig. S1A in the supplemental material for the key).
DISCUSSION
Microbial composition analysis using several alpha diversity measures revealed that infants following BLW had significantly lower alpha diversity at 12 months of age than control infants. Using mediation analysis, a novel approach in microbiota studies, to explore the association between group and alpha diversity, we were able to show that 29% and 25% of the links between group and alpha diversity at 12 months could be explained by lower intake of fruit and vegetables and dietary fiber in the BLISS group at 7 months.
Alpha diversity increased from 7 months to 12 months of age in both groups, as expected due to the introduction of sources of food for the gut microbiota other than milk (13, 25). We also noted decreases in the relative abundances of Bifidobacteriaceae, Enterobacteriaceae, and Veillonellaceae and increases in the relative abundances of Lachnospiraceae and Ruminococcaceae from 7 to 12 months. This is in agreement with data from previous studies (3, 26, 27). The substantial decrease in infant milk intake from 7 to 12 months likely explains the relative decreases in the abundances of Bifidobacteriaceae, which utilize lactose and human milk oligosaccharides that are found in milks (28), and Veillonellaceae, which have species that are able to utilize lactate, a fermentation product of Bifidobacteriaceae (29). The relative increases in the abundances of Lachnospiraceae and Ruminococcaceae at 12 months can be attributed to the introduction of solid foods, as both Lachnospiraceae and Ruminococcaceae have species that are known to play a role in polysaccharide (i.e., “complex carbohydrate”) degradation (30). They form the most abundant bacterial families of the adult fecal microbiota in Western populations.
At 12 months of age, infants in the BLISS group had lower bacterial alpha diversity than those in the control group. This was explained, at least in part, by the differential prevalence of OTUs representing members of the genus Roseburia, a taxon whose abundance in the bowel is known to be associated with the amount of dietary fiber consumed by the human host (31, 32). This observation therefore supported the association between alpha diversity and dietary fiber that was revealed by mediation analysis. The BLISS infants were encouraged to eat the same foods as the rest of the family (i.e., a more adult diet) from the start of complementary feeding, and we expected that this would result in greater alpha diversity because a recent study showed that the progression to family foods was strongly associated with increased alpha diversity (observed species and Shannon index) (19). Our results were sustained even after adjustment for parity, which has been found to be significantly associated with alpha diversity in the present study and elsewhere (33), presumably as a result of exposure to a wider range of bacteria from the range of education and other environments with which siblings interact, nor can these findings be explained by mode of delivery (34), breast milk intake, or recent use of antibiotics (35) which were shown previously to be associated with lower alpha diversity but were not significant predictors of alpha diversity in our study.
To reveal the mechanism by which the BLISS intervention was associated with lower alpha diversity, we conducted mediation analyses. Mediation analysis is increasingly being encouraged in the health literature to elucidate the underlying pathways of effects on health outcomes (36, 37). Mediation analysis enhances causal inference and allows the identification of specific components of an intervention that were effective. Mediation analysis has been used in previous randomized controlled trials, where an intervention that involved changing behaviors (such as a way of eating) was associated with an outcome (such as a difference in food intake) (38, 39). To then understand why the intervention affected the outcome, mediation analysis was used to identify the mediators (such as the use of certain maternal feeding practices or a child's access to noncore food) (36–39). Mediation analysis is therefore well suited to evaluate the specific dietary mechanisms that might explain a difference in the gut microbiota as the result of a complex behavioral intervention, such as in the BLISS study. For mediation to be present, the independent variable (in this case, randomized group) must affect both the outcome (in this case, alpha diversity) and the mediating variable (e.g., food intake), the mediating variable must affect the outcome, and the relationship between the independent variable and the outcome must be reduced in magnitude when the mediating variable is controlled for (40, 41). Temporal ordering should be present. In our study, we found that intakes of fruit and vegetables and dietary fiber were mediators explaining 29% and 25%, respectively, of the links between group and alpha diversity. Even though the intake of breads and cereals was associated with alpha diversity, the control and BLISS groups did not have significantly different intakes of this group, meaning that it was not a mediator of the association between group and alpha diversity. These findings suggest that at least one-quarter of the impact of the BLISS intervention on alpha diversity can be explained by the lower intake of fruit, vegetables, and dietary fiber in the BLISS group at 7 months of age. Dietary fiber has been shown to be positively associated with alpha diversity measures in this age group (19), presumably because the range of polysaccharides that are indigestible to the host (i.e., dietary fiber) provide growth substrates for a range of gut microbes.
The significantly lower intake of fruit and vegetables and of dietary fiber among the BLISS infants is an interesting finding. Although there has been an expectation that following a baby-led approach to infant feeding would result in the consumption of a wider variety of healthy foods (18), concerns have been expressed that the handheld foods eaten by adults are not necessarily “healthier” (42). The infants in the BLISS group were introduced to solids approximately 3 weeks later than the control infants, so at 7 months of age, they were earlier in their complementary feeding journey than controls; perhaps this resulted in them being offered less fruit and vegetables. Moreover, one small pilot study reported that parents who were following BLW with their infant had higher-than-recommended intakes of saturated fat, sodium, and sugar (43), suggesting that if infants were being given the foods that their parents were eating, they may be offered a diet that is less healthy and therefore probably lower in fruit and vegetables. However, a more likely explanation for the lower intake of fruit, vegetables, and dietary fiber is that infants who are being traditionally weaned are usually spoon-fed purées in the early months of complementary feeding, and these are commonly based on fruit and vegetables because they can be blended to a smooth consistency and are often sweet and therefore highly palatable. In fact, in the United Kingdom, about two-thirds of all baby foods have been reported to contain fruit, vegetables, or both (44).
Major strengths of our study were the use of a randomized controlled design that ensured that differences between the groups were due to the intervention rather than differences in participant characteristics and the use of 3-day weighed-diet records to provide detailed high-quality dietary data.
This study shows that infants following BLW consume a more adult-type diet and have a fecal microbiota with a less complex composition at 12 months than infants following traditional spoon-feeding. Lower intakes of fruit and vegetables and of dietary fiber are partially responsible for this lower alpha diversity. The difference in alpha diversity between groups is modest and, at this stage, cannot be related to changes in child development or health. Larger, longer-term studies are required before any conclusions can be made about the possible impact of these differences or whether infant feeding guidelines should recommend that infants following BLW consume more fruit and vegetables or dietary fiber than is currently the case.
MATERIALS AND METHODS
Study design.
The BLISS study was a 2-year randomized controlled human intervention trial (Australian New Zealand Clinical Trials Registry number ACTRN12612001133820) that compared a version of BLW (the infants feed themselves family foods) modified to address concerns about choking, iron deficiency, and growth faltering (42) with a control group (receiving usual care, predominantly traditional spoon-feeding). Because the protocol (21), pilot study (20), and main-outcomes paper (22) have been reported, only relevant information is included here. The Lower South Regional Ethics Committee of New Zealand and the University of Otago Human Ethics Committee approved the study, and written informed consent was obtained from all adult participants before randomization and before collection of the fecal samples for gut microbiota analysis. Fecal samples collected at 7 and 12 months of age and a 3-day weighed-diet record collected at 7 months were used to determine the impact of the BLISS intervention on changes in alpha diversity and the relative abundances of bacterial families in the infant gut microbiota.
Participants.
All women in late pregnancy who were booked into the only maternity hospital in Dunedin, New Zealand, from December 2012 to March 2014 were eligible to participate in the BLISS study if they were at least 16 years old and booked before 34 weeks of gestation. Infants were excluded if they were born preterm or had a congenital abnormality likely to affect feeding or growth. Participants were randomized to the control (n = 106) or BLISS (n = 108) group via random-length blocks (maximum of 7) after stratification for parity (first child or subsequent child) and maternal education (nontertiary or tertiary). Allocation was concealed by using opaque presealed envelopes. After birth, 8 infants were excluded, so the final samples included 101 control and 105 BLISS participants (Fig. 1).
Fecal samples for microbiota analysis were collected from a subset of the infants (Fig. 1). Of the 165 participants in the BLISS study at the time of fecal collection, 86 were 7 months of age or younger, 74 (86%) of whom had parents who agreed to provide a fecal sample for microbiota analysis. There were no significant differences in maternal age, body mass index (BMI), parity, or education; household deprivation; or the sex or birth weight of the child between participants who did (n = 74) and those who did not (n = 132) provide a fecal sample (statistical tests for groups as stated in “Statistical analysis,” below). Therefore, the subset was representative of the BLISS study cohort as a whole.
Intervention.
Both the control group and the BLISS group had access to government-funded routine midwifery and well-child care (45, 46). The BLISS group had eight additional contacts from pregnancy to 9 months of age. These contacts provided education and support to prolong milk feeding (ideally exclusive breastfeeding) and delay the introduction of complementary foods until 6 months of age (provided by an international board-certified lactation consultant) and specific advice at 5.5, 7, and 9 months on how to follow the BLISS approach to infant feeding (provided by trained researchers) (20, 21). The key components of the BLISS approach were to provide foods in a form that the babies can pick up themselves; to let babies feed themselves; to offer an iron-rich food, an energy-rich food, and an easy-to-eat food with every meal; and to eat as a family whenever possible (20, 21). Adherence to a baby-led approach was defined as the infants feeding themselves most or all their food in the previous week (21) and was measured by questionnaire at 6, 7, 8, 9, and 12 months of age.
Demographic data.
Information on infant sex, birth weight, parity, and level of household deprivation (47) was obtained from hospital records. Self-reported prepregnancy height and weight, maternal education attainment, and maternal age were obtained from baseline questionnaires that were completed before randomization in late pregnancy. The age when complementary foods were first introduced was determined prospectively by using data from brief feeding questionnaires administered at 2, 4, 6, 7, 8, 9, and 12 months of age (21).
Gut microbiota data.
Parents were asked to collect a fecal sample from their infant's diaper at 7 and 12 months of age. The sample was then stored in the home freezer (−18°C) in study-provided freezer containers before collection and delivery to the University of Otago Department of Microbiology and Immunology, where they were stored at −80°C until DNA extraction. DNA was extracted from 250 mg feces according to the kit protocol provided by the manufacturer (PowerSoil DNA isolation kit, catalog number 12855-100; Mo Bio). Amplification of the 16S rRNA gene V4 region, library preparation, and sequencing were carried out at Argonne National Laboratories (University of Chicago) using 2- by 250-bp paired-end reads on an Illumina MiSeq instrument. Sequences were analyzed by using a combination of QIIME version 1.9.1 (48) and vsearch version 1.9.5 (49). Taxonomy classifications were made by using the SILVA version 123 database (50).
Microbiota composition was described by using five alpha diversity measures: the number of operational taxonomic units (OTUs) (a proxy for observed species), phylogenetic diversity, the Chao1 estimator, the Simpson index, and the Shannon index. Therefore, three indices described microbial richness alone (i.e., number of species), observed species, phylogenetic diversity, and the Chao1 estimator, and two indices described richness and evenness (i.e., the equality of distribution of the species' frequencies), the Simpson index and Shannon index. The OTU table was rarefied to the minimum sample count (30,000 sequences) for calculation of the alpha diversity measures. Relative abundance at the family level was calculated by collapsing the raw OTU table based on seven-level taxonomy strings obtained from the SILVA version 123 database.
Beta diversity metrics (Bray-Curtis index, unweighted UniFrac, weighted UniFrac, and Jaccard distance) were applied by using the QIIME2 (https://qiime2.org/) command line interface (v.2018.4) and the core-metrics-phylogenetic plug-in with a sampling depth of 30,000 sequences. Group significance for each metric was measured with PERMANOVA (51).
Differential abundance testing was carried out on the full OTU table and on OTU tables that were filtered to contain data from either the 7-month samples or the 12-month samples in order to concentrate on differences between the dietary groups at distinct time points. ANCOM (52) and GNEISS (53) tests were run through the QIIME2 command line interface using default parameters. More specifically, the diet metadata group was analyzed for ANCOM, and for GNEISS analysis, “Diet+Time” was used for modeling ordinary least-squares regression (OLS-regression) on the full OTU table.
Differential abundance was also tested by using MaAsLin v.0.0.5 (54) using OTU tables filtered to 7-month and 12-month time points and on the full OTU table with “time” included as a forced predictor.
Dietary data.
Parents completed a 3-day weighed-diet record on 3 randomly assigned nonconsecutive days (1 weekend day and 2 weekdays) over 3 weeks. Parents were taught how to complete the weighed-diet record by an interviewer, given an opportunity to practice weighing a food with the interviewer's support, given detailed written instructions and examples to take home with them, and instructed to contact the researchers if they had any questions while completing the record. Breast milk intake was estimated by using total daily volumes of 750 g/day at 7 months of age and 448 g/day at 12 months (55). For infants who were fed both breast milk and infant formula, the amount of infant formula consumed was subtracted from 750 g/day at 7 months and 448 g/day at 12 months, with the remainder being entered as breast milk. Diet records were analyzed by using the Kai-culator program (version 1.13s; University of Otago), which uses data from the New Zealand Food Composition Database (FOODfiles 2010), nutrient data for commonly consumed recipes from national nutrition survey data, and nutrient data for commercial infant foods collected by the research team (21).
Nine food groups were defined based on food groups from the New Zealand Ministry of Health Food and Nutrition Guidelines for Healthy Infants and Toddlers (1) and the number of consumers (i.e., at least eight consumers were required in each food group so that there was sufficient power to perform the food group analyses): “breast milk,” “infant formula,” “dairy,” “fruit and vegetables,” “breads and cereals,” “meat, fish, and poultry,” “sweet food,” “savory food,” and “miscellaneous.” Ingredients in recipes were coded into their specific food groups instead of the whole recipe being assigned to a diverse “mixed dishes” category.
Statistical analysis.
Data were analyzed by using Stata software (version 13; StataCorp). To compare antibiotic use, feeding behaviors, and infant formula consumption at 7 months of age between randomized groups, a chi-squared test was used. A log rank test (i.e., time to events) was used to compare the ages of introduction of formula, solids, and infant cereal (Table 2). To determine differences in alpha diversity between groups at both 7 and 12 months, linear regression was used, with adjustment for parity and maternal education (i.e., the stratification variables). Mean differences, 95% confidence intervals (CI), and P values were calculated along with the proportion of variance explained (R2) by the independent variables (Fig. 2 and Table 3). Subsequent analyses for alpha diversity were then limited to one measure that described richness only and one measure that described richness and evenness. The two measures (number of OTUs and Shannon index) were chosen because they had the highest R2 values (i.e., they explained the highest proportion of the variance) and had the strongest association with randomized group (Table 3).
Group and age differences with respect to the relative abundances of bacterial families for which the median relative abundance was >1% were determined by using median regression (Fig. 3; see also Tables S1 and S2 in the supplemental material).
Linear regression was also used to assess whether demographic and feeding variables and antibiotic use in the week before fecal sampling were related to alpha diversity, with adjustment for randomized group (Table 6). Thereafter, the factor that was found to be significantly associated with alpha diversity was considered a confounder and adjusted for in subsequent analyses. To explore whether dietary components at 7 months of age predicted alpha diversity at 12 months, regression models were generated and adjusted for randomized group, parity (confounder from Table 5), and maternal education (Table 5).
Mediation analysis was then used to determine the extent to which the association between randomized group and alpha diversity was due to differences in food group intake; i.e., food group intake “mediated” the association (Fig. S1). The requirements for mediation were considered to exist (36) if randomized group predicted alpha diversity at 12 months of age, and food group intake at 7 months was both related to alpha diversity at 12 months and different between the randomized groups. The “proportion mediated” was the proportion of the effect size without the mediator (“total effect”) that was reduced when the mediator was included in the regression (“direct effect”). Decisions and explanations for mediation analysis were based on both effect sizes and P values. Residuals for all linear regression models were plotted and visually assessed for homogeneity of variance and normality.
Accession number(s).
The DNA sequence data have been deposited in the NCBI database under BioProject accession number PRJNA419227.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the members of the BLISS study team who contributed to data collection. We also thank the BLISS study participants.
We thank Lottery Health Research, Meat & Livestock Australia, the Karitane Products Society, Perpetual Trustees, The New Zealand Women's Institute, and the University of Otago, with “in kind” contributions from Heinz Watties Ltd. C.L. is supported by a University of Otago doctoral scholarship, and R.W.T. is supported by a fellowship from the Karitane Products Society that is paid via the University of Otago. The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
We declare that there is no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00914-18.
REFERENCES
- 1.Ministry of Health. 2008. Food and nutrition guidelines for healthy infants and toddlers (aged 0-2): a background paper. Ministry of Health, Wellington, New Zealand. [Google Scholar]
- 2.Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, Sears MR, Becker AB, Scott JA, Kozyrskyj AL. 2013. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ 185:358–394. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al-Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J, Wang J. 2015. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, Lieber A, Wu F, Perez-Perez GI, Chen Y, Schweizer W, Zheng X, Contreras M, Dominguez-Bello MG, Blaser MJ. 2016. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med 8:343ra82. doi: 10.1126/scitranslmed.aad7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bezirtzoglou E, Tsiotsias A, Welling GW. 2011. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe 17:478–482. doi: 10.1016/j.anaerobe.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Tannock GW, Lawley B, Munro K, Gowri Pathmanathan S, Zhou SJ, Makrides M, Gibson RA, Sullivan T, Prosser CG, Lowry D, Hodgkinson AJ. 2013. Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk-based formula, or breast milk. Appl Environ Microbiol 79:3040–3048. doi: 10.1128/AEM.03910-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. 2006. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 8.Jost T, Lacroix C, Braegger C, Chassard C. 2015. Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr Rev 73:426–437. doi: 10.1093/nutrit/nuu016. [DOI] [PubMed] [Google Scholar]
- 9.Laursen MF, Bahl MI, Michaelsen KF, Licht TR. 2017. First foods and gut microbes. Front Microbiol 8:356. doi: 10.3389/fmicb.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallani M, Amarri S, Uusijarvi A, Adam R, Khanna S, Aguilera M, Gil A, Vieites JM, Norin E, Young D, Scott JA, Dore J, Edwards CA. 2011. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 157:1385–1392. doi: 10.1099/mic.0.042143-0. [DOI] [PubMed] [Google Scholar]
- 11.Bergstrom A, Skov TH, Bahl MI, Roager HM, Christensen LB, Ejlerskov KT, Molgaard C, Michaelsen KF, Licht TR. 2014. Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Appl Environ Microbiol 80:2889–2900. doi: 10.1128/AEM.00342-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qasem W, Azad MB, Hossain Z, Azad E, Jorgensen S, Castillo San Juan S, Cai C, Khafipour E, Beta T, Roberts LJ, Friel J. 2017. Assessment of complementary feeding of Canadian infants: effects on microbiome & oxidative stress, a randomized controlled trial. BMC Pediatr 17:54. doi: 10.1186/s12887-017-0805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krebs NF, Sherlock LG, Westcott J, Culbertson D, Hambidge KM, Feazel LM, Robertson CE, Frank DN. 2013. Effects of different complementary feeding regimens on iron status and enteric microbiota in breastfed infants. J Pediatr 163:416.e4–423.e4. doi: 10.1016/j.jpeds.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morison BJ, Taylor RW, Haszard JJ, Schramm CJ, Williams Erickson L, Fangupo LJ, Fleming EA, Luciano A, Heath A-LM. 2016. How different are baby-led weaning and conventional complementary feeding? A cross-sectional study of infants aged 6-8 months. BMJ Open 6:e010665. doi: 10.1136/bmjopen-2015-010665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown A, Lee M. 2011. A descriptive study investigating the use and nature of baby-led weaning in a UK sample of mothers. Matern Child Nutr 7:34–47. doi: 10.1111/j.1740-8709.2010.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beal JA. 2016. Baby-led weaning. MCN Am J Matern Child Nurs 41:373. doi: 10.1097/NMC.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 17.D'Andrea E, Jenkins K, Mathews M, Roebothan B. 2016. Baby-led weaning: a preliminary investigation. Can J Diet Pract Res 77:72–77. doi: 10.3148/cjdpr-2015-045. [DOI] [PubMed] [Google Scholar]
- 18.Rapley G, Murkett T. 2008. Baby-led weaning: helping your baby love good food. Random House, London, United Kingdom. [Google Scholar]
- 19.Laursen MF, Andersen LBB, Michaelsen KF, Mølgaard C, Trolle E, Bahl MI, Licht TR. 2016. Infant gut microbiota development is driven by transition to family foods independent of maternal obesity. mSphere 1:e00069-15. doi: 10.1128/mSphere.00069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cameron SL, Taylor RW, Heath A-LM. 2015. Development and pilot testing of Baby-Led Introduction to SolidS—a version of baby-led weaning modified to address concerns about iron deficiency, growth faltering and choking. BMC Pediatr 15:99. doi: 10.1186/s12887-015-0422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniels L, Heath A-LM, Williams SM, Cameron SL, Fleming EA, Taylor BJ, Wheeler BJ, Gibson RS, Taylor RW. 2015. Baby-Led Introduction to SolidS (BLISS) study: a randomised controlled trial of a baby-led approach to complementary feeding. BMC Pediatr 15:179. doi: 10.1186/s12887-015-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor RW, Williams SM, Fangupo LJ, Wheeler BJ, Taylor BJ, Daniels L, Fleming EA, McArthur J, Morison B, Erickson LW, Davies RS, Bacchus S, Cameron SL, Heath AM. 2017. Effect of a baby-led approach to complementary feeding on infant growth and overweight: a randomized clinical trial. JAMA Pediatr 171:838–846. doi: 10.1001/jamapediatrics.2017.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fangupo LJ, Heath A-LM, Williams SM, Erickson Williams LW, Morison BJ, Fleming EA, Taylor BJ, Wheeler BJ, Taylor RW. 2016. A baby-led approach to eating solids and risk of choking. Pediatrics 138:e20160772. doi: 10.1542/peds.2016-0772. [DOI] [PubMed] [Google Scholar]
- 24.Daniels L, Taylor RW, Williams SM, Gibson RS, Fleming EA, Wheeler BJ, Taylor BJ, Haszard JJ, Heath A-LM. 2018. Impact of a modified version of baby-led weaning on iron intake and status: a randomised controlled trial. BMJ Open 8:e019036. doi: 10.1136/bmjopen-2017-019036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson AL, Monteagudo-Mera A, Cadenas MB, Lampl ML, Azcarate-Peril MA. 2015. Milk- and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front Cell Infect Microbiol 5:3. doi: 10.3389/fcimb.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avershina E, Storrø O, [slash]Oien T, Johnsen R, Pope P, Rudi K. 2014. Major faecal microbiota shifts in composition and diversity with age in a geographically restricted cohort of mothers and their children. FEMS Microbiol Ecol 87:280–290. doi: 10.1111/1574-6941.12223. [DOI] [PubMed] [Google Scholar]
- 27.Vallès Y, Artacho A, Pascual-García A, Ferrús ML, Gosalbes MJ, Abellán JJ, Francino MP. 2014. Microbial succession in the gut: directional trends of taxonomic and functional change in a birth cohort of Spanish infants. PLoS Genet 10:e1004406. doi: 10.1371/journal.pgen.1004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sela DA, Mills DA. 2010. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol 18:298–307. doi: 10.1016/j.tim.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shetty SA, Marathe NP, Lanjekar V, Ranade D, Shouche YS. 2013. Comparative genome analysis of Megasphaera sp. reveals niche specialization and its potential role in the human gut. PLoS One 8:e79353. doi: 10.1371/journal.pone.0079353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. 2012. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3:289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott KP, Duncan SH, Flint HJ. 2008. Dietary fibre and the gut microbiota. Br Nutr Found Nutr Bull 33:201–211. doi: 10.1111/j.1467-3010.2008.00706.x. [DOI] [Google Scholar]
- 32.Mirande C, Kadlecikova E, Matulova M, Capek P, Bernalier-Donadille A, Forano E, Bera-Maillet C. 2010. Dietary fibre degradation and fermentation by two xylanolytic bacteria Bacteroides xylanisolvens XB1AT and Roseburia intestinalis XB6B4 from the human intestine. J Appl Microbiol 109:451–460. doi: 10.1111/j.1365-2672.2010.04671.x. [DOI] [PubMed] [Google Scholar]
- 33.Laursen MF, Zachariassen G, Bahl MI, Bergström A, Høst A, Michaelsen KF, Licht TR. 2015. Having older siblings is associated with gut microbiota development during early childhood. BMC Microbiol 15:154. doi: 10.1186/s12866-015-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, Björkstén B, Engstrand L, Andersson AF. 2014. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut 63:559–566. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 35.Langdon A, Crook N, Dantas G. 2016. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med 8:39. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fairchild AJ, McDaniel HL. 2017. Best (but oft-forgotten) practices: mediation analysis. Am J Clin Nutr 105:1259–1271. doi: 10.3945/ajcn.117.152546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lockwood CM, DeFrancesco CA, Elliot DL, Beresford SAA, Toobert DJ. 2010. Mediation analyses: applications in nutrition research and reading the literature. J Am Diet Assoc 110:753–762. doi: 10.1016/j.jada.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fletcher A, Wolfenden L, Wyse R, Bowman J, McElduff P, Duncan S. 2013. A randomised controlled trial and mediation analysis of the ‘Healthy Habits’, telephone-based dietary intervention for preschool children. Int J Behav Nutr Phys Act 10:43. doi: 10.1186/1479-5868-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spence AC, Campbell KJ, Crawford DA, McNaughton SA, Hesketh KD. 2014. Mediators of improved child diet quality following a health promotion intervention: the Melbourne InFANT Program. Int J Behav Nutr Phys Act 11:137. doi: 10.1186/s12966-014-0137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baron RM, Kenny DA. 1986. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51:1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 41.Fairchild AJ, MacKinnon DP. 2009. A general model for testing mediation and moderation effects. Prev Sci 10:87–99. doi: 10.1007/s11121-008-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cameron SL, Heath A-LM, Taylor RW. 2012. Healthcare professionals' and mothers' knowledge of, attitudes to and experiences with, baby-led weaning: a content analysis study. BMJ Open 2:e001542. doi: 10.1136/bmjopen-2012-001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowan H, Harris C. 2012. Baby-led weaning and the family diet. A pilot study. Appetite 58:1046–1049. doi: 10.1016/j.appet.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 44.Garcia AL, McLean K, Wright CM. 2016. Types of fruits and vegetables used in commercial baby foods and their contribution to sugar content. Matern Child Nutr 12:838–847. doi: 10.1111/mcn.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.New Zealand College of Midwives. 2017. About lead maternity carer (LMC) services. New Zealand College of Midwives, Christchurch, New Zealand: https://www.midwife.org.nz/women-in-new-zealand/about-lead-maternity-carer-lmc-services. [Google Scholar]
- 46.Ministry of Health. 2013. Well child/Tamariki Ora visits. Ministry of Health, Wellington, New Zealand: http://www.health.govt.nz/your-health/pregnancy-and-kids/services-and-support-you-and-your-child/well-child-tamariki-ora-visits. [Google Scholar]
- 47.Atkinson J, Salmond P, Crampton C. 2014. NZDep2013 index of deprivation. University of Otago, Wellington, New Zealand. [Google Scholar]
- 48.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. 2016. VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and Web-based tools. Nucleic Acids Res 41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x. [DOI] [Google Scholar]
- 52.Mandal S, Van Treuren W, White RA, Eggesbø M, Knight R, Peddada SD. 2015. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Health Dis 26:27663. doi: 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morton JT, Sanders J, Quinn RA, McDonald D, Gonzalez A, Vázquez-Baeza Y, Navas-Molina JA, Song SJ, Metcalf JL, Hyde ER, Lladser M, Dorrestein PC, Knight R. 2017. Balance trees reveal microbial niche differentiation. mSystems 2:e00162-16. doi: 10.1128/mSystems.00162-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C. 2012. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dewey KG, Heinig MJ, Nommsen LA, Lonnerdal B. 1991. Adequacy of energy intake among breast-fed infants in the DARLING study: relationships to growth velocity, morbidity, and activity levels: Davis Area Research on Lactation, Infant Nutrition and Growth. J Pediatr 119:538–547. doi: 10.1016/S0022-3476(05)82401-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.