Abstract
Glutaric aciduria type 1 (GA1) is an autosomal recessive rare disorder caused by mutations in the GCDH gene resulting in deficiency of glutaryl-CoA dehydrogenase, leading to accumulation of the amino acids lysine, hydroxylysine and tryptophan and other metabolites. The phenotypic spectrum of disease is broad. Stress caused by infection and fever and possibly pregnancy may lead to worsening of the signs and symptoms, often with uncertain recovery.
We describe a case of a female patient with GA1 who had two clinically uneventful pregnancies.
At the age of 11 she was diagnosed with GA1 by family screening. The cultured skin fibroblast showed reduced glutaryl-CoA dehydrogenase activity (0.16 mg protein per min).
The initial diagnostic urine glutaric acid level for this patient was 1,784 μmol/mmol creatinine. Mutation analysis showed compound heterozygosity for the p.(Gly185Arg), c.553G>A in exon 7 and p.(Arg402Trp), c.1204C.T in exon 11 mutations of the GCDH.
Her pregnancy at the age of 23 was complicated by pre-eclampsia and required treatment with beta-blockers. Four years later the second pregnancy was uncomplicated. The management plan during the caesarean section included intravenous dextrose and lipid infusions. The patient rapidly recovered from both surgeries.
Both babies have had normal development to date. On newborn screening, plasma acylcarnitine showed a transient increase in glutarylcarnitine, and the urine organic acid analysis showed a trace of 3-hydroxyglutarylcarnitine, likely to be of maternal transfer.
The multidisciplinary team, consisting of metabolic, dietetic and obstetric care providers, have responsibility to ensure the risk of acute decompensation in pregnant GA1 women is minimal.
Keywords: Glutaric aciduria type 1, Maternal transfer, Pregnancy
Introduction
Glutaric aciduria type 1 (GA1) (OMIM 231670) is an autosomal recessive rare disorder caused by mutations in the GCDH gene resulting in deficiency of glutaryl-CoA dehydrogenase. Glutaryl-CoA dehydrogenase is a mitochondrial, homotetrameric, FAD-dependent mitochondrial matrix enzyme which catalyses the oxidative decarboxylation of glutaryl-CoA to crotonyl-CoA in the final common catabolic pathways of L-lysine, L-hydroxylysine and L-tryptophan (Goodman et al. 1975; Zschocke et al. 2000; Treacy et al. 2003; Kolker et al. 2006). Deficiency of the glutaryl-CoA dehydrogenase enzyme leads to accumulation of the amino acids lysine, hydroxylysine and tryptophan and the organic acid metabolites such as glutaric acid, 3-hydroxyglutaric acid, glutaconic acid and glutaryl carnitine (Boy et al. 2017a).
In the absence of detection or neonatal screening and subsequent prevention of metabolic decompensation, 80–90% of patients with GA1 develop striatal injury following intoxication with catabolic stress or intermittent illness during the first 6 years of life and consequently a predominantly dystonic movement disorder (‘acute-onset type’) (Boy et al. 2017b). The ‘late-onset’ type and insidious types are also well described, with ‘late-onset’ types diagnosed after the age of 6 years when present with non-specific and long-term symptoms associated with white matter brain MRI changes. The late-onset cases represent a smaller proportion of patients (Kolker et al. 2006; Kolker 2016; Boy et al. 2017b).
The phenotypic spectrum of disease is broad with some individuals being only mildly affected, while others have severe problems from infancy or early childhood (Boy et al. 2017b) which relates to residual enzymatic activity and also environmental risk factors. Some patients with GA1 remain asymptomatic, even in their adulthood (Kolker et al. 2006). Moreover, several maternal GA1 cases have been diagnosed based on the findings in their offspring (Crombez et al. 2008; Garcia et al. 2008; Janssen et al. 2014; Boy et al. 2017b). Often the only complaint these patients present is chronic fatigue (Janssen et al. 2014; Haworth et al. 1991; Prevett et al. 1996), and provided that they avoid severe illness, these individuals are unlikely to decompensate metabolically.
The progression of the neurological damage can be curbed by a lysine-restricted diet, carnitine supplementation and emergent intervention during an intermittent illness (Boy et al. 2017a; Mosaeilhy et al. 2017; Kolker et al. 2011).
Stress caused by infection and fever and possibly also pregnancy may lead to worsening of the signs and symptoms, often with uncertain recovery.
We describe a case of a female patient with GA1 who had two clinically uneventful pregnancies. The focus of this paper is to outline the management of her condition in the peripartum period.
Case
A 28-year-old female was diagnosed with GA1 on family screening at the age of 11. Her younger sister was identified as having GA1 during the first year of life with an acute episode of dystonia with subsequent dystonic cerebral palsy following a febrile illness. She subsequently died at the age of 3 years during an episode of metabolic decompensation. The diagnosis was confirmed in both cases, based on reduced glutaryl-CoA dehydrogenase activity (0.16 mg protein per min) in cultured fibroblasts.
The initial urine glutarate level in the affected sibling who died was 963 μmol/mmol creatinine (<2) at the age of 2 years. The initial diagnostic urine glutaric acid level for this patient was 1,784 μmol/mmol creatinine at age of 11 years. Mutation analysis showed compound heterozygosity for the p.(Gly185Arg), c.553G>A in exon 7 and p.(Arg402Trp), c.1204C.T in exon 11 mutations of the GCDH gene known to be associated with a decrease in enzyme activity (Biery et al. 1996; Christensen et al. 2004).
Although asymptomatic at the time, following her diagnosis, our current patient was commenced on a dietary management plan for GA1, with restriction of natural protein (tryptophan and lysine; 0.3 g/kg (15–22 g of natural protein/day)). Additionally she was placed on a synthetic protein substitute (to meet her daily protein and micronutrient requirements). Dietary compliance was an ongoing issue throughout late childhood into adulthood, as reflected by fluctuation and inconsistency in her level of urinary glutarates.
Past medical history was unremarkable, apart from asthma treated with inhalers and psoriasis. She remained active in her profession as a care assistant. Her medications included carnitine 1,500 mg twice daily, essential fatty acid supplements and prescribed amino acid synthetic protein supplements.
A baseline brain MRI (performed at age 13 years) showed mild dilatation of lateral ventricles, widening of the Sylvian fissures and bilateral enlargement of the fluid space anteriorly to the temporal poles that remained stable over the next 4 years. Neurological examination was normal.
Pregnancy 1
At the age of 23 a pregnancy was achieved. Dietary requirements were assessed and dietary intake adjusted throughout pregnancy to facilitate appropriate weight gain (ACOG 2013) and meet the recommended nutritional requirements for pregnancy. A detailed dietetic assessment was completed during four outpatient clinic visits from 4 to 33 weeks of gestation, with increases in weight (65–76 kg), natural protein (16–24 g/day), synthetic protein (60–68 g/day) and kcal (1,900–2,280 kcal/day) recorded (Table 1). The prescribed synthetic protein supplement was changed at week 13 of her gestation due to difficulties tolerating the original product prescribed. There were also challenges in the patient adhering to the dosage recommended at various time points throughout the pregnancy, noted post medical and dietetic review, due to ongoing nausea-related pregnancy sickness.
Table 1.
The summary of outcomes of pregnancies in GA1
| Pregnancy 1 | Pregnancy 2 | |
|---|---|---|
| Diet/supplements pre-pregnancy | Carnitine 1,500 mg bd DHA supplements: KeyOmega™ × 1 sachet daily (100 mg DHA, 200 mg AA) Synthetic amino acid protein supplements (GA Express 15™ qds) |
Carnitine 1,500 mg bd DHA supplements: KeyOmega™ × 1 sachet daily (100 mg DHA, 200 mg AA) Synthetic amino acid protein supplements (GA Express 15™ qds) |
| Diet/supplements in pregnancy | Carnitine 1,500 mg bd DHA supplements: KeyOmega™ × 1 sachet daily (100 mg DHA, 200 mg AA) |
Carnitine 1,500 mg bd DHA supplements: KeyOmega™ × 1 sachet daily (100 mg DHA, 200 mg AA) + folic acid 400 μg daily |
| Synthetic amino acid protein supplements (GA Express 15™ qds) Changed XLysLowTry Maxamum® × 140 g (54.6 g synthetic protein) from 13/40 weeks due to problems tolerating GA Express 15 and increased 175 g (68.3 g synthetic protein) from ~28/40 weeks of gestation |
Synthetic amino acid protein supplements (GA Express™ 15 qds) Dose increased to provide 70 g synthetic protein from 16/40 week of gestation; patient self-reverted back lower dose ~28/40 and increased back to 70 g synthetic protein daily from 33.5/40 weeks of gestation |
|
| Energy supplements: Lucozade® (72 kcal/100 mL) Protein free recipe using Maxijul® and Calogen® (150 kcal/100 mL) |
Energy supplements: Duocal® powder added to GA Express 15™ to augment energy (154 kcal/100 mL) SOS25™ powder added to a sugar-based beverage (110 kcal/100 mL) |
|
| Weight gain in pregnancy (kg) | 11 | 10 |
| Delivery management | IV Dextrose 10% Oral diet: Reduced half natural protein (12 g of natural protein), augmented synthetic protein (68.3 g) Augmented kcal, >2,280 kcal/day |
IV Dextrose 10% Oral diet: 2 days pre-delivery: reduced half natural protein (13 g of natural protein), augmented synthetic protein (75 g) Augmented kcal, >2,400 kcal/day Date of delivery: 0 g of natural proteins, augmented synthetic protein (75 g) Augmented kcal >2,400 kcal/day Day 1 and 2 post-delivery: Half natural protein (13 g of natural protein), augmented synthetic protein (75 g) augmented kcal >2,400 kcal/day Day 3 post-delivery: Return to usual diet, 20 g of natural protein + 60 g synthetic protein + well kcal |
| Metabolic decompensation (Y/N) | N | N |
| Post-partum management | Carnitine 6 g for 2–3 days Sick day plan for 4–5 days |
Carnitine 6 g for 2–3 days Sick day plan for 4–5 days |
| Child | Boy | Boy |
| Birth weight | 3,615 g born at 38/40 weeks | 4,470 g born at 38 + 6 weeks |
| Head circumference (cm) | 33.5 | 36 |
| Apgar scores | Apgar 9 at 1 min and 10 at 5 min | Apgar 9 at 1 min and 10 at 5 min |
| Clinical outcome | • Urine organic acids showed slight increase of 3-hydroxyglutarylcarnitine (0.16; 0.0–0.16) on day 1 of life • Plasma acylcarnitines showed glutarylcarnitine (C5DC) of 1.54 μmol/L (0.0–0.16). His plasma-free carnitine was 20.6 μmol/L (4.5–40.4 μmol/L) • Repeat testing at day 4 of life revealed no abnormalities, and it was concluded that the abnormal metabolites was most likely due to maternal transfer |
• Urine organic acid analysis a trace of 3-hydroxyglutarylcarnitine • Acylcarnitine profile showed no increase in glutarylcarnitine (C5DC) at the age of 2 and 4 days, 5 weeks and 4 months of life and free carnitine was not deficient • Repeat urine organic acids showed detectable 3-hydroxyglutarate with no increase in glutarate at day 2 of life. The metabolite subsequently cleared on day 4 of life |
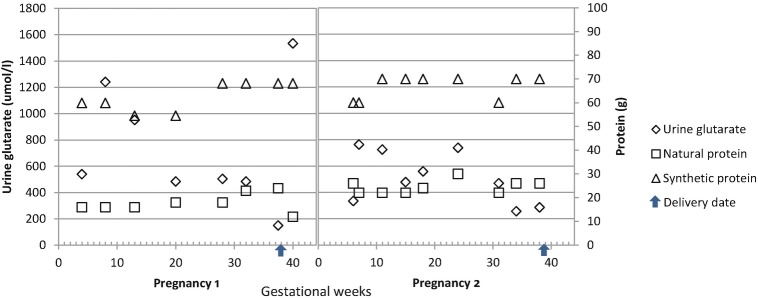
This pregnancy was also complicated by pre-eclampsia with hypertension (the highest BP 160/100 mmHg) and proteinuria (protein+) in the third trimester. Her headache and complaints of flashing lights, resolved with labetalol 200 mg twice-daily treatment in the last 6 weeks of her pregnancy. Foetal ultrasound was normal. Her plasma lysine and urine glutarate were monitored throughout the pregnancy and were well controlled (Fig. 1).
Fig. 1.
Urine glutarate and protein consumption in two pregnancies
During labour she fasted for 12 h before emergency caesarean section for failed induction of labour (on prostaglandin analogue (Carboprost) 250 μg and oxytocin 5 IU) and received intravenous dextrose 10% in the perioperative period. She required spinal anaesthesia prior to the procedure: morphine 150 μg, hyperbaric bupivacaine 12 mg and fentanyl 20 μg. Dexamethasone at the dose of 8 mg was administered to stimulate the foetal lung stimulation. Ondansetron 4 mg was administered for postoperative nausea and vomiting prophylaxis. Thereafter, she was able to take food orally and mobilise. Postoperative course was complicated by a post-partum haemorrhage for which she received 2 units of red cells.
The patient was advised to start her ‘unwell day’ plan post-delivery and continued on this for 5 days with phone support and assessment from a metabolically trained dietitian. This involved reducing natural protein intake by half (12 g of natural proteins), continuing augmented synthetic protein (68 g protein) and ensuring higher kcal >2,300 kcal daily. Additional energy was achieved using a combination of a carbohydrate beverage Lucozade® (72 kcal/100 mL) and a protein free liquid recipe using Maxijul® and Calogen® mixed with water and flavoured (150 kcal/100 mL). She continued on carnitine supplements (3 g) throughout the pregnancy, and it was increased to 6 g over 3 days after the delivery. Body weight increased by 12 kg between week 4 and 32 of her gestation.
Two months post-partum, her neurological examination showed normal gait, and the Romberg test was negative. Her reflexes were slightly reduced; right knee jerk was present with reinforcement. Sensations were normal throughout with plantar reflexes bilaterally. Pre-pregnancy BMI was 26.9 kg/m2, and the patient returned close to her pre-pregnancy weight (BMI 27.9 kg/m2). Of note, although this patient had initially talked about breast feeding, she decided to bottle-feed her baby post caesarean section.
After the first pregnancy she complained of fatigue, headache, brief episodes of ataxia and vertigo. However, there was no complaint of paresthesia, numbness, muscle spasm and dystonic posturing, cognitive deficits or falls.
Pregnancy 2
Four years later, she reported a second planned pregnancy. Additional folic acid, 400 μg, and 200 mg decosahexaenoic acid (DHA) were advised daily due to revised recommendations (RCPI 2013). A detailed dietetic assessment was completed during five outpatient clinic visits from 4 to 36 weeks of gestation, with increases in weight (69–79 kg), natural protein (20–25 g/day), synthetic protein (6,068 g/day) and kcal (1,900–2,500 kcal/day) recorded (Table 1). She developed irritable bowel syndrome-type symptoms, including heartburn and disordered eating in the early stages of this pregnancy associated with anxiety. This impacted compliance with advice to increase her synthetic protein intake at week 6 of gestation; with intermittent adherence until the later stages of pregnancy. Her general appetite was reduced throughout, and the frequency of gastrointestinal symptoms increased during the third trimester. Despite these symptoms she gained 10 kg weight from 4 to 36 weeks. At week 26 of gestation, her blood pressure was raised (153/74 mmHg) and protein-to-creatinine ratio was 12.77 mg/mmol (normal <30), treated conservatively. Foetal ultrasound was normal. Immediately following delivery, cord blood pH analyses were normal as expected for an elective caesarean section.
Following on from the Metabolic Physicians advice, she had a planned caesarean section at week 39 of her gestation, however, due to ruptured membranes prior to date she had emergency caesarean section at 38 + 4 weeks. She received 5 IU oxytocin to stimulate her labour and dexamethasone 4 mg to promote foetal lung maturation. Spinal anaesthesia was performed for her caesarean delivery using hyperbaric bupivacaine 12 mg, fentanyl 20 μg and preservative-free morphine 150 μg. A prophylactic phenylephrine infusion at a starting dose of 1,388 μg/h was administered with Hartmann’s solution 1,000 mL. Ondansetron 4 mg was administered for postoperative nausea and vomiting prophylaxis.
Four weeks before the labour, her dietary regimen included 26 g of natural proteins and a protein substitute, free from lysine and low in tryptophan, providing 70 g synthetic protein spread evenly throughout the day. L-carnitine was prescribed at a dose of 100 mg/kg/day, and she was also prescribed a DHA supplement, aiming for 2,500 kcal daily.
The diet was changed 2 days prior to emergency caesarean section to provide half of her normal natural protein (13 g) and increased formula intake with the provision of an ‘unwell day’ plan aiming for higher energy intake (>2,400 kcal daily) and augmented synthetic protein.
This involved the use of a protein-free energy supplement, added to her protein substitute daily (75 g protein, 154 kcal/100 mL) in combination with a glucose polymer, added to a sugar-based carbohydrate beverage Lucozade® for augmented kcal (110 kcal/100 mL). No natural proteins were given on the day of surgery. Perinatal plan included intravenous 10% dextrose (with electrolyte supplements 4 h before the caesarean section) and continued into the peripartum period until normal oral intake was established. Intralipid 20% infusion, to provide additional calories, was considered in the emergency plan, but ultimately not required as oral intake using the ‘unwell feed’ recipes in accordance with her updated diet plan were adequate.
Four days post-delivery, the patient was advised to return to her pre-pregnancy diet: 20 g natural protein +60 g synthetic protein and aiming for 1,800–2,000 kcal daily. Pre-pregnancy BMI was 27.6 kg/m2 and had returned to 29.2 kg/m2 6 months post-delivery. Of note, again on this occasion the patient decided to bottle-feed her baby post-caesarean section. She required analgesic agent (mefenamic acid) and antihistamine diphenhydramine after the surgery.
Discussion
We describe a case of an adult patient with a clinically mild form of GA1 who had two uneventful pregnancies. To the best of our knowledge, this is the second case report describing the management of this metabolic condition during delivery by caesarean section.
Interestingly, most women with GA1 with a documented pregnancy were diagnosed retrospectively following a positive newborn screening test in their babies. The elevated metabolites detected had crossed the placenta from maternal blood (Crombez et al. 2008). The reported cases were mainly asymptomatic or showed only mild neurologic symptoms. They were not treated and did not develop metabolic complications during pregnancy, delivery or the puerperium. Two children born to women with maternal GA1 showed transient delay of motor development (Garcia et al. 2008; Kolker 2016).
The Effect of GA1 on Pregnancy
The effect of pregnancy on GA1 remains unclear. The physiological changes of pregnancy and the catabolism associated with labour and delivery may impose a risk of a neurological ‘crisis’. Therefore, in addition to achieving optimum control during the pregnancy, we consider that good control should be emphasised during preconception. The multidisciplinary team, consisting of metabolic, dietetic and obstetrics’ care providers, has responsibility to ensure the risk of acute decompensation is minimal. Poor hydration and weight loss (which may occur due to hyperemesis, especially in the first trimester of pregnancy), prolonged preoperative fasting and restricted fluid intake in labour may trigger the metabolic crisis.
The main goals of management during labour and delivery are to minimise catabolic stress by providing effective analgesia and adequate hydration and ensuring adequate caloric intake and maintenance of acid-base status prior to and throughout the labour (Ituk et al. 2013). In our case the management plan included 10% dextrose and 20% intralipid, as caloric sources. The patient rapidly recovered from both caesarean section surgeries and resumed her normal feeding the day after the procedure. In addition, transient reduction or omission of natural protein for up to 48 h to reduce glutarate and 3-hydroxyglutarate production, as well as L-carnitine supplementation, prevented secondary carnitine depletion and enabled physiological clearance of glutaryl-CoA by conjugation with carnitine (Kolker et al. 2011).
It has been postulated that accumulating 3-hydroxyglutaric acid crosses the blood-brain barrier during infection or stress and acts as a toxin for the respiratory chain in the brain. 3-Hydroxyglutaric acid and glutaric acid have been shown to be weak neurotoxins and cause neuronal damage by inducing excitotoxic pathways and oxidative stress in neurons (Jafari et al. 2011); fronto-temporal degeneration and chronic subdural hematomas have also been described (Muhlhausen et al. 2004).
Formation of nontoxic glutarylcarnitine by conjugation of glutaryl-CoA with carnitine is considered a physiological detoxification mechanism of glutaryl excretion and regenerates intracellular CoA. Unless supplementation is given, patients can develop secondary carnitine deficiency which can lead to cardiomyopathy, muscle weakness and fatigue (Waber et al. 1982). Garcia et al. (2008) described cases of untreated maternal GA1 who were not supplemented with carnitine during pregnancy and in the post-partum period. The high glutaric acid and 3-hydroxyglutaric acid concentrations did not have any impact on pregnancy outcome (Garcia et al. 2008; Kolker et al. 2006). However, low carnitine concentration has previously been shown to influence foetal maturation (Nakano et al. 1989; Oey et al. 2005). The foetus is not able to synthesise endogenous carnitine due to the lack of butyrobetaine hydroxylase, the key enzyme catalysing the carnitine biosynthesis in the liver, kidney and brain (Bremer 1983). Previous studies have shown that carnitine has been shown to be critical in foetal growth (Genger et al. 1988; Waylan et al. 2005) and foetal maturation (Areas et al. 1998). Hypoglycaemia has been associated with intrauterine growth retardation in infants (Akisu et al. 2001). In addition, carnitine deficiency develops among very low birth weight infants who do not receive exogenous carnitine supplementation (Smith et al. 1988).
Intra-familial Variability
The prevalence of GA1 in Ireland is estimated as 1 per 50,000 newborns, an estimated carrier frequency of 1:110, including a great proportion of high excretors (Naughten et al. 2004).
The phenotypic spectrum of GA1 is broad and ranges from severely affected patients to asymptomatic adults (Straus et al. 2003; Janssen et al. 2014). Our patient was diagnosed with GA1 at the age of 11 after high-risk family screening. Both sisters shared the same causal mutations and had similarly decreased enzyme activity. However, the clinical course was significantly different. Their urine organic acids at diagnosis were high (above 100 μmol/mmol creatinine) indicating a high excretor status (Boy et al. 2017b). However, both sisters were exposed to different environmental factors such as intercurrent illnesses. Examination of the published literature described intra- and inter-familial variability in clinical presentation (Straus et al. 2003).
Both sisters presented before the newborn screening for GA1 has been established in Ireland, which underscores the importance of screening siblings of affected individuals to identify potentially affected but asymptomatic cases, so they can be closely monitored to minimise the risk of acute neurological damage that can be life limiting and can impact on intellectual prognosis. This has been our experience; our patient who had been managed immediately following diagnosis had a formal psychology review that was reported as normal, and she has worked full time in a high demanding position.
Significance of Genetic Mutations Analysis
An abnormal urine organic acid and plasma acylcarnitine profile test would require further enzymology testing and subsequently GCDH gene analysis. The mutations described in our case have both been documented previously (Wang et al. 2014; Christensen et al. 2004; Biery et al. 1996; Zschocke et al. 2000). R402W was confirmed as the commonest mutation in Europeans (Zschocke et al. 2000), whereas G185R may be a ‘private mutation’ not previously described in the Irish population.
Importantly the compound heterozygosity for the R402W and G185R mutations was associated with high excretor status in our patient.
In conclusion the management of pregnancy in women affected by GA1 requires close surveillance by a multidisciplinary team. The application of emergency regimen during the peripartum period helps to ensure an uneventful clinical course of pregnancy for mother and child.
Abbreviation
- GA1
Glutaric aciduria type 1
Synopsis
Uneventful pregnancies in a patient affected with glutaric aciduria type 1.
General Rules
Details of the Contributions of Individual Authors
KMS, GP, ET conception and design, analysis and interpretation of data, drafting the chapter, revising the chapter critically for important intellectual content.
PF and IB data interpretation.
CMcC and NK data interpretation and revising the chapter critically for intellectual content.
All authors read and approved the manuscript before submission.
The Name of the Corresponding Author
Dr. Karolina M Stepien.
National Centre for Inherited Metabolic Diseases.
The Mater Misericordiae University Hospital.
Eccles Street.
Dublin, Ireland.
Tel: 0353 1 8607065.
kstepien@doctors.org.uk.
A Competing Interest Statement
KS, GP, UH, CM, PF, NK, IB and ET have no conflict of interest for this publication.
Details of Funding
N/A.
Details of Ethics Approval
N/A.
A Patient Consent Statement
Patient’s consent was obtained.
Documentation of Approval from the Institutional Committee for Care and Use of Laboratory Animals (or Comparable Committee)
N/A.
Guarantor
ET.
Contributor Information
Karolina M. Stepien, Email: kstepien@doctors.org.uk
Collaborators: Matthias Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Akisu M, Bekler C, Yalaz M, Huseynov A, Kultursay N. Free carnitine concentrations in cord blood in preterm and full-term infants with intrauterine growth retardation. Pediatr Int. 2001;43:107–108. doi: 10.1046/j.1442-200x.2001.01366.x. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynaecologists Weight gain during pregnancy. Committee Opinion no. 548. Obstet Gynecol. 2013;121:210–212. doi: 10.1097/01.AOG.0000425668.87506.4c. [DOI] [PubMed] [Google Scholar]
- Areas J, Rubio JC, Martin MA, Campos Y. Biological roles of L-carnitine inperinatal metabolism. Early Hum Dev. 1998;53:S43–S50. doi: 10.1016/S0378-3782(98)00064-4. [DOI] [PubMed] [Google Scholar]
- Biery BJ, Stein DE, Morton DH, Goodman SI. Gene structure and mutations of glutaryl-coenzyme A dehydrogenase: impaired association of enzyme subunits that is due to an A421V substitution causes glutaric academia type I in the Amish. Am J Hum Genet. 1996;59(5):1006–1011. [PMC free article] [PubMed] [Google Scholar]
- Boy N, Mühlhausen C, Maier EM, et al. Proposed recommendations for diagnosing and managing individuals with glutaric aciduria type I: second revision. J Inherit Metab Dis. 2017;40(1):75–101. doi: 10.1007/s10545-016-9999-9. [DOI] [PubMed] [Google Scholar]
- Boy N, Heringer J, Brackmann R, Bodamer O, Seitz A, Kolker S, Harting I. Extrastriatal changes in patients with late-onset glutaric aciduria type I highlight the risk of long-term neurotoxicity. Orphanet J Rare Dis. 2017;12:77. doi: 10.1186/s13023-017-0612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J. Carnitine: metabolism and functions. Physiol Rev. 1983;63:1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- Christensen E, Ribes A, Merinero B, Zschocke J. Correlation of genotype and phenotype in glutaryl-CoA dehydrogenase deficiency. J Inherit Metab Dis. 2004;27(6):861–868. doi: 10.1023/B:BOLI.0000045770.93429.3c. [DOI] [PubMed] [Google Scholar]
- Crombez EA, Cederbaum SD, Spector E, Chan E, Salazar D, Neidich J, Goodman S. Maternal glutaric academia type I identified by newborn screening. Mol Genet Metab. 2008;94:132–134. doi: 10.1016/j.ymgme.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia P, Martins E, Diogo L, Rocha H, Marcao A, Gaspar E, et al. Outcome of three cases of untreated maternal glutaric aciduria type I. Eur J Pediatr. 2008;167:569–573. doi: 10.1007/s00431-007-0556-2. [DOI] [PubMed] [Google Scholar]
- Genger H, Enzelsberger H, Salzer H. Carnitine in therapy of placental insufficiency: initial experiences. Z Gebustshilfe Perinatol. 1988;192:155–157. [PubMed] [Google Scholar]
- Goodman SI, Markey SP, Moe PG, Miles BS, Teng CC. Glutaric aciduria: a ‘new’ disorder of amino acid metabolism. Biochem Med. 1975;12:12–21. doi: 10.1016/0006-2944(75)90091-5. [DOI] [PubMed] [Google Scholar]
- Haworth JC, Booth FA, Chudley AE. Phenotypic variability in glutaric aciduria type 1: report of 15 cases in five Canadian kindreds. J Pediatr. 1991;118:52–58. doi: 10.1016/S0022-3476(05)81843-8. [DOI] [PubMed] [Google Scholar]
- Institute of Obstetricians and Gynaecologists, Royal College of Physicians of Ireland and Directorate of Clinical Strategy and Programmes, Health Service Executive (2013) Nutrition for pregnancy. www.rcpi.ie
- Ituk US, Allen TK, Habib AS. The peripartum management of a patient with glutaric aciduria type 1. J Clin Anesth. 2013;25:141–145. doi: 10.1016/j.jclinane.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Jafari P, Braissant O, Bonafe L, Ballhausen D. The unsolved puzzle of neuropathogenesis in glutaric aciduria type I. Mol Genet Metab. 2011;104:425–437. doi: 10.1016/j.ymgme.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Janssen MCH, Kluijtmans LAJ, Wortmann SB. Screening of a healthy newborn identifies three adult family members with symptomatic glutaric aciduria type I. BBA Clin. 2014;1:30–32. doi: 10.1016/j.bbacli.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolker S. Glutaric aciduria type I. In: Hollak C, Lachmann R, editors. Inherited metabolic disease in adults. Oxford: Oxford University Press; 2016. [Google Scholar]
- Kolker S, Garbade CR, Greenberg CR, Leonard JV, Saudubray JM, Ribes A, et al. Natural history, outcome, and treatment efficacy in children and adults with glutaryl-CoA dehydrogenase deficiency. Pediatr Res. 2006;59:840–847. doi: 10.1203/01.pdr.0000219387.79887.86. [DOI] [PubMed] [Google Scholar]
- Kolker S, Christensen E, Leonard JV, Greenberg CR, Boneh A, Burlina AB, Dixon M, Duran M, Garcia Cazorla A, Goodman SI, Koeller DM, Kyllerman M, et al. Diagnosis and management of glutaric aciduria type I – revised recommendations. J Inherit Metab Dis. 2011;34(3):677–694. doi: 10.1007/s10545-011-9289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosaeilhy A, Mohamed MM, C GPD. El Abd HS, Gamal R, Zaki OK, Zayed H. Genotype-phenotype correlation in 18 Egyptian patients with glutaric acidemia type I. Metab Brain Dis. 2017;32:1417–1426. doi: 10.1007/s11011-017-0006-4. [DOI] [PubMed] [Google Scholar]
- Muhlhausen C, Ergun S, Strauss KA, Koeller DM, Crnic L, Woontner M, Goodman SI, Ullrich K, Braulke T. Vascular dysfunction as an additional pathomechanism in glutaric aciduria type I. J Inherit Metab Dis. 2004;27:829–834. doi: 10.1023/B:BOLI.0000045766.98718.d6. [DOI] [PubMed] [Google Scholar]
- Nakano C, Takashima S, Takeshita K. Carnitine concentration during the development of human tissues. Early Hum Dev. 1989;19:21–27. doi: 10.1016/0378-3782(89)90101-1. [DOI] [PubMed] [Google Scholar]
- Naughten ER, Mayne PD, Monavari AA, Goodman SI, Sulaiman G, Croke DT. Glutaric Aciduria type I, outcome in the Republic of Ireland. J Inherit Metab Dis. 2004;27:917–920. doi: 10.1023/B:BOLI.0000045777.82784.74. [DOI] [PubMed] [Google Scholar]
- Oey NA, den Boer ME, Wijburg FA, Vekemans M, Auge J, et al. Long-chain fatty acid oxidation during early human development. Pediatr Res. 2005;57:755–759. doi: 10.1203/01.PDR.0000161413.42874.74. [DOI] [PubMed] [Google Scholar]
- Prevett MC, Howard RS, Dalton RN, Olpin SE. Glutaric aciduria type 1 in adulthood. J Neurol Neurosurg Psychiatry. 1996;60:352–353. doi: 10.1136/jnnp.60.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RB, Sachan DS, Plattsmier J, Feld N, Lorch V. Plasma carnitine alterations in premature infants receiving various nutritional regimes. JPEN J Parenter Enteral Nutr. 1988;12:37–42. doi: 10.1177/014860718801200137. [DOI] [PubMed] [Google Scholar]
- Straus KA, Puffenberger EG, Robinson DL, Morton DH. Type I glutaric aciduria, part 1: natural history of 77 patients. Am J Hum Genet. 2003;121C:38–52. doi: 10.1002/ajmg.c.20007. [DOI] [PubMed] [Google Scholar]
- Treacy EP, Lee-Chong A, Roche G, Lynch B, Ryan S, Goodman SI. Profound neurological presentation resulting from homozygosity for a mild glutaryl-CoA dehydrogenase mutation with a minimal biochemical phenotype. J Inherit Metab Dis. 2003;26:72–74. doi: 10.1023/A:1024087832406. [DOI] [PubMed] [Google Scholar]
- Waber LJ, Valle D, Neill C, DiMauro S, Shug A. Carnitine deficiency presenting as familial cardiomyopathy: a treatable defect in carnitine transport. J Pediatr. 1982;101:700–705. doi: 10.1016/S0022-3476(82)80294-1. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li X, Ding Y, Liu Y, Song J, Yang Y. Clinical and mutational spectra of 23 Chinese patients with glutaric aciduria type 1. Brain and Development. 2014;36(9):813–822. doi: 10.1016/j.braindev.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Waylan AT, Kayser JP, Gnad DP, Higgins JJ, Starkey JD, Sissom EK, Woodworth JC, Johnson BJ. Effects of L-carnitine on foetal growth and the IGF system in pigs. J Anim Sci. 2005;83:1824–1831. doi: 10.2527/2005.8381824x. [DOI] [PubMed] [Google Scholar]
- Zschocke J, Quak E, Guldberg P, Hoffmann GF. Mutation analysis in glutaric aciduria type I. J Med Genet. 2000;37:177–181. doi: 10.1136/jmg.37.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]